Abstract
Vaccination with antigen-presenting cells (APCs) engineered to mimic mechanisms of immune stimulation represents a promising approach for cancer immunotherapy. Dendritic cell vaccines have entered phase 3 testing in adult malignancies, but such vaccines in children have been limited. We demonstrate that CD40-activated B cells (CD40-B) transfected with RNA may serve as an alternative vaccine that can be generated from small blood volumes regardless of patient age. CD40-B from pediatric patients are efficient APCs and can be loaded with RNA as an antigenic payload, permitting simultaneous targeting of multiple antigenic epitopes without the necessity of HLA matching. For viral and tumor antigens, CD40-B/RNA technology induced cytotoxic T lymphocytes (CTLs) from adults and children, which could be identified with peptide/major histocompatibility complex (MHC) tetramers. These CTLs secreted interferon-γ (IFN-γ) and killed targets in an MHC-restricted fashion. For pooled neuroblastoma RNA and autologous neuroblastoma RNA, CTLs that lysed neuroblastoma cell lines, including CTLs specific against the widely expressed tumor-antigen survivin, were generated. These findings support a novel platform for tumor-specific vaccine or adoptive immunotherapies in pediatric malignancies.
Introduction
The elucidation of cellular immune mechanisms that prevent and control infection and malignancy has long inspired efforts to design and deliver optimized versions of these cell-based processes to patients. Despite divergent strategies and indications, it remains the fundamental hypothesis of cell-based immunotherapy that ex vivo manipulation and reinjection of cellular products can overcome elements of immune incompetence preexisting in the host to achieve clinical responses. Physiologically, dendritic cells (DCs) assume primary cellular responsibility for T-cell priming,1 and they have been increasingly exploited as a delivery modality for vaccination strategies in cancer and other viral diseases.2 In clinical oncology, ex vivo generation and antigen loading of autologous DCs as vaccines have been used in trials of adult patients with melanoma, lymphoma, and carcinoma to show safety, feasibility, and efficacy.3-7 There has been little consensus regarding the optimal method of generating DC vaccines, though nearly all approaches have involved large-volume leukaphereses to secure sufficient precursor cells because DCs do not expand in culture.2,8
The application of these cell-based vaccine strategies to pediatric patients has been particularly challenging. DC vaccination has been attempted in older children with advanced solid tumors for whom novel therapies are critically needed.9,10 These trials produced encouraging immunologic and clinical results but involved pheresis techniques that are challenging to apply to patients younger than 5 years.
Here, we propose RNA-loaded, CD40-activated B cells (CD40-B) as an alternative antigen-presenting cell (APC) vaccine with potent T-cell stimulatory capacity and the ability to be generated from a small volume of peripheral blood. Schultze et al11,12 first reported that peptide-loaded CD40-B are as effective as DCs in inducing autologous antigen-specific T-cell responses against viral and tumor-associated antigens in vitro. Protein and retroviral loading of CD40-B has also been effective for T-cell stimulation in vitro.13,14 We explored RNA as the antigenic payload for CD40-B for several reasons. First, in vitro15,16 and in vivo17 approaches in humans have demonstrated the ability of RNA-loaded DCs to induce T-cell responses against specific tumor rejection antigens through antigen-specific mRNA. Second, using tumor RNA as the antigenic payload enables an HLA-independent, whole-antigen approach that targets a wide range of tumor proteins18-21 with the potential for identifying clinically useful tumor antigens. This is particularly applicable in pediatric oncology, where few tumor-associated antigens have been described.
In this study, we demonstrate that CD40-B from pediatric patients can be transfected with antigen-specific RNA or whole-tumor RNA to generate specific cytotoxic T lymphocytes (CTLs) that secrete interferon-γ (IFN-γ) and kill targets in a major histocompatibility complex (MHC)–restricted fashion. For pooled neuroblastoma RNA and autologous neuroblastoma RNA, CTLs that lysed neuroblastoma cell lines were generated, including CTLs specific against the widely expressed tumor antigen survivin.22,23 These findings suggest that small patient size and the paucity of defined tumor antigens in pediatric oncology can be overcome by CD40-B/RNA technology.
Materials and methods
Blood samples, tumor specimens, and cell lines
Blood samples were obtained from healthy adult volunteers or adult patients with hormone-independent prostate cancer using phlebotomy or leukapheresis after institutional review board (IRB)–approved informed consent, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll centrifugation. For children with neuroblastoma, PBMCs were obtained from peripheral blood specimens drawn after 3 courses of induction chemotherapy or after tandem stem cell transplantation. In one patient the tumor was obtained fresh at the time of surgery and was used for RNA preparation, but a cell line could not be established. CD34+ hematopoietic stem cells isolated from peripheral blood were obtained from the Stem Cell Laboratory of the Children's Hospital of Philadelphia. All patients were enrolled after informed consent on the IRB-approved Children's Hospital of Philadelphia protocol, CHP-667, for the treatment of high-risk neuroblastoma (patients with stage III or IV disease with unfavorable biology features). The carcinoma cell line (SW-480), melanoma cell lines (Malme-3M, SK-MEL-113), and the HLA-A2+ transporter associated with antigen processing (TAP)–deficient T2 cell line24 were from American Tissue Culture Collection (Manassas, VA). Neuroblastoma cell lines CHLA-90, SK-NRA, SK-NAS, and SH-SY5Y were obtained from Dr Garrett Brodeur (Children's Hospital of Philadelphia). Phytohemagglutinin (PHA) T-lymphocyte blasts were generated as described.25
CD40-activated B-cell preparation
To generate activated B-cell lines, PBMCs were stimulated with CD40 ligand using NIH-3T3 cells stably transfected with CD40 ligand (tCD40L), as described previously.11 Briefly, tCD40L were lethally irradiated (96 Gy) and plated in 6-well plates at 106 cells/well. PBMCs were added 12 to 24 hours later at 106 cells/mL (4 mL/well) in B-cell medium, consisting of Iscove modified Dulbecco medium (IMDM) supplemented with 10% human AB serum, insulin, and transferrin (1:500 of ITES supplement; Cambrex, East Rutherford, NJ), recombinant human interleukin-4 (rhIL-4) (500 U/mL), and cyclosporine A (0.625 μg/mL; Novartis, Broomfield, CO). After 7 days, cultures were replated onto irradiated tCD40L at 106 cells/mL in B-cell medium and then restimulated with tCD40L and IL-4 every 3 to 5 days for up to 40 days, and flow cytometry was performed as described.11 After 14 days in culture, CD40-B–cell populations were more than 90% CD19+ HLA-DR+, and live cell populations were negative by flow cytometry for cells expressing CD40 ligand, indicating a lack of contaminating transfected 3T3 cells.
DC preparation
Adherent cells from PBMCs were plated on day 0 in AIM-V with 500 U/mL rhIL-4 (R&D Systems, Minneapolis, MN) and 800 U/mL recombinant human granulocyte macrophage–colony-stimulating factor (rhGM-CSF; Immunex, Seattle, WA). On day 6, DCs were matured with IL-1β (10 ng/mL), tumor necrosis factor-α (TNF-α) (10 ng/mL), IL-6 (0.15 μg/mL) (cytokines from R&D), and prostaglandin E2 (PGE2; Cayman Chemical, Ann Arbor, MI) (1 μg/mL) for 24 hours before electroporation.
RNA preparation
mRNA encoding full-length green fluorescence protein (GFP), MART-1 antigen, and a portion of the FluMP gene was prepared by subcloning the appropriate cDNA into the in vitro transcription vector pGEM4Z/A64 (Merix Bioscience, Durham, NC).26 Each mRNA species was prepared from the cDNA vector by in vitro transcription using the T7 RNA polymerase promoter and the mMessage mMachine kit (Ambion, Austin, TX), treated with DNase to remove plasmid sequences, and purified by phenol/chloroform extraction and RNeasy column separation (Qiagen, Valencia, CA). Measurement of OD260 and formaldehyde-denaturing gel electrophoresis were used to determine the quantity and quality of mRNA. Total cellular RNA for reverse transcription–polymerase chain reaction (RT-PCR) and electroporation was prepared from tumor cells or PBMCs using RNeasy columns (Qiagen). Tumor RNA samples prepared from 3 neuroblastoma cell lines (CHLA90, SK-NAS, SH-SY5Y) were mixed in equal ratios by weight.
RNA electroporation
For RNA electroporation of each cell type, pulse strength and osmolarity were varied to optimize viability and transfection efficiency after electroporation with GFP mRNA, using a modified square-wave electroporator and electroporation solutions from Amaxa (Cologne, Germany). CD40-B were washed twice with phosphate-buffered saline (PBS), resuspended at 2.0 to 2.5 × 106/100 μL in B solution, and electroporated (2 μg mRNA/sample; pulse program U08). Mature DCs were harvested in cold PBS, resuspended at 1.5 to 2.0 × 106 cells/100 μL in DC nucleofection solution, and electroporated (2 μg mRNA/sample; pulse program U08). SW-480 tumor cells were harvested in trypsin-EDTA (ethylenediaminetetraacetic acid), washed in PBS, resuspended in T solution, and electroporated (2 μg mRNA/sample; pulse program T27). Antigen-specific mRNA was electroporated into DCs or CD40-B at 2 μg/sample, except for restimulations with MART-1 mRNA, which were performed with 0.5 μg/sample. For tumor RNA or lymphocyte RNA, 10 μg RNA was used for initial stimulations, and 2 μg was used for restimulation of cultures.
Establishment of T-cell cultures using RNA-electroporated CD40-B or DCs
Electroporated CD40-B were washed in T cell medium (RPMI with 10% human AB serum, 2 mM glutamine, 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], and 15 μg/mL gentamicin), and were plated at 5 × 105 cells/well with 2 × 106 peripheral blood lymphocytes (PBLs)/well in 24-well plates. Cultures were supplemented with 500 U/mL rhIL-4 and 10 ng/mL IL-7 (Sigma, St Louis, MO) on day 0 and with 20 U/mL IL-2 (Chiron, Emeryville, CA) on days 1 and 4. In some cases, T-cell cultures were restimulated on days 7 and 14 with RNA-transfected CD40-B at a ratio of 4:1. RNA-electroporated DCs were washed in T-cell medium and plated in 24-well plates (1.25 × 105 cells/well) with autologous PBLs (2.5 × 106/well). Cultures were supplemented with 10 ng/mL IL-7 on day 0 and with 20 U/mL IL-2 on days 1 and 4. In some cases, T-cell cultures were restimulated on days 7 and 14, when indicated, with RNA-transfected DCs at a ratio of 20:1.
Tetramer analysis
Peptide/MHC tetramers for HLA-A2–restricted dominant epitopes of FluMP (GILGFVFTL)27 and MART-1 (ELAGIGILTV)28 were purchased from Immunomics (San Diego, CA). Tetramers for the HLA-A2–restricted dominant epitopes of HTLV-1 tax (LLFGYPVYV)29 and survivin (LMLGEFLKL)22,23 were generated according to published methods30 using purified peptide purchased from New England Peptide (Fitchburg, MA). Tetramers were validated using peptide-specific clones or T-cell lines, as described.25 T-cell cultures were stained with phycoerythrin (PE)–labeled tetramer, fluorescein isothiocyanate (FITC)–conjugated CD8 monoclonal antibody (mAb) (Immunotech, Marseilles, France), and peridin chlorophyll protein (PerCP)–conjugated CD14 and CD4 mAbs (Becton Dickinson, Franklin Lakes, NJ) for 30 minutes at room temperature. Cells were analyzed by flow cytometry with a FACSCalibur (Becton Dickinson).
ELISPOT analysis
For IFN-γ analysis, T-cell cultures at 2.5 × 104 cells/well were added to ImmunoSpot plates (Cellular Technology, Cleveland, OH) precoated with 10 μg/mL anti–IFN-γ mAb (Mabtech, Nacka, Sweden) in the presence of stimulator cells overnight at 37°C. Stimulator cells included T2 cells at 5 × 105 cells/well with 5 μg/mL peptide (New England Peptide, Fitchburg, MA) and 1 μg/mL β2-microglobulin (Sigma, St Louis, MO) or electroporated DCs at 5 × 105 cells/well. After 24 hours at 37°C, wells were developed as previously described25 and were counted using a Prior ProScan analyzer and Image Pro Plus software (Hitech Instruments, Edgemont, PA). Blocking antibody against MHC class 1 was added at a concentration of 25 μg/mL (clone W6/32, a kind gift of Dr Carl June) and was compared with purified mouse immunoglobulin G (IgG) (Jackson Laboratories, Bar Harbor, ME). Results are shown as the mean of triplicate determinations ± 1 SD, and statistical analysis was performed using the Student t test.
Chromium release assay
Assays were performed as previously described.31 Expression of electroporated mRNA in target cells was determined by GFP expression 24 hours after electroporation) and was greater than 90% in all cells used for chromium release assays. T2 cells were loaded with 10 μg/mL peptide and 2.5 μg/mL β2-microglobulin. Standard deviation was less than 5%. Target cells were evaluated for HLA-A2 and MHC class 1 expression by fluorescence-activated cell sorter (FACS) analysis (BB7.2; DAKO, Carpenteria, CA) (anti–HLA-ABC; PharMingen, San Diego, CA).
RT-PCR
Results
CD40-B can be electroporated with mRNA for antigen transfection
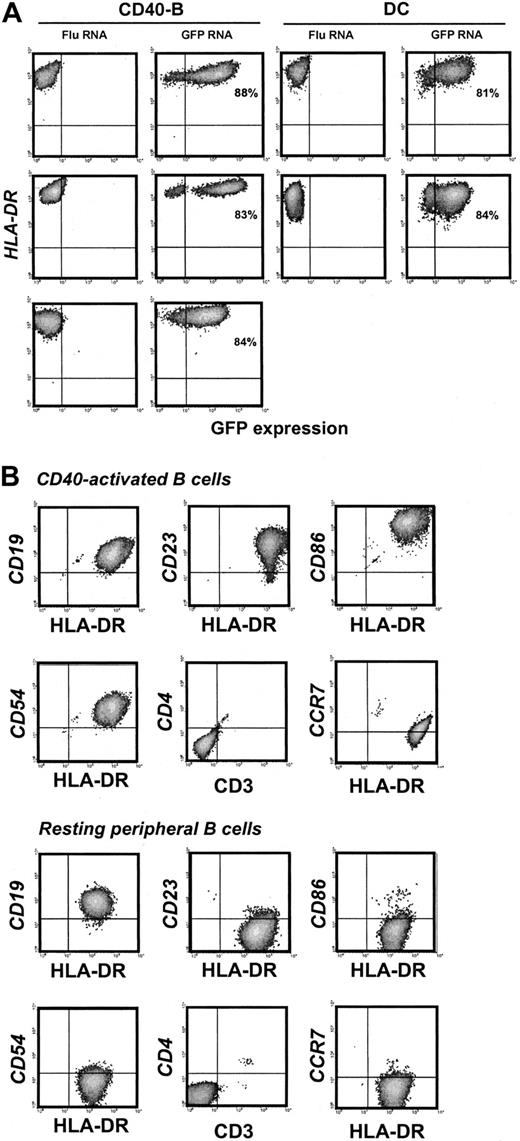
Highly enriched CD40-B and DCs generated from the blood of healthy donors and adult cancer patients were electroporated with GFP mRNA and analyzed for GFP and HLA-DR expression by flow cytometry. Transfection efficiency was greater than 80% for CD40-B or DCs from either healthy donors or cancer patients (Figure 1A). The viability of DCs or CD40-B after electroporation was similar (more than 80% at 24 hours and 50%-60% at 48 hours). PBMCs were then collected from 4 patients with high-risk neuroblastoma, with blood samples drawn after 3 rounds of induction chemotherapy or after tandem stem cell transplantation (mean age, 2.25 years; mean weight, 12.8 kg), and CD40-B cell cultures were established. For starting blood volumes of 4 to 8 mL, more than 108 CD40-B were generated in vitro in 4 weeks. CD40-B from neuroblastoma patients were efficiently electroporated with GFP mRNA (Figure 1A). These CD19+ CD40-B were highly activated based on the expression of CD23, and the level of expression of HLA-DR, CD86, and CD54 (Figure 1B) was similar to that observed for CD40-B (or DCs) from healthy donors or adult patients (data not shown). Like DCs, CD40-B were modestly CCR7+ (Figure 1B). By comparison, resting B cells in peripheral blood did not express high levels of these activation markers (Figure 1B).
CD40-B and DCs express GFP after mRNA electroporation. (A) CD40-B or matured, monocyte-derived DCs from healthy donors or adult or pediatric cancer patients were electroporated with FluMP mRNA or GFP mRNA and analyzed by flow cytometry for expression of GFP and HLA-DR 12 hours after electroporation. Upper panels, healthy donor; middle panels, 58-year-old patient with hormone-independent prostate cancer; bottom panel, (CD40-B only) 17-month-old patient with neuroblastoma. Percentages shown indicate transfection efficiency. (B) Day 14 CD40-B and resting peripheral B cells from pediatric patients were analyzed by flow cytometry for cell surface expression of CD19, CD23, HLA-DR, CD86, CD54, CD3, CD4, and CCR7. Similar results were obtained for 4 healthy donors, 2 adult patients, and 4 pediatric patients.
CD40-B and DCs express GFP after mRNA electroporation. (A) CD40-B or matured, monocyte-derived DCs from healthy donors or adult or pediatric cancer patients were electroporated with FluMP mRNA or GFP mRNA and analyzed by flow cytometry for expression of GFP and HLA-DR 12 hours after electroporation. Upper panels, healthy donor; middle panels, 58-year-old patient with hormone-independent prostate cancer; bottom panel, (CD40-B only) 17-month-old patient with neuroblastoma. Percentages shown indicate transfection efficiency. (B) Day 14 CD40-B and resting peripheral B cells from pediatric patients were analyzed by flow cytometry for cell surface expression of CD19, CD23, HLA-DR, CD86, CD54, CD3, CD4, and CCR7. Similar results were obtained for 4 healthy donors, 2 adult patients, and 4 pediatric patients.
FluMP mRNA-transfected CD40-B present influenza antigen and induce antigen-specific T-cell expansion and activation
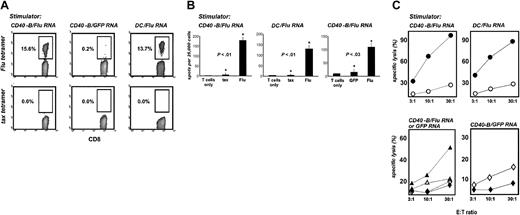
To determine whether mRNA-loaded CD40-B can induce functional T-cell responses, we first evaluated peripheral blood lymphocytes (PBLs) from HLA-A2+ healthy donors cocultured with autologous CD40-B (or DCs as a comparison) electroporated with mRNA from a FluMP mini-gene. This gene encodes an immuno-dominant influenza-derived epitope restricted to HLA-A2.27 Peptide/MHC tetramer analysis on day 7 revealed marked expansion of FluMP-specific CD8+ T cells after stimulation with FluMP mRNA–loaded CD40-B or DCs but not with GFP mRNA–loaded APCs (Figure 2A). These cells were highly functional in vitro, secreting IFN-γ in response to FluMP peptide–loaded T2 cells (but not T2 cells loaded with the irrelevant peptide tax) or FluMP mRNA–electroporated DC (but not GFP mRNA–electroporated DCs) (Figure 2B). FluMP-specific CD8+ T cells generated with either RNA-loaded CD40-B or DCs efficiently and specifically lysed FluMP peptide–loaded T2 cells or tumor cells electroporated with FluMP mRNA (Figure 2C). Control T-cell cultures generated with GFP mRNA–loaded CD40-B lysed GFP-loaded targets (Figure 2C) but exhibited minimal cytolytic (Figure 2C) or cytosecretory activity (not shown) against FluMP-loaded targets. The ability of FluMP-specific T cells to lyse tumor cells loaded with FluMP mRNA demonstrates the ability of tumor cells to take up RNA, translate and process the protein, and present peptides to specific T cells.
CD40-B and DCs loaded with FluMP mRNA induce functional, specific T cells in healthy donors. (A) CD40-B and DCs from healthy donors were electroporated with FluMP mRNA or GFP mRNA and cocultured with autologous PBL for 7 days. T-cell cultures were then harvested and stained with anti-CD8, anti-CD14, anti-CD4, and HLA-A2/FluMP or HLA-A2/tax tetramers. Cells were gated on CD8+CD4–CD14– mononuclear cells, and the percentage of CD8+ tetramer+ cells is indicated. (B) Day 7 T-cell cultures stimulated with FluMP mRNA-loaded CD40-B (right and left panels) or DCs (middle panel) were assayed by IFN-γ ELISPOT using either T2 cells (left and middle panels) pulsed with tax peptide or FluMP peptide (shown compared with T cells only) or mRNA electroporated DC (right panel) using GFP mRNA or FluMP mRNA (shown compared with T cells only). Bars represent one standard deviation. P values shown are for comparisons between starred experiments. (C) T-cell cultures were stimulated once with CD40-B loaded with FluMP mRNA (upper left and lower left panels), once with DCs loaded with FluMP mRNA (upper right panel), or once (lower left) or 3 times (lower right) with CD40-B loaded with GFP mRNA and were assayed for lysis of peptide-loaded T2 cells (• FluMP peptide; ○ tax peptide) or mRNA-loaded SW-480 carcinoma cells (lower panels: ▴ or  , FluMP mRNA–loaded targets; ▵ or ⋄, GFP mRNA–loaded targets; ▴ or ▵, FluMP mRNA–stimulated T cells;
, FluMP mRNA–loaded targets; ▵ or ⋄, GFP mRNA–loaded targets; ▴ or ▵, FluMP mRNA–stimulated T cells;  or ⋄, GFP mRNA–stimulated T cells). SW-480 cells are HLA-A2+ and MHC class II–. Similar results were obtained with 3 donors.
or ⋄, GFP mRNA–stimulated T cells). SW-480 cells are HLA-A2+ and MHC class II–. Similar results were obtained with 3 donors.
CD40-B and DCs loaded with FluMP mRNA induce functional, specific T cells in healthy donors. (A) CD40-B and DCs from healthy donors were electroporated with FluMP mRNA or GFP mRNA and cocultured with autologous PBL for 7 days. T-cell cultures were then harvested and stained with anti-CD8, anti-CD14, anti-CD4, and HLA-A2/FluMP or HLA-A2/tax tetramers. Cells were gated on CD8+CD4–CD14– mononuclear cells, and the percentage of CD8+ tetramer+ cells is indicated. (B) Day 7 T-cell cultures stimulated with FluMP mRNA-loaded CD40-B (right and left panels) or DCs (middle panel) were assayed by IFN-γ ELISPOT using either T2 cells (left and middle panels) pulsed with tax peptide or FluMP peptide (shown compared with T cells only) or mRNA electroporated DC (right panel) using GFP mRNA or FluMP mRNA (shown compared with T cells only). Bars represent one standard deviation. P values shown are for comparisons between starred experiments. (C) T-cell cultures were stimulated once with CD40-B loaded with FluMP mRNA (upper left and lower left panels), once with DCs loaded with FluMP mRNA (upper right panel), or once (lower left) or 3 times (lower right) with CD40-B loaded with GFP mRNA and were assayed for lysis of peptide-loaded T2 cells (• FluMP peptide; ○ tax peptide) or mRNA-loaded SW-480 carcinoma cells (lower panels: ▴ or  , FluMP mRNA–loaded targets; ▵ or ⋄, GFP mRNA–loaded targets; ▴ or ▵, FluMP mRNA–stimulated T cells;
, FluMP mRNA–loaded targets; ▵ or ⋄, GFP mRNA–loaded targets; ▴ or ▵, FluMP mRNA–stimulated T cells;  or ⋄, GFP mRNA–stimulated T cells). SW-480 cells are HLA-A2+ and MHC class II–. Similar results were obtained with 3 donors.
or ⋄, GFP mRNA–stimulated T cells). SW-480 cells are HLA-A2+ and MHC class II–. Similar results were obtained with 3 donors.
mRNA-loaded CD40-B generated from adult cancer patients triggered functional FluMP-specific T cells as efficiently as mRNA-loaded B cells from healthy donors. FluMP mRNA–loaded CD40-B induced the expansion of FluMP-specific CD8+ T cells (Figure 3A) that specifically secreted IFN-γ in response to T2 cells loaded with FluMP peptide or DCs loaded with FluMP mRNA (Figure 3B). Anti-class 1 antibody (but not negative control immunoglobulin) blocked IFN-γ secretion of FluMP-specific CD8+ T cells stimulated with either FluMP peptide–loaded T2 cells or FluMP mRNA–electroporated DCs (Figure 3B). To functionally compare RNA-loaded CD40-B with DCs, the capacities of these 2 cell types to induce specific T cells were compared in titration experiments, using tetramer labeling as the readout (Figure 3C). A wide range of T-cell/APC ratios was effective, but a ratio of 4:1 seemed optimal for CD40-B. FluMP T cells generated with CD40-B were capable of lysing FluMP peptide–loaded (but not tax peptide–loaded) T2 cells or FluMP mRNA–loaded (but not GFP mRNA–loaded) tumor cells (Figure 3D). FluMP mRNA–loaded DCs induced FluMP-specific T cells with similar characteristics. Control T-cell cultures generated with GFP mRNA–loaded CD40-B lysed GFP mRNA–loaded (but not FluMP mRNA–loaded) tumor cells (Figure 3D).
DCs and CD40-B loaded with FluMP mRNA induce functional specific T cells in adult cancer patients. (A) CD40-B and DCs from the same prostate cancer patient as in Figure 1A were electroporated with FluMP or GFP mRNA and were cocultured with autologous PBLs for 7 days. T-cell cultures were analyzed with tetramers as in Figure 2. (B) Day 7 T-cell cultures stimulated with FluMP mRNA-loaded CD40-B or DCs were assayed by IFN-γ ELISPOT using either T2 cells (left and middle upper panels) pulsed with tax peptide or FluMP peptide (shown compared with T cells only) or mRNA-electroporated DCs (right upper panel) using GFP mRNA or FluMP mRNA (shown compared with T cells only). For T cells stimulated with FluMP mRNA–loaded CD40-B, the effect of mouse antihuman class I mAb was compared with mouse purified IgG (each at 25 μg/mL) or T cells only (lower panels). Targets were either T2 cells (left lower panel) pulsed with tax peptide or FluMP peptide or mRNA-electroporated DCs (right lower panel) using GFP mRNA or FluMP mRNA. Bars represent 1 standard deviation. P values shown are for comparisons between starred experiments. (C) CD40-B and DCs from a second prostate cancer patient were electroporated with FluMP or GFP mRNA, cocultured with autologous PBLs for 7 days, and analyzed with tetramers as in Figure 2. A range of T-cell/APC ratios for DCs (solid line) and CD40-B (dotted line) was examined. Tetramer results for cultures stimulated with FluMP APCs are shown. The negative control tax tetramer labeled less than 0.05% in each case (not shown). Labeling of GFP mRNA stimulated cultures with the FluMP tetramer was less than 0.1% in each case (not shown). (D) T-cell cultures were stimulated once with CD40-B loaded with FluMP mRNA (left panels), once with DCs loaded with FluMP mRNA (upper right panel), or once (lower left) or 3 times (lower right) with CD40-B loaded with GFP mRNA and were assayed for lysis of peptide-loaded T2 cells (• FluMP peptide; ○ tax peptide) or mRNA-loaded SW-480 carcinoma cells (lower left panel: ▴ or  , FluMP mRNA–loaded targets; ▵ or ⋄, GFP mRNA–loaded targets; ▴ or ▵, FluMP mRNA–stimulated T cells;
, FluMP mRNA–loaded targets; ▵ or ⋄, GFP mRNA–loaded targets; ▴ or ▵, FluMP mRNA–stimulated T cells;  or ⋄, GFP mRNA–stimulated T cells). Results are representative of 1 to 3 experiments from 2 prostate cancer patients.
or ⋄, GFP mRNA–stimulated T cells). Results are representative of 1 to 3 experiments from 2 prostate cancer patients.
DCs and CD40-B loaded with FluMP mRNA induce functional specific T cells in adult cancer patients. (A) CD40-B and DCs from the same prostate cancer patient as in Figure 1A were electroporated with FluMP or GFP mRNA and were cocultured with autologous PBLs for 7 days. T-cell cultures were analyzed with tetramers as in Figure 2. (B) Day 7 T-cell cultures stimulated with FluMP mRNA-loaded CD40-B or DCs were assayed by IFN-γ ELISPOT using either T2 cells (left and middle upper panels) pulsed with tax peptide or FluMP peptide (shown compared with T cells only) or mRNA-electroporated DCs (right upper panel) using GFP mRNA or FluMP mRNA (shown compared with T cells only). For T cells stimulated with FluMP mRNA–loaded CD40-B, the effect of mouse antihuman class I mAb was compared with mouse purified IgG (each at 25 μg/mL) or T cells only (lower panels). Targets were either T2 cells (left lower panel) pulsed with tax peptide or FluMP peptide or mRNA-electroporated DCs (right lower panel) using GFP mRNA or FluMP mRNA. Bars represent 1 standard deviation. P values shown are for comparisons between starred experiments. (C) CD40-B and DCs from a second prostate cancer patient were electroporated with FluMP or GFP mRNA, cocultured with autologous PBLs for 7 days, and analyzed with tetramers as in Figure 2. A range of T-cell/APC ratios for DCs (solid line) and CD40-B (dotted line) was examined. Tetramer results for cultures stimulated with FluMP APCs are shown. The negative control tax tetramer labeled less than 0.05% in each case (not shown). Labeling of GFP mRNA stimulated cultures with the FluMP tetramer was less than 0.1% in each case (not shown). (D) T-cell cultures were stimulated once with CD40-B loaded with FluMP mRNA (left panels), once with DCs loaded with FluMP mRNA (upper right panel), or once (lower left) or 3 times (lower right) with CD40-B loaded with GFP mRNA and were assayed for lysis of peptide-loaded T2 cells (• FluMP peptide; ○ tax peptide) or mRNA-loaded SW-480 carcinoma cells (lower left panel: ▴ or  , FluMP mRNA–loaded targets; ▵ or ⋄, GFP mRNA–loaded targets; ▴ or ▵, FluMP mRNA–stimulated T cells;
, FluMP mRNA–loaded targets; ▵ or ⋄, GFP mRNA–loaded targets; ▴ or ▵, FluMP mRNA–stimulated T cells;  or ⋄, GFP mRNA–stimulated T cells). Results are representative of 1 to 3 experiments from 2 prostate cancer patients.
or ⋄, GFP mRNA–stimulated T cells). Results are representative of 1 to 3 experiments from 2 prostate cancer patients.
Tumor antigen mRNA–loaded B cells induce T-cell responses to the MART-1 tumor antigen and expand MART-1–specific cells that lyse tumor targets
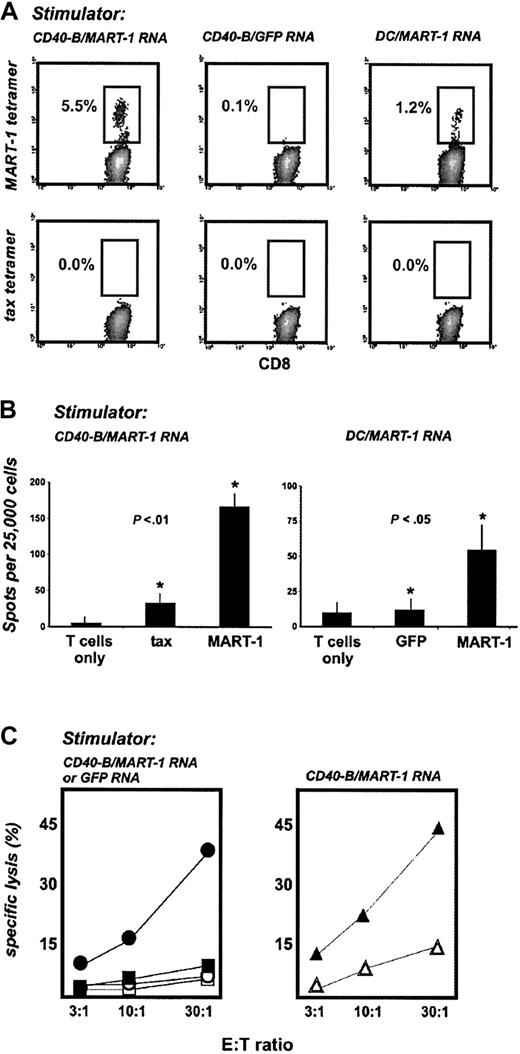
We next sought to determine whether mRNA-loaded CD40-B can trigger T-cell responses to a tumor rejection antigen, such as MART-1. HLA-A2+ PBLs from healthy donors were stimulated with autologous CD40-B (or DCs as a comparison) electroporated with full-length MART-1 mRNA. After 3 weekly stimulations, peptide/MHC tetramer analysis revealed the expansion of CD8+ T cells specific for MART-1 peptide (Figure 4A). Stimulation was antigen specific because GFP mRNA–loaded CD40-B failed to generate MART-1–specific T cells. MART-1–specific T cells generated with mRNA-loaded CD40-B specifically secreted IFN-γ in response to peptide-loaded T2 cells and MART-1 mRNA–loaded DCs (Figure 4B). MART-1–specific T cells (but not GFP-stimulated T cells) lysed the MART-1+/HLA-A2+ melanoma cell line Malme-3M, but not the MART-1+/HLA-A2– cell line SK-MEL-113 (Figure 4C). Moreover, MART-1–/HLA-A2+ SW-480 carcinoma cells transiently loaded with MART-1 mRNA by electroporation were specifically lysed by MART-1–specific T cells but not by GFP-stimulated T cells (Figure 4C).
MART-1 mRNA–loaded CD40-B and DCs generate functional antitumor T cells. (A) CD40-B and DCs from healthy donors were electroporated with MART-1 mRNA or GFP mRNA and used to stimulate autologous PBLs weekly for 3 weeks. T-cell cultures were then analyzed with tetramers as in Figure 2, but using HLA-A2/MART-1 tetramer. (B) T-cell cultures stimulated with MART-1 mRNA–loaded CD40-B were assayed by IFN-γ ELISPOT using either T2 cells (left panel) pulsed with tax peptide or MART-1 peptide (shown compared to T cells only) or mRNA electroporated autologous DC (right panel) using GFP mRNA or MART-1 mRNA (shown compared with T cells only). Bars represent one standard deviation. P values shown are for comparisons between starred experiments. (C) T-cell cultures stimulated with CD40-B loaded with MART-1 mRNA or GFP mRNA were assayed for lysis of tumor cells. Left panel: closed symbols indicate MART-1+ Malme-3M cells; open symbols, MART-1+ SK-MEL-113 cells, shown for MART-1 T cells (circles) and GFP T cells (squares). Malme-3M tumor targets matched donors only at HLA-A2; SK-MEL-113 cells and donors were HLA mismatched. Right panel: ▴ MART-1– SW-480 carcinoma cells electroporated with MART-1 mRNA. ▵ SW-480 cells electroporated with GFP mRNA, shown for MART-1 T cells. Similar results were obtained with 2 donors, except for experiments in panel B, which were performed for 1 donor.
MART-1 mRNA–loaded CD40-B and DCs generate functional antitumor T cells. (A) CD40-B and DCs from healthy donors were electroporated with MART-1 mRNA or GFP mRNA and used to stimulate autologous PBLs weekly for 3 weeks. T-cell cultures were then analyzed with tetramers as in Figure 2, but using HLA-A2/MART-1 tetramer. (B) T-cell cultures stimulated with MART-1 mRNA–loaded CD40-B were assayed by IFN-γ ELISPOT using either T2 cells (left panel) pulsed with tax peptide or MART-1 peptide (shown compared to T cells only) or mRNA electroporated autologous DC (right panel) using GFP mRNA or MART-1 mRNA (shown compared with T cells only). Bars represent one standard deviation. P values shown are for comparisons between starred experiments. (C) T-cell cultures stimulated with CD40-B loaded with MART-1 mRNA or GFP mRNA were assayed for lysis of tumor cells. Left panel: closed symbols indicate MART-1+ Malme-3M cells; open symbols, MART-1+ SK-MEL-113 cells, shown for MART-1 T cells (circles) and GFP T cells (squares). Malme-3M tumor targets matched donors only at HLA-A2; SK-MEL-113 cells and donors were HLA mismatched. Right panel: ▴ MART-1– SW-480 carcinoma cells electroporated with MART-1 mRNA. ▵ SW-480 cells electroporated with GFP mRNA, shown for MART-1 T cells. Similar results were obtained with 2 donors, except for experiments in panel B, which were performed for 1 donor.
CD40-B from pediatric oncology patients induce functional T-cell responses against viral and tumor antigens
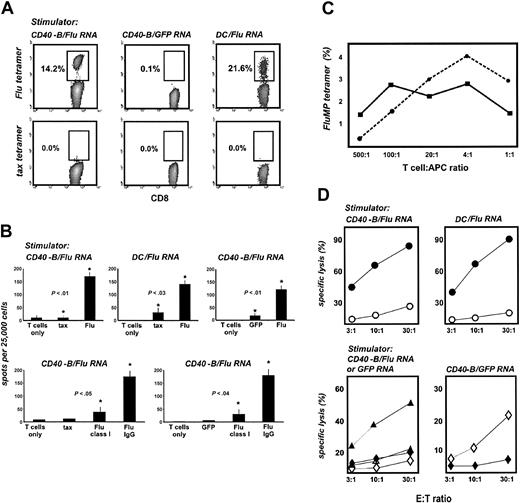
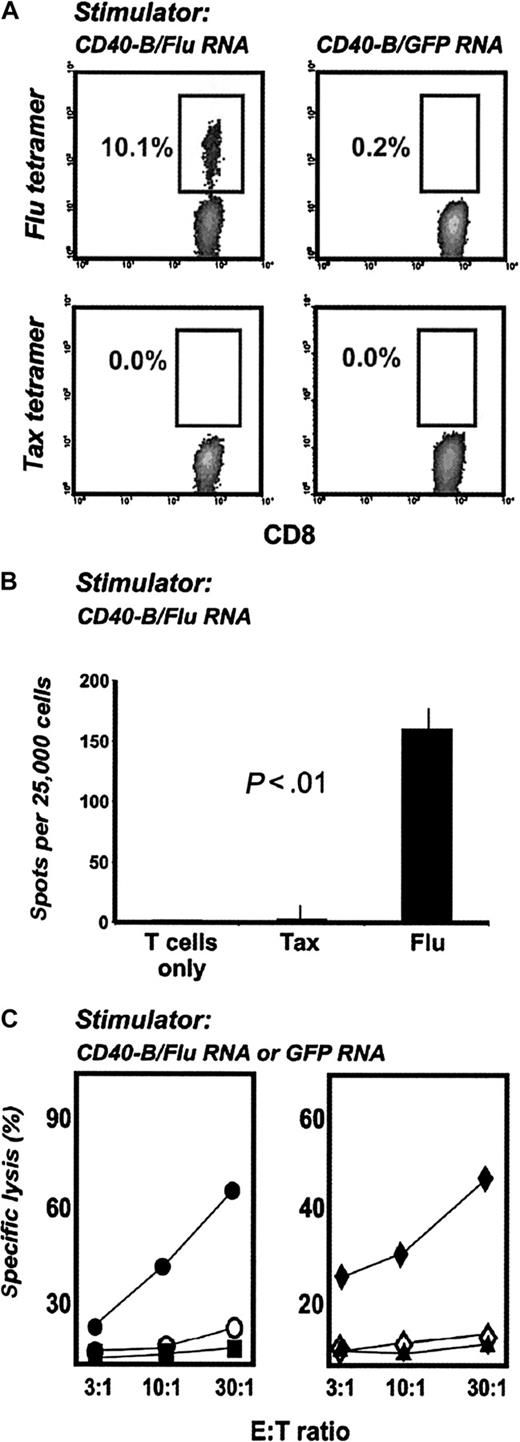
To determine whether RNA-loaded CD40-B from pediatric patients can induce functional T-cell responses, PBMCs from HLA-A2+ neuroblastoma patients were stimulated with autologous CD40-B electroporated with FluMP mRNA. After 7 days, peptide/MHC tetramer analysis revealed the expansion of FluMP-specific CD8+ T cells (Figure 5A). These T cells secreted IFN-γ in response to FluMP peptide (but not tax peptide) (Figure 5B) and lysed tumor cells transiently loaded with FluMP mRNA by electroporation (Figure 5C). T-cell cultures stimulated with GFP mRNA served as negative controls.
CD40-B loaded with FluMP mRNA induce functional specific T cells in pediatric oncology patients. (A) CD40-B from neuroblastoma patients were electroporated with FluMP mRNA or GFP mRNA and cocultured with autologous PBMC for 7 days. T-cell cultures were analyzed with tetramers as in Figure 2. (B) Day 7 T-cell cultures stimulated with FluMP mRNA-loaded CD40-B were assayed by IFN-γ ELISPOT using T2 cells pulsed with tax peptide or FluMP peptide (shown compared with T cells only). (C) T-cell cultures stimulated with FluMP mRNA or GFP mRNA were assayed for lysis of peptide-loaded T2 cells (left panel: • or ○, T2 cells loaded with FluMP peptide; ▪ or □, T2 cells loaded with tax peptide; • or ▪, FluMP mRNA–stimulated T cells; ○ or □, GFP mRNA–stimulated cells) or for lysis of SW-480 tumor cells (right panel:  or ▴, SW-480 cells loaded with FluMP mRNA; ⋄, SW-480 loaded with GFP mRNA;
or ▴, SW-480 cells loaded with FluMP mRNA; ⋄, SW-480 loaded with GFP mRNA;  or ⋄, FluMP mRNA–stimulated T cells; ▴, GFP mRNA–stimulated T cells). Similar results were obtained with 2 patients.
or ⋄, FluMP mRNA–stimulated T cells; ▴, GFP mRNA–stimulated T cells). Similar results were obtained with 2 patients.
CD40-B loaded with FluMP mRNA induce functional specific T cells in pediatric oncology patients. (A) CD40-B from neuroblastoma patients were electroporated with FluMP mRNA or GFP mRNA and cocultured with autologous PBMC for 7 days. T-cell cultures were analyzed with tetramers as in Figure 2. (B) Day 7 T-cell cultures stimulated with FluMP mRNA-loaded CD40-B were assayed by IFN-γ ELISPOT using T2 cells pulsed with tax peptide or FluMP peptide (shown compared with T cells only). (C) T-cell cultures stimulated with FluMP mRNA or GFP mRNA were assayed for lysis of peptide-loaded T2 cells (left panel: • or ○, T2 cells loaded with FluMP peptide; ▪ or □, T2 cells loaded with tax peptide; • or ▪, FluMP mRNA–stimulated T cells; ○ or □, GFP mRNA–stimulated cells) or for lysis of SW-480 tumor cells (right panel:  or ▴, SW-480 cells loaded with FluMP mRNA; ⋄, SW-480 loaded with GFP mRNA;
or ▴, SW-480 cells loaded with FluMP mRNA; ⋄, SW-480 loaded with GFP mRNA;  or ⋄, FluMP mRNA–stimulated T cells; ▴, GFP mRNA–stimulated T cells). Similar results were obtained with 2 patients.
or ⋄, FluMP mRNA–stimulated T cells; ▴, GFP mRNA–stimulated T cells). Similar results were obtained with 2 patients.
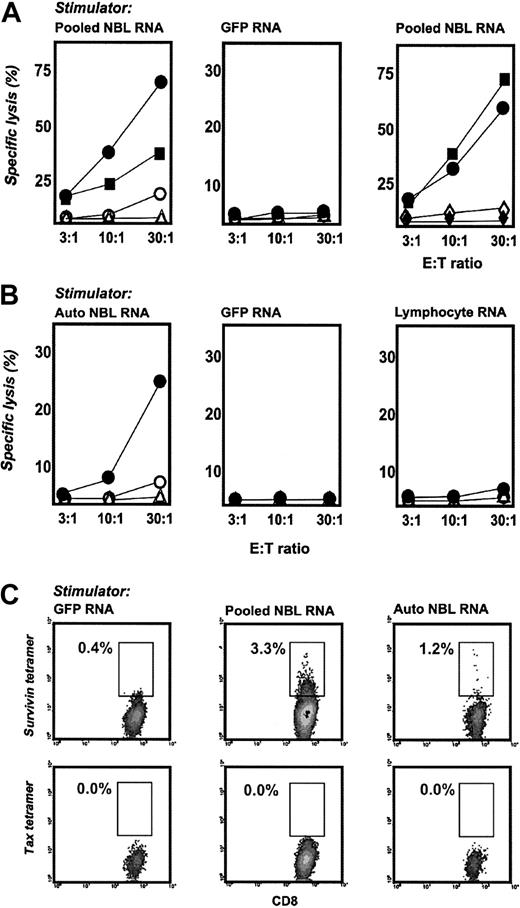
We next sought to determine whether CD40-B generated from neuroblastoma patients can induce T cells specific for neuroblastoma without targeting a particular tumor antigen during in vitro priming. To do this, CD40-B from HLA-A2+ patients were electroporated with total RNA prepared and pooled from 3 neuroblastoma cell lines (CHLA90, SK-NAS, SH-SY5Y), or with RNA prepared from autologous tumor, and cocultured with patient PBMCs obtained after induction chemotherapy. After 14 days, T-cell cultures stimulated with pooled tumor RNA lysed HLA-A2+ CHLA-90 and SK-NRA neuroblastoma cell lines but not HLA-A2– (yet HLA class I+) SK-NAS or HLA class I– SH-SY5Y cells (Figure 6A). Importantly, SK-NRA cells were not used to prepare the tumor RNA for stimulation, suggesting that cytotoxic activity against this line occurred on the basis of T cells specific for shared neuroblastoma tumor antigens. Cytotoxicity against SK-NRA and CHLA90 cells was also observed for T-cell cultures generated from PBMCs obtained after stem cell transplantation (Figure 6A). These anti-neuroblastoma T cells failed to lyse autologous PHA blasts or autologous CD34+ stem cells (Figure 6A). T cells that were stimulated with GFP mRNA failed to lyse tumor targets (Figure 6A). T-cell cultures generated with autologous tumor RNA lysed HLA-A2+ SK-NRA cells but not HLA-A2– SK-NAS cells or HLA class I– SH-SY5Y cells (Figure 6B). These T cells also failed to lyse autologous unloaded CD40-B (specific lysis less than 1% at an E/T ratio of 30:1, 10:1, or 3:1). T cells from this HLA-A2+ patient stimulated with GFP mRNA or autologous lymphocyte RNA were not lytic against any neuroblastoma target (Figure 6B).
CD40-B induce anti-neuroblastoma T cells from pediatric oncology patients. (A) PBMCs from patients with neuroblastoma were stimulated twice with CD40-B electroporated with pooled tumor RNA from 3 neuroblastoma cell lines (left and right panels) or GFP RNA(middle panel) and were assayed in chromium release assays against neuroblastoma cell lines (• SK-NRA, HLA-A2+; ▪ CHLA-90, HLA-A2+; ○ SK-NAS, HLA-A2–; ▵ SH-SY5Y, HLA-class I–); ⋄ autologous PHA blasts;  autologous CD34+ stem cells. PBMCs were obtained after induction chemotherapy (left and middle panels) or 90 days after stem cell transplantation (right panel). Similar results were obtained with 2 donors, except for experiments using PHA blasts or stem cells, which were performed once. (B) Autologous PBMCs from a patient with neuroblastoma were stimulated twice with CD40-B electroporated with autologous tumor RNA (left panel), GFP RNA (middle panel), or autologous lymphocyte RNA (right panel) and were assayed in chromium release assays against neuroblastoma cell lines. Symbols are the same as in panel A. (C) Cultures stimulated with GFP RNA (left column), pooled neuroblastoma cell line RNA (middle column), or autologous tumor RNA (right column) were labeled with anti-CD8, anti-CD14, anti-CD4, and HLA-A2/survivin or HLA-A2/tax tetramers and were analyzed as in Figure 2.
autologous CD34+ stem cells. PBMCs were obtained after induction chemotherapy (left and middle panels) or 90 days after stem cell transplantation (right panel). Similar results were obtained with 2 donors, except for experiments using PHA blasts or stem cells, which were performed once. (B) Autologous PBMCs from a patient with neuroblastoma were stimulated twice with CD40-B electroporated with autologous tumor RNA (left panel), GFP RNA (middle panel), or autologous lymphocyte RNA (right panel) and were assayed in chromium release assays against neuroblastoma cell lines. Symbols are the same as in panel A. (C) Cultures stimulated with GFP RNA (left column), pooled neuroblastoma cell line RNA (middle column), or autologous tumor RNA (right column) were labeled with anti-CD8, anti-CD14, anti-CD4, and HLA-A2/survivin or HLA-A2/tax tetramers and were analyzed as in Figure 2.
CD40-B induce anti-neuroblastoma T cells from pediatric oncology patients. (A) PBMCs from patients with neuroblastoma were stimulated twice with CD40-B electroporated with pooled tumor RNA from 3 neuroblastoma cell lines (left and right panels) or GFP RNA(middle panel) and were assayed in chromium release assays against neuroblastoma cell lines (• SK-NRA, HLA-A2+; ▪ CHLA-90, HLA-A2+; ○ SK-NAS, HLA-A2–; ▵ SH-SY5Y, HLA-class I–); ⋄ autologous PHA blasts;  autologous CD34+ stem cells. PBMCs were obtained after induction chemotherapy (left and middle panels) or 90 days after stem cell transplantation (right panel). Similar results were obtained with 2 donors, except for experiments using PHA blasts or stem cells, which were performed once. (B) Autologous PBMCs from a patient with neuroblastoma were stimulated twice with CD40-B electroporated with autologous tumor RNA (left panel), GFP RNA (middle panel), or autologous lymphocyte RNA (right panel) and were assayed in chromium release assays against neuroblastoma cell lines. Symbols are the same as in panel A. (C) Cultures stimulated with GFP RNA (left column), pooled neuroblastoma cell line RNA (middle column), or autologous tumor RNA (right column) were labeled with anti-CD8, anti-CD14, anti-CD4, and HLA-A2/survivin or HLA-A2/tax tetramers and were analyzed as in Figure 2.
autologous CD34+ stem cells. PBMCs were obtained after induction chemotherapy (left and middle panels) or 90 days after stem cell transplantation (right panel). Similar results were obtained with 2 donors, except for experiments using PHA blasts or stem cells, which were performed once. (B) Autologous PBMCs from a patient with neuroblastoma were stimulated twice with CD40-B electroporated with autologous tumor RNA (left panel), GFP RNA (middle panel), or autologous lymphocyte RNA (right panel) and were assayed in chromium release assays against neuroblastoma cell lines. Symbols are the same as in panel A. (C) Cultures stimulated with GFP RNA (left column), pooled neuroblastoma cell line RNA (middle column), or autologous tumor RNA (right column) were labeled with anti-CD8, anti-CD14, anti-CD4, and HLA-A2/survivin or HLA-A2/tax tetramers and were analyzed as in Figure 2.
To demonstrate an antigen-specific, antitumor immune response, we analyzed the specificity of CD8+ T cells in these cultures using peptide/HLA-A2 tetramers. These experiments show that pooled or autologous neuroblastoma RNA can generate CD8+ T cells specific for the tumor antigen survivin.22,23 Between 1% and 3% of CD8+ T cells from these cultures were labeled with a tetramer specific for the sur1M2 peptide from survivin. T-cell cultures stimulated with GFP mRNA bound poorly to survivin tetramer, and none of the cultures were labeled with negative control tax tetramer (Figure 6C). RT-PCR analysis demonstrated survivin expression in the neuroblastoma cell lines and autologous tumor cells used to prepare tumor RNA. Previous reports have noted tetramer+ survivin-specific T cells from cancer patients,34 but none has been observed in neuroblastoma or in cultures stimulated with multiple tumor-antigenic determinants, such as tumor RNA. These data show that CD40-B from pediatric oncology patients can be loaded with whole tumor RNA to generate and expand antitumor T cells, including some that have specificities to known tumor antigens.
Discussion
The delivery of cell-based vaccines that exploit natural mechanisms of antigen presentation represents a promising approach for the immunotherapy of cancer. In this study, we demonstrate that mRNA-loaded, CD40-activated B cells can be readily generated in vitro from less than 8 mL blood and can function as efficient APCs to drive the proliferation of T cells that specifically secrete IFN-γ and kill tumor cells in an antigen-specific and an MHC-restricted manner. These results support a novel platform for cancer vaccination or adoptive T-cell therapy, particularly in pediatric oncology patients, that addresses a need for additional antigen-presenting resources in cell-based immunotherapy and that exploits RNA as the antigenic payload to permit the targeting of multiple antigen epitopes simultaneously without the use of vectors or viruses and without regard for particular patient HLA alleles.
Although DCs have primary cellular responsibility for T-cell priming in vivo, activated B cells also present antigen professionally to T cells35,36 and have been safely piloted as part of vaccines in at least 2 small clinical trials.37,38 A far greater clinical experience with APC vaccines has been gained with blood-purified or monocyte-derived DCs, for which safety and potential efficacy have been documented.2 Feasibility, however, remains an issue, particularly in pediatric oncology patients in whom catheter placement and large-volume leukapheresis are required to obtain adequate numbers of cellular precursors.9,10 Here, FluMP and MART-1–specific T cells generated in vitro with CD40-B/RNA technology from adults or children labeled with peptide/MHC tetramers specifically secreted IFN-γ and lysed tumors expressing antigen in an MHC-restricted fashion. Moreover, whole tumor RNA derived from neuroblastoma cell lines or autologous neuroblastoma was readily transfected into CD40-B from pediatric patients and induced T cells that lysed HLA-matched neuroblastoma cells but not autologous benign cells. Our data suggest that shared tumor antigens may be targeted in this approach, including the tumor antigen survivin.
Activated B cells have been shown in several in vitro systems to activate CTL responses against viral and tumor antigen targets, including neoantigens, that are formulated as peptides, proteins, or viral vectors.11-14 Functions of antigen presentation critically depend on the activation state of the B cells, because, as are unactivated DCs,39 unactivated B cells are tolerogenic in certain settings.40,41 Activation with CD40 may be the optimal method of B-cell activation42,43 because it alters the antigen-presenting function of resting B cells44 and induces, with IL-4, marked proliferation in vitro,11,45 a feature that distinguishes CD40-B manufacturing from DC manufacturing. Although it is not yet clear how many CD40-B are needed to substitute for one activated DC, only 4 to 8 mL peripheral blood is needed to generate 108 CD40-B. This is the same number of DCs typically generated from 109 PBMCs (or roughly 1 L blood). Importantly, CD40-B—as do DCs—express chemokine receptors such as CCR7, suggesting their ability to track to regional lymph nodes after injection. In a murine model,46 adoptively transferred B cells were found in lymph nodes within 24 hours of subcutaneous injection, including nodes contralateral to the injection site. These results suggest that adoptively transferred CD40-B may reach tumor-draining lymph nodes and may be capable of stimulating antitumor T cells in vivo within their lifespan. However, the in vivo efficacy of RNA-transfected CD40-B remains untested, and a murine or large animal model would be important in justifying clinical vaccination of patients with these APCs. A method of generating CD40-B consistent with good manufacturing practice has been described12 and would facilitate the translation of these findings to the clinic.
In evaluating CD40-B/RNA technology as a vaccine modality in cancer patients, we are mindful of the risk of autoimmunity if the use of whole tumor RNA primes patients to proteins also expressed by normal cells. This has been a major consideration in many cancer vaccine formulations that use the proteome or the transcriptome of whole human tumor cells. Fortunately, there have been no serious adverse events related to this concern, even in studies in which robust antitumor responses were measurable after vaccination.47-49 For RNA as an antigenic payload, no immune responses against normal cells have been observed after in vitro or in vivo stimulation with DCs loaded with whole tumor.20,50
CD40-B/RNA technology can be applied to almost any tumor, but we chose neuroblastoma as the first model. Previous observations suggest that neuroblastoma is an immunogenic tumor: CD4+ and CD8+ tumor-infiltrating lymphocytes are found in many neuroblastoma lesions,51,52 and patients with the neuroblastoma-associated syndrome opsoclonus-myoclonus have high-titer antineuronal antibodies and germinal centers,52,53 both of which require the participation of T cells. On the other hand, data in the literature indicate that neuroblastoma cells express little to no MHC class I, suggesting that they are not susceptible to CTL lysis.54 Other authors report neuroblastoma that do express MHC, and some MHC– neuroblastomas express class I after treatment with IFN-γ.55 In our own work, 7 of 10 neuroblastoma cell lines were found by flow cytometry to express MHC class I, which can be up-regulated by IFN-γ (data not shown). The level of MHC class I expression does not approach the high levels of MHC expression typically seen in pediatric B-cell leukemia, but in our study it appears to be sufficient to trigger effector T-cell responses.
In summary, CD40-B/RNA technology has particular relevance to the development of new therapies in pediatric oncology, in which DC-based vaccine formulations have been hindered by patient size, venous access, and a paucity of characterized tumor-rejection antigens. The ability to generate CD40-B from phlebotomy samples and the feasibility of tumor RNA to target multiple tumor antigens, known and unknown, provides the opportunity for testing novel vaccines and adoptive T-cell therapies in pediatric and adult patients.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-07-2379.
Supported by a Clinical Investigator Award from the Damon Runyon Cancer Research Fund and by National Institutes of Health grant CA84050 (R.H.V.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Carl June and Richard Carroll for valuable discussions; Michael Leibowitz, Lara Schutsky, and Dih Yih Chen for excellent technical assistance; Dr Guiliana Pierson of the Stem Cell Laboratory of the Children's Hospital of Philadelphia; and MERIX Bioscience for the generous gift of GFP/pGEM4Z64A and antigen-specific mRNA.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal