Abstract
Reconstitution of cellular immunity by 3 months after hematopoietic stem cell transplantation (HSCT) is a critical determinant of the long-term success of the transplantation. We analyzed the factors affecting recovery of cytomegalovirus (CMV)–specific CD4+ and CD8+ function at 3 months after HSCT by univariate and multivariable analyses including source of stem cells (bone marrow vs peripheral blood stem cells [PBSCs]), age, sex, graft-versus-host disease (GVHD), steroid use, conditioning regimens, ganciclovir use, HLA matching, circulating CMV antigenemia, absolute CD4+ and CD8+ counts, and donor CMV serology. High-dose steroids and CD4+ count less than 100 × 109/L were significant predictors of impaired CD4+ functional recovery in the multivariable analysis. High-dose steroids, bone marrow as a source of stem cells, and CD8+ count less than 50 × 109/L were associated with impaired CD8+ function in the multivariable analysis. Steroids were found to impair both CD4+ and CD8+ function in a dose-dependent manner. In the absence of high-dose steroids, low-level subclinical CMV antigenemia was found to stimulate both CD4+ and CD8+ functional recovery in recipients of ganciclovir prophylaxis. There was no difference in immune reconstitution between those who received prophylactic ganciclovir versus antigenemia-guided pre-emptive therapy. Thus, absolute CD4+ and CD8+ counts less than 100 × 109/L and 50 × 109/L, respectively; bone marrow as the source of stem cells; and high-dose steroid use all predict delayed recovery of functional T-cell immunity at 3 months after transplantation. Subclinical CMV reactivation while on ganciclovir appears to be a potent stimulator of T-cell function. These findings have implications for vaccination and adoptive-immunotherapy strategies in this population.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) has become the preferred treatment for many hematologic malignancies and conditions arising from errors in the development of the hematopoietic stem cell.1 After HSCT, defects in cellular and humoral immunity persist for over one year.2,3 Persistent deficiencies in immune system function lead to an increased risk of death from infection,4 relapse of the initial disorder,5 and development of secondary malignancy.6 Thus, reconstitution of the immune system plays a critical role in the long-term success of the transplantation procedure.
Infection due to cytomegalovirus (CMV) remains a significant cause of morbidity and mortality after HSCT. After allogeneic transplantation 60% to 70% of patients who are CMV seropositive will experience reactivation, and without ganciclovir prophylaxis or pre-emptive therapy, 20% to 30% of these will develop end-organ disease.7 Previous animal studies using murine CMV demonstrated a major role for CD8+ CMV-specific cytotoxic T lymphocytes (CTLs) in the control of viral replication.8-10 Evaluation of CTL function after allogeneic HSCT revealed that 50% of patients, in the absence of preventive ganciclovir therapy, exhibited a detectable CTL response by 3 months after allogeneic transplantation.11 Parallel evaluation of CD4+ function showed that the proliferative response to CMV antigen was significantly depressed at 2 months after transplantation and that restoration of CTL response appeared to be dependent on CD4+ recovery.12 These studies suggest that the recovery of CMV-specific CD4+ and CD8+ T cells is essential for controlling CMV disease after HSCT.13,14 Recently, several studies using tetramers to assess CMV-specific CD8+ T-cell counts have shown a correlation between the recovery of CMV-specific CD8+ T cells and protection from CMV disease.15-17
However, the factors influencing the recovery of CMV-specific CD4+ and CD8+ function after HSCT are poorly understood. Prior studies have identified that the prophylactic use of ganciclovir early in the posttransplantation period, donor CMV-seronegativity, the use of methylprednisolone, and grades II to IV graft-versus-host disease (GVHD) inhibit immune reconstitution and lead to a more permissive milieu for CMV replication.18,19 However, these data are derived from few studies with limited sample size, which do not allow for the analysis of the relative contribution of these interrelated factors. As such, we undertook an analysis of a large database of HSCT patients who were monitored for CMV reactivation over the course of their transplantation and T-cell function between days 80 and 100 after transplantation. We chose this time point for 2 reasons. First, absence of CMV-specific immunity by 3 months after HSCT has been shown to be significantly associated with late (after day 100) CMV reactivation and increased mortality.20,21 Second, by this time the majority of patients will have recovered sufficient lymphocyte counts to allow meaningful analysis of functional recovery.
Patients, materials, and methods
Study population
Two hundred one CMV-seropositive patients who underwent non–T-cell–depleted allogeneic peripheral blood stem cell (PBSC) transplantation or bone marrow transplantation (BMT) at the Fred Hutchinson Cancer Research Center (FHCRC) between 1993 and 2000 were analyzed. Of these 201 patients, 137 were also enrolled in a study designed to evaluate risk factors for late CMV disease and mortality, the results of which have been previously published.21 Patients were followed until death or last contact, which is at least 2 years for living patients.
All patients were followed and treated according to the standard practice guidelines for allogeneic HSCT recipients at the FHCRC. This includes weekly CMV pp65 antigenemia assays to monitor for CMV reactivation.20 Ganciclovir was administered as part of a study protocol either as prophylaxis at the time of engraftment or as pre-emptive treatment for antigenemia.20 Ganciclovir prescribed at engraftment was continued until day +100. Ganciclovir prescribed pre-emptively for antigenemia was given according to 2 protocols: (1) until day +100, regardless of subsequent pp65 values; or (2) for only 21 days if no further pp65 antigen was detected. In the latter group, recurrent or persistent antigenemia prompted another round of ganciclovir therapy for a minimum of 3 weeks or until resolution of antigenemia, whichever came later. The standard dose of ganciclovir was 5 mg/kg twice daily for one week, followed by 5 mg/kg once daily until day 100 after transplantation. Dose modifications were made for impaired renal function and ganciclovir was discontinued if the absolute neutrophil count dropped below 750 × 109/L for 2 consecutive days. Acute GVHD was routinely treated with steroids, starting with 1 to 2 mg/kg/d and tapering by 10% every 5 days or longer, depending on the clinical response. The Institutional Review Board of the FHCRC approved all studies and all patients signed informed consent prior to enrollment.
Data sources
Patient demographic information, as well as clinical, microbiologic, and laboratory data, are available through the electronic database at the FHCRC. Clinical information includes underlying disease, conditioning regimens, source of stem cells (PBSCs vs bone marrow), age, sex, donor CMV-serostatus, presence of GVHD, conditioning regimen, use of steroids for GVHD, HLA matching (matched related vs unrelated/mismatched), and use of ganciclovir. These were then evaluated as potential factors affecting CMV-specific immune reconstitution.
Lymphoproliferative assays
CMV-specific and phytohemagglutinin (PHA)–stimulated CD4+ Th-cell proliferation were assessed between days 80 and 100 after transplantation as previously described.12 Briefly, peripheral blood mononuclear cells were separated by density product centrifugation and were suspended at 2 × 106 cells/mL in RPMI with 10% human blood type AB serum, glutamine (4 mM), penicillin (100 U/mL), streptomycin (0.1 mg/mL), and amphotericin B (250 ng/mL). One hundred microliters of the cell suspension was dispensed into wells of 96 round-bottomed plates. CMV antigens were prepared by sonication and glycine extraction of virus grown on foreskin fibroblasts. CMV antigen or PHA (final concentration, 10 μg/mL) was added to triplicate wells containing mononuclear cells (MNCs) on day zero. The plates were incubated at 37°C in a humidified 5% carbon dioxide atmosphere for 4 days. Eighteen to 24 hours before harvest, tritium thymidine (0.074 MBq) was added to each well. Cells were harvested by using a semiautomated harvester, and counts per minute (cpm) were determined in a β-scintillation counter. Results are expressed as a stimulation index calculated by dividing the mean cpm of cells exposed to CMV antigen or PHA by the mean cpm of cells incubated with medium alone. A stimulation index of 3 or greater was considered positive as prior analyses of CMV-seronegative individuals revealed a mean stimulation index of 0.9 (range, 0.5-1.2) under identical conditions.12
Cytotoxic T-cell assays
CTL assays were performed at day 80 after transplantation to assess CD8+ T-cell function. Short-term CMV-specific CTL lines were generated as previously described.12 Briefly, skin biopsies were obtained from the marrow or PBSC donor to establish a fibroblast line for use as stimulator and target cells. Fibroblasts were propagated in Waymouth media supplemented with 20% fetal calf serum, penicillin (50 U/mL), and streptomycin (50 μg/mL). Peripheral blood mononuclear cells (PBMCs) were obtained from the transplant recipient and cultured at a responder-to-stimulator ratio of 20:1 with HLA-identical fibroblasts derived from the donor and infected for 6 hours with CMV (AD169 strain) at a multiplicity of infection (MOI) of 5. T-cell cultures were restimulated at 7 days with fresh CMV-infected fibroblasts and supplemented with autologous irradiated (33 Gy [3300 rad]) PBMCs as feeder cells, then fed with interleukin-2 (2 U/mL) at 2 and 4 days after restimulation. T-cell cultures were assayed for cytolytic activity in a 5-hour chromium release assay 6 to 7 days after restimulation at effectortarget (e/t) ratios of 10:1, 5:1, and 2.5:1. Each assay included CMV-infected and mock-infected fibroblasts, which were preincubated for 48 hours with 100 U/mL recombinant interferon γ (to increase the sensitivity of detection of CMV-specific CTLs) before infection with CMV (MOI of 5) and labeling with Cr51 for 16 to 20 hours. A positive CTL response was defined as lysis of HLA-identical CMV-infected targets at a level at least 10% greater than lysis of HLA-identical mock-infected and HLA class I–mismatched CMV-infected and mock-infected target cells. This definition of a positive response is based on the finding that cultures from CMV-seronegative individuals show less than 2% lysis of CMV-infected target cells over mock-infected cells.12
Determination of lymphocyte counts
CD4+ and CD8+ T-cell lymphocyte counts were determined by labeling PBMCs with specific monoclonal antibodies and subsequent analysis by 3-color flow cytometry as previously described.23
Statistics and data analysis
The primary end point of this study was the recovery of CMV-specific CD4+ and CD8+ function at 3 months after transplantation as determined by lymphoproliferative response (LPR) assays and CTL assays, respectively. Ganciclovir use was initially analyzed simply according to whether or not a patient received the medication; additional analysis was then performed according to whether ganciclovir was administered prophylactically at engraftment or pre-emptively for antigenemia, and by duration of ganciclovir use. Steroid use was evaluated according to the largest dose (0, 1 mg/kg/d, or 2 mg/kg/d) the patient received in the 2 weeks prior to sample collection for the immune assays as well as according to the cumulative dose (expressed in mg/kg) received in the 30 days prior to sample collection. Summary statistics including frequency counts and percentages for categorical variables and median for age were calculated. Comparisons from 2 × 2 tables were made using chi-squared and Fisher exact tests, as appropriate. Tests for trend in 2 × 3 tables were made using the Mantel-Haenszel chi-squared test. Comparisons of age at transplantation were performed using the Wilcoxon rank sum test. Multivariable logistic regression models were used to investigate the existence of independent, adjusted effects for the risk of CD4+ and CD8+ responses. Variables for the multivariable models were selected with backward stepwise elimination. Only those factors whose likelihood ratio P < .05 were retained. Profile likelihood confidence intervals were calculated.
Results
Patient characteristics
Patient characteristics are shown in Table 1. One hundred ninety-three patients had day-80 CD4+ LPR assays and 61 patients had day-80 CD8+ CTL assays available for analysis.
Host factors affecting immune reconstitution
The presence of grade II or greater GVHD, prednisone administration, the use of antithymocyte globulin (ATG; 10 mg/kg total dose) or total body irradiation (TBI) as part of the conditioning regimen, a CD4+ count less than 100 × 109/L, a CD8+ count of less than 100 × 109/L, and HLA-mismatched or unrelated transplant pairings were associated with delayed CD4+ functional recovery at 3 months after transplantation by univariate analysis (Table 2). Bone marrow as the source of stem cells, HLA mismatching or unrelated transplants, a CD8+ count of less than 50 × 109/L, and prednisone administration were associated with delayed CD8+ functional recovery at 3 months by univariate analysis (Table 2). Neither ganciclovir use nor donor CMV serology had any impact on CD4+ or CD8+ functional recovery (Table 2).
Based on the univariate analysis, we analyzed the use of steroids at a dose of more than 1 mg/kg/d, GVHD of grade II or higher, use of ganciclovir, HLA matching, source of stem cells, lymphopenia (a CD4+ count < 100 × 109/L or CD8+ count < 50 × 109/L), use of TBI, and use of ATG in a multivariable model since these were the factors with P ≤ .10 when compared with either CD4+ or CD8+ function in the univariate analysis. Low CD4+ count was not included in the CD8+ function model nor was low CD8+ count included in the CD4+ function model. The use of steroids and CD4+ lymphopenia remained statistically significant predictors of CD4+ functional recovery (Table 3). The use of steroids, the source of stem cells, and a CD8+ count less than 50 × 109/L remained significant with regards to CD8+ function, with recipients of PBSCs exhibiting better recovery by 3 months compared with those receiving bone marrow. As CD8+ CTL data were available in a minority of patients studied, in order to exclude a bias we compared steroid use, lymphopenia, and the source of stem cells between those who had the assay performed and those who did not. There was no difference in the source of stem cells (BM versus PBSCs) or in the incidence of lymphopenia between these 2 groups (data not shown). When the maximum steroid dose was categorized as 0 mg/kg, between 0 and 1 mg/kg, and more than 2 mg/kg, patients missing CTL data were on higher doses of steroids compared with those who had the assay performed (P = .01). This fact is notable in that even though fewer patients having the assay performed were on high-dose steroids compared with those missing CTL data, we still observed a significant effect of steroid dose on CTL response.
Effect of ganciclovir administration on immune reconstitution
A total of 154 patients (76.6%) received ganciclovir prior to day 80 after transplantation. LPR and CTL results were available for 149 (96.8%) and 51 (33.1%) patients, respectively, of those who received ganciclovir and for 44 (95.7%) and 10 (21.7%) patients, respectively, of those who did not receive ganciclovir (Table 2). Overall, the receipt of ganciclovir did not predict recovery of CD4+ or CD8+ function after transplantation (Table 3).
In order to assess whether ganciclovir strategy impacted CMV-specific immune reconstitution, we analyzed immune recovery in 60 patients who received ganciclovir prophylactically at engraftment as well as 94 patients who received pre-emptive ganciclovir for antigenemia. Overall, there was no significant difference between those who received prophylactic versus preemptive therapy; 35.3% and 44.1% of those receiving prophylactic ganciclovir recovered CD8+ and CD4+ function, respectively, by day 80, compared with 50.0% and 44.4%, respectively, of those receiving pre-emptive therapy (P not significant for both comparisons). We also analyzed whether the duration of ganciclovir treatment affected CD4+ or CD8+ functional recovery. There was no difference in either CD4+ or CD8+ functional recovery between those who received less than 6 weeks of pre-emptive ganciclovir therapy compared to those who received at least 6 weeks ganciclovir (data not shown).
Role of antigenemia in immune reconstitution
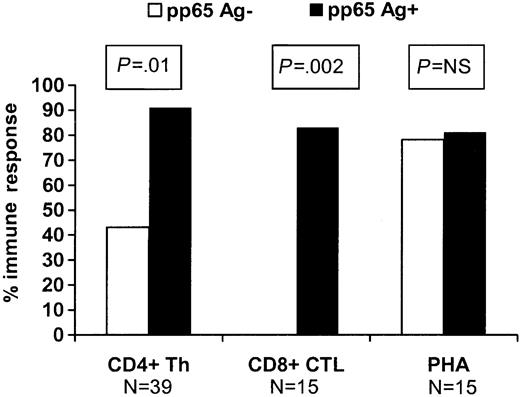
In order to evaluate whether circulating CMV antigenemia was associated with the more rapid appearance of a CMV-specific immune response in this population, we evaluated patients who received ganciclovir prophylactically at engraftment and compared the immune responses in 37 patients who remained antigenemianegative to 22 patients who developed “breakthrough” antigenemia of at least 1 cell/slide while on ganciclovir. Day-80 CD4+ and CD8+ responses were significantly better in those who developed breakthrough antigenemia compared with those who did not (P ≤ .01 for both comparisons; Figure 1). There was no difference in the incidence of GVHD, use of high-dose steroids (≥ 1 mg/kg/d), source of stem cells, HLA matching, and donor CMV serology between the 2 groups (data not shown).
Impact of subclinical pp65 antigenemia (≥ 1cell/slide) during the first 80 days on recovery of CMV-specific CD4+ Th responses and CD8+ CTL responses at 3 months among patients receiving prophylactic ganciclovir at engraftment. PHA indicates phytohemagglutinin and represents a nonspecific antigen recognized by CD4+ cells.
Impact of subclinical pp65 antigenemia (≥ 1cell/slide) during the first 80 days on recovery of CMV-specific CD4+ Th responses and CD8+ CTL responses at 3 months among patients receiving prophylactic ganciclovir at engraftment. PHA indicates phytohemagglutinin and represents a nonspecific antigen recognized by CD4+ cells.
In order to determine whether steroid use can suppress the stimulatory effect of circulating CMV antigenemia, we evaluated the steroid doses of those who experienced breakthrough antigenemia while on prophylactic ganciclovir. Of patients who were not receiving any steroids, 100% recovered CD4+ function, while only 62.5% of those on 1 mg/kg/d and 14.3% of those on 2 mg/kg/d of steroids recovered CD4+ function (P = .01). Therefore, it appears that high-dose steroids can override the inducing effect of CMV antigenemia on the recovery of the CMV-specific CD4+ functional response. Unfortunately a similar analysis could not be performed with regards to CD8+ function due to the low number of data points in this subgroup.
Impact of steroid dose on immune recovery
In the HSCT population, the dosage of steroids resulting in maximal immune suppression is unknown. In order to assess whether immune reconstitution was affected in a dose-dependent manner by steroids, we grouped patients according to whether they received no steroids, 1 mg/kg/d or less (maximum dose), or 2 mg/kg/d (maximum dose) within 2 weeks of the day-80 LPR and CTL analyses.
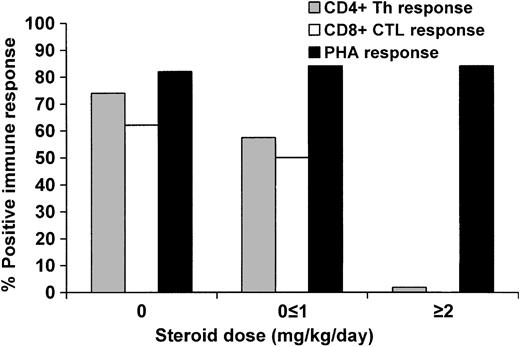
The use of steroids impaired CMV-specific CD4+ functional recovery at 3 months in a dose-dependent manner (Figure 2). Of those receiving 2 mg/kg/d of steroids, only 1 individual of 57 (1.8%) exhibited recovery of CD4+ function, compared with 35 of 61 (57.4%) on a dose of 1 mg/kg/d or less and 54 of 73 (74.0%) who received no steroids (P < .0001). In the multivariable model, only steroid use and CD4+ lymphopenia exhibited significant independent effects (Table 3). The use of steroids early in the posttransplantation course (by day 30) did not impact later recovery of CD4+ function, as 12 of 15 (80.0%) of those who were receiving 1 to 2 mg/kg/d on day 30 but were on less than 1 mg/kg/d of steroids by day 66 (2 weeks prior to sample collection for the LPR assay) had positive LPRs, compared with 20 of 22 (90.9%) of those who never received steroids (P not significant).
Proportion of patients who reconstituted CMV-specific CD4+ Th and CD8+ CTL function at 3 months when receiving 0, less than or equal to 1 mg/kg/d steroids, or greater than or equal to 2 mg/kg/d in the 2 weeks preceding sample collection.P < .0001 for CD4+ and P = .002 for CD8+ (test for trend).
Proportion of patients who reconstituted CMV-specific CD4+ Th and CD8+ CTL function at 3 months when receiving 0, less than or equal to 1 mg/kg/d steroids, or greater than or equal to 2 mg/kg/d in the 2 weeks preceding sample collection.P < .0001 for CD4+ and P = .002 for CD8+ (test for trend).
Similar results were observed when evaluating CD8+ functional response (Figure 2), although fewer data points were available for analysis. No individual of 10 receiving 2 mg/kg/d of steroids mounted a CD8+ response, compared to 11 of 22 (50.0%) receiving 1 mg/kg/d or less and 18 of 29 (62.1%) among those who were not receiving steroids (P = .002). The use of high-dose steroids, bone marrow as the source of stem cells, and CD8+ count less than 50 × 109/L had important negative effects with regards to CD8+ functional recovery in the multivariable model (Table 3). Five of 9 (55.6%) of those who received 1 to 2 mg/kg/d of steroids by day 30 but were on less than 1 mg/kg/d of steroids by day 80 exhibited CD8+ functional recovery, compared to 9 of 9 (100%) of those who never received steroids (P = .08).
We also analyzed the impact of the cumulative steroid dose received on T-cell functional recovery. We categorized the cumulative dose of steroids per kilogram body weight received during the 1-month period prior to specimen collection as no steroids, 10 mg/kg or less, or more than 10 mg/kg. High cumulative steroid dose predicted impaired CD4+ functional recovery (P < .0001) and there was a trend toward impaired CD8+ functional recovery (P = .06). Thus, both the maximum steroid dose as well as the cumulative amount of steroids received appear to negatively influence the recovery of both CD4+ and CD8+ function.
Relationship between absolute CD4+ and CD8+ counts and immune function
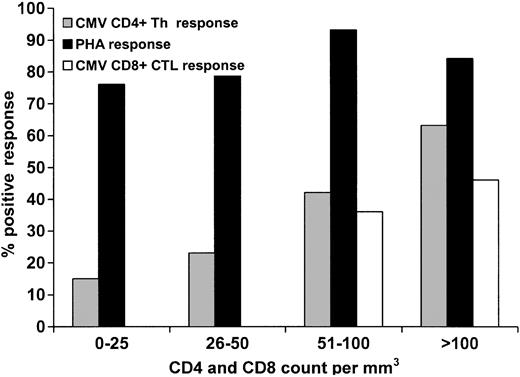
There was a direct correlation between positive CD4+ and CD8+ functional responses as the day 80 absolute CD4+ and CD8+ counts increased (Figure 3). A CD4+ count of less than 100 × 109/L was associated with impaired CMV-specific CD4+ functional response, while a CD8+ count of less than 100 × 109/L did not predict impaired CMV-specific CD8+ functional response (Table 2). However, no patient with a CD8+ count of less than 50 × 109/L exhibited a positive response, compared with 41.7% of those with a CD8+ count of at least 50 × 109/L (Figure 3). It appears that the restoration of the CMV-specific CD4+ Th cellular immune response is also dependent on the quantitative recovery of CD8+ cells, as those with low CD8+ counts had significantly worse CD4+ Th function (Table 2). This relationship was not significant when analyzing CD4+ counts and CTL responses, although there was a trend toward this direction in a group containing a small sample size (Table 2).
Effect of absolute CD4+ counts on CD4+ Th function (CMV and PHA) and CD8 counts on CMV-specific CD8+ CTL response. Patients with a CD4 cell count of at least 100 × 109/L had significantly higher likelihood of demonstrating a positive CMV-specific Th response than those with a count less than 100 × 109/L (P = .0006). There was no difference in CTL response between those who had a CD8+ count of at least 100 × 109/L versus those with a CD8+ count less than 100 × 109/L (P = .24); however, those with a CD8+ count of at least 50 × 109/L had a significantly higher likelihood of demonstrating a positive response versus those with a CD8+ count less than 50 × 109/L (P = .04).
Effect of absolute CD4+ counts on CD4+ Th function (CMV and PHA) and CD8 counts on CMV-specific CD8+ CTL response. Patients with a CD4 cell count of at least 100 × 109/L had significantly higher likelihood of demonstrating a positive CMV-specific Th response than those with a count less than 100 × 109/L (P = .0006). There was no difference in CTL response between those who had a CD8+ count of at least 100 × 109/L versus those with a CD8+ count less than 100 × 109/L (P = .24); however, those with a CD8+ count of at least 50 × 109/L had a significantly higher likelihood of demonstrating a positive response versus those with a CD8+ count less than 50 × 109/L (P = .04).
Discussion
Our analysis revealed several new observations regarding the reconstitution of CMV-specific T-cell immunity and the reactivation of CMV after HSCT. We found that steroids have a discernable dose-dependent effect on T-cell function and, somewhat surprisingly, did not find a significant effect of ganciclovir administration on late immune function when our cohort was evaluated according to usage strategy (prophylaxis versus pre-emptive). Other host factors affecting long-term functional immune restoration in a univariate analysis include the absolute CD4+ and CD8+ counts, the use of ATG or TBI in the conditioning regimen, grade II or greater GVHD, HLA matching, and the source of stem cells. Multivariable modeling revealed only steroids and CD4+ lymphopenia as predictive of impaired CD4+ function, whereas steroid use, severe CD8+ lymphopenia, and the source of stem cells remained significant for CD8+ functional recovery.
Our findings of steroid-induced immunosuppression are consistent with previous studies demonstrating that the risk of infection (and by inference the degree of immunosuppression) is directly related to the dose and duration of steroid administration.24,25 Steroids act in numerous ways to inhibit immune function26,27 and specifically block T-cell activation via their direct negative effects on interleukin-1 and -6 and thereby indirectly on interleukin-2.25 Our study demonstrates a dose-dependent effect of steroids on T-cell function as determined by CMV-specific CD4+ and CD8+ responses. These findings have implications regarding the tapering of steroids in patients with active infections normally controlled by T-cell responses, such as CMV disease and invasive fungal infections. The most common scenario where this would happen would be in the treatment of GVHD in the setting of an acute infection, in which case it is a balance between maintaining a dose of steroids sufficient to control GVHD and lowering the dose to boost immune function. Given the extremely high mortality rates of such infections, reducing steroids to levels at or below 1 mg/kg/d should be given serious consideration in this decision analysis.
Whether the effect of steroids is due purely to functional suppression versus steroid-induced T-cell depletion is an important question. Our evidence suggests that both CD4+ and CD8+ function return fairly quickly after discontinuing steroids, which supports the hypothesis that the dysfunction induced by steroids is due mainly to repression of function as opposed to deletion of T cells. Additionally, using tetramer studies combined with analyses of intracellular cytokine production, Ozdemir et al28 showed that steroid administration resulted in a significant impairment in CD8+ tumor necrosis factor α (TNFα) production but not a decrease in the frequency of CMV-specific CD8+ T cells.
Surprisingly, we did not find a significant delay in immune reconstitution attributable to ganciclovir administration strategy. It is thought that ganciclovir prophylaxis may inhibit the return of CMV-specific cellular immunity by suppressing CMV reactivation, resulting in a loss of immune stimulation. This effect was seen in a study that compared ganciclovir prophylaxis with culture-based pre-emptive therapy.12 The present study suggests that pre-emptive ganciclovir therapy based on antigenemia does not result in significantly improved immune reconstitution compared with prophylactic ganciclovir. Both the type of pre-emptive therapy (culture- vs antigenemia-based) and the slightly more intense prophylactic regimen in the study by Li et al12 (7 vs 6 days per week of ganciclovir maintenance) may explain this finding. Indeed, there was at least 30% lower rate of CMV-specific immune reconstitution by day 80 with ganciclovir prophylaxis in the study by Li et al12 compared with our study. If, as our data seems to indicate, reconstitution of functional CMV-specific immunity is driven by subclinical antigenemia, it is possible that ganciclovir prophylaxis, especially when given at lower doses, is not as effective at completely suppressing CMV antigenemia as we believe. Approximately one third of patients who received prophylactic ganciclovir at engraftment in this study developed breakthrough subclinical antigenemia. We hypothesize that the relatively high frequency of breakthrough subclinical CMV reactivation may stimulate CMV-specific immunity. Indeed, among recipients of ganciclovir at engraftment, patients who had breakthrough antigenemia had a significantly better recovery of T-cell function at 3 months compared with patients who remained antigenemia negative. Of note, this effect was only seen in the absence of high-dose steroids and all cases of breakthrough antigenemia were of low levels and of short duration; no patient developed CMV end-organ disease while receiving prophylaxis.20 However, all of these patients were reinduced with twice-daily ganciclovir. Similar findings have been shown with the subclinical reactivation of varicella-zoster virus after BMT.29
Cases of subclinical reactivation are thought to herald the development of ganciclovir resistance and lead to an increased incidence of CMV disease in the solid–organ transplant population30,31 ; no such data linking subclinical reactivation to ganciclovir resistance exists in the stem cell transplantation literature. Our data suggest that in the setting of HSCT and the absence of high-dose steroids, low-level, short-term antigenemia may, in fact, have a protective effect by enhancing late immune function. Although we did not analyze the incidence of late CMV infection and mortality in this study, undetectable CMV-specific CD4+ function at 3 months has been shown to be an independent risk factor for mortality after HSCT.21 While we do not know how durable or protective an immune response that develops while on antivirals is, our finding represents an interesting avenue for further investigation.
The finding that antigenemia results in a significant boost in cellular immunity provides support for the development of CMV vaccine strategies in this setting. Vaccination in this population is generally underused due to a number of factors, including a paucity of information regarding immunocompetence after transplantation.32-34 Current CMV vaccine strategies include recombinant CMVgB to induce neutralizing antibody, DNA vaccines, peptide vaccines, and whole-protein vaccines.35 The success of these will likely be determined as much by host factors as by the immunogenicity of the vaccine itself. Thus, knowledge of which recipient is most likely to mount an immune response to a given vaccine would be valuable information prior to attempts at vaccinating individuals after transplantation. The same principle can be applied to another method aimed at enhancing cellular immunity after transplantation, namely adoptive immunotherapy. Our results indicate a vaccination strategy might produce an immune response in the presence of early antiviral prophylaxis, which may result in both maximal protection against CMV disease early after transplantation when a vaccine alone is unlikely to be protective against disease, and a durable protection late after transplantation. However, our results also suggest that both adoptive transfer of T cells or vaccination is unlikely to be effective in patients who are receiving 2 mg/kg/d of steroids.
Also striking was the prolonged immunosuppressive effect of ATG on CD4+ functional recovery when used as part of the conditioning regimen. This finding supports those of preliminary reports indicating that high-dose ATG (> 10 mg/kg total dose) may result in delayed T-cell function.36,37 Studies evaluating the use of ATG in the conditioning regimen have shown an increased risk of Epstein-Barr virus reactivation and adenovirus disease only at high doses (15 mg/kg total dose compared with 7.5 mg/kg),36 but no effect on CMV reactivation.22,38 In the setting of solid organ transplantation, ATG has been associated with increased risk of CMV infection and disease when used primarily for acute organ rejection or delayed graft function.39 Other agents designed to delete T cells, such as alemtuzumab, have also been reported to be complicated by a high rate of CMV reactivation.40 While our findings need to be confirmed in larger studies, we feel that the use of these agents should be considered a risk factor for infections caused by organisms normally controlled by T-cell function, even up to months after their administration.
The significant correlation between CD4+ and CD8+ lymphopenia and lack of CMV-specific immune responses is consistent with the clinical observation that the vast majority of CMV disease in AIDS patients occurs when the CD4+ count drops below 50 × 109/L. The relative ease of measuring CD4+ and CD8+ counts makes this an inexpensive way to monitor for those at increased risk for CMV disease. A CD4+ count less than 100 × 109/L or a CD8+ count less than 50 × 109/L may entail more frequent pp65 or polymerase chain reaction (PCR) monitoring, extension of ganciclovir (whether pre-emptive, prophylactic, or therapeutic) treatment until the period of lymphopenia has resolved, or the identification of candidates for adoptive immunotherapy.
We could not find a significant impact of donor CMV serostatus on CMV-specific T-cell immunity at 3 months after transplantation. An effect of donor serostatus on posttransplantation CMV-specific immune reconstitution has been described in previous studies.12,41 However, this effect was observed either in a setting of T-cell depletion41 or at one month after transplantation.12 A possible explanation for this discrepancy is that our patients were not T-cell depleted and therefore had a high risk of GVHD. Thus at 3 months after transplantation, there was a significant use of high-dose corticosteroids that would be expected to diminish the effect of adoptively transferred immunity from the donor.
Our findings that recipients of peripheral blood stem cells have better functional CD8+ immune reconstitution than recipients of bone marrow, albeit in a relatively small subgroup, are in agreement with previous studies showing better quantitative and qualitative T-cell restoration after PBSCT compared with BMT.42-46 As we have shown that quantitative recovery of CD8+ populations is a factor influencing functional recovery, this difference is likely due to the fact that PBSC product contains roughly 10 times more lymphocytes than bone marrow.42,44,45 To test this hypothesis, tetramers can be used to assess the CMV-specific CD8+ T-cell content of bone marrow and PBSC product, which can be quantitatively compared and then correlated with recipient CMV-specific immune reconstitution and occurrence of CMV disease. Indeed, the presence of CMV-specific CD8+ T lymphocytes in CD34+ T-cell–depleted stem cell grafts as determined by tetramers has been associated with protection from CMV disease after transplantation in a small study.16
We chose to evaluate CMV-specific immunity with assays that measure the functional response of CD4+ and CD8+ cells to a broad range of CMV antigens. Recent studies using tetramers have proven very useful for assessing the frequency of antigen-specific CD8+ T cells and correlating this with CMV disease.15-17 However, it is important to acknowledge that tetramer-staining CD8+ T cells are not necessarily functional in vivo.47 Ozdemir et al28 used both tetramer staining and TNFα production as a marker of CD8+ T-cell function and found that the risk of CMV antigenemia after stem cell transplantation was more dependent on the functional recovery of CD8+ T cells not the absolute numbers of CMV-specific T cells. Thus, while tetramer staining provides a very useful method of monitoring quantitative T-cell recovery, it cannot be assumed that these T cells are functional and therefore protective against CMV replication and disease.
In summary, we identified several factors that significantly impair the recovery of CMV-specific T-cell immunity after HSCT and demonstrate that a subgroup of these patients are able to mount an immune response in response to circulating antigenemia. This information will help clinicians in terms of assessing the risk of infections normally mediated by T-cell responses as well as the management of steroids during episodes of active infection. Additionally, this information can be used to help optimally target vaccination or adoptive immunotherapy strategies in this population.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2002-11-3472.
Supported by CA18029 and 15704 from the National Institutes of Health, Bethesda, MD; 94-52 from the Milheim Foundation for Cancer Research, Denver, CO; and the Joel Meyers Endowment Fund, Seattle, WA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.