Abstract
Hydroxyurea improves hematologic parameters for children with sickle cell disease (SCD), but its long-term efficacy at maximum tolerated dose (MTD) has not been determined. Between 1995 and 2002, hydroxyurea therapy was initiated for 122 pediatric patients with SCD including 106 with homozygous sickle cell anemia (HbSS), 7 with sickle hemoglobin C (HbSC), 7 with sickle/β-thalassemia (HbS/ β-thalassemia [6 HbS/β0, 1 HbS/β+]), and 2 with sickle hemoglobin OArab (HbS/OArab). Median age at initiation of therapy was 11.1 years. Hydroxyurea was escalated to MTD, with an average dose of 25.4 ± 5.4 mg/kg per day; the average duration of hydroxyurea therapy has been 45 ± 24 months (range, 6-101 months). Hydroxyurea was discontinued for 15 (12%) children with poor compliance. Mild transient neutropenia occurred, but no hepatic or renal toxicity was noted. Hydroxyurea therapy led to significant increases in hemoglobin level, mean corpuscular volume, and fetal hemoglobin (HbF) level, whereas significant decreases occurred in reticulocyte, white blood cell, and platelet counts and serum bilirubin levels. Children with variant SCD genotypes also had hematologic responses to hydroxyurea. HbF induction has been sustained for up to 8 years without adverse effects on growth or increased numbers of acquired DNA mutations. Long-term hydroxyurea therapy at MTD is well tolerated by pediatric patients with SCD and has sustained hematologic efficacy with apparent long-term safety.
Introduction
Low levels of fetal hemoglobin (HbF) in patients with sickle cell anemia (SCA) are associated with a variety of vaso-occlusive complications and an increased risk for early death.1-3 Hydroxyurea is the prototype of drugs known to induce HbF, because it is administered orally with once-daily dosing, has no immediate adverse effects, and is generally effective for patients with SCA.4 Hydroxyurea enhances HbF parameters, including the percentage HbF and percentage F cells, in children and adults with SCA.5-8 In adults, hydroxyurea is well tolerated and leads to increases in hemoglobin concentration, mean corpuscular volume (MCV), and percentage HbF and to decreases in white blood cell and reticulocyte counts.5 The phase 1/2 Pediatric Hydroxyurea Group (HUG-KIDS) trial of hydroxyurea therapy in school-aged children with SCA demonstrated safety and hematologic efficacy similar to that seen in adults.7 In a randomized, double-blind, placebo-controlled phase 3 trial involving severely affected adults with SCA, hydroxyurea therapy was associated with significantly fewer painful vaso-occlusive events, episodes of acute chest syndrome, erythrocyte transfusions, and hospitalizations.6 Clinical efficacy of hydroxyurea therapy in children has also been reported in several small series.9-13
The short-term toxicities of hydroxyurea treatment in patients with SCA include primarily transient and reversible myelosuppression, typically mild neutropenia. Minimal, if any, renal or hepatic toxicity has been observed.5,7 Importantly, the myelosuppression and HbF response to hydroxyurea are dose dependent,5 such that escalating hydroxyurea to the maximum tolerated dose (MTD) may be important for maximizing the effects of hydroxyurea therapy.5,7,8 In the HUG-KIDS trial, children who tolerated higher MTDs achieved significantly higher percentage HbF levels, which were associated with greater treatment-associated decreases in white blood cell (WBC) and reticulocyte counts and greater increases in hemoglobin concentration and MCV.8 A critical but unanswered question is whether escalating hydroxyurea therapy to the MTD is necessary to achieve a sustained clinical and hematologic response. In most US studies of hydroxyurea therapy for patients with SCA, the hydroxyurea dose has been increased to MTD, typically defined as a maximum of 30 to 35 mg/kg per day.5-8 In contrast, European reports describe hydroxyurea responses at lower doses of 20 to 25 mg/kg per day, with no attempt to achieve MTD.10,12,14
The issues of sustained response and safety with long-term hydroxyurea therapy have also not been resolved. In adults with SCA treated with hydroxyurea, marrow exhaustion has been proposed as the explanation for HbF responses that decline over time,15 but this phenomenon may not occur in pediatric patients treated with hydroxyurea.7 Leukemia has not developed in adults with SCA taking hydroxyurea for up to 9 years.16 Furthermore, long-term in vivo exposure to hydroxyurea was not associated with an increased number of acquired DNA mutations.17
In a recently published report from the Multicenter Study of Hydroxyurea in Sickle Cell Anemia (MSH) trial, adult patients treated with hydroxyurea had a 40% reduction in overall mortality after 9 years of follow-up,16 suggesting that more young patients should be considered candidates for hydroxyurea therapy. The current analysis was undertaken to provide additional data on the long-term use of hydroxyurea in sickle cell disease (SCD), particularly regarding sustained hematologic efficacy and safety in pediatric patients. Between 1995 and 2002, the Duke Pediatric Sickle Cell Program has treated 122 pediatric SCD patients with hydroxyurea. We describe the long-term, sustained hematologic efficacy of hydroxyurea when prescribed at MTD—with minimal toxicity—in this large cohort of pediatric patients.
Patients, materials, and methods
Indications for hydroxyurea therapy
Between 1995 and 2002, pediatric patients followed in the Duke Pediatric Sickle Cell Program were offered hydroxyurea therapy. Initially, only patients with severe sickle-related clinical events were eligible to receive hydroxyurea as part of an institutional review board (IRB)–approved research protocol; Duke patients were included in the multicenter phase 1/2 HUG-KIDS safety trial of hydroxyurea in children7 and later in the phase 1/2 Hydroxyurea Study of Organ Function and Toxicity (HUSOFT) infant pilot trial.18 Another IRB-approved institutional protocol allowed the use of hydroxyurea and phlebotomy to prevent secondary stroke in children with SCA and previous stroke who are unable to continue chronic erythrocyte transfusion prophylaxis.19 Over time, additional patients with SCD have been offered hydroxyurea based on clinical indications of disease severity, including recurrent or severe vaso-occlusive painful events or episodes of acute chest syndrome. Moreover, patients with variant sickle genotypes, including HbSC, HbS/OArab, or HbS/β+thalassemia, have also been considered eligible for hydroxyurea therapy if the clinical course of disease was severe. The cohort included in this analysis of long-term efficacy of hydroxyurea includes all Duke pediatric SCD patients for whom hydroxyurea therapy was initiated for any reason and who continued therapy for at least 6 months. Patients receiving therapy for less than 6 months were not included because the hydroxyurea dose had not yet been escalated to MTD.
Prehydroxyurea evaluation
Before the initiation of hydroxyurea therapy, all patients underwent baseline laboratory evaluation that included a complete blood count with manual WBC differential, reticulocyte count, and serum chemistry profiles including alanine aminotransferase (ALT) and creatinine. Serologies to parvovirus B19, HIV, and hepatitis A, B, and C were obtained, along with serum ferritin, folate, and vitamin B12 levels and baseline percentage HbF.
Several members of the Duke Pediatric health care team met with each family on at least 2 different occasions to discuss reasons for starting hydroxyurea therapy. The risks, benefits, and adverse effects of hydroxyurea, including the unknown risk for malignancy with long-term treatment, were presented. In addition, the importance of strict compliance with prescribed daily medication and follow-up visits with blood counts at least every 1 to 2 months was stressed. If there were concerns about a family's ability to comply with the treatment regimen, hydroxyurea therapy was not started. For females of childbearing potential, use of contraception or monthly serum pregnancy tests during hydroxyurea therapy was strongly encouraged.
Hydroxyurea dose escalation to MTD
For children in the HUG-KIDS trial, the oral dose of hydroxyurea started at 15 mg/kg per day and was escalated every 8 weeks to the MTD or a maximum dose of 30 mg/kg per day, with careful monitoring of blood counts every 2 weeks. If hematologic toxicity occurred twice at the same dose, the MTD was set at 2.5 mg/kg below the toxic dose.7 Infants enrolled in the HUSOFT trial received 20 mg/kg per day for 2 years, then had dose escalation to 30 mg/kg per day or MTD.18 For all other patients in this cohort, oral hydroxyurea was started at 15 to 20 mg/kg per day, either as a capsule or as a liquid preparation (100 mg/mL) given as a single daily dose, usually at bedtime. The hydroxyurea dose was then increased every 8 weeks as tolerated to the MTD, defined as an absolute neutrophil count (ANC) of approximately 2.0 × 109/L. Irrespective of clinical improvement, the hydroxyurea dose was increased to MTD with a maximum dose of 30 mg/kg per day. In a few patients with good compliance, whose ANCs remained above 4.0 × 109/L with relatively low HbF responses, the hydroxyurea dose was increased as high as 35 mg/kg per day. For all patients, the hydroxyurea dose was periodically adjusted for weight gain.
During the dose-escalation phase, patients were evaluated monthly until MTD was reached, then subsequently every 2 months after reaching a stable hydroxyurea dose. Laboratory monitoring during hydroxyurea therapy included a complete blood count with WBC differential and reticulocyte count at each visit and periodic monitoring of serum chemistry profiles with liver function tests and percentage HbF. Compliance was assessed by history taking and laboratory test results, including review of the peripheral blood smear, and suggestions were offered to improve compliance.
Toxicity and dose adjustments
For children who participated in the HUG-KIDS trial, hematologic toxicity was defined by a hemoglobin concentration less than 5 g/dL or a 20% decline from baseline, an absolute reticulocyte count less than 80 × 109/L unless the hemoglobin concentration was more than 9 g/dL, an ANC less than 2.0 × 109/L, or a platelet count less than 80 × 109/L.7 Similar parameters were used for the HUSOFT study except that an ANC greater than 1.5 × 109/L was acceptable to continue hydroxyurea therapy.18 Over time, toxicity thresholds for patients treated with hydroxyurea outside these 2 research studies have been relaxed, and patients on stable MTDs were occasionally allowed to have ANCs as low as 1.0 to 1.5 × 109/L without stopping therapy. Other nonhematologic toxicity thresholds included ALT twice the upper limit of normal for age or serum creatinine twice the baseline value and greater than 1.0 mg/dL. If there was evidence of drug toxicity, hydroxyurea was stopped for 1 week, then restarted at the previous dose after count recovery.
Data collection
Baseline demographic data including age, sex, diagnosis, and laboratory values were abstracted from clinical charts and entered into an Access database (Microsoft Access; Microsoft, Bellevue, WA). Baseline values were recorded for patients on chronic transfusion therapy for a history of stroke only if data were available before the transfusion regimen was initiated. For ongoing and long-term follow-up of the effects of hydroxyurea therapy, similar data, including weight and height, hydroxyurea dose, laboratory parameters, and duration of hydroxyurea therapy, were collected on an annual basis. Data were collected and analyzed for all patients, including those who initially responded to hydroxyurea but were subsequently noncompliant. Data to document the numbers and types of individual vaso-occlusive clinical events before and after hydroxyurea therapy were not recorded prospectively.
In vitro assay of acquired DNA mutations
To assess the potential mutagenic effects of long-term hydroxyurea therapy, the frequency of “illegitimate” interlocus VDJ recombination events between the T-cell–receptor γ (TCR-γ) variable gene and the TCR-β junctional gene loci on chromosome 7 was measured in patients with SCD and prolonged hydroxyurea exposure. As previously described,17 genomic DNA was obtained from peripheral blood mononuclear cells, and a 2-step nested polymerase chain reaction (PCR) protocol was used to amplify rare gene rearrangements between these TCR regions. PCR products were resolved on a 6% polyacrylamide gel and exposed to x-ray film overnight; by analysis of serial dilutions of DNA, scoring was based on the minimum amount of DNA necessary to yield a positive band.17
Statistical analysis
Descriptive statistics were performed using the Primer of Biostatistics (McGraw-Hill, New York, NY). The Student t test was used to compare means between groups, and linear regression models were used to describe the correlations between baseline variables and percentage HbF (Microsoft Excel; Microsoft). No adjustments were made for multiple comparisons.
Results
Patient characteristics
Between 1995 and 2002, a total of 122 pediatric patients with SCD monitored by the Duke Pediatric Sickle Cell Program were treated with hydroxyurea for at least 6 months, including 106 patients with HbSS, 7 with sickle hemoglobin C (HbSC), 7 with sickle/β-thalassemia (HbS/β-thalassemia [6 HbS/β0, 1 HbS/β+]), and 2 with sickle hemoglobin OArab (HbS/OArab). All patients were treated with hydroxyurea to ameliorate the signs and symptoms of sickle cell disease, including 15 children with recurrent vaso-occlusive events who were enrolled in the phase 1/2 HUG-KIDS safety trial, 7 infants in the phase 1/2 HUSOFT infant pilot trial, 33 patients with previous stroke for the prevention of stroke recurrence, and 67 patients treated outside these research studies.
A total of 71 males and 51 females were treated, with a median age at initiation of hydroxyurea therapy of 11.1 years (range, 0.5-19.7 years). Patients received hydroxyurea therapy for an average duration of 45 ± 24 months (range, 6-101 months), with an average hydroxyurea dose of 25.4 ± 5.4 mg/kg per day. Of the 122 patients, 21 (17%) received hydroxyurea doses higher than 30 mg/kg per day (median dose, 31.1 mg/kg per day; range, 30.1-36.1 mg/kg per day). No patient was found to be deficient in iron, folate, or vitamin B12 before the start of therapy. Similarly, no unexpected infectious serologic exposures were identified. Parvovirus immunoglobulin G (IgG) antibodies indicating past exposure were found in more than 50% of patients, as we recently reported.20
Hematologic effects of hydroxyurea therapy
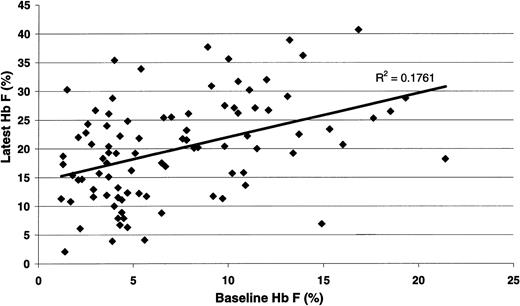
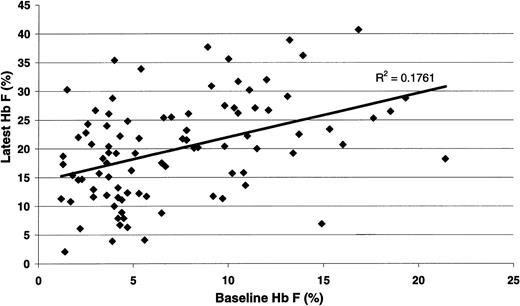
For all 122 Duke pediatric patients with SCD (Table 1), the hematologic efficacy of hydroxyurea when prescribed at MTD included highly significant increases in hemoglobin concentration, MCV, and percentage HbF. All patients responded to hydroxyurea therapy; there were no nonresponders. Significant decreases in reticulocyte count, WBC count, ANC, platelet count, and serum bilirubin concentration were also noted. The HbF response to hydroxyurea therapy at MTD (Figure 1) was significantly associated with baseline percentage HbF (R = 0.419; P < .001). When the data were analyzed using change in percentage HbF as the outcome variable, the correlation between MTD and change in percentage HbF approached significance (P = .056) (data not shown).
Statistical association between the percentage HbF value at baselineand at hydroxyurea MTD. There was a significant positive correlation between the baseline value and the treatment percentage HbF (R = 0.419; P < .001).
Statistical association between the percentage HbF value at baselineand at hydroxyurea MTD. There was a significant positive correlation between the baseline value and the treatment percentage HbF (R = 0.419; P < .001).
The hematologic effects of hydroxyurea at MTD were then analyzed according to SCD genotype (Table 2). Although most patients had HbSS, the 16 children with other sickle cell variants also had excellent hematologic responses to hydroxyurea therapy. In patients of all sickle genotypes, MCV and percentage HbF increased from baseline, with MTD HbF values of approximately 20% in all patients except those with HbSC disease. Patients with severe forms of SCD (HbSS, HbS/β0, HbS/OArab) had significant increases in hemoglobin concentration, whereas the 8 with HbSC or HbS/β+ had minimal changes in hemoglobin values. Hydroxyurea treatment led to decreases in the rate of hemolysis for all genotypes, as demonstrated by significantly lower reticulocyte counts and lower total serum bilirubin concentrations. Predictable myelosuppression occurred as well, with significant decreases in WBC and ANC values. In all patients, the hydroxyurea dose was increased to MTD to achieve modest myelosuppression, but the change from baseline WBC count and ANC was greatest in those with severe forms of SCD.
The 7 patients with HbSC disease tolerated lower doses of hydroxyurea before reaching MTD, as we previously reported.21 Perhaps in part because of this dose-limiting myelosuppression, patients with HbSC disease had lower maximal percentage HbF responses than patients with other genotypes. However, the change from baseline values was still highly significant, with an average increase in percentage HbF from 1.8% ± 0.7% to 8.6% ± 3.1% (P < .001).
Short-term toxicities of hydroxyurea therapy
The primary toxicity observed in our patients was mild, reversible bone marrow suppression, but the thresholds for toxicity occurred in only approximately 5% of blood counts (data not shown). Usually mild neutropenia was observed, which recovered within one week of temporary discontinuation of hydroxyurea treatment. Anemia and thrombocytopenia as toxicities of hydroxyurea were more rare. Among the 7 children with HbSC disease, there were 3 episodes of transient thrombocytopenia (less than 80 × 109/L) that required temporary discontinuation of hydroxyurea therapy over a total of 24.3 patient-years. Among the 7 children with HbS/β-thalassemia, there were 4 episodes of thrombocytopenia over a total of 20.3 patient-years, all occurring in the same patient. There were no episodes of platelet toxicity in the 21 HbSS patients who received more than 30 mg/kg per day of hydroxyurea. After a patient reached MTD, hydroxyurea dose reduction for recurrent myelosuppression was rarely required.
Gastrointestinal irritation from hydroxyurea was occasionally described, but this adverse effect almost always resolved when the dose was given at bedtime. No renal or hepatic toxicity based on serum creatinine, ALT, or bilirubin measurement was noted. As we previously reported,22 melanonychia or other changes in skin pigmentation were observed in approximately 10% of patients, but no alopecia or hair loss was noted. These skin and nail changes were mild and did not lead to the discontinuation of hydroxyurea therapy for any patient.
Long-term effects of hydroxyurea therapy
To determine whether the hematologic efficacy of hydroxyurea was sustained over time, annual laboratory values for the children in this cohort were then analyzed (Table 3). The hematologic effects of hydroxyurea were maintained over 7 years of follow-up. At year 1, there were significant increases in hemoglobin concentration, MCV, and percentage HbF and significant decreases in WBC count, ANC, reticulocyte count, and serum bilirubin concentration. These changes were then maintained from year 1 through year 7 (P = NS for each year-to-year comparison). The mean percentage HbF values were sustained at approximately 20%, even after 7 years of hydroxyurea therapy.
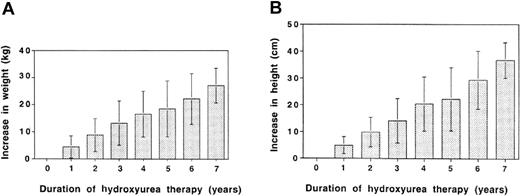
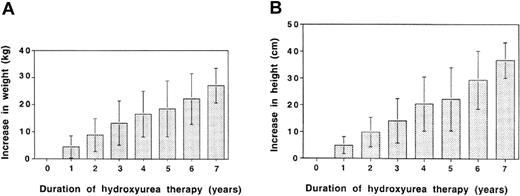
Serial measurements of weight and height showed no adverse effects of hydroxyurea therapy at MTD on growth. As shown in Figure 2, our cohort of patients had ongoing increases in weight (panel A) and height (panel B) with up to 7 years of follow-up. The range of annual increases was broad because some patients were older and were past puberty when they started hydroxyurea therapy, whereas others were very young and experienced rapid rates of growth. On average, however, weight increased by about 3 to 4 kg per year, and height increased by 5 to 7 cm per year, indicating no adverse effects of hydroxyurea at MTD on growth in these patients. Indeed, many children experienced improved growth while on hydroxyurea therapy; some even crossed percentiles.
Growth parameters in 122 pediatric patients with SCD during 7 years of hydroxyurea therapy. (A) Change in weight (kg) and (B) change in height (cm) in our cohort of patients. Because some patients were already pubertal when hydroxyurea therapy was initiated, values for weight and height ranged widely. However, there was no evidence of adverse effects on growth rates; rather, steady increases were observed during the 7 years of therapy. Error bars represent standard deviation.
Growth parameters in 122 pediatric patients with SCD during 7 years of hydroxyurea therapy. (A) Change in weight (kg) and (B) change in height (cm) in our cohort of patients. Because some patients were already pubertal when hydroxyurea therapy was initiated, values for weight and height ranged widely. However, there was no evidence of adverse effects on growth rates; rather, steady increases were observed during the 7 years of therapy. Error bars represent standard deviation.
To address the issues of mutagenicity and potential carcinogenicity associated with long-term hydroxyurea therapy, acquired somatic mutations were measured using the highly sensitive and quantitative VDJ assay, as previously described.17 In 26 of 34 patients with SCD who had 5 years of in vivo exposure to hydroxurea at MTD, paired testing was performed and revealed no increase in the average number of acquired illegitimate VDJ rearrangements detected (Table 4). No child on hydroxyurea therapy acquired myelodysplasia, leukemia, or any other malignancy.
Compliance with hydroxyurea therapy was an important focus during follow-up clinic visits. At each visit, patients and families were asked about adherence to the daily dosing regimen. In addition, patients were asked to return medication bottles for pill counts. Using laboratory values such as MCV, percentage HbF, and WBC and review of the peripheral smear for the presence of sickled forms, clinical evidence of compliance was also assessed at each visit. In 15 (12%) children, hydroxyurea therapy was discontinued by health care providers because of poor compliance. Children who were declared noncompliant had initial hematologic responses to hydroxyurea therapy, but then their laboratory profiles reverted toward baseline values.
Seventy-six of the 122 patients are still receiving hydroxyurea therapy at MTD and are monitored through the Duke Pediatric Sickle Cell Program. Another 17 patients are no longer on hydroxyurea therapy; 15 were the noncompliant patients for whom hydroxyurea therapy was discontinued, and 2 patients died. The remaining 29 patients have transferred from the Duke Pediatric Program; 19 were transitioned to the Adult Service, and 10 moved to other programs.
Serious adverse events during hydroxyurea therapy
Few serious adverse events were noted in this cohort of patients during 455 patient-years of follow-up. No strokes occurred in the 89 children who started hydroxyurea therapy without a history of previous stroke. Recurrent stroke did occur in several patients who had a history of stroke, as previously described.19 No child on hydroxyurea experienced acute chest syndrome requiring exchange transfusion or mechanical ventilation. Similarly, no child developed a serious infection, including bacteremia, in association with neutropenia.
Two children on hydroxyurea therapy died during the follow-up period. One 4-year-old girl with HbSS disease died of pneumococcal sepsis. She received hydroxyurea from age 6 months as part of the HUSOFT infant pilot trial18 and underwent elective splenectomy at 2 years of age after an acute, life-threatening splenic sequestration crisis. Her death occurred before the availability of pneumococcal 7-valent conjugate vaccine (Prevnar; Wyeth Pharmaceuticals, Philadelphia, PA), but she was on penicillin prophylaxis and had received the polysaccharide pneumococcal vaccine before splenectomy. A routine blood count in the clinic 2 days before she died revealed a hemoglobin concentration of 9.6 g/dL, WBC 9.8 × 109/L, ANC 4.1 × 109/L, and platelet count 584 × 109/L. She had a fever the evening before her death, and she was taken to the local emergency room after collapsing at home. Diplococci were noted on the peripheral blood smear, and, despite an adequate neutrophil count and prompt administration of intravenous antibiotics, she died of presumed pneumococcal sepsis. Postmortem examination was otherwise unrevealing.
The second child who died while receiving hydroxyurea therapy was a heavily alloimmunized 15-year-old boy with HbSS disease who received hydroxyurea for 5.4 years as part of the phase 1/2 HUG-KIDS safety trial.7 He had excellent clinical and hematologic response to hydroxyurea. His family relocated from the Duke Pediatric Sickle Cell Program in October 2000, and local providers reportedly continued hydroxyurea therapy. Eight months later, by his father's report, the patient received 2 erythrocyte transfusions locally to improve the healing of a cut on his foot. He died of complications, including disseminated intravascular coagulation and massive hemorrhage, resulting from an acute transfusion reaction.
Benefits of escalating hydroxyurea therapy to MTD
To determine the effects of escalating the hydroxyurea dose to MTD regardless of clinical response, hematologic results from the Duke cohort of children with sickle cell anemia were compared with results, reported by Ferster et al,12 of patients from the Belgian Registry. The primary difference between the 2 cohorts was that the hydroxyurea doses of Duke patients were increased to MTD, whereas the Belgian patients received 20 to 25 mg/kg or less per day.12 At baseline, there were no significant differences in hemoglobin concentration, MCV, or percentage HbF, but throughout 5 years of hydroxyurea therapy, Duke patients had significantly higher values (Table 5). Although not statistically different each year, the ANC was also lower in Duke patients whose hydroxyurea doses were escalated to MTD.
Discussion
Previous studies of hydroxyurea in patients with SCA have documented its usefulness for increasing HbF parameters, improving hematologic values, and ameliorating clinical severity.5-14,18 For some adults with SCA, however, the effectiveness of hydroxyurea has not been sustained, raising questions of compliance or the possibility of marrow exhaustion.15 Our large cohort of pediatric patients includes some children who have been on hydroxyurea therapy for up to 8 years, contributing to the cohort total of 455 patient-years of follow-up. Hematologic benefits of hydroxyurea therapy at MTD include significant increases in hemoglobin concentration, MCV, and HbF parameters (Table 1) that appear to be long-lasting (Table 3). HbF levels were maintained at approximately 20%, a common therapeutic goal and threshold level suggested to provide clinical benefit.23 We did not observe any statistically significant declines in HbF or other hematologic parameters to support evidence of marrow exhaustion.
Our cohort also included pediatric patients with genotypic variants of SCD, such as HbSC, HbS/β-thalassemia, and HbS/OArab. All responded to hydroxyurea therapy and experienced improvements in hemoglobin concentration, MCV, and percentage HbF. These data suggest that at least some patients with variant forms of SCD may be able to respond to hydroxyurea therapy with hematologic and clinical improvement. For example, the 6 patients with HbS/β0 responded to hydroxyurea therapy with similar increases in hemoglobin concentration, MCV, and percentage HbF as seen in patients with HbSS. Because there was only one patient with HbS/β+ in the cohort, it is difficult to comment on the changes in hematologic parameters in this genotype. Her baseline hemoglobin concentration was relatively high and did not change much in response to hydroxyurea therapy, but her MCV increased from 69 fL to 86 fL, her percentage HbF increased from 11.5% to 17.1%, and her clinical response to treatment was remarkable. For the 7 patients with HbSC disease, MTD was achieved at a lower dose of hydroxurea (19.6 ± 4.2 mg/kg per day) than it was in patients with other sickle genotypes. Persistent splenomegaly with relative hypersplenism and WBC and platelet sequestration might have contributed to this lower MTD, but patients with HbSC also have lower baseline WBC counts, ANCs, and platelet counts than children and adults with HbSS. The maximum percentage HbF was lower in patients with HbSC but was still increased significantly from starting values. This is in contrast to the experience in adults with HbSC, in whom hemoglobin concentration and MCV increased in response to hydroxyurea therapy, but the percentage HbF did not statistically change from baseline values, and patients did not have clinical responses.24
In some previous studies, hydroxyurea dosing has been escalated based on acceptable laboratory parameters and clinical response, with no deliberate attempt to achieve myelosuppression as a treatment goal. In our large cohort of pediatric patients with SCD, we increased hydroxyurea therapy to a predefined MTD, even after a clinical response was observed. Based on a comparison between our patients and those reported by Ferster et al,12 dose escalation to MTD, as defined by mild neutropenia, resulted in significantly better hematologic parameters (Table 5). Our HbF values at MTD were sustained at 20% on average and were significantly higher at each year of therapy, despite potential differences in HbF measurement techniques. Determining the clinical significance of this difference in percentage HbF would require a prospective, randomized, controlled trial. The potential benefits of higher HbF, however, seem to outweigh the risk from mild neutropenia in this patient population. Indeed, lowering the WBC count and the ANC may be an appropriate therapeutic goal of hydroxyurea treatment for children with SCD.
Compliance with hydroxyurea therapy is an important part of achieving a sustained hematologic effect in patients with SCD. In addition to careful assessment of the family's commitment to hydroxyurea therapy at baseline, we provided ongoing clinical assessments based on laboratory parameters, review of the peripheral blood smear, and pill counts when possible. All patients initially responded to hydroxyurea therapy, but some lost that response because of noncompliance with the treatment regimen. Achieving good compliance with long-term hydroxyurea therapy requires a coordinated effort by the medical team and frequent contact with patients and families to provide support and encouragement. Of the 122 patients, hydroxyurea therapy was discontinued because of noncompliance in only 12%. Adolescents and young adults were particularly at risk and were the most likely to discontinue hydroxyurea therapy despite parental or medical advice to the contrary.
Our data support the long-term efficacy of hydroxyurea therapy in pediatric patients with SCD, but concerns over long-term safety, particularly in young children who require indefinite treatment with hydroxyurea therapy, remain incompletely answered. The lack of serious shortor long-term toxicities in our cohort—with more than 450 patient-years of follow-up—is encouraging. Furthermore, children receiving hydroxyurea therapy had sustained or improved growth (Figure 2), possibly because therapy led to fewer clinical complications with resultant effects on nutritional intake and metabolism. Using weight and height data from the Cooperative Study of Sickle Cell Disease (CSSCD), the growth rate for children with HbSS can be approximated.23 Considering the 8- to 13-year age range, which approaches the mean age of 11.1 years in our patients, the children in our cohort had growth rates exceeding those of children with HbSS and resembling those of healthy children. To date, several groups of children treated with hydroxyurea have not shown any adverse effects on growth.7,9,18,26 In the HUG-KIDS trial, significant increases in weight and height were noted at each 6-month interval,7 but follow-up extended only to 18 months. A formal statistical analysis of the growth rate of children with SCA on hydroxyurea therapy, compared with those enrolled in the CSSCD, has recently been published.27 That analysis documented that hydroxyurea had no adverse effects on growth in a 2-year period, and our current data extend those findings. Patients in our cohort had sustained growth for up to 7 years, and they achieved puberty normally during treatment with hydroxyurea. The potential carcinogenicity of hydroxyurea is more difficult to address, but our in vitro data document no increase in acquired mutations (Table 4). In the adult MSH follow-up report, no cases of leukemia have been observed in up to 9 years of follow-up,16 and we have had no cases of malignancy in our patient population. The true answer regarding the risk for carcinogenicity will require long-term follow-up of large numbers of patients with SCD treated with hydroxyurea. Until then, pediatric hematologists should remain cautious about the use of hydroxyurea in children with SCD.
The recently published long-term clinical efficacy report of hydroxyurea, which indicates a 40% reduction in mortality in patients receiving hydroxyurea,16 suggests that hydroxyurea should be considered for all patients with SCD. The lack of significant short- or long-term toxicity in our large cohort of patients with SCD provides further support for this recommendation. Our data also support escalating the dose of hydroxyurea to MTD to achieve maximum and sustained beneficial hematologic effects on percentage HbF and WBC count. The potential for using long-term hydroxyurea therapy to reduce the morbidity and mortality of all children with sickle cell disease requires additional and careful investigation, but hydroxyurea currently provides the best available strategy to achieve hematologic and clinical improvements in the disease.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-07-2475.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Thomas R. Kinney for his involvement in organizing the HUG-KIDS study, in which the first Duke pediatric patients were treated with hydroxyurea therapy, and Shannon F. MacKeigan for providing excellent clinical care to many of these patients.