Abstract
The chemokine receptor CX3CR1 (CX3C chemokine receptor 1) is expressed in mouse blood on natural killer (NK) cells and on monocytes. Because interleukin-15 (IL-15) is an essential cytokine for NK cell development and maintenance, we hypothesized that it may induce CX3CR1 expression on this cell type. In contrast, we found that in primary mouse bone marrow-derived NK cells IL-15 specifically inhibited CX3CR1 protein and mRNA accumulation, whereas the related cytokine IL-2 did not inhibit but instead increased CX3CR1 expression. Consistent with this finding, intravenous injection of a single dose of recombinant IL-15 into C57BL/6 mice decreased steady-state CX3CR1 levels 24 hours after injection in freshly isolated peripheral blood mononuclear cells (PBMCs), splenocytes, and bone marrow cells, and treatment of mouse PBMCs with IL-15 in vitro inhibited CX3CL1 (ligand for CX3CR1)-induced chemotaxis. These data suggest that IL-15 may be a negative regulator of innate immunity by inhibiting CX3CR1 expression. These data also suggest that IL-15 inhibition of CX3CR1 may subvert potential cell immunotherapy strategies in which IL-15 is used to expand NK cell populations in vivo or ex vivo. Finally, our results provide additional evidence for differential signaling by IL-2 and IL-15, despite usage of common βγc receptor chains. (Blood. 2003;102:3494-3503)
Introduction
Differential expression of chemokine receptors coordinates specific leukocyte trafficking, which is important for development, distribution, and deployment of the immune system.1 Lymphocyte subsets differentially express most of the 18 known human chemokine receptors and may be marked by them. For example, CXC chemokine receptor 3 (CXCR3) is preferentially expressed on T helper type 1 (Th1) effector T cells, and CC chemokine receptor 7 (CCR7) marks naive T cells that home to secondary lymphoid tissue.2,3 In contrast to lymphocytes, neutrophils and eosinophils more uniformly express smaller subgroups of chemokine receptors. The interleukin-8 (IL-8) receptors CXCR1 and CXCR2 are strongly expressed on human neutrophils4,5 and CCR3 is characteristic of human eosinophils.6 CX3CR1 (CX3C chemokine receptor 1) is unusual among chemokine receptors because it is not expressed on mouse T cells but is expressed on 5% to 30% of mouse natural killer (NK) cells.7 In humans, this receptor is expressed on cytotoxic CD8+ and CD4+ T cells,8 as well as on most cells in the major subset of human NK cells, which have the immunophenotype CD56dimCD16+.9,10 In addition, CX3CR1 is expressed on mouse and human monocytes,7,11 neurons, and microglia.7,12,13
CX3CR1, like all chemokine receptors, is a 7-transmembrane domain G protein-coupled receptor (GPCR).1 Fractalkine (CX3CL1) is its only known ligand, and CX3CR1 is fractalkine's only known mammalian receptor (the viral receptor US28 also binds CX3CL1).14 CX3CR1 is classified among the inflammatory subgroup of chemokine receptors because it is selectively expressed on effector leukocytes.8,11,15 In addition to its expression pattern, it is unusual compared with other chemokine receptors in 2 major ways. First, it is one of the few inflammatory chemokine receptors that has only one ligand.16 Second, unlike most other chemokine receptors it functions as an adhesion molecule,11 able to mediate integrin- and G protein-independent adhesion of receptor-bearing leukocytes to CX3CL1-expressing endothelial cells under both static and physiologic flow conditions.17 The latter property is due in part to the unusual structure of CX3CL1, which in addition to having a typical chemokine domain has 3 other modules: a transmembrane domain that tethers the molecule to the plasma membrane, a mucin-like stalk which extends the chemokine domain from the cell surface, and a cytosolic domain of unknown function.18 The receptor is also able to function as a classic Gi-coupled integrin-dependent chemotactic receptor in response to soluble CX3CL1, which is released from the cell surface by proteolytic cleavage.19 Further, CX3CR1 triggering can induce degranulation of CX3CR1-expressing cytotoxic lymphocytes and NK cells in a G protein-dependent manner.15,20 Thus, expression of CX3CR1 on cytotoxic T cells and NK cells may be important not only for cell adhesion and migration but also for killer function.
The precise biologic role of CX3CR1 has not yet been delineated. CX3CR1 knock-out mice appear healthy and fertile, have reduced susceptibility to atherosclerosis,21 and in the presence of subtherapeutic doses of cyclosporin A are resistant to cardiac allograft rejection.22,23 CX3CL1 neutralization in vivo protects rodents from glomerulonephritis.24,25 In humans, a CX3CR1 polymorphism named CX3CR1-M280 that impairs ligand binding has been associated with the rate of HIV disease progression26,27 and, consistent with the mouse CX3CR1 knock-out model,21 with reduced risk of atherosclerotic coronary artery disease.28,29
Regulation of CX3CR1 expression has not been defined. Because IL-15 is essential for NK cell development30 and maintenance in vivo31,32 and because CX3CR1 is expressed at high levels on a major subset of freshly isolated human NK cells,9 we hypothesized that IL-15 may be a positive regulator. Likewise, because IL-15 signals through the βγc chains of the IL-2 receptor,33,34 and because IL-2 induces NK cell proliferation and killer function,35 we hypothesized that it may also positively regulate CX3CR1 expression. Consistent with this hypothesis, IL-2 has been reported to up-regulate several chemokine receptors on human NK cells, including CCR2, CCR4, CCR5, CCR7, and CCR8,10 but its effect on CX3CR1 is unknown. IL-15 up-regulates expression of CC but not CXC chemokine receptors on human T cells,36 but its effects on NK cell chemokine receptors and on CX3CR1 on any cell type have not been reported. Here, we test these hypotheses in vitro and in vivo in the mouse.
Materials and methods
Materials
Sources of recombinant proteins were as follows: human IL-2, Roche Diagnostics (Indianapolis, IN); human IL-15 and mouse CXCL12, Peprotech (Rocky Hill, NJ); and mouse CX3CL1 (chemokine domain), R&D Systems (Minneapolis, MN). Monoclonal antibodies neutralizing IL-15, IL-2, and isotype-matched control antibody were all from R&D Systems. Mouse recombinant IL-15 was tested in our system but failed to support growth and differentiation of NK1.1+ bone marrow-derived NK cells. Thus, for all studies presented we used recombinant human IL-15 (IL-15), which has been reported to be active on mouse NK cells. The following monoclonal antibodies (mAbs) and isotype-matched controls were purchased from Pharmingen BD Biosciences (Palo Alto, CA): mouse NK1.1-PE (phycoerythrin) and -FITC (fluorescein isothiocyanate), Pan NK-PE (specific for DX5), Gr-1-PE, CD3-FITC, CD8-FITC, and Ly49G2-FITC. A rabbit polyclonal antiserum raised against the N-terminal segment of human CX3CR1 was purchased from ProSci (Poway, CA). This reagent is able to recognize both mouse and human CX3CR1 by Western blot analysis, but it fails to recognize the receptor by flow cytometry. A goat polyclonal antiserum directed against a chemokine domain of rat CX3CL1 and able to recognize human, mouse, and rat CX3CL1 was purchased from R&D Systems. An mAb directed against β-actin was purchased from Abcam Limited (Cambridge, United Kingdom). Horseradish peroxidase (HRP)-linked secondary antibodies (rabbit, mouse, rat) were from Amersham Biosciences (Piscataway, NJ). Development of an HEK 293 cell line stably expressing mouse CX3CR1 and creation of a CX3CR1-/- mouse have been reported previously.37
Tissue preparation, cell separation, and immunophenotyping
Mice were first injected with heparin (1000 U/mouse) by tail vein, then injected with xylazine-ketamine to induce anesthesia. Thoracotomy was then performed, and peripheral blood was collected by cardiac puncture. Bone marrow was collected by flushing the femoral cavity with sterile phosphate-buffered saline (PBS). Spleens were harvested, minced, and mechanically homogenized into single cell suspensions using a 20-mL syringe. Mouse blood, spleen, and bone marrow cell suspensions were separated by density gradient centrifugation (1800 rpm, 20 minutes, room temperature) using lympholyte-M (spleen, bone marrow) and lympholyte-mammal (blood) (Cedarlane Laboratories, Ontario, ON, Canada). An intermediate “lymphocyte” fraction was collected and stained with NK1.1-FITC and Gr-1-PE antibodies using standard 2-color flow cytometry methods. Nonspecific staining was defined using isotype antibody controls. Sample fluorescence was measured using a FACScan I instrument, and data were analyzed using CELLQuest software (Becton Dickinson, San Jose, CA).
Analysis of IL-15 and IL-2 action in vivo
Female C57BL/6 mice (4.5 weeks) were purchased from Taconic (Germantown, NY). Mice were separated into 10 groups of 5 and quarantined for 10 days prior to injection. Mice were injected by tail vein with a single dose of recombinant human IL-15 (2 μg in 200 μL PBS per mouse) or the same volume of sterile PBS. To monitor effects of IL-2 on CX3CR1 expression in vivo mice were injected with a single dose of recombinant human IL-2 (50 000 U/mouse). Mice were killed at 1, 5, and 7 days after injection, and bone marrow cells, splenocytes, and peripheral blood mononuclear cells (PBMCs) were prepared. The presence of CX3CR1-expressing populations (NK1.1 and Gr-1) was confirmed in all tissues examined, and samples were evaluated for expression of mouse chemokine receptors CCR5, CXCR4, and CX3CR1 by reverse transcription-polymerase chain reaction (RT-PCR) in the case of IL-15-treated animals. Expression of mouse CX3CR1 protein was evaluated by Western blot for both IL-2- and IL-15-treated animals.
Establishment of bone marrow-derived NK cell cultures
Bone marrow cells were separated and immunophenotyped as described in “Tissue preparation, cell separation, and immunophenotyping.” Cells (2 × 107) were further cultured in RPMI 1640 (American Type Culture Collection [ATCC], Manassas, VA) supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin, 0.1 mM 2-mercaptoethanol, and recombinant human IL-2 or IL-15. In all conditions tested, the bone marrow cells were first cultured and stabilized for 3 days in IL-15 (100 ng/mL). After this step, the cells were washed and placed in fresh medium containing IL-2, IL-15, or IL-2 + IL-15. Expression of NK-specific surface antigens NK1.1 and Ly49G2, as well as mouse CD3 and CD8, was monitored.
Chemotaxis
Freshly isolated PBMCs were harvested from peripheral blood of C57Bl/6 mice, then cultured according to the method of Campbell et al.9 In brief, cells were incubated for 12 hours in RPMI 1640 media supplemented with 10% FCS, 100 U/mL of penicillin, 100 μg/mL streptomycin with or without 50 ng/mL IL-15. Prior to assay, cells were washed twice in chemotaxis buffer (RPMI 1640 containing 10% FBS and 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]). Cells (5 × 105) in 100 μL chemotaxis buffer were loaded into the top of each well of a transwell chemotaxis plate (CoStar, Cambridge, MA) with 5-μm pore inserts. The bottom of each well contained 600 μL prewarmed chemotaxis buffer with the indicated concentration of chemoattractant. The plate was incubated for 90 minutes at 37°C in an atmosphere containing 5% CO2 and 100% humidity. Chemotaxis is expressed as a number of cells that migrated. All conditions were tested in triplicate.
RNA isolation and analysis
Total RNA was extracted from 2 × 106 freshly isolated mouse bone marrow cells, splenocytes, and PBMCs, as well as from bone marrow-derived NK cells using the Qiagen RNeasy Mini kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Prior to reverse transcription, RNA was treated with RNase-free DNase I following the manufacturer's protocol. Equal amounts of total RNA (1 μg) were used for the synthesis of cDNA using the Superscript First Strand Synthesis System from Gibco (Invitrogen, Carlsbad, CA) in the presence of 40 U/μL recombinant RNasin (Promega, Madison, WI). Reverse transcription was performed for 120 minutes at 37°C and stopped by heating the samples for 10 minutes at 72°C. Unique primer sets for mouse β2-microglobulin, CCR5, CXCR4, and CX3CR1, designed on the basis of sequences deposited with the National Center for Biotechnology Information (NCBI), were synthesized by Invitrogen. PCR was performed using AmpliTaq Gold DNA Polymerase with the GeneAmp PCR Gold buffer system (Applied Biosystems, Foster City, CA). PCR conditions for β2-microglobulin and CX3CR1 were as follows: 95°C for 9 minutes, followed by 30 cycles of 1 minute at 95°C, 45 seconds at 61°C, and 1.5 minutes at 72°C, with a final extension at 72°C for 10 minutes. PCR conditions for CCR5 and CXCR4 were the same with the exception of an annealing temperature of 56°C. PCR conditions were optimized to allow for semiquantitative comparison of the results. The plateau for amplification of chemokine receptor messages was 35 cycles. Positive controls were as follows: CCR5, total mouse spleen RNA; CXCR4, total mouse thymus or brain RNA; and CX3CR1, total mouse brain RNA (Pharmingen BD Biosciences Clontech, Palo Alto, CA). A negative-control RNA without reverse transcriptase added was included for each analysis. Expected sizes of amplified fragments were 220 bp for β2-microglobulin, 362 bp for CCR5, 400 bp for CXCR4, and 410 bp for CX3CR1.
Detection of mouse CX3CR1 by Western blotting
Freshly isolated bone marrow and spleen cells, PBMCs, and lymphocytes, as well as mouse bone marrow-derived NK cells (107), were lysed by sonication in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (BioRad, Hercules, CA). Samples were loaded onto a 10% polyacrylamide gel, and proteins were separated using a Hoefer SE 600 electrophoresis unit (Amersham Biosciences, Piscataway, NJ) and transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Membranes were blocked overnight at 4°C in Tris (tris(hydroxymethyl)aminomethane)-buffered saline containing 0.05% Tween 20 (TTBS) and 5% skim milk. CX3CR1-directed antibody was diluted to 1/1000 and incubated with membranes at room temperature for 3 hours. HRP-linked secondary antibody was incubated with membranes for an hour, and protein bands were visualized by chemiluminescence (Amersham Biosciences). Equal protein loading was confirmed by re-probing membranes with anti-β-actin antibody, which was diluted as recommended by the manufacturer.
Neutralization studies
PBMCs (107) were incubated for 12 hours in 1 mL RPMI 1640 media supplemented with 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin with or without 50 ng/mL IL-15 and with or without 50 U/mL IL-2. The capacity of IL-15- and IL-2-directed mouse monoclonal antibodies to block the activity of these 2 cytokines was controlled by isotype-matched control Ab (mouse immunoglobulin G1 [IgG1]). After 12-hour incubation cells were collected, washed twice in PBS, and lysed in SDS-PAGE sample buffer. The amount of CX3CR1 protein was assessed by Western blot using anti-CX3CR1 Ab.
Results
IL-2 and IL-15 differentially affect CX3CR1 expression on bone marrow-derived NK1.1+ cells
To test the effects of IL-2 and IL-15 on CX3CR1 expression, we first developed a cytokine-dependent primary mouse bone marrow-derived NK1.1+ cell culture system ex vivo, in which stroma was excluded. This culture system allows for the production of large numbers of enriched NK1.1+ Ly49G2+ cells for analysis and is a modification of previously published methods.38,39 Cell type was assessed by surface marker expression.
In freshly harvested uncultured bone marrow cells, we observed less than 1% CD3+ cells, approximately 3% NK1.1+ DX5+ double-positive cells, and approximately 2% and 3% DX5 and NK1.1 single-positive cells, respectively (Figure 1A). When cultured ex vivo in basic medium (RPMI + 10% FCS) in the absence of added cytokines, freshly harvested bone marrow cells underwent apoptosis within 3 days. In contrast, freshly harvested cells placed immediately in either IL-2 or IL-15 proliferated robustly, with outgrowth of a mixed population of T cells, NK cells, and NK-T cells in different proportions, depending on the cytokine and the time in culture.
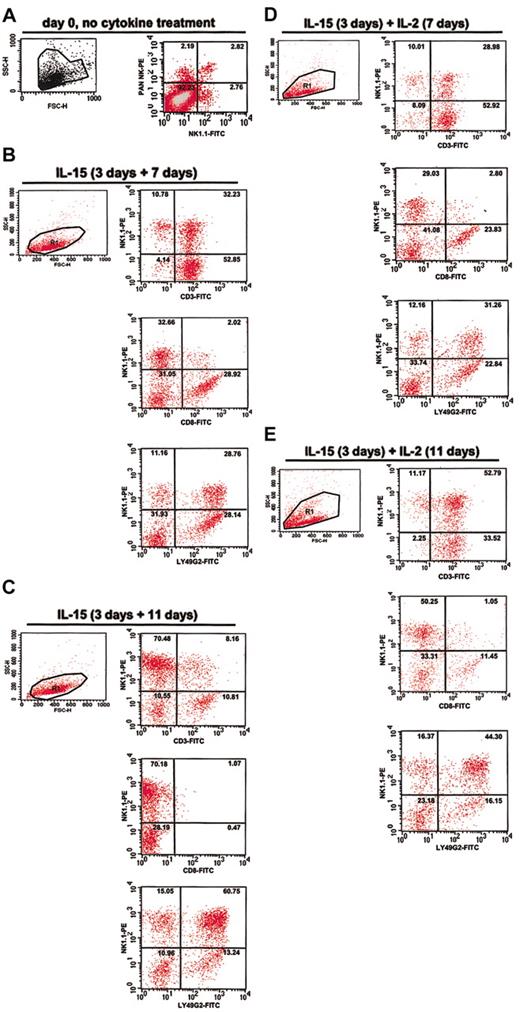
Characterization of IL-2- and IL-15-dependent mouse bone marrow-derived cultures enriched in NK1.1+ cells. Freshly harvested bone marrow from C57Bl/6 mice was immediately analyzed by fluorescence activated cell sorting (FACS) (A) or after cytokine treatment (B-E). Cultured cells were first treated with IL-15 for 3 days (culture initiation). Cells were then washed and cultured further in the presence of IL-15 for an additional 7 days (B) or 11 days (C), or else IL-2 for a period of 7 days (D) or 11 days (E), as indicated at the top of each panel. Cells from each culture were analyzed for side scatter (SSC-H) and forward scatter (FSC-H) and by 2-color FACS using mAbs to the surface markers NK1.1, Ly49G2, CD3, and CD8. The number in the upper right corner of each quadrant indicates the percentage of cells in that quadrant.
Characterization of IL-2- and IL-15-dependent mouse bone marrow-derived cultures enriched in NK1.1+ cells. Freshly harvested bone marrow from C57Bl/6 mice was immediately analyzed by fluorescence activated cell sorting (FACS) (A) or after cytokine treatment (B-E). Cultured cells were first treated with IL-15 for 3 days (culture initiation). Cells were then washed and cultured further in the presence of IL-15 for an additional 7 days (B) or 11 days (C), or else IL-2 for a period of 7 days (D) or 11 days (E), as indicated at the top of each panel. Cells from each culture were analyzed for side scatter (SSC-H) and forward scatter (FSC-H) and by 2-color FACS using mAbs to the surface markers NK1.1, Ly49G2, CD3, and CD8. The number in the upper right corner of each quadrant indicates the percentage of cells in that quadrant.
In the presence of IL-2 alone for 10 days the cultures consisted mainly of T cells with only a small proportion of NK-T cells and NK cells (data not shown). These cultures were not studied further. IL-2 was able to stimulate selective outgrowth of NK1.1+ LY49G2+ cells if the freshly isolated bone marrow cells were first cultured in IL-15. We, therefore, adopted as standard procedure a 3-day incubation in IL-15 (100 ng/mL) prior to comparing effects of different cytokine treatments. We designate this 3-day period in IL-15 as cell culture initiation. Initiated cultures were washed and incubated further in basic media containing IL-2 and/or IL-15. On the basis of CD3 and NK1.1 expression, both cytokines appeared to induce a similar proportion of NK and NK-T cells at 7 days after initiation, approximately 10% and approximately 30%, respectively, and expression of Ly49G2 was similar at this time point (Figure 1B,D). Most cells in the IL-15-dependent culture at 7 days and in the IL-2-dependent culture at both 7 and 11 days after initiation were firmly adherent and were morphologically indistinguishable (data not shown). In contrast, cells in the IL-15-dependent culture at 11 days after initiation were rounder and less firmly adherent in tissue culture (data not shown).
In the presence of IL-15 alone there was a preferential outgrowth of NK1.1+ LY49G2+ NK cells (Figure 1B-C), reaching a level of 70% 11 days after initiation; the remainder of the population consisted of approximately 10% each of T cells and NK-T cells at that time point (Figure 1C). In the presence of IL-2 there was an outgrowth of NK1.1+ LY49G2+ cells after initiation (Figure 1D-E); however, most of these cells (53% after 11 days in IL-2) were also CD3+ and, therefore, NK-T cells. Approximately 11% of the cells were NK cells and 34% of the cells were T cells at that time (Figure 1E).
There are no available antibodies that recognize mouse CX3CR1 by flow cytometry; however, we found that anti-CX3CR1-NT, a rabbit polyclonal antiserum raised against the N-terminus of human CX3CR1, is able to recognize the mouse receptor at the total cell protein level by Western blot in bone marrow, spleen, and PBMCs. The antiserum recognized a major immunoreactive species 50 kDa in mass in mouse brain, which has been previously reported to express CX3CR1 mRNA in large amounts, whereas mouse skeletal muscle, which has previously been reported to lack CX3CR1 mRNA, was negative (Figure 2A). The specificity of the antiserum was tested further by demonstrating that the peptide which served as immunogen for rabbit injections could block the immunoreactivity (data not shown). The commercial supplier and we have verified that this antiserum also recognizes a major 50-kDa band by Western blot in human tissues, including spleen (data not shown). Finally, recombinant mouse CX3CR1 expressed in HEK 293 cells was also detected as a specific 50-kDa immunoreactive band by Western blot using this antiserum (data not shown).
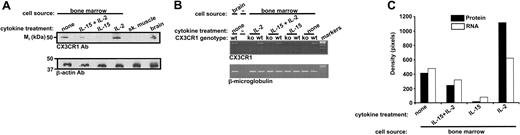
Opposite effect of IL-15 and IL-2 on CX3CR1 expression in cytokine-dependent mouse bone marrow-derived cells. (A) Total cellular CX3CR1 protein. Wild-type C57Bl/6 mouse bone marrow cell differentiation was initiated ex vivo for 3 days in IL-15 for all cultures. Cells were then washed and incubated in the presence of IL-2 (100 U/mL) or IL-15 (100 ng/mL) for an additional 11 days, giving a total of 14 days of cytokine treatment. Cells were also treated for a total of 14 days with a combination of the 2 cytokines according to the following protocol: IL-15 for 3 days → wash → IL-15 for 7 days → wash → IL-15 plus IL-2 for 4 days. Protein extracts were analyzed by Western blot using antibodies for CX3CR1 or β-actin. Control lanes contained a commercial source of protein extracts from C57Bl/6 mouse skeletal muscle and brain. (B) RNA analysis. Total RNA was isolated from bone marrow cells grown according to exactly the same protocol as in panel A. To control for specificity, cells were also cultured according to this protocol from both CX3CR1-/- knock-out (ko) and wild-type (wt) mice. RT-PCR using primers for the genes listed at the lower left of each panel was performed. (C) Densitometry of results shown in panels A and B. The results shown are from a single experiment representative of at least 3 separate experiments.
Opposite effect of IL-15 and IL-2 on CX3CR1 expression in cytokine-dependent mouse bone marrow-derived cells. (A) Total cellular CX3CR1 protein. Wild-type C57Bl/6 mouse bone marrow cell differentiation was initiated ex vivo for 3 days in IL-15 for all cultures. Cells were then washed and incubated in the presence of IL-2 (100 U/mL) or IL-15 (100 ng/mL) for an additional 11 days, giving a total of 14 days of cytokine treatment. Cells were also treated for a total of 14 days with a combination of the 2 cytokines according to the following protocol: IL-15 for 3 days → wash → IL-15 for 7 days → wash → IL-15 plus IL-2 for 4 days. Protein extracts were analyzed by Western blot using antibodies for CX3CR1 or β-actin. Control lanes contained a commercial source of protein extracts from C57Bl/6 mouse skeletal muscle and brain. (B) RNA analysis. Total RNA was isolated from bone marrow cells grown according to exactly the same protocol as in panel A. To control for specificity, cells were also cultured according to this protocol from both CX3CR1-/- knock-out (ko) and wild-type (wt) mice. RT-PCR using primers for the genes listed at the lower left of each panel was performed. (C) Densitometry of results shown in panels A and B. The results shown are from a single experiment representative of at least 3 separate experiments.
The antiserum also recognized a 50-kDa band in freshly isolated mouse bone marrow cells (Figure 2A,C). Relative to this benchmark, bone marrow cells cultured in IL-2 (100 U/mL) for 11 days after initiation expressed a 3-fold higher level of CX3CR1 immunoreactivity, whereas in striking contrast bone marrow cells cultured for the same duration in IL-15 (100 ng/mL) had almost no detectable expression (Figure 2A,C). When IL-2 (100 U/mL) was added to the IL-15-dependent culture, so that the cells were grown in both IL-2 and IL-15 for 4 days, the CX3CR1 protein level was intermediate, 41% lower then the benchmark present in freshly isolated bone marrow cells (Figure 2A,C). Cells cocultured with IL-2 and IL-15 had an immunophenotype identical to cells cultured in IL-15 or IL-2 for 7 days after initiation (pattern identical to those in Figure 1).
The same general pattern was observed for IL-2 and IL-15 regulation of steady-state CX3CR1 mRNA expression. Thus, compared with freshly isolated bone marrow cells, IL-2 induced a 33% increase, whereas IL-15 induced an 84% decrease in CX3CR1 mRNA (Figure 2B-C). Again, when the cytokines were combined, the level of CX3CR1 mRNA was intermediate, 32% lower then receptor message levels in primary freshly isolated bone marrow (Figure 2B-C).
To further address the suppressive effect of IL-15 on CX3CR1 expression in bone marrow-derived NK cells we carried out a more detailed dose-response analysis. We initiated the study at 100 ng/mL IL-15 because, although the cells survived in 50 ng/mL IL-15, they failed to proliferate. As shown in Figure 3A and C, we observed a dose-dependent reduction in mouse CX3CR1 protein levels compared with initial levels expressed in mouse bone marrow. At 300 ng/mL, IL-15 completely abolished steady-state CX3CR1 protein accumulation. Consistent with the effect of IL-15 on CX3CR1 protein levels, we found that the cytokine was also able to induce a dose-dependent decrease of mouse CX3CR1 mRNA in mouse bone marrow-derived NK cell cultures (Figure 3B,D).
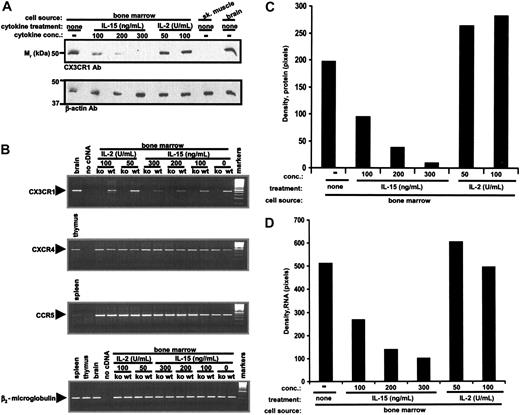
IL-15 suppresses CX3CR1 expression in mouse bone marrow-derived NK cells in a dose-dependent manner. Freshly harvested bone marrow from wild-type C57Bl/6 mice (A-B) and from CX3CR1-/- C57Bl/6 mice (B) was cultured according to the protocol in Figure 2, varying the concentration of cytokine. Samples were then analyzed for CX3CR1 and control protein and RNA content as in Figure 2. The same cell cultures were also examined for accumulation of CCR5 and CXCR4 transcripts. (B) The legend for the CXCR4 and CCR5 panels is identical to the CX3CR1 panel, with the exception of the positive control tissue, as noted. (C-D) Densitometric analysis of results shown in panels A and B, respectively. Results shown are from a single experiment representative of at least 3 separate experiments.
IL-15 suppresses CX3CR1 expression in mouse bone marrow-derived NK cells in a dose-dependent manner. Freshly harvested bone marrow from wild-type C57Bl/6 mice (A-B) and from CX3CR1-/- C57Bl/6 mice (B) was cultured according to the protocol in Figure 2, varying the concentration of cytokine. Samples were then analyzed for CX3CR1 and control protein and RNA content as in Figure 2. The same cell cultures were also examined for accumulation of CCR5 and CXCR4 transcripts. (B) The legend for the CXCR4 and CCR5 panels is identical to the CX3CR1 panel, with the exception of the positive control tissue, as noted. (C-D) Densitometric analysis of results shown in panels A and B, respectively. Results shown are from a single experiment representative of at least 3 separate experiments.
In our system, the minimum concentration of IL-2 necessary for production of NK1.1+ cell-containing cultures from mouse bone marrow ex vivo was 50 U/mL. At this concentration, CX3CR1 protein (Figure 3A,C) and mRNA (Figure 3B,D) were highly expressed and did not increase further in response to 100 U/mL.
To address the specificity of IL-2 and IL-15 action on CX3CR1 expression in this culture system, we examined their effects on CCR5 and CXCR4. Regardless of the dose or culture time investigated, neither cytokine influenced the expression of mouse CXCR4 or CCR5 mRNA (Figure 3B). Thus, the differential effect of IL-2 and IL-15 on CX3CR1 expression appears to be specific relative to these other chemokine receptors.
Effects of IL-15 on mouse CX3CL1 expression
We next tested whether IL-15 could induce expression of mouse CX3CL1, because this could conceivably provide a molecular mechanism for downregulation of CX3CR1. IL-2 and IL-15 stimulation of peripheral blood-derived human NK cells has previously been reported to induce and up-regulate the expression and secretion of other chemokines.10 Moreover, IL-15 specifically induces expression of CC chemokine ligand 2 (CCL2), CCL3, CCL4, CCL5, CXCL8, and XCL1 in human T lymphocytes in a dose- and time-dependent manner.36
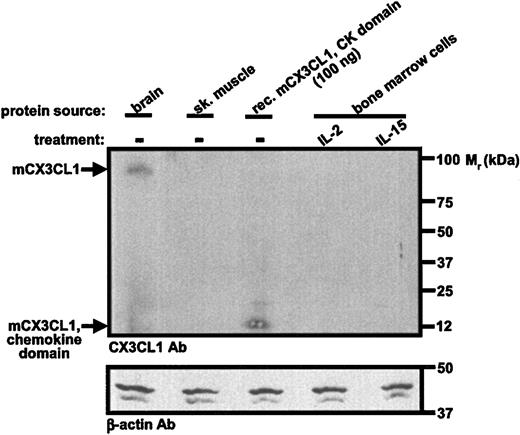
We were unable to demonstrate expression of CX3CL1 protein using an antibody directed against the chemokine domain of rat CX3CL1. In control studies, this antibody was able to detect immunoreactive full-length native CX3CL1 in a lysate from 20 million cells from mouse brain. By comparison, we analyzed 10 million cytokine-stimulated bone marrow-derived NK cells. Further, the antibody was able to detect the recombinant chemokine domain of mouse CX3CL1 by Western blot with a threshold between 50 and 100 ng (Figure 4). These data imply that IL-15 down-regulates CX3CR1 expression by a mechanism independent of CX3CL1.
Mouse CX3CL1 protein is present in both IL-2- and IL-15-dependent bone marrow-derived NK1.1+ cells. Freshly isolated bone marrow cells from C57Bl/6 mice were cultured for 3 days in IL-15, washed, and then cultured for 11 days in either IL-2 (100 U/mL) or IL-15 (100 ng/mL). Protein extracts were made and analyzed by Western blot with antibodies listed at the lower left of each panel. Protein extracts from brain and skeletal muscle were from a commercial vendor. Data are from a single experiment representative of 3 independent experiments.
Mouse CX3CL1 protein is present in both IL-2- and IL-15-dependent bone marrow-derived NK1.1+ cells. Freshly isolated bone marrow cells from C57Bl/6 mice were cultured for 3 days in IL-15, washed, and then cultured for 11 days in either IL-2 (100 U/mL) or IL-15 (100 ng/mL). Protein extracts were made and analyzed by Western blot with antibodies listed at the lower left of each panel. Protein extracts from brain and skeletal muscle were from a commercial vendor. Data are from a single experiment representative of 3 independent experiments.
IL-15, but not IL-2, decreases mouse leukocyte CX3CR1 expression in vivo
To test whether IL-15 can also negatively regulate CX3CR1 expression in vivo, we injected C57BL/6 mice by tail vein with IL-15 and measured CX3CR1 protein and mRNA in freshly isolated PBMCs, bone marrow cells, and splenocytes 24 hours, 5 days, and 7 days after injection. In previous studies, injection of exogenous recombinant human IL-15 (2 μg) into mice has been reported to increase incorporation of 5-bromo-2-deoxy-Uridine (BrdU) in bone marrow and CD44+ CD8+ T cells, reflecting increased cell proliferation of memory CD8+ T cells.40
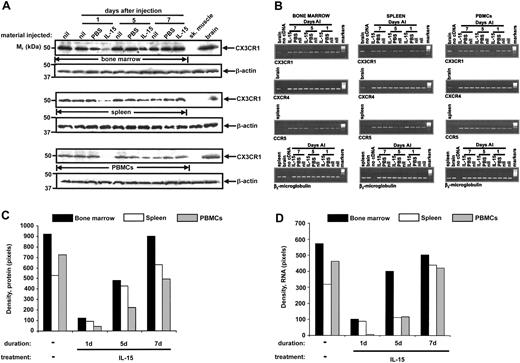
Mice injected by tail vein with a single dose of IL-15 (2 μg) were killed 1, 5, and 7 days after injection. Expression of mouse CX3CR1 protein was confirmed in the noninjected control group in bone marrow, spleen, and PBMCs. We observed a dramatic decrease of both CX3CR1 protein (Figure 5A,C) and mRNA (Figure 5B,D) in bone marrow, spleen, and PBMCs to levels barely detectable above background 1 day after injection of IL-15, as compared with levels found in tissues from uninjected and PBS-injected mice at the same time point. CX3CR1 mRNA and protein levels in bone marrow, spleen, and PBMCs were partially restored to baseline by day 5 and completely restored to baseline by day 7 after IL-15 injection (Figure 5).
Exogenous IL-15 negatively regulates expression of endogenous mouse CX3CR1 in vivo. Freshly isolated bone marrow, splenocytes, and PBMCs from PBS-injected (PBS), IL-15-injected (IL-15), and noninjected (nil) C57Bl/6 mice were examined for CX3CR1 protein (A) and mRNA (B) expression 1, 5, and 7 days after injection (AI). (B) CCR5, CXCR4, and β2-microglobulin mRNA expression was also analyzed. (C-D) Results in panels A and B, respectively, were quantitated by densitometry. Protein and RNA analysis was performed on samples pooled from 5 mice in each group, to have sufficient material for protein analysis in the PBMCs.
Exogenous IL-15 negatively regulates expression of endogenous mouse CX3CR1 in vivo. Freshly isolated bone marrow, splenocytes, and PBMCs from PBS-injected (PBS), IL-15-injected (IL-15), and noninjected (nil) C57Bl/6 mice were examined for CX3CR1 protein (A) and mRNA (B) expression 1, 5, and 7 days after injection (AI). (B) CCR5, CXCR4, and β2-microglobulin mRNA expression was also analyzed. (C-D) Results in panels A and B, respectively, were quantitated by densitometry. Protein and RNA analysis was performed on samples pooled from 5 mice in each group, to have sufficient material for protein analysis in the PBMCs.
The effect of IL-15 on CX3CR1 expression in vivo was specific because it had no effect on CXCR4 gene expression in mouse bone marrow, spleen, and PBMCs. Furthermore, expression of mouse CCR5 mRNA was slightly increased 1 day after IL-15 administration (Figure 5B). Moreover, IL-2 injection did not reduce CX3CR1 protein expression on PBMCs or in spleen or bone marrow (Figure 6).
Exogenous IL-2 does not negatively regulate expression of endogenous mouse CX3CR1 in vivo. (A) Freshly isolated bone marrow, splenocytes, and PBMCs from PBS-injected (PBS), IL-2-injected (IL-2), and noninjected (nil) C57Bl/6 mice were examined for CX3CR1 protein expression 1, 5, and 7 days after injection using methods identical to those for Figure 5. (B) Results in panel A were quantitated by densitometry. Protein analysis was performed on samples pooled from 5 mice in each group, to have sufficient material for protein analysis in the PBMCs.
Exogenous IL-2 does not negatively regulate expression of endogenous mouse CX3CR1 in vivo. (A) Freshly isolated bone marrow, splenocytes, and PBMCs from PBS-injected (PBS), IL-2-injected (IL-2), and noninjected (nil) C57Bl/6 mice were examined for CX3CR1 protein expression 1, 5, and 7 days after injection using methods identical to those for Figure 5. (B) Results in panel A were quantitated by densitometry. Protein analysis was performed on samples pooled from 5 mice in each group, to have sufficient material for protein analysis in the PBMCs.
Effect of IL-15 on responsiveness of PBMCs to CX3CL1
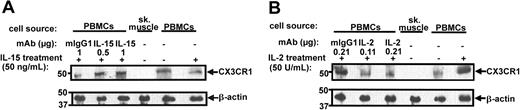
Because IL-15 was able to rapidly suppress CX3CR1 expression in PBMCs in vivo, we next measured its effect on CX3CR1 function in freshly harvested peripheral blood-derived mouse PBMCs treated ex vivo for only 12 hours in a CX3CL1-dependent chemotaxis assay. IL-15 was tested at 50 ng/mL, a concentration that stabilizes and supports survival of bone marrow-derived NK cells and PBMCs but does not promote cell proliferation. Under these conditions, IL-15 treatment induced a 50% reduction in total cell CX3CR1 protein by Western blot analysis, and this reduction could be specifically prevented by a mouse mAb directed against human IL-15 (Figure 7A). In contrast, incubation in IL-2 for 12 hours induced a marked increase in total cell CX3CR1 protein which could be specifically prevented by a mAb directed against human IL-2 (Figure 7B).
IL-15 and IL-2 differentially regulate CX3CR1 expression in mouse peripheral blood-derived PBMCs. PBMCs were cultured in vitro for 12 hours in the presence or absence of the indicated cytokines and neutralizing mAbs or isotype controls. Skeletal muscle protein extract was used as a negative control. Cells were then lysed and analyzed by Western blot using the antibody indicated to the right of each panel. Each lane represents a pool of PBMCs from 5 C57Bl/6 mice. Results are representative of 2 independent experiments.
IL-15 and IL-2 differentially regulate CX3CR1 expression in mouse peripheral blood-derived PBMCs. PBMCs were cultured in vitro for 12 hours in the presence or absence of the indicated cytokines and neutralizing mAbs or isotype controls. Skeletal muscle protein extract was used as a negative control. Cells were then lysed and analyzed by Western blot using the antibody indicated to the right of each panel. Each lane represents a pool of PBMCs from 5 C57Bl/6 mice. Results are representative of 2 independent experiments.
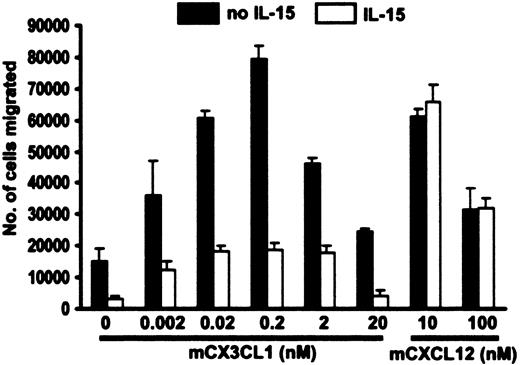
Control cells incubated for 12 hours in the absence of IL-15 migrated in response to CX3CL1 in a typical biphasic dose-dependent manner, giving an optimal response at 0.2 nM (Figure 8). In contrast, and consistent with the protein data in Figure 7, the chemotactic response of cells treated with IL-15 was markedly reduced at every concentration tested. The inhibitory effect of IL-15 appears to be specific for CX3CR1 because it did not interfere with cell responsiveness to the mouse CXCR4 agonist CXCL12 (Figure 8).
IL-15 suppresses chemotaxis of mouse PBMCs to CX3CL1. PBMCs pooled from 5 C57Bl/6 mice were incubated for 12 hours in 50 ng/mL IL-15 or basic medium alone, washed, and then analyzed for their ability to chemotax in response to various concentrations of mouse recombinant CX3CL1 (chemokine domain) and mouse CXCL12. Data are presented as mean ± SEM and are pooled from 3 independent experiments.
IL-15 suppresses chemotaxis of mouse PBMCs to CX3CL1. PBMCs pooled from 5 C57Bl/6 mice were incubated for 12 hours in 50 ng/mL IL-15 or basic medium alone, washed, and then analyzed for their ability to chemotax in response to various concentrations of mouse recombinant CX3CL1 (chemokine domain) and mouse CXCL12. Data are presented as mean ± SEM and are pooled from 3 independent experiments.
Discussion
In the present study we have demonstrated that IL-15 potently suppresses expression of the chemokine receptor CX3CR1 in diverse mouse leukocytes. This suppression was observed in vivo in all 3 compartments investigated (bone marrow, spleen, and blood), in vitro with PBMCs, and was modeled in vitro in an IL-15-dependent culture system able to direct preferential NK cell outgrowth from primary mouse bone marrow cells. The effect was specific for CX3CR1 because IL-15 did not down-regulate expression of β2-microglobulin or 2 other chemokine receptors tested, CXCR4 and CCR5, and because the related cytokine IL-2 did not inhibit CX3CR1 expression on any of these cell types in any of the conditions tested. In contrast IL-2 appeared to induce CX3CR1 expression, at least in PBMCs and NK-T cells in vitro. IL-15 inhibition of CX3CR1 expression was functionally significant because it was associated with a marked reduction in PBMC chemotaxis in response to the CX3CR1 agonist CX3CL1 in vitro. Suppression appeared to occur at the transcriptional level, because CX3CR1 mRNA levels were absent to extremely low in the presence of IL-15, and it did not appear to involve an autocrine or paracrine effect mediated by CX3CL1.
Although the results are clear and consistent, they are highly unexpected, for 3 main reasons. First, IL-15 is an essential factor for the development of NK cells,30 which when freshly harvested from the blood may express CX3CR1 in up to 30% of cells.9 Second, to date no chemokine receptor tested, in any cell type, has been reported to be down-regulated by IL-15. Finally, IL-2, which is similar to IL-15 in structure and receptor usage, did not suppress, but instead modestly enhanced CX3CR1 expression.
A resolution to the paradox of how IL-15 can support NK cell development, yet suppress CX3CR1 expression, may lie in the details of when and where IL-15 and CX3CR1 are expressed in vivo. CX3CR1 is expressed in freshly isolated mouse bone marrow, splenocytes, and 5% to 30% of NK cells present in mouse peripheral blood.7 However, it has not been established exactly when during development of the NK cell lineage IL-15 is active or when CX3CR1 gene transcription occurs. IL-15 is produced by a variety of cell types during both homeostatic and inflammatory conditions. It appears to undergo unusual regulation because it is expressed at the mRNA level in numerous healthy human tissues in a broad range of cell types. Yet at the protein level IL-15 can be isolated as a secreted protein from only a restricted subset of these cell types,34 including dendritic cells, osteoblasts, fibroblasts, and activated monocytes/macrophages.34,41-43
Bone marrow and spleen, in which NK cells develop and mature, both produce IL-15. Bone marrow stromal cells and spleen stromal fibroblastic cells are the main cell sources in these organs. In the spleen, IL-15 is produced as both a membrane-associated and soluble form,41 whereas in bone marrow the structural form has not been precisely delineated.44,45 During the immune response, IL-15 is made by activated macrophages and monocytes, and by antigen-presenting cells such as dendritic cells.46,47 Moreover, several human malignancies, including myeloma, rhabdomyosarcoma, osteosarcoma, and colon cancer, as well as adult T-cell leukemia induced by T-cell lymphotrophic virus type I (HTLV-I), have been suggested to produce IL-15.48-51 In addition to inhibiting apoptosis and supporting proliferation and activation of mature NK cells in vitro, IL-15 is also chemotactic for these cells and stimulates their adhesion to vascular endothelium.52
On the basis of this expression pattern, there is no obvious time when CX3CR1 expression in the NK lineage would be sequestered from IL-15 action. IL-15 secreted by activated macrophages could conceivably act in a paracrine manner through its suppression of CX3CR1 to negatively regulate the innate immune response, by inhibiting NK cell migration and effector function in traumatized tissues. Moreover, during development the IL-15 activity we have described may account in part for why 70% to 95% of mouse blood-derived NK cells are CX3CR1 negative.7
A limitation of our study is that we have only examined the effects of exogenously added soluble IL-15 and, therefore, can only make inferences regarding the physiologic and pathologic roles of endogenous soluble and membrane-bound IL-15 on CX3CR1 expression and function. Addressing this limitation more directly will be difficult, because mice with homozygous deletion of the IL-15 gene, or with mutations in IL-2 receptor β or γc, which are shared by the IL-15 receptor, show profound defects of NK cell development and survival.30,53
This points to the second paradox of our study. IL-2, despite sharing βγc receptor signaling chains with IL-15, lacked the ability to suppress CX3CR1 expression and, in contrast, was able to modestly increase it in all contexts tested (in vivo in blood, bone marrow, and spleen; in vitro in PBMCs; and in vitro in an IL-15-dependent culture system able to direct preferential NK-T cell outgrowth from primary mouse bone marrow cells). Our finding is consistent with that of Lauwerys et al,54 who also found that these cytokines are capable of differential gene expression. Moreover, IL-15 and IL-2 have profoundly different immunoregulatory roles: IL-2 promotes peripheral tolerance through activation-induced cell death, whereas IL-15 is antiapoptotic and promotes NK cell development.33,55 Despite low overall primary sequence homology, both cytokines belong to a family characterized by the presence of a 4-α-helical bundle.56 IL-2 has been shown to activate resting mature NK cells. It stimulates cytotoxicity of NK cells and supports up-regulation of several adhesion molecules and receptors, including many chemokine receptors.10,35 Although IL-2 has frequently been used to support NK cell differentiation and activation, the absence of the IL-2 gene product within bone marrow stroma and the presence of NK cells in IL-2-/- mice clearly indicate that cytokines other then IL-2, such as IL-15, drive NK cell development.57 Classic IL-2 and IL-15 receptors composed of βγc signaling chains have both been shown to activate JAK1 (Janus kinase 1), JAK3, Stat3 (signal transducer and activator of transcription 3), and Stat5. Precisely where the pathways diverge is not yet clear; however, there is evidence for a second IL-15 receptor subtype, IL-15RX on mast cells, with distinct signaling features.56 Additional work will be needed to define the precise receptor and postreceptor signaling pathways activated by IL-2 and IL-15 in the primary cell systems used in this study.
IL-15 has previously been shown to up-regulate expression of many chemokines and other chemokine receptors, including CCR2, CCR4, CCR5, CCR7, and CCR8, on freshly isolated human T lymphocytes, but to have no effect on expression of CXC chemokine receptors.36 These findings may not be relevant in the mouse, however, because analysis of a CX3CR1 knock-in mouse expressing green fluorescent protein (GFP) suggested that CX3CR1 is not expressed in mouse T cells.7 With regard to NK cells, the effects of IL-15 on chemokine receptor expression have not been previously studied, and the effects on chemokine expression have not been extensively surveyed. Given the unusual regulation by IL-15 of CX3CR1 as compared with other chemokine receptors apparently occurring at the transcriptional level, it will be important in future studies to compare the promoters of these receptors in detail for IL-15 responsive elements. In this regard, the human CX3CR1 promoter has been identified recently; however, specific transcription factors that regulate CX3CR1 expression have not yet been identified.58
CX3CR1 has been implicated in the immune response on the basis of its powerful CX3CL1-dependent adhesive activity and its ability to support leukocyte chemotaxis, as well as NK cell degranulation and killer function.15,17 Furthermore, a recent report by Nishimura et al8 suggests that CX3CL1 plays a predominant role in recruiting cytotoxic perforin and granzyme B-containing lymphocytes such as circulating cytotoxic CD8+ T cells (CTLs), most γδ T lymphocytes, granzyme B-positive CD4+ T cells, and NK cells. Moreover, high levels of CX3CR1 have been detected on CTLs with immediate cytotoxic activity.8 Although mice lacking CX3CR1 and people homozygous for a dysfunctional mutant of CX3CR1 named CX3CR1-M280 both appear healthy, specific roles for the receptor have been identified in several diseases, including allograft rejection in a mouse model and atherosclerosis in both mouse and humans22,23,28,29 in which it may mediate the response of not only NK cells but also monocytes, macrophages, and effector T cells. Thus, it will be important in future work to extend the scope of the present study to human and to other CX3CR1+ leukocyte subsets. The precise roles of NK cell CX3CR1 in these models have not yet been clearly delineated.
In conclusion, our results indicate that IL-2 and IL-15 have opposite effects on CX3CR1 expression in mouse NK1.1+ cells. Given the importance of IL-15 in NK cell development, these data imply that positive regulators of CX3CR1, potentially including IL-2, may operate in vivo to counterbalance the putative inhibitory effect of IL-15 in the CX3CR1-expressing NK cell subset. Moreover, our data show clearly that these 2 cytokines, which use the same IL-2Rβγc heterodimer signaling chains, differentially signal in vivo at the level of regulation of a single gene. These results raise new and fundamental questions regarding the basic mechanisms of chemokine receptor regulation in leukocytes. Moreover, at the translational level, they have important implications for any attempt to use IL-15 as a single agent in cell therapeutics, because the cell product is likely to have crippled chemotactic responses to CX3CL1 and, therefore, may be less able to traffic to disease targets.
While this manuscript was in revision Hanna et al59 reported that IL-15 induced down-regulation of CX3CR1 in human peripheral blood CD56+CD16- NK cells,59 in agreement with our work in mice.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-03-0946.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ji-Liang Gao for help and advice with the CX3CR1-/- mouse.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal