Abstract
Fourteen adults with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) were studied to evaluate the role of imatinib prior to allogeneic stem cell transplantation (SCT). Of these, 12 patients were in complete hematologic response (CHR), and 2 were refractory. Imatinib was administered as an interim schedule after each chemotherapy course. After the first imatinib cycle, 11 patients remained in sustained CHR with a decrease in the BCR-ABL/ABL ratios (0.89 logs), and one refractory patient achieved CHR. Meanwhile, 2 patients were resistant to imatinib. Ten patients receiving a second imatinib cycle following consolidation showed sustained CHR, including 2 molecular CR, with a further decrease in the BCR-ABL/ABL ratios (0.19 logs). Twelve patients underwent SCT in a favorable status, and of these, 11 are still alive in a leukemia-free status at 9 to 28+ months after SCT. First-line imatinib interim therapy appears to be a useful strategy to bridge the time to SCT for patients with Ph+ ALL.
Introduction
The Philadelphia chromosome (Ph) is the most frequent cytogenetic abnormality (20%-40%) in adult acute lymphoblastic leukemia (ALL).1-4 To date, only allogeneic stem cell transplantation (SCT) performed early in the remission status has resulted in long-term survival (35%-65%).5-12 Because the SCT results correlate with the pretransplantation leukemia burden, improved treatment strategies are needed. Recently, imatinib was demonstrated to induce overall responses in 60% to 70% of patients with relapsed/refractory Ph+ ALL.13-15 Unfortunately, the median time to progression is only 2 to 3 months, which reflects the development of resistance to imatinib. Considering the frequency and kinetics of resistance, it is possible that imatinib monotherapy is not sufficient as a first-line treatment for Ph+ ALL. We conducted a prospective phase 2 study to evaluate the minimal residual disease (MRD)–based role of imatinib as a first-line interim therapy prior to SCT. This study speculated that the achievement of a low MRD level with this strategy would improve the transplantation outcome.
Study design
Between September 2000 and February 2002, 18 (27.7%) of the 65 adults with ALL had Ph at the time of diagnosis. Of these, 14 patients who completed induction chemotherapy9 and intended to undergo allogeneic SCT were enrolled in this study. Four patients were excluded because of death during induction (n = 1) or lack of donors (n = 3). All patients in the study provided written informed consent that had been approved by the institutional review board of The Catholic University of Korea. After induction with recovery of white blood cell counts (≥ 3 × 109/L) and platelet counts (≥ 60 × 109/L), the first imatinib cycle (400-600 mg/d for 4 weeks) was started at the attending physician's discretion. Subsequently, most patients received consolidation with a high-dose cytarabinecontaining regimen followed by a second imatinib cycle bridging the time to SCT. The preparative regimen consisted of total body irradiation (1320 cGy) and cyclophosphamide (120 mg/kg). Cyclosporine plus methotrexate was used to prevent graft-versus-host disease (GVHD).16 The hematologic and molecular responses were defined using the standard criteria.14,17
For MRD monitoring, 118 marrow samples from all patients were analyzed by real-time quantitative polymerase chain reaction (RQ-PCR). The samples were collected at diagnosis, before and after imatinib therapy, and then at 3-month intervals after SCT. Total RNA was extracted using an RNAqueous kit (Ambion,Austin, TX), and reverse transcription was performed using 1 μg RNA. The plasmid standard titrations with the defined copy numbers for BCR-ABL and reference ABL were analyzed simultaneously with the patient samples. As previously described,16 we designed one set of primers for each type of BCR-ABL transcript, ABL, and TaqMan probes. RQ-PCR was performed in triplicate reactions using iCycler software 2.1 (Bio-Rad, Hercules, CA) with the standard conditions (95°C for 10 minutes, 50 cycles at 95°C for 15 seconds, and 60°C for 1 minute). The 50-μL PCR reaction mix contained 5 μL 1 × PCR buffer (4.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphate (dNTP), 0.2 μM primer, 140 nM TaqMan probe, 1.25 U AmpliTaq gold DNA polymerase, and 4 μL target cDNA). The quantity of the BCR-ABL transcript was normalized for ABL expression (sensitivity, 10–5).
Results and discussion
Table 1 summarizes the characteristics and treatment outcomes for the patients. Their median age was 38.5 years (range, 20-44 years). Karyotype analysis revealed additional chromosomal changes in 10 patients (71.4%). Eight patients (57.1%) had the p190BCR-ABL transcript, and the other 6 (42.9%) had the p210BCR-ABL transcript. At the time of enrollment, 12 patients (85.7%) had achieved a complete hematologic response (CHR) with median BCR-ABL/ABL ratios of 2.96 (0.26-7.90) × 10–3, whereas the other 2 (14.3%) were refractory.
Patient characteristics and treatment outcomes
. | . | . | . | . | . | Response to imatinib* . | . | . | GVHD (grade) . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age, y/sex . | BCR-ABL isoform . | Karyotype . | Status before imatinib . | Imatinib dose . | After first imatinib . | After second imatinib . | Pre-SCT status . | Acute . | Chronic . | Current LFS status; cause of death . | ||
| 1 | 39/F | p190 (e1a2) | Additional | CHR | 600 mg/d | CHR (-0.20) | Mol CR (UD) | Mol CR | + (II) | + (E) | Alive 28 + mo | ||
| 2 | 29/M | p210 (b3a2) | Additional | CHR | 400 mg/d | CHR (-0.89) | CHR (-0.27) | CHR | - | - | Alive 27 + mo | ||
| 3 | 40/M | p210 (b2a2) | Additional | CHR | 600 mg/d | CHR (-0.40) | CHR (-0.79) | CHR | - | + (L) | Alive 25 + mo | ||
| 4 | 39/F | p190 (e1a2) | Additional | CHR | 400 mg/d | CHR (-0.35) | Mol CR (UD) | Mol CR | - | - | Alive 24 + mo | ||
| 5 | 26/M | p190 (e1a2) | Ph alone | CHR | 400 mg/d | CHR (-1.22) | CHR (-0.31) | CHR | - | - | Alive 21 + mo | ||
| 6 | 20/M | p210 (b2a2) | Additional | CHR | 600 mg/d | CHR (-0.97) | CHR (-0.08) | CHR | - | + (L) | Alive 19 + mo | ||
| 7 | 43/F | p210 (b3a2) | Additional | CHR | 600 mg/d | CHR (-1.85) | CHR (-0.09) | CHR | + (III) | NA | Died 3 mo; TRM | ||
| 8 | 40/F | p190 (e1a2) | Ph alone | CHR | 600 mg/d | CHR (-0.50) | CHR (-1.09) | CHR | - | - | Alive 16 + mo | ||
| 9 | 31/M | p210 (b3a2) | Ph alone | CHR | 400 mg/d | CHR (-0.93) | CHR (-0.11) | CHR | - | - | Alive 14 + mo | ||
| 10 | 27/M | p190 (e1a2) | Additional | CHR | 600 mg/d | Relapse (+0.93) | Relapse (+1.63) | Relapse | NA | NA | Died before SCT; sepsis | ||
| 11 | 38/F | p190 (e1a2) | Additional | CHR | 400 mg/d | CHR (-0.20) | CHR (-0.07) | CHR | + (II) | + (E) | Alive 11 + mo | ||
| 12 | 39/M | p210 (b3a2) | Ph alone | Refractory | 600 mg/d | CHR (-0.89) | NA | CHR | - | + (L) | Alive 10 + mo | ||
| 13 | 30/M | p190 (e1a2) | Additional | CHR | 400 mg/d | CHR (-1.48) | NA | CHR | - | - | Alive 9 + mo | ||
| 14 | 44/M | p190 (e1a2) | Additional | Refractory | 600 mg/d | Refractory (+0.54) | NA | Refractory | + (IV) | NA | Died 4 mo; TRM | ||
. | . | . | . | . | . | Response to imatinib* . | . | . | GVHD (grade) . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age, y/sex . | BCR-ABL isoform . | Karyotype . | Status before imatinib . | Imatinib dose . | After first imatinib . | After second imatinib . | Pre-SCT status . | Acute . | Chronic . | Current LFS status; cause of death . | ||
| 1 | 39/F | p190 (e1a2) | Additional | CHR | 600 mg/d | CHR (-0.20) | Mol CR (UD) | Mol CR | + (II) | + (E) | Alive 28 + mo | ||
| 2 | 29/M | p210 (b3a2) | Additional | CHR | 400 mg/d | CHR (-0.89) | CHR (-0.27) | CHR | - | - | Alive 27 + mo | ||
| 3 | 40/M | p210 (b2a2) | Additional | CHR | 600 mg/d | CHR (-0.40) | CHR (-0.79) | CHR | - | + (L) | Alive 25 + mo | ||
| 4 | 39/F | p190 (e1a2) | Additional | CHR | 400 mg/d | CHR (-0.35) | Mol CR (UD) | Mol CR | - | - | Alive 24 + mo | ||
| 5 | 26/M | p190 (e1a2) | Ph alone | CHR | 400 mg/d | CHR (-1.22) | CHR (-0.31) | CHR | - | - | Alive 21 + mo | ||
| 6 | 20/M | p210 (b2a2) | Additional | CHR | 600 mg/d | CHR (-0.97) | CHR (-0.08) | CHR | - | + (L) | Alive 19 + mo | ||
| 7 | 43/F | p210 (b3a2) | Additional | CHR | 600 mg/d | CHR (-1.85) | CHR (-0.09) | CHR | + (III) | NA | Died 3 mo; TRM | ||
| 8 | 40/F | p190 (e1a2) | Ph alone | CHR | 600 mg/d | CHR (-0.50) | CHR (-1.09) | CHR | - | - | Alive 16 + mo | ||
| 9 | 31/M | p210 (b3a2) | Ph alone | CHR | 400 mg/d | CHR (-0.93) | CHR (-0.11) | CHR | - | - | Alive 14 + mo | ||
| 10 | 27/M | p190 (e1a2) | Additional | CHR | 600 mg/d | Relapse (+0.93) | Relapse (+1.63) | Relapse | NA | NA | Died before SCT; sepsis | ||
| 11 | 38/F | p190 (e1a2) | Additional | CHR | 400 mg/d | CHR (-0.20) | CHR (-0.07) | CHR | + (II) | + (E) | Alive 11 + mo | ||
| 12 | 39/M | p210 (b3a2) | Ph alone | Refractory | 600 mg/d | CHR (-0.89) | NA | CHR | - | + (L) | Alive 10 + mo | ||
| 13 | 30/M | p190 (e1a2) | Additional | CHR | 400 mg/d | CHR (-1.48) | NA | CHR | - | - | Alive 9 + mo | ||
| 14 | 44/M | p190 (e1a2) | Additional | Refractory | 600 mg/d | Refractory (+0.54) | NA | Refractory | + (IV) | NA | Died 4 mo; TRM | ||
LFS indicates leukemia-free survival; F, female; M, male; Mol CR, molecular complete remission; UD, undetectable; +, presence of GVHD; —, absence of GVHD; E, extensive; L, limited; NA, not available; and TRM, transplant-related mortality.
Values in parentheses indicate the changes of BCR-ABL/ABL ratio between each paired sample (before and after imatinib level).
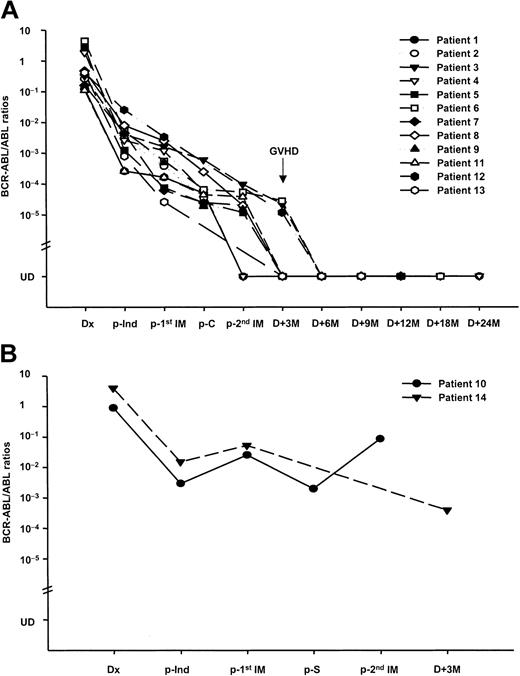
This study found that the kinetics of the BCR-ABL transcript correlated well with the patients' clinical course. After the first imatinib cycle, 11 of the 12 patients with CHR remained in sustained CHR, and their median BCR-ABL/ABL ratios decreased by 0.89 (0.20-1.85) logs. Meanwhile, one patient with CHR (no. 10) had a relapse with an increase in the MRD. In the remaining 2 refractory patients, one (no. 12) achieved a CHR, and the other patient (no. 14) showed no response with an increase in the MRD. Overall, the BCR-ABL/ABL ratios were decreased in 12 (85.7%) and increased in 2 (14.3%) patients after the first imatinib cycle (Figure 1). To date, this is the first report regarding the MRD-triggered role of the first-line imatinib therapy in Ph+ ALL. Recently, Scheuring et al17 reported the utility of MRD analysis using RQ-PCR in relapsed/refractory Ph+ ALL. They found that 40 (71%) of the 56 patients achieved a good response with a significant decrease in the MRD (1.37 logs in marrow) after the imatinib monotherapy, and the BCR-ABL levels had a predictive relevance to the response and progression-free survival. According to other reports and our experience,16,18-21 the BCR-ABL transcripts in Ph+ ALL have been monitored to assess the effect of therapy. Our findings should be explored in future large clinical trials.
Sequential assessment of the BCR-ABL kinetics. The 118 bone marrow samples from 14 Ph+ ALL patients treated with a first-line interim therapy of imatinib prior to allogeneic SCT were assessed by RQ-PCR. (A) Plot shows quantitative BCR-ABL values for 12 responders. After the first imatinib cycle, 11 patients remained in sustained CHR with a decrease in their BCR-ABL/ABL ratios (0.89 logs), and one patient (no. 12) who was refractory achieved a CHR. Of these, 10 patients received the second imatinib cycle following consolidation and showed a sustained CHR, including 2 molecular CRs, with a further decrease in the BCR-ABL/ABL ratios (0.19 logs). All patients underwent SCT in a favorable status, and of these, 11 are alive in a leukemia-free status 9 to 28+ months after SCT. An interesting phenomenon involving a decreased BCR-ABL/ABL ratio to a PCR-negative status after the development of GVHD induced by rapid cyclosporine withdrawal was observed in patient nos. 3, 6, and 12. (B) Plot shows quantitative BCR-ABL values for 2 nonresponders. One patient (no. 10) showing a CHR after chemotherapy developed an overt hematologic relapse with an increase in the BCR-ABL/ABL ratio after each imatinib cycle and died of sepsis prior to SCT. The other patient (no. 14) showed no response to the first imatinib cycle and proceeded directly to SCT. The patient died of refractory grade IV acute GVHD. The patients' characteristics and treatment outcomes are listed in Table 1. Dx indicates diagnosis; p, post; Ind, induction; C, consolidation; IM, imatinib; S, salvage chemotherapy; M, months; and UD, undetectable.
Sequential assessment of the BCR-ABL kinetics. The 118 bone marrow samples from 14 Ph+ ALL patients treated with a first-line interim therapy of imatinib prior to allogeneic SCT were assessed by RQ-PCR. (A) Plot shows quantitative BCR-ABL values for 12 responders. After the first imatinib cycle, 11 patients remained in sustained CHR with a decrease in their BCR-ABL/ABL ratios (0.89 logs), and one patient (no. 12) who was refractory achieved a CHR. Of these, 10 patients received the second imatinib cycle following consolidation and showed a sustained CHR, including 2 molecular CRs, with a further decrease in the BCR-ABL/ABL ratios (0.19 logs). All patients underwent SCT in a favorable status, and of these, 11 are alive in a leukemia-free status 9 to 28+ months after SCT. An interesting phenomenon involving a decreased BCR-ABL/ABL ratio to a PCR-negative status after the development of GVHD induced by rapid cyclosporine withdrawal was observed in patient nos. 3, 6, and 12. (B) Plot shows quantitative BCR-ABL values for 2 nonresponders. One patient (no. 10) showing a CHR after chemotherapy developed an overt hematologic relapse with an increase in the BCR-ABL/ABL ratio after each imatinib cycle and died of sepsis prior to SCT. The other patient (no. 14) showed no response to the first imatinib cycle and proceeded directly to SCT. The patient died of refractory grade IV acute GVHD. The patients' characteristics and treatment outcomes are listed in Table 1. Dx indicates diagnosis; p, post; Ind, induction; C, consolidation; IM, imatinib; S, salvage chemotherapy; M, months; and UD, undetectable.
Ten patients received the second imatinib cycle following consolidation chemotherapy. After the second imatinib cycle (Figure 1), the median BCR-ABL/ABL ratios decreased by 0.19 (0.07-1.09) logs in 8 patients. In addition, the BCR-ABL transcript was not detected in 2 patients (nos. 1 and 4). Scheuring et al17 also experienced molecular CR (albeit rare) after imatinib monotherapy. Meanwhile, one relapsed patient (no. 10) after the first imatinib cycle was also resistant to the second imatinib cycle and died of sepsis prior to SCT. The remaining 3 patients (nos. 12-14) proceeded directly to SCT after the first imatinib cycle. These findings suggest that imatinib therapy allows SCT in a more favorable status. Indeed, 12 of the 13 patients undergoing SCT had CHR (including 2 molecular CR) before SCT. Considering the high frequency of relapse or refractoriness to chemotherapy alone prior to SCT, the first-line imatinib therapy appears to be promising.
The conditioning regimen was started with a median interval of 7 days from the last day of the imatinib cycle. There are no data regarding the influence of imatinib on the outcome of transplantation and the optimal timing of the imatinib discontinuation. This study could not observe any detrimental effects of imatinib on the engraftment, GVHD, and transplant-related organ toxicity. After the SCT, 8 patients showed molecular CR, while the remaining 3 (nos. 3, 6, and 12) had residual leukemia at 3 months (Figure 1). However, their BCR-ABL/ABL ratios rapidly decreased to an undetectable status after the development of GVHD induced by cyclosporine withdrawal. To date, 11 patients are alive in a leukemia-free status 9 to 28+ months after SCT; the other 2 patients died of transplant-related complications. Previously, we demonstrated the possibility of GVHD-related antileukemic activity in Ph+ ALL.16 Other investigators have also reported a similar relationship between GVHD and the BCR-ABL transcript level in chronic myeloid leukemia.22,23 Within the limitations imposed by the small number of study patients, our data suggest that postgrafting immunomodulation based on the MRD level might have a possible role in controlling MRD in Ph+ leukemia.
In summary, first-line imatinib interim therapy appears to be a useful strategy to bridge the time to SCT for patients with Ph+ ALL. Further follow-up with a sizable population is needed to define the impact of imatinib on the long-term outcome of transplantation. If monitoring the MRD has the future potential to define the role of imatinib and to identify patients at risk of relapse or resistance, more tailored therapeutic interventions might be undertaken.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2003-04-1180.
Supported by grant no. R21-2002-000-00010-0 from the Basic Research Program of the Korea Science and Engineering Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to acknowledge the dedicated nurses of our chemotherapy and stem cell transplantation program, our fellows and house-staff, and the medical technicians for their expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal