Abstract
Liver injury is a frequent, serious complication of allogeneic hematopoietic cell transplantation (HCT) following myeloablative preparative regimens. We sought to determine the frequency and severity of hepatic injury after nonmyeloablative conditioning and its relationship to outcomes. One hundred ninety-three consecutive patients who received 2 Gy total body irradiation with or without fludarabine were evaluated for end points related to liver injury. Patients with diseases treatable by HCT who were ineligible for conventional myeloablative allogeneic HCT because of advanced age and/or comorbid conditions were included. Fifty-one patients (26%) developed hyperbilirubinemia of 68.4 μM (4 mg/dL) or greater, most commonly resulting from cholestasis due to graft-versus-host disease (GVHD) or sepsis. Pretransplantation factors associated with liver dysfunction were a diagnosis of aggressive malignancy (hazard ratio [HR] 1.9; P = .04) and the inclusion of fludarabine in the conditioning regimen (HR 1.8; P = .07). Overall survival at 1 year was superior for patients who had maximal serum bilirubin levels in the normal (78%) or minimally elevated (22.23-66.69 μM [1.3-3.9 mg/dL]) ranges (69%) compared with those in the 68.4 to 117.99 μM (4-6.9 mg/dL; 20%), 119.7 to 169.29 μM (7.0-9.9 mg/dL; 17%), and 171.0 μM (10 mg/dL; 19%) or greater groups. In summary, significant jaundice occurred in 26% of patients and was predominantly due to cholestasis resulting from GVHD and/or sepsis. Aggressive malignancies (mainly advanced disease) and later development of jaundice after transplantation predicted inferior survival.
Introduction
Conventional allogeneic hematopoietic cell transplantation (HCT) for patients with hematologic malignancies involves conditioning with myeloablative doses of chemotherapeutic drugs with or without total body irradiation (TBI). The regimen-related toxicities of myeloablative conditioning have limited this therapeutic approach by excluding older patients and those with underlying organ dysfunction from receiving allografts. Liver injury, a common and potentially fatal complication after myeloablative conditioning therapy, is caused by many different processes: toxic injury to hepatic sinusoids (ie, sinusoidal obstruction syndrome [SOS], also known as venoocclusive disease [VOD]); cholestasis related to septicemia (cholangitis lenta); graft-versus-host disease (GVHD) or drug injury; and hepatocellular necrosis caused by infection, ischemia, or drug injury.1
SOS is a clinical syndrome characterized by hyperbilirubinemia, tender hepatomegaly, and sudden weight gain caused by fluid accumulation. This syndrome develops in 5% to 60% of patients after HCT and ranges in severity from mild, reversible disease to progressive liver injury associated with multiorgan failure. In some reported series, the case fatality rate is as high as 28%, with established severe SOS having a mortality rate of higher than 90% by day +100 after HCT.2-6 SOS is the dose-limiting toxicity for myeloablative conditioning regimens.2 The transplant-related morbidity and mortality associated with myeloablative conditioning has provided the impetus to develop less intensive conditioning regimens. A variety of regimens ranging from reduced-intensity to minimally myelosuppressive regimens has been reported.7-10 The preparative regimens used for the patients in the current study are minimally myelosuppressive and based on preclinical canine studies using 2 Gy TBI given on day 0,11 with or without preceding fludarabine (Flu), followed by HCT from either related or unrelated donors. Our objectives were to (1) determine the frequency and severity of liver injury in patients receiving these nonmyeloablative regimens, (2) determine the outcome of patients with serious liver dysfunction after transplantation, and (3) identify whether pretransplantation variables or the composition of the conditioning regimen influenced the development of liver injury.
Patients and methods
Patient selection
Data from one hundred ninety-three consecutive patients treated with allogeneic HCT after nonmyeloablative conditioning between December 1997 and February 2002 at the Seattle Cancer Care Alliance (SCCA) were evaluated. The SCCA comprises the clinical hematology and oncology activities of the Fred Hutchinson Cancer Research Center (FHCRC), the University of Washington Medical Center, and Children's Hospital Regional Medical Center. Medical records were reviewed under protocols approved by the FHCRC Institutional Review Board. Included in this study were patients with hematologic diseases who were ineligible for conventional myeloablative allogeneic HCT because of advanced age and/or comorbid conditions or preceding extensive therapies. Patient characteristics are outlined in Table 1. Sixty percent of the patients were male and median patient age was 53 years (range, 1-72 years). Most patients had a hematologic malignancy (n = 168), renal cell carcinoma (n = 8), or an immunodeficiency disorder (n = 7). Patients with malignancies were considered to have either “indolent” or “aggressive” disease according to the criteria outlined in Table 1. Thirty-eight patients had a history of at least one previous failed autologous transplantation, and 5 patients had a prior failed allogeneic transplantation, including one patient who had a previous failed autologous and allogeneic transplantation.
Conditioning regimens were based on canine studies, which suggested that 2 Gy TBI followed by cyclosporine/mycophenolate mofetil (CSP/MMF) could secure engraftment. Initial human studies using HLA-matched donors were initiated using 2 Gy TBI alone on day 0 (n = 48).9 After it became apparent that the (nonfatal) graft rejection rate was 17%,9,12 predominantly due to patients with chronic myeloid leukemia (CML) and myelodysplastic syndrome (MDS) who had not received extensive prior chemotherapy, the regimen was modified to include Flu. One hundred forty-two patients received 2 Gy TBI, preceded by Flu (30 mg/m2) daily from day –4 to day –2. This resulted in a reduction of the rejection rate to 3% leading to the adoption of Flu/TBI as our new standard regimen. A subgroup of patients with multiple myeloma or lymphoma was treated with a combined autologous transplantation followed by nonmyeloablative allogeneic transplantation. Thirty-one patients (including 3 patients with a prior failed autologous transplantation) received a planned autologous transplant followed by nonmyeloablative allogeneic transplantation as part of a combined autologous-allogeneic transplantation strategy, 22 of these patients received TBI alone and 9 received Flu/TBI for the nonmyeloablative allogeneic transplantation. In this group of patients the addition of Flu was found to be unnecessary in patients with related donors as no patients rejected the graft.12 For patients with unrelated donors, fludarabine was always given in view of the increased risk of rejection.13,14 Three patients with immunodeficiency diseases did not receive any preparative regimen. Postgrafting immunosuppression consisted of CSP and MMF, the duration of which varied depending on whether the donor was related or unrelated and on the risk of disease progression. For related donor transplantations, CSP was administered from day –3 to day +35 or day +56 followed by a taper. MMF was administered from day 0 to day +28. For unrelated donor transplantations, CSP was given from day –3 to day +100 followed by a taper, and MMF was given from day 0 to day +40 followed by a taper.
Assessment of liver dysfunction
Before transplantation, patients were evaluated for the presence of liver disease by history, physical examination, and laboratory tests (serum levels of aspartate aminotransferase [AST], alanine aminotransferase [ALT], bilirubin, albumin, prothrombin time, and tests for hepatitis viruses). Patients with evidence of liver disease underwent liver imaging and biopsy as clinically indicated. After transplantation, frequent examination and laboratory tests were required by protocol. Patients with symptoms, signs, or laboratory findings of liver dysfunction were evaluated by additional laboratory tests, liver imaging, and hepatology consultation as clinically indicated. The severity of liver injury was assessed by maximal total serum bilirubin measurements, prolongation of the prothrombin time, and development of hepatic encephalopathy. Patients whose maximal total serum bilirubin level was 68.4 μM (4 mg/dL) or greater were considered to have severe liver dysfunction based on the impact of this level of jaundice on nonrelapse mortality following myeloablative allogeneic HCT.1 The attribution of jaundice was made by an experienced hepatologist (G.B.M.) who reviewed data from all 51 patients whose peak total serum bilirubin level exceeded 68.4 μM (4 mg/dL). Of 51 patients, 18 had liver histology that was reviewed (4 patients had autopsy specimens and 14 transvenous biopsy specimens). The attribution of causes of jaundice was based on the clinical presentation; laboratory results; comorbid events such as sepsis, hypotension, or hepatotoxic drug exposure; liver imaging studies such as ultrasound or computed tomography (CT); liver histology if available; and histology from other organs affected by GVHD if hepatic GVHD was a possibility (eg, gastric, small intestine, colon, skin, or lip). A primary cause of each patient's liver injury was identified, followed by additional causes supported by evidence when liver injury was thought to be multifactorial. As cholangitis lenta frequently occurred in patients with active GVHD and these 2 factors appeared codependent, these 2 etiologies were combined under the term “cholestatic liver disease related to GVHD or sepsis” for the purposes of assigning causation of liver injury.
Statistical analysis
Survival was estimated by the Kaplan-Meier method. Evaluations of risk factors for survival following increase of total serum bilirubin level of 68.4 μM (4 mg/dL) or greater and for time to bilirubin level of 68.4 μM (4 mg/dL) or greater were performed using the proportional hazards regression model. For the latter end point, death prior to total serum bilirubin level of 68.4 μM (4 mg/dL) or greater was considered a competing event. Multivariate models were constructed in a stepwise fashion. P values in the multivariate models reflected the significance of the variable after adjusting for other variables in the model. All P values were derived from likelihood ratio statistics and were 2-sided.
Results
Frequency and severity of hepatic dysfunction
One hundred sixty-three (84%) of 193 patients developed some degree of hyperbilirubinemia (total serum bilirubin level > 20.5 μM [1.2 mg/dL]. Of these 163 patients, 112 had mild jaundice, that is, maximal total serum bilirubin levels < 68.4 μM (4 mg/dL) and 51 had maximal bilirubin levels ≥ 68.4 μM (4 mg/dL) (Table 2). More severe degrees of hyperbilirubinemia tended to occur later after transplantation, due in part to the progressive increase of serum bilirubin levels over time in patients with liver dysfunction.
Causes of hyperbilirubinemia
Hyperbilirubinemia with a peak less than 68.4 μM (4 mg/dL) was very common, particularly immediately after transplantation (median 11 days). We did not assign specific etiologies to episodes of mild transient hyperbilirubinemia, as clear-cut etiologies were often not apparent, and this phenomenon was not associated with a poor outcome. Of 51 patients with jaundice (bilirubin level ≥ 68.4 μM [4 mg/dL]), 18 had liver histology. The most common primary cause of bilirubin levels ≥ 68.4 μM (4 mg/dL) was cholestatic liver injury from GVHD or cholangitis lenta (n = 27). In 21 of these patients, GVHD appeared more prominent, while in 6 patients cholangitis lenta dominated the clinical picture. Other causes of hyperbilirubinemia ≥ 68.4 μM (4 mg/dL) included cholestasis related to CSP (n = 10), hemolysis (n = 5), decompensation of chronic liver disease (n = 3), ischemia (n = 3), metastatic carcinoma (n = 2), and labetalol hepatitis (n = 1). A diagnosis of CSP-related jaundice was made if CSP blood levels were high, if the timing of hyperbilirubinemia was temporally related to elevated blood levels, if no alternative explanation for jaundice was forthcoming, and if bilirubin levels declined coincident with lower CSP blood levels. No patient met diagnostic criteria for SOS/VOD. Table 2 shows the primary causes of jaundice according to the severity of hyperbilirubinemia.
Risk factors for developing serious liver dysfunction
Patients who developed hyperbilirubinemia ≥ 68.4 μM (4 mg/dL) were more likely to have received transplants for aggressive malignancies (hazard ratio [HR] 1.9; [95% confidence interval (CI), 1.0-3.4], P = .04) and to have received the Flu-containing regimen (HR 1.8; 95% CI, 0.9-3.7]; P = .07) by univariate analysis. Thirty-four patients had received fludarabine-based chemotherapy prior to the conditioning regimen. This did not correlate with the subsequent development of hyperbilirubinemia (HR 1.2; [95% CI, 0.6-2.3]; P = .66). The incidence of hyperbilirubinemia ≥ 68.4 μM (4 mg/dL) was significantly less in the patients who had received a prior transplant either independent of the nonmyeloablative HCT or as part of an auto-allo strategy (12/70, 17%), compared with patients with no prior transplantation (39/123, 31%; HR 0.5, P = .05). The proportion of patients classified as having aggressive disease was similar in the patients with a prior transplantation compared with those without such a history (54% vs 55%, respectively). However, of the 70 patients who had either a prior failed transplantation or a planned autologous transplantation preceding the nonmyeloablative conditioning, 58 (83%) had B-cell malignancies (ie, lymphoma, chronic lymphocytic leukemia [CLL], multiple myeloma [MM], amyloidosis) compared with only 35% in the nontransplantation group. Conversely, there was a paucity of patients with acute leukemia and myeloproliferative disorders in the prior transplantation group (17% vs 50%). In contrast, patient sex (HR 1.0; [95% CI, 0.6-1.8]; P = .92) and related versus unrelated donors (HR 1.1; [95% CI, 0.6-2.0]; P = .88) did not have any impact on the development of hyperbilirubinemia.
Three patients with known chronic liver disease before transplantation developed serious liver dysfunction after transplantation. A 43-year-old man with a history of exposure to hepatitis B and C viruses and excessive alcohol use was conditioned with 2 Gy TBI alone. Pretransplantation liver biopsy had demonstrated chronic hepatitis with moderate inflammatory activity, increased portal fibrosis, and portal-portal bridging. Maximal serum bilirubin level (188.1 μM [11 mg/dL]) occurred on day +5 after conditioning, and the patient remained persistently jaundiced until he died at day +183 of liver failure and relapsed myelodysplastic syndrome (RAEB-T). A 47-year-old man with Philadelphia chromosome–positive acute lymphocytic leukemia (ALL) in first complete remission and a history of hepatitis B and C had a pretransplantation biopsy showing severe necroinflammatory changes with portal-portal bridging related to chronic hepatitis. Lamivudine therapy was commenced prior to Flu/TBI conditioning and continued during the peritransplantation period; however, acute hepatic failure developed on day +52. A transjugular liver biopsy was performed on day +54 revealing a wedged hepatic venous pressure gradient of 28 mm Hg (normal ≤ 6 mm Hg). The necroinflammatory changes noted before transplantation were virtually absent in the posttransplantation biopsy; however, there was marked canalicular cholestasis associated with apoptotic bile duct changes consistent with GVHD. Maximum bilirubin level (666.9 μM [39 mg/dL]) occurred preterminally on day +57. A 54-year-old man with MDS/acute myeloid leukemia (AML) who had a history of excessive alcohol intake had abnormal serum AST levels before transplantation but no pretransplantation biopsy was available. After conditioning with Flu/TBI, total bilirubin level increased to 208.62 μM (12.2 mg/dL) by day +9. A liver biopsy was obtained that demonstrated diffuse hepatic fibrosis consistent with alcohol-induced chronic liver disease. The patient died on day +20 with hepatic failure.
Overall survival and nonrelapse mortality (NRM) as a function of hyperbilirubinemia
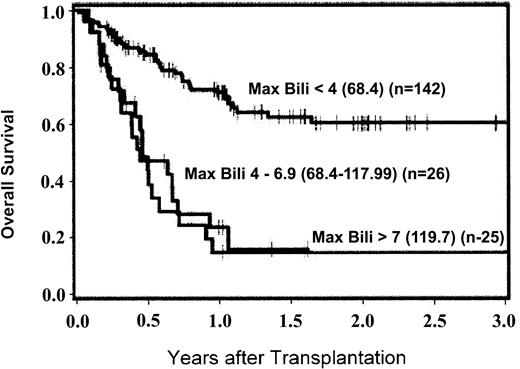
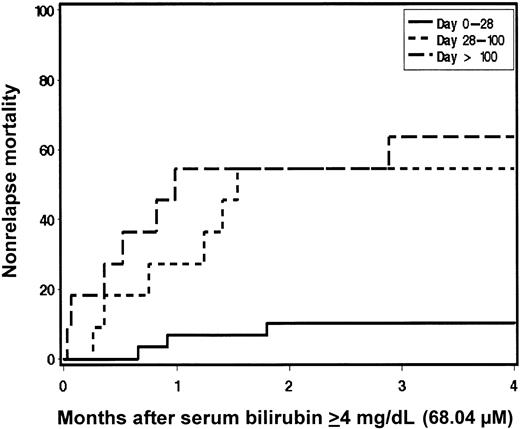
Kaplan-Meier estimates of survival were generated with patients stratified according to maximum total serum bilirubin level occurring in the first 200 days after HCT (Figure 1). Overall survival at 200 days and 1 year was superior for patients who had maximal serum bilirubin levels in the normal (88%, 78%, respectively) or minimally elevated (22.23-66.69 μM [1.3-3.9 mg/dL]) ranges (83%, 69%) compared with those with maximal serum bilirubin levels in the 68.4 to 117.99 μM (4-6.9 mg/dL; 44%, 20%), 119.7 to 169.29 μM (7.0-9.9 mg/dL; 69%, 17%), and ≥ 171.0 μM (10 mg/dL; 25%, 19%) groups. In general, patients who developed hyperbilirubinemia ≥ 68.4 μM (4 mg/dL) had a greater risk of mortality; however, hyperbilirubinemia occurring as a result of hemolysis appeared to have a favorable outcome with 4 of 5 patients surviving (HR 0.2; [95% CI, 0.0-1.4]; P = .03) (Table 3). In patients with bilirubin levels ≥ 68.4 μM (4 mg/dL), risk factors for death included peak bilirubin level occurring later in the posttransplantation course (HR 1.5 per month; [95% CI, 1.2-1.8]; P < .0001) and aggressive underlying malignancy (HR 3.0; [95% CI, 1.3-6.8]; P = .005) by multivariate analysis. Multivariate analysis of risk factors for NRM after developing bilirubin levels of ≥ 68.4 μM (4 mg/dL) was also performed. Delayed development of jaundice remained prominent as a risk factor for NRM (HR 1.9 per month; [95% CI, 1.4-2.5]; P < .0001). While exposure to Flu during conditioning may be associated with the development of hyperbilirubinemia, it was not an independent predictor of mortality after jaundice developed. The relationship between time of onset of hyperbilirubinemia and NRM is demonstrated in Figure 2. Patients who developed bilirubin levels ≥ 68.4 μM (4 mg/dL) later after transplantation had an increased risk of NRM compared with those who developed jaundice in the first 28 days (bilirubin levels ≥ 68.4 μM [4 mg/dL] first occurring between day 0 and day +28 [HR = 1.0]), between day +28 and day +100 (HR 4.8), and after day +100 (HR 7.2; P = .0007). The lower incidence of NRM in patients developing serum bilirubin levels ≥ 68.4 μM (4 mg/dL) early after HCT correlates with the lack of SOS/VOD while the occurrence of liver injury with bilirubin levels ≥ 68.4 μM (4 mg/dL) later after transplantation, commonly due to GVHD and cholangitis lenta, carried a poor prognosis.
Kaplan-Meier estimates of overall survival according to maximum total serum bilirubin in the first 200 days after transplantation. Bilirubin levels are in μM (mg/dL). Max Bili indicates maximum total serum bilirubin level.
Kaplan-Meier estimates of overall survival according to maximum total serum bilirubin in the first 200 days after transplantation. Bilirubin levels are in μM (mg/dL). Max Bili indicates maximum total serum bilirubin level.
Relationship between the timing of jaundice and nonrelapse mortality after transplantation.
Relationship between the timing of jaundice and nonrelapse mortality after transplantation.
Discussion
One of the expectations from less toxic, nonmyeloablative regimens was that the frequency of hepatic sinusoidal injury would be far less. A number of reports describe the frequency and severity of liver injury occurring after reduced-intensity conditioning regimens. Andrews et al8 described the toxicity of reduced-intensity conditioning consisting of Flu (180 mg/m2), busulfan (8 mg/kg), and rabbit antithymocyte globulin (40 mg/kg) in 26 patients undergoing allogeneic hematopoietic cell transplantation. SOS/VOD was observed in 13 (50%) patients and was graded as severe in 2 patients, moderate in 2 patients, and mild in 9 patients. In a subsequent report15 of 12 patients undergoing a second transplantation for relapsed or secondary malignancies, no case of SOS/VOD was observed using the same conditioning regimen. Schetelig et al16 described 50 patients with either hematologic malignancies (n = 44) or solid tumors (n = 6) also treated according to this regimen. HLA-identical siblings (n = 25), non–HLA-identical family members (n = 6), and unrelated volunteers (n = 19) donated hematopoietic cell grafts. GVHD prophylaxis consisted of CSP alone (n = 17) or in combination with methotrexate (n = 18) or MMF (n = 15). Six patients (12%) developed VOD with this moderately myelosuppressive regimen.
The frequency of bilirubin level greater than 68.4 μM (4 mg/dL) by 200 days in the current study was 26%. For comparison, data from 1419 consecutive recipients of allogeneic HCT after cyclophosphamide-based myeloablative conditioning from our institution have been analyzed, and the frequency of bilirubin level greater than 68.4 μM (4 mg/dL) by 100 days was 48%.17 We found that, although the development of a bilirubin level of greater than 68.4 μM (4 mg/dL) occurred less frequently in patients undergoing nonmyeloablative conditioning, the prognosis was equally poor once this degree of jaundice developed. Our data suggest that the incidence of SOS/VOD using minimally myelosuppressive conditioning is further reduced, as we could not identify a single patient who met published criteria for SOS/VOD. The predominant cause of hepatic dysfunction in our study was cholestatic liver injury, especially cholestasis related to GVHD and cholangitis lenta. Many patients with GVHD affecting the liver had concomitant sepsis, and both factors likely contributed to liver injury in these patients. Histologic findings of acute GVHD in the liver include pleomorphism and drop-out of epithelial cells of small bile ducts, mild hepatocyte necrosis, and cholestasis, often with minimal inflammatory infiltrate.18,19 There may be loss of recognizable bile ducts in severe cases. Cholangitis lenta, a common cause of jaundice following persistent fever or sepsis syndrome, is characterized histologically by dilated, peripherally located ductules that contain inspissated bile.20 Systemic and local infections are associated with the release of endotoxin and inflammatory cytokines (interleukin-1, interleukin-6, and tumor necrosis factor α), which can then act as potent cholestatic agents by inhibiting canalicular bile secretion.21,22 Our analysis cannot discriminate between jaundice as a biomarker of serious underlying problems (eg, GVHD, sepsis) and liver dysfunction as a contributor to death. Jaundice, per se, even in the absence of liver failure, is associated with a poor prognosis in numerous medical and surgical conditions. One hypothesis is that cholestasis leads to translocation of bacteria and endotoxin across gut mucosa, a phenomenon readily observed in animal models of cholestasis.23-26
The presence of Flu in the conditioning regimen was marginally associated with an increased risk of developing jaundice after HCT. Phase 1 studies of Flu outside of the transplantation setting generally do not report hepatotoxicity as a serious problem. A phase 1 study of Flu (80-260 mg/m2) as a single dose identified the dose-limiting toxicity as myelosuppression, which occurred at 160 mg/m2 in patients with solid tumors. Overall, there were 6 hepatic toxicity episodes (confined to minimal reversible elevation of AST and ALT) of a total of 41 evaluable courses administered. When Flu was given on a 5-day schedule at doses ranging from 15 to 40 mg/m2/day, there were 4 hepatic toxicity episodes (again confined to minimal reversible elevation of AST and ALT) of a total of 35 evaluable courses.27 These findings are confirmed in additional studies.28-30
The demonstration of Flu as a risk factor for the development of liver injury after TBI-based conditioning followed by allogeneic HCT raises the possibility that Flu may predispose patients to cholestatic liver injury from immunosuppressive drugs or allogeneic cells. Fludarabine facilitates rapid engraftment of donor cells, potentially predisposing to visceral GVHD. It is well documented that Flu does have a radio-sensitizing effect,31-36 which has been demonstrated to increase neurologic toxicity in animals after spinal radiation.37 To date, there are no reports of increased hepatotoxicity as a result of synergism between TBI and Flu. However, F-Ara (2-fluoroadenine 9-β-D-arabinofuranoside), an active metabolite of Flu, damages human microvascular endothelial cells and dermal and alveolar epithelial cell lines at pharmacologically relevant concentrations.38 In addition, intracellular adhesion molecule 1 (ICAM1) and major histocompatibility complex (MHC) class I molecules are up-regulated. Microvascular endothelial cells that are pretreated by F-Ara were more susceptible to lysis by allogeneic MHC class I–restricted cytotoxic T lymphocytes. This is consistent with the previously reported finding of increased nonrelapse mortality in patients receiving a Flu-containing regimen.12 Although no cases of hepatic sinusoidal injury were observed in our study population, it is possible that the addition of Flu to the conditioning regimen could contribute to subsequent hepatic toxicity by sensitizing the liver to injury from either TBI or allogeneic cells.
A less frequent cause of jaundice included cholestasis caused by CSP. This was not unexpected and may be due in part to a deliberate effort to target CSP levels at a higher therapeutic level (∼500 ng/mL) to facilitate engraftment in patients who received nonmyeloablative conditioning. CSP inhibits canalicular bile transport at pharmacologic doses in a dose-related manner39 and commonly causes increases in serum bilirubin levels.40 At very high blood levels, CSP can result in liver injury with hepatocyte necrosis and elevated serum hepatic aminotransferase enzyme levels. Patients with acute liver GVHD may have impaired elimination of CSP,41 and, in addition, CSP may contribute to compromised renal function, reducing the renal clearance of conjugated bilirubin and resulting in further elevation of total serum bilirubin level out of proportion to the degree of underlying liver dysfunction.
Because most patients with jaundice in this series had cholestatic liver injury (and not sinusoidal damage or hepatocellular necrosis), it may be possible to lessen the impact of cholestasis by use of ursodeoxycholic acid (ursodiol) as prophylaxis. The primary bile salts chenodeoxycholate and cholate normally comprise approximately 90% of bile salts in humans, while ursodiol is a hydrophilic bile acid that constitutes a small fraction of normal human bile. With oral administration of ursodiol this proportion can be increased to 40% to 50%, thereby replacing hydrophobic bile salts and creating a more favorable bile salt composition that is less toxic to biliary epithelium.42,43 In addition, ursodiol may have immunomodulatory properties that reduce immune-mediated liver damage.41 Ruutu et al44 reported a randomized trial using prophylactic ursodiol in 242 patients undergoing allogeneic HCT. There was a reduction in grades III to IV acute GVHD including a reduction in grades II to IV liver and gut GVHD. Overall survival was significantly better in the ursodiol-treated group, 71% versus 55%, (P = .02) with lower nonrelapse mortality, 19% versus 34% (P = .01). As expected, the incidence of SOS was not different between the 2 groups. This study provides evidence to support the use of ursodiol as a relatively nontoxic prophylaxis against cholestatic jaundice.
In conclusion, jaundice (total serum bilirubin level 68.4 μM [4 mg/dL] or greater) occurred in 26% of patients. Peak serum bilirubin level in the first 200 days after transplantation was a significant predictor of mortality. Development of jaundice was associated with a diagnosis of “aggressive” malignancy and marginally with the presence of Flu in the conditioning regimen. The combination of GVHD and cholangitis lenta was the most common cause of jaundice. No patient met published criteria for SOS despite the inclusion of patients who were ineligible for myeloablative HCT due to advanced age or medical comorbidities. Patients with established hepatic fibrosis were at high risk of fatal liver decompensation after transplantation, either because of exacerbation of underlying liver disease or because of the burden of additional cholestatic injury. Barely compensated chronic liver disease should be considered a contraindication even for minimally myelosuppressive conditioning regimens. Factors associated with reduced survival after established liver injury include aggressive malignancy and development of jaundice later after HCT. The prophylactic use of protective agents such as ursodiol should probably be considered in an effort to further reduce transplant-related mortality related to cholestatic jaundice.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-04-1311.
Supported in part by grants CA78902, CA18029, CA18221, CA15704, CA92058, and HL36444 awarded by the National Institutes of Health, Department of Health and Human Services (DHHS), Bethesda, MD. R.S. also received support from the Laura Landro Salomon Endowment Fund and a prize from the Josef Steiner Krebsstiftung, Bern, Switzerland. W.J.H. received support from a Mayo Foundation Scholarship, Division of Hematology, Mayo Medical Center, Rochester, MN.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.