Abstract
The neoplastic cells in classical Hodgkin disease (Reed-Sternberg cells) are of B-lymphoid origin, but they lack many markers of this cell lineage, for example, immunoglobulin, CD20, and B-cell–associated transcription factors. In contrast, the neoplastic cells (“L&H” cells) in lymphocyte predominance Hodgkin disease retain the molecular profile of germinal center B cells. In this paper, we investigated the expression in Hodgkin disease (45 cases and 3 cell lines) of 5 intracellular signaling molecules found in B cells. The Src family kinase Syk, the B-cell adaptor protein BLNK, and phospholipase C (PLC)–γ2 were consistently absent from Reed-Sternberg cells, whereas 2 other Src kinases (Lyn and Fyn) were heterogeneously expressed in a proportion of cases (12% and 42%, respectively). In contrast, the tumor cells in all cases of lymphocyte predominance Hodgkin disease were positive for Fyn, Syk, BLNK, and PLC-γ2, and Lyn immunostaining was seen in a minority of biopsies. These results indicate that in Reed-Sternberg cells, the defect in B-cell lineage marker expression includes a spectrum of molecules involved in intracellular signaling, a finding in keeping with recent gene expression profiling studies. Furthermore, the clear difference in expression of signaling proteins between the 2 major subtypes of Hodgkin disease may be of diagnostic value.
Introduction
It is widely recognized that the neoplastic cells in classical Hodgkin disease (Reed-Sternberg cells) are of B-cell origin, but it is also evident that they lack the most important functional molecule associated with this lineage, namely immunoglobulin. A possible cause for this defect was suggested by observations that they also lack B-cell–associated transcription factors (eg, BOB.1, OCT-2, and PU.1) that govern the expression of immunoglobulin.1,2 However, more recent studies have shown that Reed-Sternberg cells have a much broader defect in the expression of B-cell–associated molecules.3
These recent observations were based principally on micro-array profiling of gene expression in Hodgkin cell lines, and data concerning molecular abnormalities at the protein level are still limited. We recently have found that 5 intracellular molecules involved in B-cell signaling can be detected by immunohistologic labeling of routine tissue biopsies. These molecules tend to be selectively expressed in normal B cells (staining pattern comparable to CD20 labeling), and they are also expressed in most B-cell lymphomas (M.P. et al, unpublished data, August 2003). In the present paper, we report the expression profile of these novel B-cell markers in classical Hodgkin disease and compare these results with the patterns seen in lymphocyte predominance disease (in which the neoplastic cells are known to retain the expression of many B-cell–associated molecules).
Our results support the concept of a major disruption in the expression of functional B-cell–associated molecules in Reed-Sternberg cells that involves not only cell surface constituents and transcription factors but also proteins involved in intracellular B-cell signal transduction. The markers we report provide further evidence for the biologic differences between the 2 forms of Hodgkin disease and may also be of value for the diagnosis of Hodgkin disease and other lymphoid neoplasms.
Materials and methods
Tissue specimens
Paraffin-embedded biopsies of classical and lymphocyte predominance Hodgkin disease were retrieved from the files of the routine diagnostic service of the authors' institutions. In each case the diagnosis had been made on the basis of conventional histologic and immunohistologic examination, according to the criteria of the World Health Organization (WHO) classification.4 In all cases of classical Hodgkin disease, Reed-Sternberg cells expressed CD30 and CD15 but were negative for B- and/or T-cell markers. In contrast, the L&H cells in all cases of lymphocyte predominance Hodgkin disease expressed CD20 and CD79a but were CD30- and CD15-negative.
Immunohistochemistry
Four-micrometer sections were cut from paraffin blocks, coated on electrically charged slides (Surgipath Europe, Peterborough, United Kingdom), dewaxed, and rehydrated. Slides were subjected to conventional antigen retrieval protocol for 2 minutes using a pressure cooker in a solution (pH 9) containing Tris [tris(hydroxymethyl)aminomethane] (Trizma base) 50 mM/EDTA (ethylenediaminetetraacetic acid) (Sigma-Aldrich, Poole, United Kingdom) 2 mM. After this treatment, slides were placed in 1% hydrogen peroxide/methanol for 30 minutes to block endogenous peroxidase and then rinsed in Tris-buffered saline (TBS), pH 7.4, before incubation for 30 minutes at room temperature with antibodies to the following markers: CD3, CD15, CD20, CD30, Epstein-Barr virus–associated latent membrane protein-1 (LMP1), epithelial membrane antigen (EMA) and MIB-1 (Ki-67) (DakoCytomation, Glostrup, Denmark); CD79a (author's laboratory, Oxford, United Kingdom); Lyn, Fyn, Syk, BLNK, and phospholipase C (PLC)–γ2 (Santa Cruz Biotechnology, Santa Cruz, CA). Antibodies were diluted with 10% human serum (deactivated for 15 minutes at 50°C) in order to prevent nonspecific binding. An indirect immunoperoxidase technique (EnVision kit, DakoCytomation, Glostrup, Denmark) was used to detect sites of primary antibody binding.5
Cell pellets and cytospin preparations of cell lines were stained by the peroxidase-based EnVision+ method.5
Three authors of this study (T.M., M.-L.H., and D.Y.M.) independently evaluated the immunohistochemistry staining results. In each stained tissue section, normal cells (for example, lymphoid follicles, reactive lymphocytes) served as positive internal controls. Sections were stained in parallel without primary antibody to provide a negative control for each reaction.
Double labeling
Double immunofluorescence was performed by a previously described protocol6 using fluorochrome-conjugated anti-Ig reagents (species and/or subclass-specific) from Molecular Probes (Eugene, OR).
Cell lines
The B- and T-cell lymphoma cell lines (Raji, CCRF/CEM, and Jurkat) used in this study, as well as their sources, are detailed elsewhere.7 Hodgkin disease cell lines were obtained from the DSMZ cell collection, Braunschweig, Germany (cell lines KM-H2 and L428), or from Prof V. Diehl, University of Cologne, Germany (cell line L1236).
Cells were maintained in culture in RPMI 1640 containing 10% fetal calf serum (Invitrogen, Paisley, Scotland) and 1% streptomycin/penicillin at 37°C in 5% CO2. Aliquots of cell lines were centrifuged, washed in phosphate-buffered saline (PBS), and fixed for 48 hours in PBS/formalin buffer at room temperature. The cell pellets were then embedded in paraffin wax, as previously described.8 Cytospin preparations of cell lines were fixed in acetone for 10 minutes, air dried for 1 hour at room temperature, and stored at –80°C.
Results
Tissue sections
The immunocytochemical labeling reactions for the 5 molecules involved in intracellular B-cell signaling were assessed in paraffin-embedded biopsies from 45 cases of Hodgkin disease, and the results are summarized in Table 1. The antibodies stained mainly the cytoplasm of positive cells, although weak nuclear labeling was also seen with one of these markers (Syk) and Lyn tended to show a peripheral “membrane-associated” staining pattern.
Classical Hodgkin disease
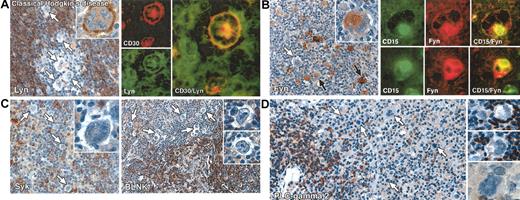
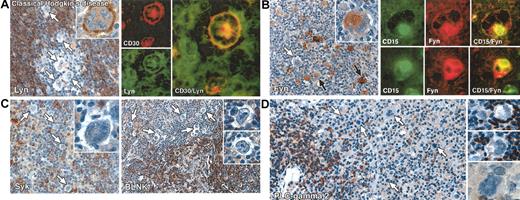
Immunostaining for 3 molecules, Syk, BLNK, and PLC-γ2, was consistently negative in Reed-Sternberg cells in all cases of classical Hodgkin disease (Figure 1). Lyn kinase also was essentially undetectable in most cases (21 of 24). The exceptions comprising 3 cases (8, 9, and 17) in which a substantial number of Lyn-positive Reed-Sternberg cells (Table 1; Figure 1) were seen, and 3 cases (1, 5, and 15) in which occasional Lyn-positive tumor cells were observed (Table 1; Figure 1). In contrast to the absence or reduced expression of these molecules, immunostaining for Fyn kinase was detected in Reed-Sternberg cells in 17 of 26 classical Hodgkin disease cases (Table 1); in 11 of these cases, plentiful positive cells were seen (from 30% to almost 100%) (Figure 1); in the remaining 6 cases, Fyn was found only in scattered Reed-Sternberg cells. Double immunofluorescent staining for Fyn in conjunction with CD15 in a representative case confirmed that the kinase was indeed expressed in Reed-Sternberg cells (Figure 1).
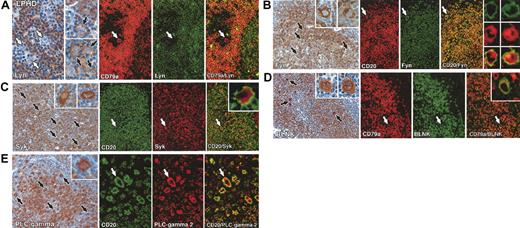
Immunolabeling for B-cell–associated signaling molecules in classical Hodgkin disease. All staining was performed on paraffin biopsies by immunoperoxidase or double immunofluorescent techniques. (A) Left: Reed-Sternberg cells are negative for Lyn kinase (white arrows), which is confined to reactive lymphocytes. A single Reed-Sternberg cell that shows cell-membrane–associated staining is seen in the inset. Right: Double immunofluorescent staining in an exceptional case of mixed cellularity disease (no. 17 in Table 1) in which Lyn was expressed (CD30, red; Lyn, green) confirms that this kinase is present in Reed-Sternberg cells. (B) Left: Heterogeneous immunostaining for Fyn in Reed-Sternberg cells in a case of mixed cellularity (no. 16 in Table 1), comprising both positive (black arrows) and negative tumor cells (white arrow). The inset shows clear cytoplasmic staining in a multinucleated Reed-Sternberg cell. Right: Double immunofluorescent labeling for CD15 (green) and Fyn (red) confirms the expression of Fyn by Reed-Sternberg cells. (C) Left: Reed-Sternberg cells (white arrows) and accompanying T cells are Syk negative, contrasting with Syk-positive reactive B cells. The inset shows a Syk-negative multinucleated Reed-Sternberg cell. Right: Reed-Sternberg cells are consistently BLNK negative (white arrows), as highlighted in the insets, contrasting with positive-reactive B cells. (D) Left and right: Reed-Sternberg cells are PLC-γ2 negative (white arrows), as highlighted in the insets. Reactive B cells are strongly PLC-γ2 positive. Original magnifications: × 20 (C, right panel), × 40 (A, left panel; B, left panel; C, left panel and right panel insets; and D), and × 60 (A, left panel inset and right panels; B, left panel inset and right panels; and C, left panel inset).
Immunolabeling for B-cell–associated signaling molecules in classical Hodgkin disease. All staining was performed on paraffin biopsies by immunoperoxidase or double immunofluorescent techniques. (A) Left: Reed-Sternberg cells are negative for Lyn kinase (white arrows), which is confined to reactive lymphocytes. A single Reed-Sternberg cell that shows cell-membrane–associated staining is seen in the inset. Right: Double immunofluorescent staining in an exceptional case of mixed cellularity disease (no. 17 in Table 1) in which Lyn was expressed (CD30, red; Lyn, green) confirms that this kinase is present in Reed-Sternberg cells. (B) Left: Heterogeneous immunostaining for Fyn in Reed-Sternberg cells in a case of mixed cellularity (no. 16 in Table 1), comprising both positive (black arrows) and negative tumor cells (white arrow). The inset shows clear cytoplasmic staining in a multinucleated Reed-Sternberg cell. Right: Double immunofluorescent labeling for CD15 (green) and Fyn (red) confirms the expression of Fyn by Reed-Sternberg cells. (C) Left: Reed-Sternberg cells (white arrows) and accompanying T cells are Syk negative, contrasting with Syk-positive reactive B cells. The inset shows a Syk-negative multinucleated Reed-Sternberg cell. Right: Reed-Sternberg cells are consistently BLNK negative (white arrows), as highlighted in the insets, contrasting with positive-reactive B cells. (D) Left and right: Reed-Sternberg cells are PLC-γ2 negative (white arrows), as highlighted in the insets. Reactive B cells are strongly PLC-γ2 positive. Original magnifications: × 20 (C, right panel), × 40 (A, left panel; B, left panel; C, left panel and right panel insets; and D), and × 60 (A, left panel inset and right panels; B, left panel inset and right panels; and C, left panel inset).
Lymphocyte predominance Hodgkin disease
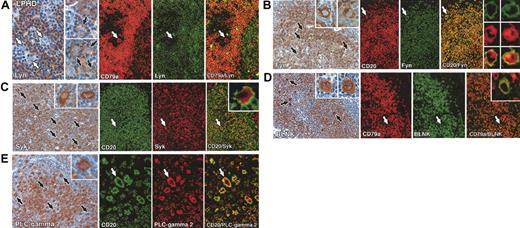
The staining patterns in this subtype were clearly different from those seen in classical Hodgkin disease. Fyn, Syk, BLNK, and PLC-γ2 were found in the great majority of L&H cells in all cases tested, in contrast to their absence (Syk, BLNK, PLC-γ2) or heterogeneous expression (Fyn) in the classical subtype. However, Lyn kinase, expressed by at least a minority of Reed-Sternberg cells in 25% of classical Hodgkin disease biopsies, was absent in 15 of 19 lymphocyte predominance disease cases (Figure 2; Table 1). Double immunofluorescent staining of a representative case confirmed that L&H cells (detected by labeling for CD20 or CD79a) coexpressed Fyn, Syk, BLNK, and PLC-γ2, but not Lyn (Figure 2).
Immunolabeling for B-cell–associated signaling molecules in lymphocyte predominance Hodgkin disease. All staining was performed on paraffin biopsies by immunoperoxidase or double immunofluorescent techniques. (A) Left: Two cases of lymphocyte predominance Hodgkin disease (nos. 4 and 7 in Table 1), showing Lyn-negative (white arrows) and Lyn-positive (black arrows) L&H cells. Right: Double immunofluorescent staining of another case (no. 6 in Table 1) shows an L&H cell (white arrow) that is CD79a positive but Lyn negative. (B) Left: Many L&H cells are Fyn positive (black arrows) in a case of lymphocyte predominance Hodgkin disease (no. 14 in Table 1) as seen at higher magnification in the insets. Right: Double immunofluorescent staining for CD20 (red) and Fyn (green) confirms coexpression of these markers in L&H cells (white arrows). The numerous mantle zone B cells in this field are also strongly CD20/Fyn positive. (C) Left: Strong reactivity of L&H cells (black arrows) for Syk, as seen at higher magnification in the insets. Right: Double immunofluorescent labeling confirms the coexpression of CD20 (green, membrane associated) and Syk (red, cytoplasmic) in L&H cells (white arrows). (D) Left: Numerous strongly BLNK-positive L&H cells (black arrows), seen at higher magnification in the insets, lie within nodules of BLNK-positive B cells. Right: Double immunofluorescence for CD79a (red) and BLNK (green) confirms coexpression of these markers in L&H cells (white arrows and inset). (E) Left: L&H cells are strongly PLC-γ2 positive (as seen at higher power in the inset). Right: Double immunofluorescence shows coexpression of CD20 (green, membrane associated) and PLC-γ2 (red, intracellular) in L&H cells (arrows). Original magnifications: × 20 (A-D, E left panel), × 40 (A-E insets, E right panel).
Immunolabeling for B-cell–associated signaling molecules in lymphocyte predominance Hodgkin disease. All staining was performed on paraffin biopsies by immunoperoxidase or double immunofluorescent techniques. (A) Left: Two cases of lymphocyte predominance Hodgkin disease (nos. 4 and 7 in Table 1), showing Lyn-negative (white arrows) and Lyn-positive (black arrows) L&H cells. Right: Double immunofluorescent staining of another case (no. 6 in Table 1) shows an L&H cell (white arrow) that is CD79a positive but Lyn negative. (B) Left: Many L&H cells are Fyn positive (black arrows) in a case of lymphocyte predominance Hodgkin disease (no. 14 in Table 1) as seen at higher magnification in the insets. Right: Double immunofluorescent staining for CD20 (red) and Fyn (green) confirms coexpression of these markers in L&H cells (white arrows). The numerous mantle zone B cells in this field are also strongly CD20/Fyn positive. (C) Left: Strong reactivity of L&H cells (black arrows) for Syk, as seen at higher magnification in the insets. Right: Double immunofluorescent labeling confirms the coexpression of CD20 (green, membrane associated) and Syk (red, cytoplasmic) in L&H cells (white arrows). (D) Left: Numerous strongly BLNK-positive L&H cells (black arrows), seen at higher magnification in the insets, lie within nodules of BLNK-positive B cells. Right: Double immunofluorescence for CD79a (red) and BLNK (green) confirms coexpression of these markers in L&H cells (white arrows and inset). (E) Left: L&H cells are strongly PLC-γ2 positive (as seen at higher power in the inset). Right: Double immunofluorescence shows coexpression of CD20 (green, membrane associated) and PLC-γ2 (red, intracellular) in L&H cells (arrows). Original magnifications: × 20 (A-D, E left panel), × 40 (A-E insets, E right panel).
Cell lines
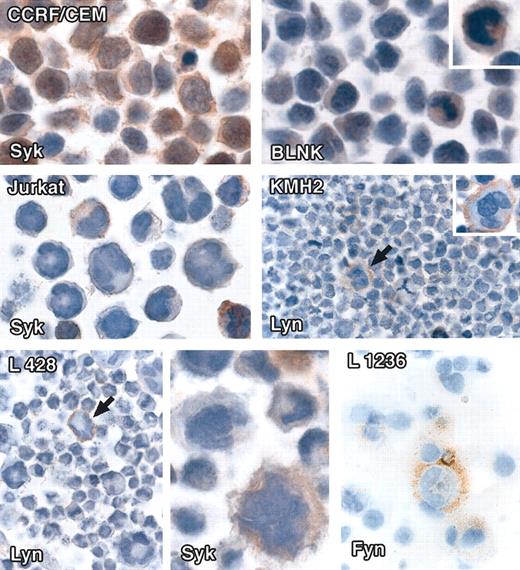
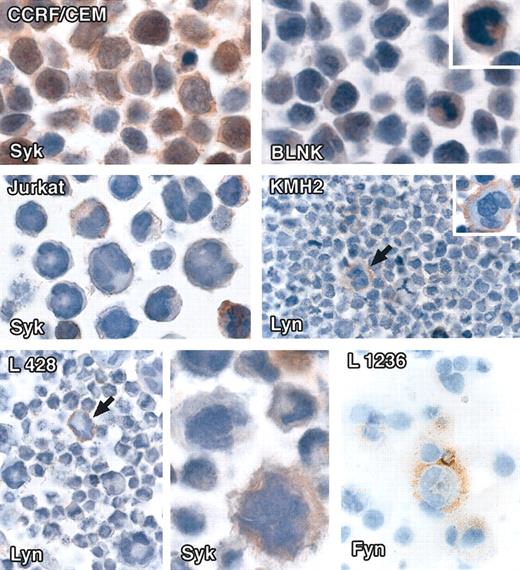
Three Hodgkin disease cell lines (KMH2, L428, and L1236) were essentially negative for all markers (Table 2), with the exception of rare single cells (that were usually only weakly labeled). In contrast, the B-cell lymphoma cell line Raji reacted strongly for all the 5 signaling molecules, whereas one T-cell lymphoma cell line, CCRF/CEM, showed nuclear and cytoplasmic immunostaining for Syk and BLNK in occasional cells and another (Jurkat) revealed cytoplasmic immunostaining for Syk in scattered cells (Table 2; Figure 3).
Immunostaining of cell lines for intracellular signaling molecules. All staining was performed on cell pellets and/or cytospins by the immunoperoxidase technique. (Top row) Left: The T-cell lymphoma line CCRF/CEM shows cytoplasmic and nuclear staining with anti-Syk antibody. Right: Scattered cells stain weakly with anti-BLNK. (Middle row) Left: Scattered cells in the Jurkat T-cell lymphoma line show weak Syk-cytoplasmic labeling. Right: Lyn membrane-associated labeling of rare cells in the Hodgkin disease cell line KMH2 (arrowed). (Bottom row) Left and center: Single cells in the L428 Hodgkin disease cell line show membrane-associated staining for Lyn (arrowed) and weak cytoplasmic labeling for Syk. Right: The periphery of a single cell in the L1236 Hodgkin disease cell line is stained for Fyn. Original magnifications: × 40 (top, middle, and left and right panels of bottom row), × 60 (bottom row, center panel).
Immunostaining of cell lines for intracellular signaling molecules. All staining was performed on cell pellets and/or cytospins by the immunoperoxidase technique. (Top row) Left: The T-cell lymphoma line CCRF/CEM shows cytoplasmic and nuclear staining with anti-Syk antibody. Right: Scattered cells stain weakly with anti-BLNK. (Middle row) Left: Scattered cells in the Jurkat T-cell lymphoma line show weak Syk-cytoplasmic labeling. Right: Lyn membrane-associated labeling of rare cells in the Hodgkin disease cell line KMH2 (arrowed). (Bottom row) Left and center: Single cells in the L428 Hodgkin disease cell line show membrane-associated staining for Lyn (arrowed) and weak cytoplasmic labeling for Syk. Right: The periphery of a single cell in the L1236 Hodgkin disease cell line is stained for Fyn. Original magnifications: × 40 (top, middle, and left and right panels of bottom row), × 60 (bottom row, center panel).
Discussion
The transduction of signals from the cell surface to the interior in lymphoid cells involves a complex interaction between multiple intracytoplasmic molecules.9 In the present study, we have explored the expression patterns of 5 signaling molecules (the tyrosine kinases Lyn, Fyn, and Syk, the linker protein BLNK, and the phospholipase PLC-γ2) in classical and lymphocyte predominance Hodgkin disease. Three of these molecules (Lyn, Syk, and BLNK) are involved in early signaling events initiated by antigen binding to the B-cell receptor (BCR). Following this step, tyrosine residues in the “immunoreceptor tyrosine-based activation motifs” (ITAMs) in the cytoplasmic portion of CD79 are phosphorylated by Lyn and Syk. This promotes the subsequent binding of the linker molecule BLNK to these ITAM regions.10-14 The adapter protein BLNK is a key molecule that couples Syk and Btk (Bruton tyrosine kinase), either in concert or sequentially, leading to activation of another molecule (PLC-γ2)15 investigated in this study. The Btk/PLC-γ2 signaling axis activates NF-κB, thereby regulating genes involved in cell proliferation and survival.16 The importance of PLC-γ2 in signaling17 is demonstrated by the impairment in knock-out mice of both B-cell maturation and the response to BCR ligation.18,19 The fifth molecule we studied, Fyn kinase, plays a role similar to that of Lyn and Syk in T cells (ie, by associating with the T-cell receptor20,21 ), but it has not been implicated previously in B-cell signaling.
We recently have shown that antibodies to 8 different molecules involved in intracellular signaling in human white cells can be used to label distinct leukocyte subpopulations in paraffin-embedded tissue sections (M. P. et al, unpublished data, August 2003). In the present paper, we performed immunostaining of Reed-Sternberg cells for 5 of these markers that are selectively expressed in B cells. Each of these markers gives a “pan–B-cell” staining pattern in normal/reactive lymphoid tissue (ie, comparable to a conventional marker such as CD20), with the exception of anti-Fyn, which selectively immunostains B cells of the mantle zone, showing little or no reactivity with germinal center B or “monocytoid” B cells (M. P. et al, unpublished data, August 2003). In addition, some plasma cells are positive for Syk and PLC-γ2. The immunoreactivity of these antibodies also has been tested on B-cell lymphomas. Preliminary data from more than 100 samples indicate that 3 of the markers (Syk, BLNK, and PLC-γ2) are expressed on the great majority of mature B-cell neoplasms, with the exception of plasmacytomas/myelomas (7 cases tested), which tended to be negative for Syk and BLNK but were often positive for PLC-γ2. Antibodies to Lyn and Fyn also stained many mature B-cell neoplasms and about half of the 7 plasmacytoma/myeloma samples. Furthermore, each of the latter 2 markers was absent in about 50% of diffuse large B-cell neoplasms, and it was noted that mantle cell lymphomas and chronic lymphocytic leukemia were often Fyn negative or only weakly labeled (M. P. et al, unpublished data, August 2003).
The immunostaining patterns for these 5 markers in Hodgkin disease reported in this paper should be interpreted in the light of previous immunohistologic and molecular studies, as summarized in Table 3. From these data it is evident that Reed-Sternberg cells, despite the clear evidence from molecular biology studies that they are of B-lymphoid origin,22-24 lack many of the markers associated with this lineage. However, it is also clear that this loss is selective: for example, 3 transcription factors (BOB.1, OCT-2, and PU.1) involved in the expression of B-cell–associated molecules (eg, immunoglobulin, CD20, CD79a) are commonly absent,1,2,25 whereas 2 other B-cell–associated transcription factors (BSAP/PAX-5 and MUM-1/IRF4), and also a marker of terminally differentiated B cells (CD138/syndecan-1), are usually preserved.26-30
The findings we present in this paper further confirm the aberrant phenotype of Reed-Sternberg cells, since we found absence (Syk, BLNK, PLC-γ2, and Lyn) or heterogeneous expression (Fyn) of molecules involved in intracellular B-cell signaling. Thus, Reed-Sternberg cells show a unique and partial defect in the expression of B-cell–associated signaling molecules, and this is in keeping with a recent RNA profiling study that also demonstrated absent or reduced expression of a number of genes encoding signal transduction proteins (Table 3), including components of BCR signaling pathways.3 These observations have been interpreted as indicating a major disruption of the B-cell gene expression program in Reed-Sternberg cells, possibly secondary to inactivation of a “master” gene. It would obviously be of considerable interest to identify the causal genetic defect, but the investigation of downstream consequences also may be of importance: the molecular alterations that transform cells into neoplastic Reed-Sternberg cells are presumably to be found among these abnormalities, and they may include targets susceptible to therapeutic intervention. In this context, the demonstration that BLNK and PLC-γ2 were absent from Reed-Sternberg cells may be of interest. One of the major functions of BLNK is to orchestrate the activation of PLC-γ2, a molecule intimately associated with intracellular Ca2+ mobilization and activation of NF-κB.16 The absence of BLNK and PLC-γ2 in Reed-Sternberg cells might therefore be relevant to the constitutionally enhanced activity of NF-κB that has been reported in these cells.31
It could be argued that the loss of B-cell–associated markers by Reed-Sternberg cells is not an acquired defect, but rather a physiologic phenomenon, reflecting down-regulation of these molecules in B cells undergoing terminal differentiation. In keeping with this is the expression by Reed-Sternberg cells of 2 plasma cell–associated markers (MUM-1/IRF4 and CD138). However, the former of these molecules is expressed in a wide spectrum of lymphoid neoplasms,32 including T-cell lymphomas, and cannot therefore be considered (in the context of lymphoid malignancy) specific for terminal B-cell differentiation, while CD138 is only variably expressed by Reed-Sternberg cells.33-37 It also may be noted that neither the morphologic features of Reed-Sternberg cells nor their anatomic location suggests a relationship to plasma cells. Reed-Sternberg cells lack many molecular hallmarks of plasma cells (ie, immunoglobulin, EMA, CD27, CD38, J-chain, VS38c antigen), and retain at least 2 B-cell–associated functional molecules that are down-regulated in plasma cells (namely, the PAX-5 transcription factor and the major histocompatibility complex [MHC] Class II antigen). In addition, as described above, plasma-cytomas/myelomas retain to some degree the expression of several of the signaling molecules we studied, and we also have noted that each of these molecules was present in most of 6 putative “plasmablastic” tumors that we investigated (T.M. et al, unpublished data, July 2003). This again argues that down-regulation of proteins because of plasma cell differentiation does not explain the loss of B-cell markers in Reed-Sternberg cells. Finally, it may be added that gene expression studies have shown that loss of many B-cell–associated molecules by Reed-Sternberg cells3 is not accompanied by appearance of a plasma cell–associated profile.38,39
In this study, we also investigated lymphocyte predominance Hodgkin disease, a disorder in which (in contrast to classical Hodgkin disease) the neoplastic cells retain many of the molecular markers of normal B cells (and show features in common with germinal center cells) (Table 3).1,40,41 Our data confirmed the clear difference between the 2 subtypes of Hodgkin disease,42 since L&H cells expressed 4 markers (Fyn, Syk, BLNK, and PLC-γ2) that tended to be lost from Reed-Sternberg cells. However, it was of interest that immunostaining for Lyn kinase was frequently negative, in contrast to the frequent positivity of normal B cells and B-cell lymphomas (M.P. et al, unpublished data, August 2003). This kinase aids in the recruitment of downstream kinases43 after its activation by antigen binding to BCR. B cells in Lyn-deficient knock-out mice are over-responsive to BCR cross-linking, and this is associated with activation of several kinases (eg, ERK1, ERK2, MEK1, and c-Jun) and the production of circulating autoantibodies.44-46 It will be of interest in the future to see if the absence of Lyn in L&H cells can be directly implicated in the genesis of lymphocyte predominance Hodgkin disease.
In conclusion, our results represent the first study in which data on abnormalities in Reed-Sternberg cells identified by gene expression profiling have been correlated with an immunohistologic study of protein expression in vivo using a panel of antibodies to B-cell–associated signaling molecules. It may be noted that our studies were performed on routine biopsies and could hence be extended to a much larger number of cases, whereas gene expression studies are feasible only when fresh tissue is available. Our findings indicate that a number of B-cell signal transducing molecules are absent or reduced in Reed-Sternberg cells, whereas they are largely preserved in L&H cells and in non-Hodgkin B-cell lymphomas, further supporting the idea that quite distinct oncogenic mechanisms underlie the 2 forms of Hodgkin disease. Furthermore, the immunohistologic detection of Syk, BLNK, and PLC-γ2 in routine processed tissue sections may be of value not only for studies of the nature of these 2 subtypes, but also for the differential diagnosis between Hodgkin disease and other lymphoma types.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-05-1487.
Supported by The Leukaemia Research Fund, United Kingdom (grant 9970, D.Y.M.), Alliance Recherche en Cancer (ARECA) network Proteomic and Cancer (G.D.), and Italian Association for Cancer Research (AIRC)-Milan (S.A.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors express their gratitude to Ralf Küppers for his help in the discussion of the data, Mrs Bridget Watson for assistance with the preparation of this manuscript, and Mr Ralph Liebertz for his technical help.