Abstract
Bone marrow (BM) contains hematopoietic stem cells (HSCs), which differentiate into every type of mature blood cell; endothelial cell progenitors; and marrow stromal cells, also called mesenchymal stem cells (MSCs), which can differentiate into mature cells of multiple mesenchymal tissues including fat, bone, and cartilage. Recent findings indicate that adult BM also contains cells that can differentiate into additional mature, nonhematopoietic cells of multiple tissues including epithelial cells of the liver, kidney, lung, skin, gastrointestinal (GI) tract, and myocytes of heart and skeletal muscle. Experimental results obtained in vitro and in vivo are the subject of this review. The emphasis is on how these experiments were performed and under what conditions differentiation from bone marrow to epithelial and neural cells occurs. Questions arise regarding whether tissue injury is necessary for this differentiation and the mechanisms by which it occurs. We also consider which bone marrow subpopulations are capable of this differentiation. Only after we have a better understanding of the mechanisms involved and of the cells required for this differentiation will we be able to fully harness adult stem cell plasticity for clinical purposes. (Blood. 2003; 102:3483-3493)
Introduction
By definition, stem cells are capable of both self-renewal and differentiation into at least one mature cell type. Stem cells are subclassified based on their species of origin, tissue of origin, and potential to differentiate into one or more specific types of mature cells. Some stem cells are more pluripotent than others. For example, the zygote and its immediate daughter cells are thought to be the only single cells capable of differentiating into any cell type, including all cells of the embryo, developing fetus, and adult organism, and extra-embryonic tissues such as the placenta. Thus, a fertilized egg is totipotent. Adult (postnatal) stem cells, although still pluripotent, have been thought to have even more limited differentiation ability and to be organ specific. For example, gastrointestinal crypt cells, oval cells of the liver, and type 2 pneumocytes of the lung all contribute to local tissue regeneration.
During the past 5 years, a series of exciting reports has challenged the hematopoietic specificity of marrow-derived stem cells.1 Studies on animals and humans document an expanding repertoire of potential for these cells (Table 1). This flexibility is termed “plasticity.” A widely accepted definition of plasticity has yet to be established, but, in general, this term refers to the newly discovered ability of adult stem cells to cross lineage barriers and to adopt the expression profiles and functional phenotypes of cells unique to other tissues. Many of the findings in this new field are controversial, in part because (1) few of the techniques used thus far to assess in vitro and in vivo plasticity are convincing, (2) existing paradigms of cellular differentiation do not yet include postnatal switching of cell fate, and (3) it is unclear how this phenomenon can be safely and reasonably exploited for therapeutic use in humans. This review considers these issues and presents the available data regarding stem cells resident in adult marrow.
Types of marrow-derived stem cells
Hematopoietic stem cells
The only true assay for the presence of hematopoietic stem cells (HSCs) is their ability to reconstitute the hematopoietic system of a myeloablated host. This is because hematopoietic reconstitution requires extensive self-renewal of the transplanted HSCs and their differentiation into every mature blood cell type. In mice and humans, multiple approaches have been used to isolate and characterize HSCs. For mice, many isolation protocols start with a lineage-depletion step in which all cells bearing lineage-specific markers (eg, CD11b for macrophages and granulocytes, CD3 for T cells, B220 for B cells, and Ter-119 for red blood cells) are removed. The resultant population, referred to as Lin-, is enriched 10- to 100-fold for HSCs. Further purification of HSCs from the Lin- population can be performed in several ways. Lin- cells that exclude rhodamine and Hoechst dyes (Hoechstlorhodaminelo)22 are greatly enriched for HSCs, as are Lin-, CD34+,23 and Lin-Sca+kit+Thy1lo populations.24 Certain populations of CD34-/Lin- cells also reconstitute hematopoiesis.25 In humans, the CD34+CD38- cell population is enriched for HSCs in humans. Side population (SP) cells are also enriched for HSCs. They are called SP cells because they have a unique ability to extrude Hoechst dye and because, when examined by fluorescence-activated cell sorter (FACS) analysis, they fall within a separate population to the side of the rest of the cells on a dot plot of emission data in the blue rather than the red spectrum. SP cells express the ABCG2 transporter, a transmembrane protein, which allows them to actively exclude Hoechst dye and fluoresce in this specific manner.26 SP cells are also present in other tissues, including skeletal muscle. Data are conflicting; some suggest that SP cells can be tissue-specific stem cells within these organs,27 and others suggest that they are actually bone marrow (BM)-derived SP cells lodged within these tissues.28-30
It has been hypothesized that cell subpopulations that home rapidly to the BM may be enriched for HSCs. When lineage-depleted donor BM cells are selected first by size fractionation and then by ability to home rapidly to the BM of a lethally irradiated host, a highly enriched HSC population can be obtained.31 A single cell provides long-term hematopoietic reconstitution 20% of the time when injected into lethally irradiated recipients.32
Marrow stromal cells
Marrow stromal cells (MSCs) derived in different laboratories using different techniques share 2 features: growth in culture as adherent cells with a finite life span and ability to differentiate into osteoblasts, chondroblasts, and adipocytes in response to appropriate stimuli. One of the main hindrances to our understanding of the full potential of MSCs has been confusion in the literature regarding what specifically defines an MSC and how it should be isolated and grown in vitro. A wide array of cytokines (eg, fibroblast growth factor 2 [FGF2], FGF4, platelet-derived growth factor-BB [PDGF-BB], leukemia inhibitory factor) and isolation techniques (eg, immunomagnetic and physical) have been used to identify and expand MSCs. Furthermore, no specific constellation of surface markers has been agreed on for these cells. Some of the surface antigens reported to be on these cells are Stro 1,33-35 CD13, α-integrins (CD49a and CD49b), β1-integrins (CD29), CD44 (hyaluronate), CD71 (transferrin), CD90 (thy-1), CD106 (vascular cell adhesion molecule-1 [VCAM-1]), and CD124 (interleukin-4 [IL-4] receptor).36 MSCs uniformly lack antigens such as CD45 that typically identify hematopoietic cells. Variations in the isolation techniques and culture media used to grow MSCs in different laboratories has led to variable findings regarding the differentiation potential of these cells. For example, some report that MSCs can differentiate in vitro into neuronal-type cells, whereas others cannot obtain this phenotype.37 To resolve these inconsistencies regarding MSCs, laboratories must collaborate to compare and contrast findings using uniform cell populations. Efforts are under way to disperse MSC populations that have been isolated to researchers outside each laboratory.
Multipotent adult progenitor cells
A population of highly plastic, adult-derived BM cells, referred to as multipotent adult progenitor cells (MAPCs), can be grown in vitro from postnatal marrow (and other organs38 ) of mice, rats, and humans.39 They copurify initially with MSCs and grow as adherent cells in vitro. However, unlike MSCs, MAPCs can be cultured indefinitely in a relatively nutrient-poor medium. Specific changes in growth factors induce differentiation of MAPCs into cells bearing endodermal, mesodermal, or ectodermal markers. MAPCs also display their broad differentiation potential in vivo. For these assays, MAPCs were derived from ROSA 26 mice, which express β-galactosidase under a ubiquitous promoter so that cells from these animals can be tracked. ROSA-26-derived MAPCs injected into murine blastocysts resulted in chimeric mice with ROSA-26 cells contributing to nearly all somatic tissues, including brain, lung, myocardium, liver, intestine, and kidney. After intravenous administration into a sublethally irradiated immunodeficient mouse, MAPCs differentiate to varying degrees into hematopoietic cells in the marrow, blood, and spleen and into epithelial cells in liver, lung, and intestine. Consistent with the ability to grow indefinitely in vitro and to self-renew in vivo, MAPCs express telomerase; telomere length is maintained after many cell doublings.40 It is not yet clear whether MAPCs are a distinct, rare subpopulation of MSCs normally present in humans or whether their in vivo potential is a phenomenon developed under unique in vitro cell culture conditions. Efforts to isolate naive, uncultured MAPCs are under way. In either case, the discovery of these highly plastic cells opens many promising new avenues of research.
Bone marrow stem cells
Several recent studies indicate that the same BM populations enriched for HSCs are also enriched for these highly plastic BM-derived stem cells (BMSCs). It is unclear whether BMSC populations are enriched for prehematopoietic cells that maintain greater pluripotentiality than HSCs or whether HSCs can transdifferentiate. An additional possibility is that a differentiated hematopoietic cell, such as a macrophage, may be able to take on the gene expression pattern of a different cell type by fusion with that cell type. Potential mechanisms of stem cell plasticity are addressed in further detail in this review. In order not to bias our understanding of their differentiation state at this early stage in our understanding, here we refer to the highly plastic cells in the BM as BMSCs. In addition, with the caveat that commitment may actually be reversible, we reserve the term “hematopoietic stem cells” (HSCs) for those cells already committed to a hematopoietic phenotype. Here we review data regarding the plasticity of BMSCs, assess methods for optimal study design, and discuss potential therapeutic applications of BMSC plasticity.
Considerations of study designs for assessment of bone marrow plasticity
To demonstrate BMSC plasticity, it is necessary to define as precisely as possible the stem cell population used (comprehensively outlined above), to target the stem cells to a tissue of interest and induce plasticity (see “Demonstration of bone marrow plasticity”), to identify and confirm that marrow-derived cells have the genome of the donor while sharing phenotypic and functional properties of the recipient organ, and to evaluate whether fusion has occurred (see “Mechanism of homing of bone marrow cells to the liver”).
Approaches for identifying donor-derived cells in target organs
To track the fate of BMSCs, researchers usually transplant BMSCs from a donor to a recipient who differs genotypically or phenotypically. An alternative to bone marrow transplantation is to link the circulatory system of 2 distinguishable mice without myeloablation or immunosuppression. This is referred to as parabiosis, and its advantage is that it causes minimal disturbance to the target tissues of the recipient mice. To date, the most commonly used donor-specific markers are the Y chromosome (in sex-mismatched transplantations) and transgenes. Another approach to identify donor-derived cells is to use normal mice with genetic polymorphisms that can be detected in all daughter cells.
Each of these approaches has advantages and disadvantages. Advantages to male-to-female transplantation for the detection of donor-derived cells are that the Y chromosome is present in every intact donor-derived cell and that whole-chromosome paint probes make it relatively easy to image the Y chromosome. Disadvantages to using the Y chromosome are that it cannot be visualized in all male-derived cells if thin tissue sections that only partially sample the nuclei are assessed and that it is technically difficult to use Y-chromosome fluorescence in situ hybridization (FISH) on thicker sections that contain the full thickness of most cells (larger than 10 microns). In addition, Y-chromosome staining can cause false-positive results because of cell overlay41 and potentially because of nonspecific binding of the probe. With experience and the use of proper negative controls, the latter problem can be avoided. Cell overlay is of particular concern because nonhematopoietic cells may be incorrectly identified as donor derived when small blood cells, which of course are marrow derived, overlie the cell of interest. Several approaches can be taken to rule out cell overlay, including analysis of serial sections, isolation of single cells, and inclusion of proper staining to identify blood cells. For transgene markers, cells from transgenic donor mice that express β-galactosidase or green fluorescence protein (GFP) are transplanted into recipients lacking the transgene. Alternatively, retroviral infection of donor cells can be used to “mark” individual donor cells. Because retroviruses stably insert into the genomic DNA of infected cells, they can be used to identify and track cell clones that will have the same insertion site. Cell-type-specific transgene expression not only identifies the cells as donor derived, it indicates that the donor-derived cell has initiated the gene expression pattern of a specific cell type. Care must be taken when interpreting the data of experiments using transgenic models because transgene silencing can occur42,43 and promiscuous gene expression, also known as gene priming, can potentially cause transgene expression in a wide variety of cells in which the cell-type-specific promoter may not generally be active.44,45
Identification of differentiated cell phenotype and function
Immunohistochemistry, immunofluorescence, and cell-type-specific functional assays can be used to identify and characterize the differentiated cells. When antibody-based approaches are used, it must be confirmed that cell labeling is not caused by nonspecific antibody staining or microscopic artifact. This is generally accomplished by performing appropriate isotype controls using adequate antibody concentrations, costaining with hematopoietic markers to rule out overlay with blood cells where relevant, and examining cells of interest in multiple tissue planes or as isolated single cells.
When possible, functional assays should be performed to further verify the identities of the BMSC-derived nonhematopoietic cells. This can be achieved by harvesting single donor-derived cells and assessing their function in vitro. However, the relatively small number of donor-derived cells makes it difficult to culture these cells successfully. Additionally, without a selective growth advantage in vitro, these cells may not be detectable. Therefore, the function of these cells is often inferred from cell-type-specific gene expression. Ideally, function should be demonstrated in vivo, such as by restoring the function of a nonhematopoietic organ in an injured or a mutant mouse after engrafting marrow-derived cells.
Demonstration of bone marrow plasticity
Bone marrow to liver
BMSC engraftment as hepatocytes using male-to-female BM transplantation in rats,46 mice,47 and humans48,49 (for a review, see Austin and Lagasse50 ) was first demonstrated in response to liver damage, which may promote BMSC-to-hepatocyte transition. In rats, a combination of hepatotoxin, which induces widespread liver damage, and 2-acetylaminofluorine, which prevents endogenous liver repair,51 was used. In these rats, a combination of Y chromosome FISH and transgene expression was used to confirm that BMSC cells were the source of the resultant hepatocytes. In mouse BM transplantation studies, the irradiation or chemotherapy used for myeloablation before BM transplantation caused liver damage, and donor-derived hepatocytes were identified using Y-chromosome FISH. The effect of other forms of liver damage could be assessed in liver samples from men who received orthotopic liver transplants from female donors. In these patients, the degree of subsequent damage to the transplanted liver correlated with the extent of male (host-derived) hepatocyte engraftment.49
In perhaps the most exciting demonstration of BMSC plasticity, transplantation of Lin-kit+Sca+Thy1lo (KTLS) BM cells to irradiated hosts was used to treat an inborn error of hepatic metabolism. Mice lacking the gene for fumarylacetoacetate hydrolase (FAH) develop progressive liver and kidney failure from tyrosinemia unless they are treated with NTBC (2-(2-nitro-4-trifluoro-methylbenzyol)-1,3-cyclohexanedione), a drug that prevents the breakdown of tyrosine to its toxic metabolites. Lethal irradiation followed by transplantation of as few as 50 purified KTLS cells from ROSA-26 (β-galactosidase+) mice allowed for the animals to be weaned from NTBCs and to survive with nearly normal liver function because of the ability of transplanted wild-type (FAH+) KTLS BMSCs to engraft as a renewable supply of functional hepatocytes.52 Donor-derived cells were identified by the expression of β-galactosidase and FAH, neither of which was present in the genome of the recipients. A major strength of this study is that the hepatocytes derived from BMSCs were shown to be functional. In this model, there is tremendous selective pressure for differentiating donor-derived cells into hepatocytes in livers with highly disordered architecture. Only a small number of BMSCs actually differentiated into hepatocytes, but expansion of these hepatocytes created a significant mass of donor-derived hepatocytes visualized by β-galactosidase staining. The mechanism by which BMSCs became functional hepatocytes in this system may be cell-cell fusion (see below).
Several studies have examined human liver after sex-mismatched orthotopic liver or BM transplantation. Male recipients of female livers and female recipients of male marrow have hepatocytes containing the Y chromosome,48,53 which, unless fusion has occurred between a Y+ marrow cell and an XX hepatocyte, can only be marrow derived. It has yet to be shown that these human hepatocytes are functional, and, given that a heterogeneous population of marrow cells was used, the identity of the human marrow-derived liver progenitor cannot be determined. These data are exciting and suggest that we may in future be able to provide in vivo replacement of diseased tissue without the need for whole organ transplantation.
We still do not know how cells are instructed to exit the marrow and engraft as nonhematopoietic cells. Control of this differentiation of BMSCs into hematopoietic and nonhematopoietic cell types likely involves the local microenvironment of the cells—first to mobilize the cells out of the marrow and then to recruit them to the damaged tissue. The prototypical stem cell environment is a niche in which the programming of pluripotent cells depends on the adjacent cells and the surrounding soluble and membrane-bound ligands. Many aspects of the BM microenvironment that control hematopoietic stem cell homing and adhesion have been elucidated,54-56 and it is possible that many of the same chemokines, growth factors, and adhesion molecules that regulate HSCs in the BM also play a role in regulating BMSCs. For example, stromal derived factor-1α (SDF-1α) and its receptor, CXCR4, are known to play a role in the homing of HSCs to the BM. Consistent with this, SDF-1α is expressed by hepatocytes in response to liver injury in which oval cell repair is induced.57 Because oval cells can be derived from the BM and HSCs and oval cells express CXCR4, it is possible that an SDF-1α gradient from the liver plays a role in mobilizing BMSCs from the marrow to the circulation with a homing effect to the injured liver.57 These experiments represent the early stage of our studies to elucidate the mechanism by which BMSC-to-epithelial cell differentiation occurs.
Bone marrow to heart/cardiac muscle
The functional pluripotentiality of BM-derived cells includes cardiac muscle. Therapeutic benefit has been demonstrated in mice with experimentally induced myocardial infarcts that receive intracardiac injection of whole marrow (or kit+ BM cells) during the initial period after infarction. BMSCs may improve outcomes after infarction by at least 3 pathways: increased vascularity from BM cells differentiating into endothelial cells, myogenic repair from differentiation of BMSCs into cardiac myocytes, and production of cytokines or other factors that promote myogenic repair and prevent fibrosis.58 A related study demonstrates that stem cell factor (SCF) and granulocyte-colony-stimulating factor (G-CSF), which increase the circulating pool of BMSCs 40-fold, can lead to improved cardiac outcome after infarction when administered during the peri-infarction period.59 Although it may be that these growth factors exert ameliorative effects on the infarcted heart that are unrelated to stem cell engraftment, this possibility is intriguing and warrants further investigation.
In humans, after orthotopic transplantation of female hearts into males, up to 15% of cardiac myocytes can be donor derived.60 Two recent phase 1 studies have shown the safety of injecting autologous BM cells into the human heart after infarction. In patients in whom autologous BM cells were injected directly into damaged myocardium, some improvement in cardiac function was documented based on medication use, quality of life, and magnetic resonance imaging (MRI)-based studies of function at the site of injection.61 In a separate study, after autologous AC133+ BM cells were injected into infarction borders after coronary artery bypass grafting, improved perfusion and cardiac function might have occurred.62 (AC133 is another marker of hematopoietic stem and progenitor cells). Caution must be taken in interpreting the results of these phase 1 studies because small numbers of patients were assessed and no control subjects were compared. In addition, it is not possible to assess whether patient outcomes resulted from the generation of BM-derived myocytes because these were autologous transplants. Ongoing studies will continue to assess whether bone marrow administration or mobilization from the bone marrow should be added to the armamentarium of therapies available to treat ischemic heart disease.
Bone marrow to skeletal muscle
Injection of marrow cells into damaged muscle leads to marrow-derived cells with myocyte-specific gene expression.63 Furthermore, BM transplantation restores low levels of dystrophin expression to the muscles of mdx mice, which lack the gene for dystrophin and are a mouse model of human muscular dystrophy.64 Functionality of the marrow-derived myocytes is unclear because, despite muscle degeneration and regeneration in the mdx mice, they do no have a clear “clinical” phenotype. A case report65 describes a boy with relatively mild Duchenne muscular dystrophy (DMD) diagnosed when he was 12. He underwent allogeneic BM transplantation for X-linked severe combined immunodeficiency syndrome (SCID) when he was 1 year of age. The report suggests that healthy muscle fibers forming from the donor marrow might have decreased the severity of DMD. At age 14, 13 years after allogeneic BM transplantation, rare donor-derived nuclei expressed normal dystrophin (0.5%-0.9%) in the skeletal muscle fibers.65 Because patients with DMD have a wide range of disease severity, it cannot be determined based on this one case whether donor-derived myocytes improved muscle function and allowed for the relatively mild DMD phenotype.
An elegant study using the transplantation of GFP-positive marrow cells documents the engraftment kinetics of BM-derived myocytes after the transplantation of whole marrow. Using this approach, confocal microscopy confirms that early engraftment of small numbers of donor-derived myocytes increases up to approximately 3.5% of the muscle fibers in response to muscle damage with exercise.66 In addition, this study documents the progression from donor-derived uninucleate cells to multinucleate muscle cells, thereby demonstrating that the behavior of marrow-derived myocytes is similar to that of healthy muscle cells. However, it is unclear whether the level of engraftment observed can be amplified for therapeutic benefit.
Bone marrow to central nervous system
Two different systems show that BM-derived stem cells can serve as progenitors of nonhematopoietic cells in the murine central nervous system (CNS). In one study, lethally irradiated adult mice that received whole marrow intravenously developed donor-derived brain cells bearing the neuronal antigens NeuN and class 3 b-tubulin.67 In a separate study, after marrow cells were injected into nonirradiated newborn mice, they migrated to the brain where they expressed NeuN.68 These studies speak to the plasticity of marrow in adults and in developing animals. Morphologically the cells are immature neural cells that lack axons. A functional role for these neuronal cells has yet to be shown.
Complementing the findings with bone marrow, MSCs also may be helpful in treating CNS disease. Niemann-Pick disease is a lysosomal storage disorder resulting from the lack of acid-sphingomyelinase. The phenotype of this disease is profound neurologic dysfunction related to the loss of Purkinje cells in the CNS. In a mouse model (acid-sphingomyelinase-null animal), the onset of neurologic defects and death were delayed after the direct injection of MSCs that had been genetically modified to express acid-sphingomyelinase into the hippocampi and cerebella of 3-week-old mice. MSC-derived sphingomyelinase-expressing Purkinje cells were identified.69 MSCs can also be induced to differentiate into neuronlike cells in vitro. These neuronal cells express neuron-specific antigens, but their electrophysiologic (functional) characteristics have not yet been assessed.70
In addition to their ability to differentiate into neuronlike cells, MSCs differentiate into oligodendrocytes in vivo. When MSCs from GFP-expressing mice are microinjected into a demyelinated spinal cord71 or fresh BM mononuclear cells are injected intravenously,72 remyelination occurs because of the transplanted cells. Donor cells were identified by GFP expression, and oligodendrocyte phenotype was identified based on the appearance of the cells under electron microscopy and the expression of myelin basic protein. Function was inferred on the basis of improved conduction velocity of the axons. Other studies have also documented a therapeutic benefit from MSC administration to the spinal cord after injury.73 In these studies, however, the effect is likely to be indirect because no donor-derived oligodendrocytes were found. Only donor-derived stromal support cells were identified, and it is these cells that were likely responsible for the beneficial effect of MSC administration. These promising data raise hope that the cells may be useful for treating developmental and neurodegenerative disorders of the CNS.
Bone marrow to kidney
BMSCs differentiate into epithelial and nonepithelial cell types in the kidney. Two human studies have demonstrated that when female kidneys were transplanted into male recipients, Y-chromosome-positive epithelial cells develop in the transplanted kidneys.74,75 Additional studies have shown engraftment of BMSCs into nonepithelial mesangial cells and interstitial cells within the kidney.76-78
A functional benefit for BMSC differentiation into renal tubular cells has been demonstrated in a model of ischemic renal disease.79 When wild-type mice underwent transplantation with whole marrow from ROSA-26 mice after sublethal irradiation, rare β-galactosidase+ renal tubule cells developed in the recipients' kidneys. Because flow cytometric analysis of peripheral blood after ischemic injury to the kidney showed a large increase in circulating Lin-Sca-1+ cells, the investigators predicted that this progenitor population may be mobilized to repair the damaged kidney. Further experiments proved this hypothesis. Renal ischemia was induced in wild-type mice that underwent BM transplantation using Lin-/Sca-1+ ROSA-26 BM by surgical clamping of the renal artery followed by reperfusion. The normal rise in blood-urea-nitrogen (BUN) concentrations induced by renal ischemia 48 hours after lethal irradiation was significantly reduced in mice after transplantation with Lin-Sca+ BM cells compared with mice that did not receive such cells, and β-galactosidase+ renal tubule epithelial cells were present as early as 48 hours after ischemic injury, which correlated with the protective effect.
In contrast, no β-galactosidase+ renal tubules were present in mice whose renal ischemia was preceded by the transplantation of Lin+ (already committed to specific blood lineages) cells. The epithelial phenotype of the β-galactosidase+ cells was confirmed by immunohistochemistry for megalin (a surface marker of tubular epithelia) and the lack of CD45 expression. These data provide the first definitive in vivo evidence that BMSCs can play a beneficial role in renal tissue repair.
Bone marrow to pancreas
BMSCs can also differentiate in vivo into islet cells.80,81 In one study, 4 to 6 weeks after the transplantation of male GFP-positive BM into female recipients, GFP-positive cells were isolated from the pancreatic islets of recipient mice after digestion into single-cell slurries and fluorescence-activated cell sorting (FACS).80 Immunohistochemistry for insulin and FISH for Y chromosome on the isolated cells confirmed that the GFP-positive pancreatic cells were donor-derived β cells. Furthermore, reverse transcription-polymerase chain reaction (RT-PCR) confirmed the expression of many islet cell markers—including insulin I, II, GLUT-2, IPF-I, HNF1a, HNF1b, and PAX6—while remaining uniformly negative for CD45. Overall, 1.7% to 3% of islet cells in the recipients were donor derived. When grown in vitro under conditions standard for islet cells, the marrow-derived cells had normal morphology and secreted insulin in response to glucose and exendin (a glucagonlike peptide). In addition, the authors ruled out the possibility that this BMSC-to-pancreatic β-cell transition resulted from cell fusion (see “Possible mechanisms for plasticity”).
In a separate but related study, streptozotocin-induced diabetes was ameliorated by bone marrow transplantation.81 In this model, mice became hyperglycemic within 10 days of streptozotocin administration due to destruction of pancreatic β cells. If they were irradiated and receive transplanted wild-type bone marrow within 10 days of streptozotocin (STZ) administration, glucose and insulin levels normalized. This ameliorative effect was not due to the irradiation. Mice that were irradiated and given phosphate-buffered saline (PBS) showed hyperglycemia and hypoinsulinemia similar to that of STZ-treated control animals.81 Over time, 2.5% of the insulin-producing cells in the pancreas were donor derived (GFP+). However, before any insulin-expressing BMSC-derived cells were detectable, the mice that underwent transplantation had nearly normal levels of endogenous insulin-positive cells in contrast to STZ-treated animals that had not undergone BMT, and many BMSC-derived cells that did not express insulin were present in the pancreases. Therefore, bone marrow transplantation seemed to indirectly contribute to maintaining insulin production (and pancreatic β-cell survival) in the STZ-treated mice. This indirect support may be attributed to the engraftment of BMSCs as endothelial cells, which protect endogenous tissue from damage. This is analogous to the protective effect of MSC administration to injured spinal cords.73
Bone marrow to lung
In the lung, Clara cells are the stem and progenitor cells for airway epithelia cells, and type 2 pneumocytes are the stem cells of the alveoli because they can self-renew to produce new type 2 pneumocytes and differentiate into type 1 pneumocytes. Unfractionated whole BM or CD34+Lin- cells can differentiate into bronchiolar epithelia and type 2 pneumocytes after transplantation into lethally irradiated female mice.32 Donor-derived epithelial cells were documented using colocalization of the Y chromosome and cytokeratin, and the identity of type 2 pneumocytes was confirmed by colocalization of the Y chromosome and surfactant B (SP-B) mRNA, both assayed by FISH. (Although SP-B can also be found in the bronchial epithelium, in alveoli it is specific to type 2 cells.82 ) Cells with the morphology of type 1 pneumocytes were Y+, but the identification of these cells as type 1 pneumocytes was not confirmed because of the lack of an ideal marker for them.
The immediate early response to irradiation in the lung is extensive alveolar breakdown, which is repaired within 2 weeks of BM transplantation. The kinetics of engraftment suggest that the high degree of BMSC engraftment as type 2 pneumocytes is the result of a contribution of BMSC to repair of extensive irradiation-induced damage. Within the first 2 weeks of transplantation, the number of donor-derived pneumocytes gradually increases, and after 2 months, 1% to 20% of type 2 pneumocytes can be donor derived.83
Based on these data, it was not clear whether the marrow harbored separate stem cells for the hematopoietic system and for nonhematopoietic tissues and organs or whether a subpopulation of marrow cells had the ability to differentiate into hematopoietic and nonhematopoietic cell types. In a study designed to distinguish between these possibilities, a single marrow-derived cell that had been selected by size fractionation, lineage depletion, and ability to home rapidly to the marrow was transplanted into a lethally irradiated host.32 This single male-derived BMSC engrafted not only the hematopoietic system of a lethally irradiated recipient but also type 2 pneumocytes in the lung and epithelial cells of the GI tract and skin.
MSCs may also contribute to lung repair. ROSA-26-derived MSCs injected intravenously into mice after bleomycin-induced lung injury can differentiate into type 1 pneumocytes.84 This was demonstrated by the detection of β-galactosidase-expressing type 1 cells, which were identified by their ability to bind to the lectin Lycopersicon esculentum. No β-galactosidase-expressing type 2 pneumocytes were found, which challenges the currently accepted model in which type 1 cells arise solely from type 2 cells.84,85 These findings may be analogous to data showing that donor-derived keratinocytes in the skin do not appear to differentiate into skin-specific stems (see below). Functional studies have not yet been performed on marrow-derived lung epithelial cells.
Bone marrow to skin
In mice and humans, Y+ cytokeratin-positive cells are present in the skin of female recipients after BM transplantation from a male donor.32,53,86 In the human studies, donor-derived keratinocytes were cytokeratin positive and CD45-.53 However, even though 4% to 14% of keratinocytes in human skin were Y+, keratinocytes grown in vitro from the same skin biopsies failed to demonstrate any Y+ donor cells even when sensitive PCR analysis for sequences specific to the Y chromosome was used. These findings can be viewed in at least 2 ways: Either the donor-derived keratinocytes require different culture conditions than those used or the donor-derived stem cells became keratinocytes without passing through an intervening tissue-specific stem cell (self-renewing) state.86 These data may be analogous to those showing engraftment of type 1 but not type 2 pneumocytes, which are functional alveolar stem cells.
After the skin is wounded, BMSCs may engraft as epithelial and endothelial cell types within the healing wound.87 Ongoing studies in our laboratory have demonstrated that BMSCs engraft as proliferating (Ki67+) keratinocytes at the wound edges, then migrate to the wound area and become part of the scar tissue. These findings suggest that BMSCs contribute to wound healing. However, future studies will have to be performed to determine whether BMSCs can engraft as self-renewing skin stem cells.
Bone marrow to gastrointestinal tract
Injecting a single marrow-derived stem cell with long-term repopulating ability in mice leads to low numbers of donor-derived esophageal and bowel epithelial cells.32 Unlike BMSCs, MAPCs administered intravenously can engraft as GI crypt cells, the functional stem cells of the gastrointestinal (GI) epithelium.39 BMSCs also engraft as epithelial cells in the human GI tract after allogeneic BM transplantation.53 In women who underwent BM transplantation with male BM, Y+ epithelia can be detected in the esophagus and stomach and in the small and large bowel. Areas of chronic inflammation, such as gastric ulcers and those secondary to graft-versus-host disease, have higher percentages of Y+, cytokeratin-positive, CD45- cells.88 These data suggest that tissue injury may increase the engraftment of donor-derived tissue cells. Based on these data, it is unclear whether the BM cells homed to damaged tissue or were themselves the cause of the damage and ongoing inflammation.
Possible mechanisms for plasticity
As seen in this review, all the studies documenting plasticity have used models of tissue injury to induce homing and differentiation of BMSCs. Tissue damage likely creates a favorable environment for the crossing of lineage barriers. It is likely that the extra-hematopoietic tissue microenvironment resulting from apoptosis or necrosis (eg, cytokine milieu, extracellular matrix characteristics) enables efficient engraftment of circulating stem cells. Currently unknown is whether BMSCs pass through a tissue-specific stem cell phase before final maturation, either through differentiation or fusion. Carefully designed studies using the identification and injury techniques described here will ascertain the cellular sources and kinetics of this transformation.
Direct and indirect differentiation
Several possible mechanisms may be speculated for BMSC plasticity (Figure 1), and proof of one mechanism does not necessarily preclude other possibilities. One mechanism could be that BM cells that differentiate into these diverse cell types represent a previously unsuspected population of highly pluripotent stem cells located in the BM that have not “committed” to becoming blood (Figure 1A). Alternatively, they are committed HSCs that can transdifferentiate (Figure 1B-C). Committed cells have begun a path of terminal differentiation, likely through irreversible changes in DNA conformation. Transdifferentiation refers to the ability of one committed cell type to change its gene expression pattern to that of a completely different cell type. Putative mechanisms for this change in potency include indirect transdifferentiation (Figure 1B), requiring dedifferentiation followed by maturation down an alternative pathway, and direct transdifferentiation (Figure 1C), in which there is a direct transition in the gene expression pattern. However, the distinction between direct and indirect transdifferentiation may be artificial; it may simply be that our existing paradigms of cellular differentiation do not encompass the true plasticity of these cells in vivo.
Mechanisms of differentiation. The 4 models shown represent mechanisms of differentiation from BM-derived cells into an alternative nonhematopoietic phenotype (green). (A) Consistent with our existing paradigm that cells always travel from a less differentiated to a more differentiated state, this model predicts that there is a highly pluripotent cell (red) that has not yet committed to the hematopoietic lineage and maintains the ability to differentiate into multiple diverse cell types. (B) With indirect transdifferentiation, an HSC changes its gene expression pattern to that of an alternative cell type through a dedifferentiation/redifferentiation pathway that presumably passes through an as yet unidentified intermediate cell type, shown in white. (C) In direct transdifferentiation, an HSC may be able to directly change its gene expression pattern from that of a hematopoietic stem cell to an alternative cell type. (D) If fusion is the mechanism by which BMSCs acquire a nonhematopoietic phenotype, a marrow-derived cell, perhaps a macrophage (blue), fuses with a nonhematopoietic cell (yellow), and the nucleus of the marrow-derived cell takes on the gene expression pattern of the nonhematopoietic cell type. The 2 nuclei do not have to fuse. Note that these models are not mutually exclusive and may all reflect the in vivo mechanisms involved. These models apply equally well to MSCs and MAPCs, which may directly transdifferentiate into multiple cell types, dedifferentiate through an intermediate cell type, represent highly pluripotent stem cells with the ability to differentiate directly into multiple cells types, or have the ability to fuse with different cell types.
Mechanisms of differentiation. The 4 models shown represent mechanisms of differentiation from BM-derived cells into an alternative nonhematopoietic phenotype (green). (A) Consistent with our existing paradigm that cells always travel from a less differentiated to a more differentiated state, this model predicts that there is a highly pluripotent cell (red) that has not yet committed to the hematopoietic lineage and maintains the ability to differentiate into multiple diverse cell types. (B) With indirect transdifferentiation, an HSC changes its gene expression pattern to that of an alternative cell type through a dedifferentiation/redifferentiation pathway that presumably passes through an as yet unidentified intermediate cell type, shown in white. (C) In direct transdifferentiation, an HSC may be able to directly change its gene expression pattern from that of a hematopoietic stem cell to an alternative cell type. (D) If fusion is the mechanism by which BMSCs acquire a nonhematopoietic phenotype, a marrow-derived cell, perhaps a macrophage (blue), fuses with a nonhematopoietic cell (yellow), and the nucleus of the marrow-derived cell takes on the gene expression pattern of the nonhematopoietic cell type. The 2 nuclei do not have to fuse. Note that these models are not mutually exclusive and may all reflect the in vivo mechanisms involved. These models apply equally well to MSCs and MAPCs, which may directly transdifferentiate into multiple cell types, dedifferentiate through an intermediate cell type, represent highly pluripotent stem cells with the ability to differentiate directly into multiple cells types, or have the ability to fuse with different cell types.
It is important to point out that in vivo transdifferentiation is not necessarily pathologic; it occurs normally in plants and animals.89,90 For example, transdifferentiation occurs in amphibians such as Urodeles (newts) during limb regeneration. In vitro, somatic terminally differentiated cells such as pancreatic epithelium can be switched to a hepatic phenotype.91,92 Oligodendrocyte progenitors can be reprogrammed into neural stem cell when maintained in a low-density, serum free medium,93 and fibroblasts can be reprogrammed to express T-cell-specific genes using T-cell protein extracts.94
Fusion
An alternative mechanism for plasticity could be the fusion of a BM-derived cell with a nonhematopoietic cell to form a heterokaryon, thereby converting the gene expression pattern of the original BM cell type to that of the fusion partner (Figure 1D). For example, in vitro fusion of fibroblasts with myoblasts is known to result in the expression of muscle-specific mRNA by the fibroblast nuclei.95 The question of whether the apparent differentiation of marrow-derived cells into nonhematopoietic cells is the result of cell fusion has been raised based on recent studies of coculture of embryonic stem (ES) cells with adult somatic cells.96,97 In one study, primary neuronal stem cells cocultured with ES cells fused with the ES cells and took on some of the phenotypic properties of ES cells.97 A similar study showed the previously unexpected possibility that BM cells grown with ES cells in the presence of LIF and IL-3 can also develop into ES-like cells after fusion.96 In both cases the resultant progeny were tetraploid and hexaploid. Although both studies found fusion to be a relatively rare event (on the order of 1/104-1/106 cells), they open the possibility that cells fuse without apparent fusogenic stimulation. Therefore, investigators in this field should, when possible, test whether fusion may be responsible for changes in the gene expression patterns (differentiation) of BMSCs to those of epithelial cells and other nonhematopoietic cell types. When cell-cell fusion is responsible for reprogramming the gene expression pattern of an adult cell, this still represents plasticity, but the cells involved need not be stem cells.
Although we still do not have optimal methods for testing whether fusion has occurred, several studies have used chromosomal analysis to show that BM-derived lung, muscle,66 and kidney98 are 2N, which suggests, but does not prove, that they do not result from fusion. Cell ploidy is not a foolproof approach because aneuploid and tetraploid cells can be present normally in some tissues, and it is possible that a 4N cell formed by fusion could subsequently become 2N, particularly if the 2 nuclei did not fuse.
In a study designed to assess whether BMSCs fuse with recipient cells to become pancreatic β cells, BMSCs from male stop-lox-GFP mice, in which the cells express enhanced GFP (EGFP) only after recombination by Cre recombinase, were transplanted into female recipient animals that expressed Cre recombinase in all their cells. If fusion were to occur, the Cre recombinase from the donor cell would induce recombination and subsequent GFP expression from the donor cell nuclei. Y+ pancreatic β cells were found as expected. However, none expressed EGFP, suggesting that fusion had not occurred.80
Despite data in muscle, kidney, and pancreas suggesting that fusion is not the underlying cause of BMSC differentiation into mature nonhematopoietic cells, recently published papers find just the opposite in the case of severely injured liver.99,100 In both studies, donor-derived BMSCs were transplanted into FAH-/- mice, and engraftment into hepatocytes occurred after the FAH-/- mice were weaned from the drug NTBC, which allows them to survive in the absence of the FAH enzyme. In the mice that underwent transplantation and survived NTBC withdrawal, most hepatocytes that were FAH+ (donor derived) also had markers of the recipient cells, suggesting that fusion had occurred.
We do not know whether fusion is responsible for much of the plasticity data. Even if it is, research in this field should not be abandoned or ignored. Fusion of cells may be a naturally occurring phenomenon or an abnormal response to intense selective pressure for circulating BMSCs to fuse with the epithelia of multiple tissues. If the resultant cells are functional and healthy, these cells could be of great physiologic significance or may represent benign random events. The concern, of course, would be that the resultant cells carry high potential for malignant transformation. Such avenues of research will require extensive investigation to see whether the fusion data represent an even more profound challenge to our existing paradigms of cell differentiation and development.101,102
Controversies
These studies showing a previously unsuspected differentiation potential for adult BMSCs have spawned controversy. Two primary controversies include the suggestion that plasticity data are all caused by artifacts of the detection methods and that the findings are not reproducible in other laboratories.
Overlay
Because detection methods have been largely 2 dimensional, microscopic artifacts may responsible for the appearance of donor-derived epithelium after transplantation. One study documents this phenomenon, using confocal microscopy to prove that what appears to be a donor-derived glial cell after ex-mismatched primate transplantation is actually 2 superimposed nuclei, one of which is likely from a blood cell.41 Termed overlay, this significant challenge to the data necessitates the use of additional detection techniques, such as confocal microscopy or single-cell assays for more convincing proof of BM-derived tissue cells. To date, assays of isolated single cells have confirmed the lung and liver findings in mice (E.L.H. and D.S.K., unpublished data, 2002), pancreas,80 and kidney,98 and confocal microscopy has been used to confirm the murine muscle data.66
Irreproducibility of data
Late in 2002, a study was published in which a single Lin-kit+Sca+Thy1lo BM-derived cell from a GFP transgenic mouse was transplanted into lethally irradiated hosts.103 In contrast to a previous report using transplantation of a single male BMSC into female recipients in which BMSCs differentiated into epithelial cells at a level of 1 of 100 to 1 of 1000 cells in the GI tract, lung, liver, and skin, the study, using a single KTLS cell, showed no GFP-positive donor-derived cells engrafting as epithelial cells in the GI tract or lung and only 1 of 70 000 liver cells. The authors concluded there was “little evidence for plasticity of BM-derived stem cells.”103 However, significant differences in the study designs used may be responsible for the different findings. The primary differences are in the donor cell subpopulations used, the ages of the donor and recipient mice, and the different methods of detection of donor-derived cells. Regarding the detection methods, one study used Y-chromosome FISH and the other used GFP transgene expression. It is possible that transgene silencing, which has been recognized as a cause for false-negative data,42,43 led to the inability to detect some donor-derived cells that had differentiated into epithelial cells. At this stage in our understanding of BMSC plasticity, these seemingly contrasting results could highlight important differences in the conditions required for these differentiation events to occur, in the degree of plasticity among BM subpopulations, and in the potential sensitivity of different detection methods.
Potential clinical applications
The potential clinical applications of BM differentiation into nonhematopoietic cell types are limited only by our imaginations and by the potential of the cells. Pluripotent marrow cells could be used to treat tissue injury and multiple diseases of nonhematopoietic tissue in at least 4 ways: (1) transplantation of normal autologous cells, (2) enhancement or mobilization of endogenous marrow-derived stem cells, (3) transplantation of gene-modified autologous marrow cells, and (4) transplantation of allogeneic BM cells. For some applications, cells could be administered directly to nonhematopoietic tissues, and for others the clinical benefit may be best achieved by replacing the endogenous marrow. If BMSCs do not engraft as self-renewing, tissue-specific stem cells in the nonhematopoietic tissues, a long-term therapeutic effect of BMSC administration would require engraftment first in the bone marrow, where they can self-renew as BMSCs.
Engrafting BMSCs as epithelial cells is likely to be of most benefit in response to acute damage such as that caused by infarction or a toxin. The animal models in which BMSC administration has been beneficial include renal ischemia, acute myocardial ischemia, and acute liver damage (in FAH-null mice). Endogenous BMSCs may normally play a role in the repair of low-level injuries, and administering exogenous BMSCs may be advantageous for tissue repair only when the body's endogenous repair mechanisms are overwhelmed by the extent of the injury or when the BMSCs do not have access to the injured site, as in severe wounds or ischemia when the vasculature is compromised.
When treating genetic diseases, transplanting genetically modified autologous marrow would avoid many of the risks of allogeneic transplantation. A number of studies indicate that such a goal is feasible. Mouse models of sickle cell disease104 and glycogen storage disease105,106 have been successfully treated with retrovirally mediated gene transfer to host marrow followed by autologous transplantation. More relevant to the ability of BMSCs to differentiate to epithelial cells, after transduction with GFP encoding retrovirus, BMSCs transplanted into irradiated syngeneic hosts can engraft as lung epithelia107 and astroglia108 that express GFP. Future studies will have to determine whether this approach can be used to stably express transgenes in a cell-type-specific manner in target cells. One challenge will be maintaining stable gene expression while minimizing the risk for vector-associated malignancies. There is also some concern that the stem cells themselves may have malignant potential because of telomere maintenance or other mechanisms. Thus, we must be cautious and perform appropriate preclinical studies in animals, including nonhuman primates, before translating these potential therapies to patients.
Critics of plasticity have voiced concern that the relatively small number of marrow-derived epithelia limits the significance and the usefulness of the data. In addition, if fusion were the sole mechanism by which marrow-derived cells change to a nonhematopoietic gene expression pattern in vivo, the potential repercussions of providing selective growth advantage to fused cells would have to be assessed in depth. These criticisms emphasize the need for more studies; the only way we can determine whether the plasticity of BMSCs can be harnessed for potential clinical benefit is to first better understand how this occurs. Only then can we knowledgeably develop approaches and methods to enhance differentiation to levels that could be clinically beneficial.
Where the future lies
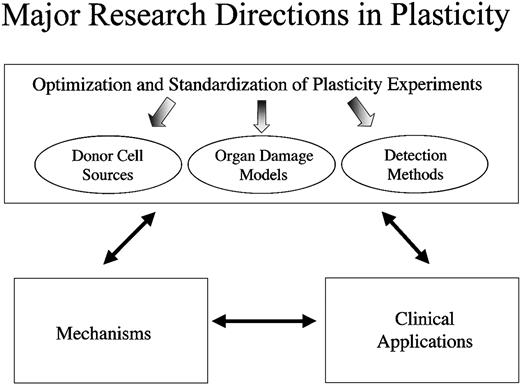
Ultimately, we want to apply our understanding of adult stem cell plasticity to treating disease and tissue injury in patients. For any field of biomedical inquiry, there is a complex interplay between developing effective clinical strategies, improving our understanding of the underlying cellular mechanisms, and optimizing and standardizing ongoing experiments (Figure 2), and this holds true for the field of adult stem cell plasticity. To better understand the mechanisms responsible for the differentiation of BMSCs into mature functional nonhematopoietic cell types, progress will have to be made on multiple fronts. It is critical that researchers optimize and standardize experiments. For standardization, we must clearly indicate the donor cell sources and the specific cell subpopulations analyzed. Because different injuries and diseases will likely select for different cell types, tissue damage models and animal disease models will have to be optimized. In addition, investigators must continue to improve detection methods so that cell source, cell phenotype, and cell function can be assessed unequivocally. It is important to acknowledge, however, that developing clinical applications can occur concurrently with studies to elucidate the underlying cellular mechanisms. In fact, findings in the clinic will likely reveal critical information regarding the underlying mechanisms of plasticity and which model systems are most applicable.
Discerning mechanisms underlying plasticity. Progress in the field of bone marrow stem cell plasticity must be made on multiple fronts simultaneously. To discern the mechanisms underlying plasticity, we must optimize and standardize the experimental approaches used so that the data obtained are as reproducible and definitive as possible. While making progress in our understanding of the underlying mechanisms, however, we must not lose sight of the potential therapeutic applications of these findings. By assessing the potential clinical benefits of stem cell administration in different disease models, we will gain insight not only into their therapeutic potential but also into the mechanisms by which plasticity occurs.
Discerning mechanisms underlying plasticity. Progress in the field of bone marrow stem cell plasticity must be made on multiple fronts simultaneously. To discern the mechanisms underlying plasticity, we must optimize and standardize the experimental approaches used so that the data obtained are as reproducible and definitive as possible. While making progress in our understanding of the underlying mechanisms, however, we must not lose sight of the potential therapeutic applications of these findings. By assessing the potential clinical benefits of stem cell administration in different disease models, we will gain insight not only into their therapeutic potential but also into the mechanisms by which plasticity occurs.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-05-1664.
Supported by National Institutes of Health (NIH) grants DK61846 and HL63357, the Yale Liver Center, and the Leukemia Lymphoma Society. E.L.H. and L.C. were supported by NIH T32-HL07778 and NIH T32-HL07974.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We apologize to colleagues whose work was not cited due to space limitations.