Abstract
Reduced-intensity conditioning (RIC) regimens are increasingly used for allogeneic stem cell transplantation (allo-SCT). RIC has been shown to allow engraftment with minimal early transplantation-related mortality (TRM). However, in the context of RIC, predictive factors for acute and chronic graft-versus-host disease (aGVHD and cGVHD, respectively) and their effect on outcome remain unknown. In this report, we analyzed the outcome of 101 high-risk patients (70 hematologic and 31 nonhematologic malignancies) who received an HLA-identical sibling allo-SCT after RIC, including fludarabine, busulfan, and antithymocyte globulin (ATG). The cumulative incidence of grade II-IV aGVHD was 36% (95% confidence interval [CI], 27%-45%), whereas the cumulative incidence of cGVHD at 2 years was 43% (95% CI, 33%-53%). In multivariate analysis, the incidence of aGVHD was significantly associated with the ATG dose infused during conditioning (P = .0005), whereas peripheral blood as stem cell source was the only predictive factor for the development of cGVHD (P = .0007). The 1-year cumulative incidences of disease progression or relapse in patients with (n = 69) and without (n = 31) GVHD (whatever its form or grade) were 30% (95% CI, 19%-41%) and 55% (95% CI, 37%-72%), respectively (P = .02), suggesting that a potent graft-versus-tumor (GVT) effect can be achieved in high-risk patients following RIC. Moreover, the GVT effect was closely associated with GVHD without an increased risk of TRM (cumulative incidence of TRM, 18% [95% CI, 10%-25%]). Collectively, these results provide a framework for the refinement of RIC approaches designed to enhance the GVT effect with an acceptable risk of GVHD.
Introduction
Myeloablative chemoradiotherapy followed by allogeneic stem cell transplantation (allo-SCT) from HLA-identical siblings has become a curative treatment option for selected patients with a number of hematologic malignancies. Extensive clinical and experimental data support an important role for a graft-versustumor (GVT) effect in eradicating tumor cells in patients who receive allo-SCT.1,2 However, because of the high incidence of procedure-related toxicity, this procedure is often limited to younger patients in good medical condition, with graft-versus-host disease (GVHD) with older age and poorer pre–allo-SCT performance status being the major limiting factor.3-5 In an attempt to reduce procedure-related toxicity in elderly or heavily pretreated patients, or in patients with medical comorbidities precluding the use of myeloablative preparative regimens, or in patients with nonhematologic malignancies not eligible for myeloablative regimens, different reduced-intensity conditioning (RIC) regimens have been investigated. Several groups have shown that administration of highly immunosuppressive drugs, including fludarabine or antithymocyte globulin (ATG), or low-dose total body irradiation can result in durable donor cell engraftment.6-13 In the myeloablative allo-SCT setting, GVHD, both in its acute (aGVHD) and chronic (cGVHD) form, remains the major cause of nonrelapse mortality and morbidity in long-term survivors.4,14 However, it has also been shown that GVHD is associated with a powerful GVT effect protecting patients against relapse.4,15-20 Although promising preliminary results have been reported in regard to low early toxicity after RIC allo-SCT, only a few studies have specifically focused on the incidence and severity of GVHD in this setting. Our current knowledge of clinical GVHD is based primarily on results of analyses performed in the myeloablative allo-SCT setting. Risk factors for aGVHD and cGVHD are still poorly defined after RIC, and limited information is available on the association between the GVT effect and GVHD in this setting. This report describes the results of 101 patients with hematologic and nonhematologic malignancies who received an ATG, fludarabine, and busulfan–based RIC for allo-SCT from HLA-identical siblings. The aim of this analysis was to identify potential risk factors predicting for the development of aGVHD and cGVHD and to assess its effect on clinical outcome.
Patients and methods
Patients and donors
Study design. One hundred one consecutive patients who received a RIC allo-SCT from HLA-identical donors for hematologic and nonhematologic malignancies were included in this study. Patients were treated in a joint program between the Institut Paoli-Calmettes, Marseille, France (n = 75), and the Centre Jean-Perrin, Clermont-Ferrand, France (n = 26) between April 1998 and June 2002. Written informed consent was obtained from each patient and donor. The study was approved by the institutional review board of each participating center. All donors were HLA-A-, HLA-B-, and HLA-DR-identical siblings. All patients were treated with a RIC before allo-SCT because of high-risk clinical features that made them ineligible for our standard transplantation program. High risk was defined by the presence of one or more of the following features that precluded the use of standard myeloablative allo-SCT: (1) patient age older than 50 years; (2) patients with high-risk diagnoses for allo-SCT such as lymphoma and myeloma; (3) heavily pretreated patients with more than 2 lines of chemotherapy before allo-SCT, including patients with metastatic solid tumors; and (4) patients with poor performance status because of significant medical comorbidities. All patients received the preparative regimen as inpatients in private rooms and remained hospitalized until hematopoietic and clinical recovery. The primary aim of the study was to analyze engraftment, toxicity, and transplantation-related mortality (TRM). Other objectives included incidence of GVHD and disease response.
Conditioning regimen. The preparative regimen was adapted from that reported by Slavin et al,7 with fludarabine (Fludara; Schering AG, Lys-Lez-Lannoy, France) 30 mg/m2 for 6 consecutive days (administered intravenously over 30 minutes), oral busulfan 4 mg/kg per day for 2 consecutive days, and ATG (Thymoglobuline; IMTIX-Sangstat, Lyon, France) 2.5 mg/kg per day for 4, 3, or 1 days as indicated hereinafter (administered intravenously over 6 to 8 hours between day –4 to –1). As part of the protocol, although the setting of the study was not designed as a dose-finding setting, the ATG dose administered during conditioning was progressively decreased from initially 10 mg/kg to 2.5 mg/kg. The first 25 patients received the higher total ATG dose of 10 mg/kg, whereas the next 21 patients received a total dose of 7.5 mg/kg. The remaining last 55 patients received the lower total ATG dose of 2.5 mg/kg. For comparison of low versus high ATG dose, the limit of 2.5 mg/kg was defined as the median ATG dose received by the patients. Therefore, patients receiving 10 or 7.5 mg/kg ATG were considered as the high ATG dose group, whereas patients receiving 2.5 mg/kg represented the low ATG dose group.
Supportive care. Supportive care was similar to that reported previously in the myeloablative allo-SCT setting21 and included antibacterial prophylaxis with intravenous vancomycin at 2 g daily starting at day –2 (vancomycin was stopped as soon as the absolute neutrophil count [ANC] exceeded 0.5 × 109/L). Pneumocystis carinii prophylaxis included trimethoprim/sulfamethoxazole administered before transplantation and as soon as ANC exceeded 0.5 × 109/L twice weekly. Prophylaxis against herpes simplex virus included intravenous acyclovir or oral valacyclovir. Empiric broad-spectrum antibiotics were begun for temperature more than 38.5°C or clinical signs of infection. Hemoglobin was maintained through packed red blood cell transfusions at a level of 70 g/L, and the platelet count was maintained at 10 × 109/L. All blood products were filtered and irradiated. All patients received intravenous heparin (100 UI/kg) until ANC reached 0.5 × 109/L, to prevent venoocclusive disease (VOD).22
GVHD prophylaxis. GVHD prophylaxis included cyclosporine A (CsA) only at a dose of 3 mg/kg per day by continuous intravenous infusion starting from day –2 and changed to twice-daily oral dosing as soon as tolerated. CsA doses were adjusted to achieve blood levels between 150 and 250 ng/mL and to prevent renal dysfunction. CsA was tapered starting on day 90 if no GVHD appeared. CsA delivery (initial dose and route of administration) was comparable between the 2 participating centers.
Graft source. Forty-seven patients (47%) received a bone marrow (BM) graft collected under general anesthesia, whereas 54 patients (53%) received peripheral blood stem cells (PBSCs). For PBSC collection, donors were treated with granulocyte colony-stimulating factor (G-CSF) (Filgrastim; Amgen, Neuilly-sur-Seine, France) at a dose of 10 μg/kg per day for 5 days. Apheresis procedures were performed starting from day 5 of G-CSF treatment. The day of BM or PBSC infusion was designated as day 0. The graft was analyzed for content of hematopoietic progenitors (CD34+ cells) and CD3+ lymphoid cells using standard flow cytometry procedures.
Clinical outcomes and GVHD assessment
Clinical outcomes after transplantation that were recorded included time of neutrophil and platelet engraftment, time to start, and severity of aGVHD; cGVHD; disease relapse or progression and progression-free survival (PFS). Time to neutrophil engraftment was defined as the first of 3 consecutive days in which the ANC exceeded 0.5 × 109/L. Time to platelet engraftment was defined as the first of 3 days with 20 × 109/L without a need for platelet transfusion during a 5-day period. Acute GVHD was evaluated according to standard criteria.23 The diagnosis of cGVHD was made on the basis of both clinical and histology criteria of skin and other affected sites as previously described.24,25 Chronic GVHD was defined as any GVHD present after day 100. Extensive cGVHD was defined according to standard criteria.26 Chronic GVHD had a progressive onset if it followed as a direct extension of aGVHD. Quiescent-onset cGVHD developed after the resolution of aGVHD, whereas de novo cGVHD was not preceded by aGVHD.27 On diagnosis of grades II-IV aGVHD or extensive cGVHD, all patients were primarily treated with CsA and a corticosteroid-based regimen (2 mg/kg per day). Patients were considered off immunosuppression after being able to tolerate at least 15 days without the need for reinstitution of therapy. Second-line immunosuppressive regimen was defined as the initiation of secondary systemic immunosuppressive treatment replacing or being in addition to primary first-line systemic therapy because of refractory or progressive GVHD. Patients received various second-line therapies such as higher doses of corticosteroids (> 2 mg/kg per day), ATG, mycophenolic acid mofetil, extracorporeal photopheresis, or low-dose total lymphoid irradiation. For the purpose of this analysis, data relating to GVHD were captured on designated report forms from medical charts by M.M. and J.O.B.
Donor leukocyte infusions (DLIs)
Patients who relapsed or who showed evidence of disease progression or persistent disease without any sign of GVHD after immunosuppressive therapy withdrawal were candidates for DLI, ranging between 1 × 105 and 1 × 107 CD3+ cells/kg. Patients with mixed hematopoietic chimerism beyond 3 months after allo-SCT were also candidates for preemptive DLI. Donors underwent a leukapheresis without cytokine mobilization for donor lymphocyte procurement.
Assessment of response
Disease progression or relapse was defined as reemergence of the underlying disease (if a complete remission had been reached), increase in disease volume from a prior stable condition, or development of new areas of disease. TRM was defined as death without evidence of disease progression.
Statistics
All data were computed using SPSS for Windows (SPSS, Chicago, IL). The Mann-Whitney test was used for comparison of continuous variables. Categorical variables were compared using the chi-square test. The probability of developing aGVHD or cGVHD was depicted by calculating the cumulative incidence28 with relapse and death without relapse or aGVHD or cGVHD as competing risks.29 Cumulative incidence estimates were also used to measure the probability of relapse or progression.28 Probabilities of PFS and duration of immunosuppressive therapy were estimated from the time of transplantation using the Kaplan-Meier product-limit estimates.30 Differences between groups were tested using the log-rank test when Kaplan-Meier analysis was performed. The association of time to aGVHD, cGVHD, or disease progression or relapse, with the ATG dose infused during conditioning and other variables (age, sex, cytomegalovirus [CMV] serologic status, graft source [BM versus PBSC], risk of disease [standard risk versus advanced disease; standard risk was defined as chronic myeloid leukemia in first chronic phase, or acute leukemias in first complete remission], and ABO mismatch) was evaluated in a multivariate analysis, with the use of Cox proportional hazards regression model.31
Results
Patient characteristics and engraftment
The baseline characteristics and GVHD risk factors according to the ATG dosage group (recipient and donor age, sex, diagnosis, disease status, ABO mismatch, CMV status, graft source) are shown in Table 1. Briefly, the median age of recipients was 50 years (range, 17-61 years). Fifty-four donor-recipient pairs (53%) were sex-mismatched. Twenty-five patients received transplants for myeloid malignancies, whereas 45 patients were diagnosed with lymphoid malignancies. The remaining 31 patients were treated for metastatic nonhematologic malignancies (9 breast cancers, 8 renal cell cancers, 5 ovarian cancers, 4 melanoma, and 5 others). For the first 47 patients from this series, donor BM was infused rather than PBSCs because of concerns about the increased rate of cGVHD associated with PBSCs,32 in particular in this group of elderly and heavily pretreated patients.3 However, following data suggesting that PBSC transplantation may correlate with an increased GVT effect in high-risk patients,33,34 the graft source was switched to PBSCs for the remaining 54 patients. As expected, a higher number of CD34+ hematopoietic stem cells and CD3+ lymphoid cells were obtained and infused by peripheral collection of the donor hematopoietic stem cells (data not shown). As part of the protocol, the total ATG dose administered during conditioning was progressively decreased from initially 10 mg/kg to 2.5 mg/kg with the aim of modulating GVHD, and thereby the putative GVT effect that might be associated with GVHD. Forty-six patients received the high total ATG dose of 10 or 7.5 mg/kg, whereas the remaining 55 patients received the low ATG dose of 2.5 mg/kg. Overall, patients in this series received a median ATG dose of 2.5 mg/kg, giving a median total ATG dose of 200 mg (range, 75-1000 mg). All but one patient engrafted. This patient, who had refractory Hodgkin disease, died 7 days after allo-SCT from a progressive pneumonia. The remaining 100 patients reached a sustained ANC of more than 0.5 × 109/L at a median of 15 days (range, 9-23 days). Six patients failed to reach a sustained platelet count of more than 20 × 109/L prior to their death. Platelet engraftment occurred at a median of 14 days (range, 0-99 days).
GVHD
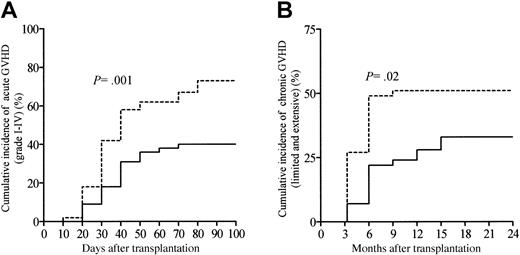
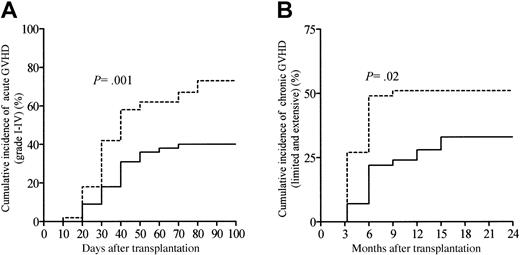
Table 2 summarizes transplantation-related events according to the ATG dosage group. One hundred patients in this series were evaluable for aGVHD. Twenty-two patients (22%; 95% confidence interval [CI], 14%-30%) developed grade I aGVHD. The cumulative incidences of grades II-IV and grades III-IV aGVHD were 36% (95% CI, 27%-45%) and 17% (95% CI, 10%-24%), respectively. The median time to onset of aGVHD was 30 days (range, 8-74 days) for the whole study population, with this being comparable between patients receiving the high ATG dose and patients receiving the low ATG dose (P = NS; Table 2). Overall, the cumulative incidence of grades I-IV aGVHD among all 100 patients at day 100 was 58% (95% CI, 48%-68%). In addition, the cumulative incidence of aGVHD was significantly lower in patients receiving the high ATG dose as compared with patients receiving the low ATG dose (P = .001; Figure 1A). The skin was the most common target of aGVHD, being involved in 57 patients (98%); the gastrointestinal tract was involved in 17 patients (29%), and the liver in 13 patients (22%). Sixty-eight patients (67%; 95% CI, 58%-76%) survived beyond day 100 and were evaluable for cGVHD. Overall, cGVHD developed in 43 patients at a median time of 118 days (range, 100-450 days) after allo-SCT, with a significantly later onset in patients receiving the high ATG dose as compared with patients receiving the low ATG dose (P = .008; Table 2). In the 68 patients evaluable for cGVHD, 17 developed limited cGVHD, whereas 26 developed clinical extensive cGVHD. Twelve patients (28%) experienced de novo cGVHD, whereas 31 patients (72%) had progressive or quiescent cGVHD (Table 2). The cumulative incidence of cGVHD (both limited and extensive cGVHD) at 2 years was 43% (95% CI, 33%-53%) with this being significantly different between patients receiving the high ATG dose as compared with patients receiving the low ATG dose (P = .02; Figure 1B). Immunosuppressive treatment duration and types were assessed in all patients included in this study. At time of last follow-up, 58 patients (58%) were off immunosuppressive therapy, and the median time for immunosuppressive treatments withdrawal was 96 days after allo-SCT (range, 10-810 days). By the first year following allo-SCT, 29% of the patients were still under immunosuppressive therapy. Among the 69 patients who experienced acute or cGVHD or both, 15 patients (22%) received high-dose corticosteroids (5 mg/kg per day or more) because of refractory aGVHD to the primary immunosuppressive regimen, including CsA and 2 mg/kg per day of steroids. Moreover, 23 GVHD patients (33%) needed a second-line immunosuppressive regimen replacing or being in addition to primary first-line systemic therapy, because of steroid-refractory or clinically worsening aGVHD (n = 14) or cGVHD (n = 9).
Cumulative incidence of GVHD according to the ATG dosage group. (A) Grades I-IV acute GVHD and (B) chronic GVHD (extensive and chronic). Patients receiving 10 or 7.5 mg/kg ATG were considered the high ATG dosage group (solid line), whereas patients receiving 2.5 mg/kg represented the low ATG dosage group (dashed line).
Cumulative incidence of GVHD according to the ATG dosage group. (A) Grades I-IV acute GVHD and (B) chronic GVHD (extensive and chronic). Patients receiving 10 or 7.5 mg/kg ATG were considered the high ATG dosage group (solid line), whereas patients receiving 2.5 mg/kg represented the low ATG dosage group (dashed line).
Risk factors for acute or chronic GVHD
Table 3 presents the results of univariate analysis of risk factors for development of aGVHD. Stem cell source and the ATG dose received during conditioning were the only variables associated with the risk of aGVHD in univariate analysis (Table 3). In the multivariate analysis, only a decreasing ATG dose infused during conditioning was associated with an increased risk of aGVHD (Table 4). We also assessed risk factors for development of cGVHD. Table 5 presents the results of univariate analysis of risk factors for development of cGVHD. Acute GVHD, ATG dose, and the stem cell source were the only variables significantly associated with an increased risk of cGVHD in univariate analysis (Table 5). In multivariate analysis, the use of PBSCs as stem cell source was the only factor associated with an increased probability of developing cGVHD (Table 4).
Donor leukocyte infusions
DLIs were given to establish full donor chimerism (n = 4) or to patients who relapsed or who showed evidence of disease progression or persistent disease without any sign of GVHD after immunosuppressive therapy withdrawal (n = 33). Overall, 37 patients (37%) in this series received up to 3 escalating doses of DLIs, starting at a median time of 135 days (range, 38-412 days) after allo-SCT. GVHD occurred in 12 patients (32%) among the 37 who received DLIs (2 grade I, 1 grade II, 9 grades III-IV). Among patients receiving DLIs, 9 (24%; 95% CI, 10%-38%) subsequently achieved complete or partial remission.
Transplantation-related mortality and outcome
Of the 101 patients included in this study, 52 died during the follow-up period, and 49 are still alive at a median follow-up of 413 days (range, 60-1326 days), of these 35 (71%; 95% CI, 58%-84%) achieved and remained in complete or partial remission (Table 6). Disease progression or relapse occurred in 40 patients (40%; 95% CI, 30%-50%) at a median time of 76 days (range, 6-781 days) after RIC allo-SCT. Thirty-four deaths were directly attributed to disease progression or relapse. Twelve deaths were attributed to aGVHD or cGVHD, whereas 5 patients died of infections. The overall cumulative incidence of transplantation-related mortality (n = 18) was 18% (95% CI, 10%-25%) at a median of 138 days (range, 7-420 days) after RIC allo-SCT. However, it is noteworthy that among these 18 patients, 2 patients died from DLI-related GVHD following disease progression. In addition, among the 18 patients who died from TRM, most (n = 13; 72%) were older than 50 years. Only 5 patients (5%; 95% CI, 1%-9%) died of transplantation-related toxicity before day 100. Among patients who died of TRM, 11 were in the group of 46 patients who received the higher ATG dose of 10 mg/kg or 7.5 mg/kg, whereas 7 were in the group of 55 patients who received the lower ATG dose of 2.5 mg/kg (P = NS).
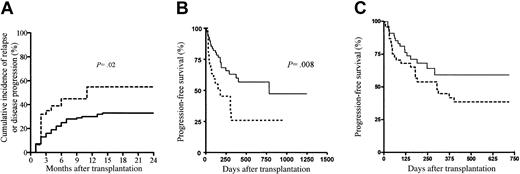
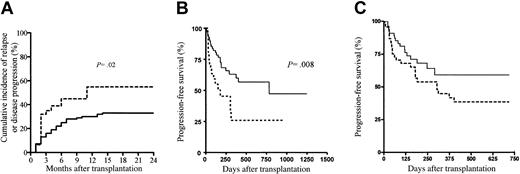
The Kaplan-Meier estimates of PFS at 2 years was 57% (95% CI, 42%-70%) in patients receiving a transplant for hematologic malignancies. Only 29% (95% CI, 14%-51%) of the patients with nonhematologic malignancies survived progression free in the first year after allo-SCT. The 1-year cumulative incidences of disease progression or relapse in patients with (n = 69) and without (n = 31) GVHD (whatever its form or grade) were 30% (95% CI, 19%-41%) and 55% (95% CI, 37%-72%), respectively (P = .02) (Figure 2A). When comparing the group of patients with GVHD and the group of patients without GVHD, apart from the ATG dose infused, all other relevant prognostic factors were not significantly different between the 2 groups. In multivariate analysis, only GVHD (whatever its form or grade) showed a protective effect on disease progression or relapse (Table 4). The latter finding translated into a significantly better PFS for patients with GVHD as compared with patients without GVHD (P = .008; Figure 2B) and, although not statistically significant, a tendency toward better PFS for patients receiving the low ATG dose as compared with patients receiving the high ATG dose (Figure 2C), thereby further confirming the beneficial role of GVHD on disease control following RIC allo-SCT.
Disease progression and progression-free survival. (A) Disease progression or relapse after allo-SCT in patients with (n = 69; solid line) and without (n = 31; dashed line) GVHD (whatever its form or grade), (B) progression-free survival according to GVHD, and (C) progression-free survival according to ATG dose. Dashed line indicates high ATG dose; solid line, low ATG dose.
Disease progression and progression-free survival. (A) Disease progression or relapse after allo-SCT in patients with (n = 69; solid line) and without (n = 31; dashed line) GVHD (whatever its form or grade), (B) progression-free survival according to GVHD, and (C) progression-free survival according to ATG dose. Dashed line indicates high ATG dose; solid line, low ATG dose.
Discussion
In this study, we have analyzed the incidence and effect of aGVHD and cGVHD in high-risk patients given HLA-identical allo-SCT following ATG-based RIC. Overall TRM did not exceed 18%, confirming the significantly lower TRM associated with RIC when compared with the 30% to 40% TRM rate reported with conventional myeloablative allo-SCT.3,5,35 Our results suggest that in a busulfan, fludarabine, and ATG–based RIC, the ATG dose infused during the conditioning is the major determinant for the likelihood of developing aGVHD. The incorporation of ATG as part of the RIC provided a powerful tool to modulate aGVHD without an increased risk of graft failure, TRM, or compromising disease response or occurrence of B-cell lymphoproliferative disorders that are usually observed after T-cell depletion for allo-SCT.36,37 Although previous studies have shown that removal of T cells from the graft by ex vivo T-cell depletion resulted in a dramatic decrease in aGVHD, this has been shown to be associated with a significant increase in graft failure and the risk of relapse,16,18-20,38,39 even in studies in which T-cell add-back have been investigated.40-43 An alternative strategy is to provide for in vivo T-cell depletion by using ATG, as part of the preparative regimen. In HLA-identical sibling allo-SCT, ATG that consists of polyclonal antibodies against a variety of cell subtypes has been shown to facilitate a sustained engraftment in patients with severe aplastic anemia.44 In this study, we show a dose-dependent effect of ATG on aGVHD, that is likely related to a dose-dependent in vivo T-cell depletion, efficiently modulating the risk of aGVHD.
The stem cell source (BM versus PBSC) was the only predictive factor for the development of cGVHD. Despite some variances in the incidence of cGVHD following PBSC transplantation, this is in accordance with data from the myeloablative setting, showing that the use of allogeneic PBSCs may be associated with a higher incidence of cGVHD.32,45-48 The biologic mechanisms accounting for this observed increase of cGVHD when using allogeneic PBSCs in both myeloablative and RIC allo-SCT remain unclear, and only minimum data are available to explain the higher incidence of cGVHD observed with allogeneic PBSCs as compared with BM. In addition, we did not find a significant association between aGVHD (whatever its grade) and the risk of cGVHD in multivariate analysis. Although the risk of cGVHD was found to be reduced by approximately 50% after ex vivo T-cell depletion,20 it has been reported that cGVHD was still noted in 85% of long-term survivors who received T-cell–depleted BM from unrelated donors,49 suggesting that dose-dependent in vivo T-cell depletion using ATG might not completely abrogate the risk of long-term cGVHD. In our series, the cumulative incidence of clinical extensive cGVHD did not exceed 26% at 2 years. This probability is at least half of the estimated probability of 40% to 70% described with myeloablative allo-SCT,29,50,51 and that probability might be expected to be significantly higher in elderly high-risk patients.3-5
In addition to a significant reduction in the rates of procedure-related toxicities, our study documents a strong protective GVT effect that is directly correlated to GVHD whatever its clinical form. Our results support the concept that a potent GVT effect can be induced following low-intensity allo-SCT, but this GVT effect cannot yet be reliably dissociated from GVHD. As already established in the myeloablative setting,2,4,52,53 and although further large specific studies are needed to identify the type of disease (eg, myeloid versus lymphoid malignancies) that is more likely to benefit from this GVT effect, GVHD appears to be a major determinant associated with lower progression or relapse achieved concurrent with the development of GVHD in the context of ATG-based RIC.
Overall, the results from our study provide a framework for the refinement and further development of low-intensity approaches designed to enhance the GVT effects of allo-SCT. Subtle changes such as in the ATG dose, the choice of the stem cell source, and the type of post–allo-SCT immunosuppression may have a significant effect on the probability of a favorable outcome. An increased knowledge of the effectors and targets of GVT and GVHD, significant clinical improvement, especially harnessing the GVT potential of allogeneic donor cells without clinically significant GVHD, are obvious aspects for investigations into improving the safety and outcome of low intensity or nonmyeloablative allo-SCT.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-12-3629.
Supported by grants from the Societé Francaise de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC), the Fondation de France, and the Fondation pour la Recherche Médicale (M.M).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ``advertisement'' in accordance with 18 U.S.C. section 1734.
We thank F. B. Petersen, MD (University of Utah Health Sciences Center, Salt Lake City) for helpful discussions and for critical reading of the manuscript. We thank the nursing staff for providing excellent care for our patients. We thank A. G. Le Coroller (INSERM U379, Marseille, France) for help with statistical analysis. We also thank the following physicians at the Institut Paoli-Calmettes for their important study contributions and dedicated patient care: J. A. Gastaut, F. Viret, R. T. Costello, J. M. Schiano de Collela, A. Charbonnier, R. Bouabdallah, T. Aurran, G. L. Damaj, V. Ivanov, G. Novakovitch, and P. Ladaique.