Abstract
We previously reported that interleukin-10 (IL-10) and transforming growth factor (TGF)–β treatment of primary mixed lymphocyte reaction (MLR) cultures resulted in secondary alloantigen-specific hyporesponsiveness and protection from graft-versus-host disease (GVHD) lethality. Here, we report that CD4+ T cells recovered from the IL-10– and TGF-β–treated primary MLR cultures have immunoregulatory function. Tolerized cells significantly inhibited proliferation of naive alloreactive CD4+ T cells in a primary MLR. Inhibition of the naive alloresponse was observed with as few as 1 tolerized cell to 10 naive responder cells. Tolerized cells were able to significantly reduce GVHD lethality when injected with naive alloreactive CD4+ T cells into major histocombatibility class (MHC) II disparate recipients. Rigorous CD25 depletion of the primary MLR had no effect on generation of a regulatory capacity, suggesting that the regulatory cells likely originated from CD4+CD25– T cells. Immune suppression was mediated independently of IL-10 and TGF-β production, as neutralizing antibodies for IL-10, IL-10R, and TGF-β were unable to revert suppression, and IL-10– deficient CD4+ T cells were able to mediate in vitro and in vivo suppression. The generation of immunoregulatory cells from a CD4+CD25– population during tolerization with IL-10 and TGF-β provides an additional mechanism to prevent GVHD lethality by T cells that may escape full tolerance induction.
Introduction
Graft-versus-host disease (GVHD) remains a significant cause of morbidity and mortality following bone marrow transplantation (BMT). Rigorous T-cell depletion of the donor graft and nonspecific immunosuppressive strategies are commonly used to successfully control T-cell alloreactivity after BMT. However, prolonged use of immunosuppressive regimens can eliminate beneficial T-cell functions, including antiviral responses, antileukemic responses, and engraftment. In a BMT setting, ex vivo tolerization strategies that selectively target the fraction of potentially alloreactive cells while leaving beneficial T-cell functions intact have significant clinical potential for GVHD prevention. Furthermore, tolerization strategies that enhance the immunoregulatory potential of tolerized cells may also be clinically useful for the dual purposes of GVHD prevention and therapy.
Regulatory T cells are able to suppress pathogenic T-cell responses in a variety of murine disease models, including GVHD.1 Regulatory T cells can be assigned to different subsets based on the expression of cell surface markers, production of cytokines, and mechanisms of action. CD4+CD25+ regulatory T cells are one important subset that has been carefully studied by many investigators.2 CD4+CD25+ regulatory T cells were initially identified by Sakaguchi and colleagues, based on their unique capacity to prevent the induction of certain autoimmune diseases in mice.3 Subsequently, Suri-Payer demonstrated that these cells could inhibit proliferation of autoantigen-specific T-cell clones in vivo.4 In addition to autoimmunity, recent evidence indicates that CD4+CD25+ regulatory cells participate in the modification of other immune responses. For example, we have demonstrated that CD4+CD25+ regulatory cells were critical for induction of alloantigen-specific tolerance by costimulatory pathway blockade.5 Others also have found that CD4+CD25+ regulatory cells could inhibit interleukin-2 (IL-2) production and facilitate T-cell anergy induction.6,7 Recently, it has been shown by our group and others that CD4+CD25+ regulatory cells could attenuate murine GVHD lethality.1,8,9 Despite accumulating evidence that underscores the importance of CD4+CD25+ cells in immune regulation, there is still very little known about the development and differentiation of these cells. In addition, their mechanisms of action remain elusive.
Unlike the endogenous CD4+CD25+ regulatory cell subset, which is derived from a subset of thymic emigrants,4,10-12 T regulatory type 1 cells (Tr1 cells) are derived by repeated in vitro stimulation of mature CD4+ T cells in the presence of IL-1013 or by chronic antigenic stimulation in vivo.14 Tr1 cells mediate their regulatory function by producing a distinct profile of immunosuppressive cytokines including IL-10 and transforming growth factor (TGF)–β, with little or no IL-4 or IL-2 production.13 Tr1 cells have the capacity to inhibit colitis in mice.13 Another regulatory cell subset, TGF-β–secreting Th3 cells, may be generated by in vitro stimulation in the presence of IL-415 or in vivo by oral administration of low-dose antigen.16 Th3 cells have the capacity to prevent autoimmune disease in several animal models.16-18 Yamagiwa and colleagues have recently reported the in vitro generation of a human CD4+CD25+ regulatory cell subset by culture with TGF-β that can inhibit alloresponses by impairing cytotoxic effector cell activation.19 The generation of Tr1, Th3, and other regulatory cell subsets testifies that naive CD4+ T cells may acquire regulatory functions.
Previously, we reported that alloantigen specific T-cell hyporesponsiveness and GVHD prevention resulted from treatment of a primary mixed lymphocyte reaction (MLR) with both IL-10 and TGF-β, whereas responses to nominal antigen remained intact.20 Since IL-10 has been shown to play a primary role in generation of Tr1 cells in vitro,21 we tested T cells recovered from IL-10/TGF-β–treated primary MLR cultures for immune regulatory cell function. In this report, we show that CD4+ T cells tolerized to alloantigen by culture with IL-10/TGF-β significantly inhibited proliferation of freshly isolated alloreactive CD4+ T cells in a primary MLR culture. IL-10/TGF-β–treated CD4+ T cells could reduce GVHD lethality in major histocombatibility class (MHC) II–disparate recipients when co-injected with naive, disease-causing allogeneic CD4+ T cells. Interestingly, IL-10/TGF-β–treated T cells mediated their inhibitory function independent of their capacity to make IL-10 and TGF-β, distinguishing these cells from the Tr1 and Th3 regulatory cell subsets. Furthermore, we report that the induction of alloantigen-specific tolerance and generation of regulatory cells by IL-10/TGF-β treatment does not require the presence of endogenous CD4+CD25+ regulatory T cells. Collectively, these data suggest a unique approach to generate alloreactive immune regulatory T cells in vitro by IL-10 and TGF-β, which may have clinical application for prevention of GVHD and other T-cell–mediated immune disorders.
Materials and methods
Mice
B6.C-H2bm12/KhEg (termed bm12) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Congenic B6.SJL-Ly5aPep3b (termed B6-CD45.1) and C57BL/6 (B6)–CD45.2 mice were purchased from the National Institutes of Health (NIH; Bethesda, MD). bm12 and B6 (both H-2b) mice differ at 3 amino acids due to mutations in the I-A region. Mice were used at 6 to 8 weeks of age. B6 IL-10 deletional mutant (IL-10–/–) mice were purchased from The Jackson Laboratory. OT-II transgenic mice were generated as described22 and provided by Dr Marc Jenkins (University of Minnesota, Minneapolis, MN) with permission from Dr William Heath (The Walter and Eliza Hall Institute of Medical Research, Australia). All mice were housed in a specific pathogen-free facility in micro-isolator cages according to the NIH guidelines.
In vitro MLR cultures
Axillary, mesenteric, cervical, and inguinal lymph nodes (LNs) were collected from mice into phosphate-buffered saline (PBS) containing 2% fetal calf serum (FCS). CD4+ T cells were isolated by mashing the LNs and passing through wire mesh to obtain a single-cell suspension. Cell preparations were depleted of natural killer (NK) cells (hybridoma PK136, rat IgG2a; provided by Dr Koo, Rahway, NJ) and CD8+ T cells (hybridoma 2.43, rat IgG2b; provided by Dr Sachs, Charlestown, MA) by coating with monoclonal antibodies (mAbs) and passing through a goat anti–mouse and goat anti–rat-Ig–coated column (Cedarlane Laboratories, Hornby, ON, Canada). The purity of the preparation was determined by flow cytometric analysis and was routinely at least 95% CD4+ T cells. CD4+ T cells from IL-10–/– mice were similarly prepared. To enrich CD4+CD25– T cells, purified CD4+ T cells were stained with phycoerythrin (PE)–conjugated anti-CD25 mAb (PC61) (BD/Pharmingen, San Diego, CA) and subjected to FACS (fluorescence-activated cell-sorter) (FACS Vantage; Becton Dickinson, San Jose, CA). The final collection was at least 99.6% CD4+CD25– T cells.
Responder CD4+ T cells were mixed with irradiated (30 Gy) anti-Thy 1.2 mAb (hybridoma 30H-12, rat IgG2b) and anti-NK1.1 mAb plus rabbit complement (Nieffenegger, Woodland, CA)–treated bm12 splenic stimulators. Responders and stimulators were cultured at a final concentration of 0.5 × 106/mL in 24-well plates (Costar, Acton, MA) in RPMI 1640 (BioWhittaker, Walkersville, MD) containing 10% FCS (Hyclone, Logan, UT), 50 mM 2-mercaptoethanol (2-ME; Sigma, St Louis, MO), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer, 1 mM sodium pyruvate (Life Technologies, Grand Island, NY), amino acid supplements (1.5 mM l-glutamine, l-arginine, l-asparagine) (Sigma), and antibiotics (100 U/mL penicillin; 100 mg/mL streptomycin) (Sigma). Human IL-10 (Schering-Plough Research Institute, Kenilworth, NJ) (specific activity, 3.1 × 107 U/mg) and TGF-β2 (Genzyme, Framingham, MA) (specific activity, 5 × 107 U/mg) were added at final concentrations of 1000 U/mL and 1 ng/mL, respectively. Plates were incubated at 37°C and 5% CO2 for 8 days. To monitor primary responses, 96-well round-bottom microtiter plates (Costar) were set up to contain 105 responders and stimulators per well. To monitor secondary responses, 5 × 104 washed responders and irradiated (30 Gy) non–T-cell–depleted stimulators were plated. IL-10 and TGF-β were not added to the secondary MLR. Microtiter wells were pulsed with tritiated thymidine (1 μCi [0.037 MBq]/well) (Amersham Life Sciences, Buckinghamshire, United Kingdom) on the indicated days for 16 to 18 hours prior to harvesting and counted in the absence of scintillation fluid on a β-plate reader (Packard Instruments, Meriden, CT). Samples were analyzed in triplicate.
To assay regulatory T-cell function, IL-10/TGF-β–treated cells were recovered on day 8 of a primary MLR culture. They were extensively washed and then added at specified concentrations into MLR cultures consisting of 105 freshly isolated CD4+ T-cell responders and bm12 splenocyte stimulators in 96-well round-bottom microtiter plates. Viability of MLR cultured cells was determined by trypan blue exclusion to be more than 95%. For experiments with neutralizing mAbs, anti–IL-10 mAb (hybridoma SXC1/SXC2, rat IgM, kindly provided by Dr Kevin Moore, DNAX, Palo Alto, CA), anti–IL-10R mAb (hybridoma 1B1.2, rat IgG1; kindly provided by Dr Moore), and anti–TGF-β mAb (hybridoma 1D11.16.8, mouse IgG1; American Type Culture Collection, Manassas, VA) were each added to cultures at a final concentration of 100 μg/mL on day 0.
To determine specificity of suppression, OT-II T-cell receptor (TCR) transgenic CD4+ T cells with specificity to chicken ovalbumin peptide (OVAp) 323-339 (ISQAVHAAHAEINEAGR) were used.22 Freshly isolated splenocytes (containing syngeneic antigen-presenting cells and transgenic T cells) from OT-II mice were cultured in 96-well plates at 104 OT-II CD4+ T cells/well in the presence or absence of an equal number of alloreactive B6 CD4+ T cells tolerized to bm12 alloantigen by IL-10/TGF-β. OVAp was added to a final concentration of 0.5 or 5 μg/mL to specified wells.
Flow cytometric analysis
The purity of freshly isolated CD4+ T cells and CD25-depleted CD4+ T cells were determined by staining with fluorescein isothiocyanate (FITC)–conjugated anti-CD4 mAb and PE-conjugated anti-CD25 mAb (3c7). Staining was performed according to manufacturer's instruction. All conjugated mAbs were from Pharmingen (San Diego, CA). Data were obtained and analyzed with CellQuest software on a FACSCalibur (Becton Dickinson).
In some experiments 5-(and -6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) was used to label freshly isolated CD4+ T cells or IL-10/TGF-β–treated cells to monitor cell division. CFSE (Molecular Probes, Eugene, OR) was added at a concentration of 2 μM to cells that had been resuspended to a concentration of 107/mL in PBS at 37°C for 10 minutes with shaking. Cells were washed with cold 2% FCS in PBS prior to use. CFSE intensity change was measured in the FL1 channel using a FACSCalibur. To distinguish naive from tolerized T-cell populations, tolerized cells were from B6 CD45.1 mice, and freshly isolated CD4+ T cells were from B6 CD45.2 congenic mice. Naive cells were positively identified for CFSE analysis by costaining with PE-conjugated anti-CD45.2 mAb and CyChrome-conjugated anti-CD4 mAb. Quantification of responder frequency and proliferative capacity of CFSE-labeled cells was done as described.23
In vivo alloresponses and GVHD induction
bm12 recipient mice were sublethally irradiated by exposure to 6 Gy total body irradiation (TBI) from a 137Cs source 4 hours before adoptive transfer of cells. 105 freshly isolated B6 CD4+ T cells were injected with or without 105 day-8 MLR-cultured cells at a 1:1 ratio into the lateral tail veins. Peripheral blood was obtained by retro-orbital venipuncture for measurement of day 14 and 28 hematocrit (HCT) values as an indicator of the bone marrow destructive effects of infused T cells. Mice were monitored daily for survival. Survival data were analyzed by life-table methods, and actuarial survival rates are shown in the figures. Group comparisons were made by log-rank test statistics.
Results
IL-10/TGF-β–tolerized CD4+ T cells down-regulate a naive alloresponse both in vitro and in vivo
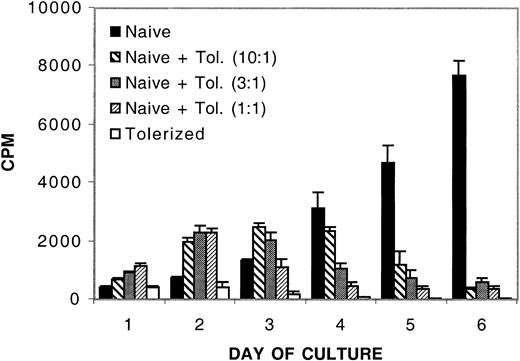
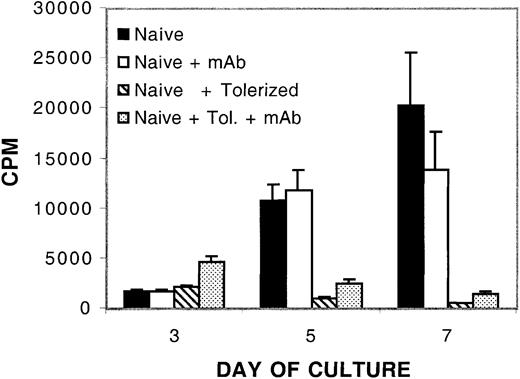
An 8-day treatment of murine CD4+ T cells with IL-10 and TGF-β in an MLR results in profound alloantigen-specific hyporesponsiveness.20 Although each cytokine is able to independently reduce primary, and transiently inhibit secondary, MLR responses, only the combination of these cytokines in an MLR culture protects most of the recipients from GVHD-induced lethality when cultured cells are adoptively transferred in vivo.20 Other reports suggest that treatment of human CD4+ T cells with immune regulatory cytokines such as IL-10 or TGF-β can result in the acquisition of regulatory function.13,19,24 Therefore, we investigated whether the IL-10/TGF-β–treated murine CD4+ T cells might have gained regulatory or suppressor cell function as assessed by analyzing the capacity of treated cells to regulate a naive alloresponse. B6 CD4+ T cells, previously tolerized to bm12 alloantigen by an 8-day MLR culture with IL-10 and TGF-β, were washed to remove soluble cytokines and added to a naive MLR culture of B6 CD4+ T cells and bm12 splenic stimulators. Figure 1 shows that by day 4 of culture, the addition of as few as 1 tolerized cell to 10 naive CD4+ T cells inhibited the naive alloresponse. By day 6, at the peak of control T-cell proliferation, there was more than a 90% reduction in the naive alloresponse when naive CD4+ T cells were cultured with tolerized cells up to a ratio of 10 naive cells to 1 tolerized cell. No inhibition of the naive alloresponse was observed at any time point when cells were cultured at a ratio of 1 tolerized to 100 naive cells (data not shown). The suppression was specifically due to the presence of tolerized cells, because removal of apoptotic cells and debris from the tolerized cells prior to culture with naive CD4+ T cells had no effect on suppressive capacity (data not shown).
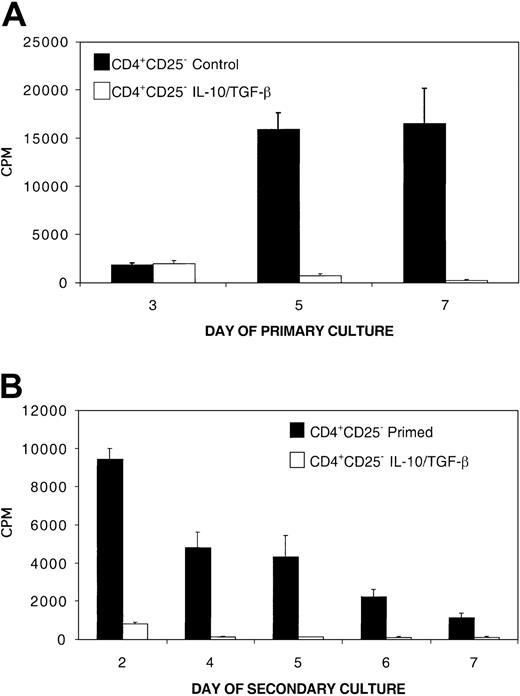
In vitro inhibition of naive alloresponses by the addition of IL-10/TGF-β –tolerized cells. The addition of tolerized cells down-regulates a naive alloresponse in vitro. MLR culture consisted of 105 naive B6 CD4+ T-cell responders per well and 105 bm12 splenic stimulators per well plated with no, 104,3 × 104,or105 B6 CD4+ T cells that had been previously tolerized to bm12 alloantigen by an 8-day culture with IL-10 and TGF-β. Tolerized cells were washed free of cytokines prior to plating in regulatory cultures. To verify secondary hyporesponsiveness of tolerized cells, 105 tolerized cells were plated with 105 bm12 splenic stimulators per well. The y-axis presents the mean cpm ± 1 SE. On the x-axis are days in primary culture. Shown is 1 of 10 representative experiments.
In vitro inhibition of naive alloresponses by the addition of IL-10/TGF-β –tolerized cells. The addition of tolerized cells down-regulates a naive alloresponse in vitro. MLR culture consisted of 105 naive B6 CD4+ T-cell responders per well and 105 bm12 splenic stimulators per well plated with no, 104,3 × 104,or105 B6 CD4+ T cells that had been previously tolerized to bm12 alloantigen by an 8-day culture with IL-10 and TGF-β. Tolerized cells were washed free of cytokines prior to plating in regulatory cultures. To verify secondary hyporesponsiveness of tolerized cells, 105 tolerized cells were plated with 105 bm12 splenic stimulators per well. The y-axis presents the mean cpm ± 1 SE. On the x-axis are days in primary culture. Shown is 1 of 10 representative experiments.
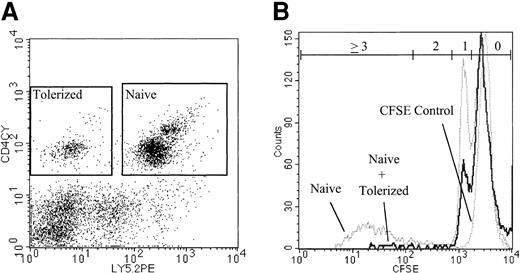
To directly visualize the suppressive effect of IL-10/TGF-β–treated cells on the proliferation of naive CD4+ T cells, naive CD4+ B6 CD45.2 responders were labeled with CFSE before coculture with IL-10/TGF-β–treated B6 CD45.1 congenic CD4+ T cells. After 6 days of coculture, the cells were recovered and analyzed by flow cytometry for proliferation status as indicated by CFSE intensity (Figure 2). On day 6, in the absence of IL-10/TGF-β–treated cells, 18% of the naive B6 CD4+ cells in the culture had undergone at least 2 cell divisions and had diluted CFSE intensity (Table 1). In contrast, only 3% of the naive CD4+ cells cocultured with IL-10/TGF-β–treated CD4+ cells had undergone 2 or more cell divisions, demonstrating a significant inhibition of naive CD4+ proliferation mediated by IL-10/TGFβ–treated cells. This reproducible result directly demonstrates that addition of tolerized cells to the culture inhibits cell division of the naive B6 CD4+ responders.
Inhibition of cell division of naive CD4+ T cells cocultured with tolerized CD4+ cells. The addition of tolerized cells inhibits cell division of naive CD4+ T cells in vitro. CFSE-labeled naive B6 CD45.2 CD4+ T cells were cultured with bm12 stimulators with or without the addition of tolerized B6 CD45.1 CD4+ cells at a ratio of 4 naive cells to 1 tolerized cell. On day 6, CD45.2 CD4+ cells were identified (Figure 2A) and analyzed for CFSE dilution by flow cytometry (Figure 2B). 17.5% of naive cells cultured alone and 3.2% of naive cells cocultured with tolerized cells had undergone 2 or more cell divisions. Gates for each number of cell divisions were calculated based on comparison with undivided control cells (Table 1). Data are representative of 2 replicate experiments with similar results.
Inhibition of cell division of naive CD4+ T cells cocultured with tolerized CD4+ cells. The addition of tolerized cells inhibits cell division of naive CD4+ T cells in vitro. CFSE-labeled naive B6 CD45.2 CD4+ T cells were cultured with bm12 stimulators with or without the addition of tolerized B6 CD45.1 CD4+ cells at a ratio of 4 naive cells to 1 tolerized cell. On day 6, CD45.2 CD4+ cells were identified (Figure 2A) and analyzed for CFSE dilution by flow cytometry (Figure 2B). 17.5% of naive cells cultured alone and 3.2% of naive cells cocultured with tolerized cells had undergone 2 or more cell divisions. Gates for each number of cell divisions were calculated based on comparison with undivided control cells (Table 1). Data are representative of 2 replicate experiments with similar results.
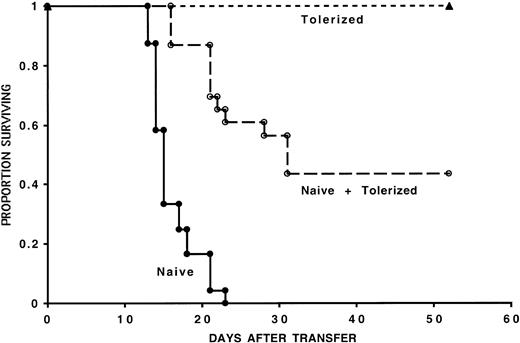
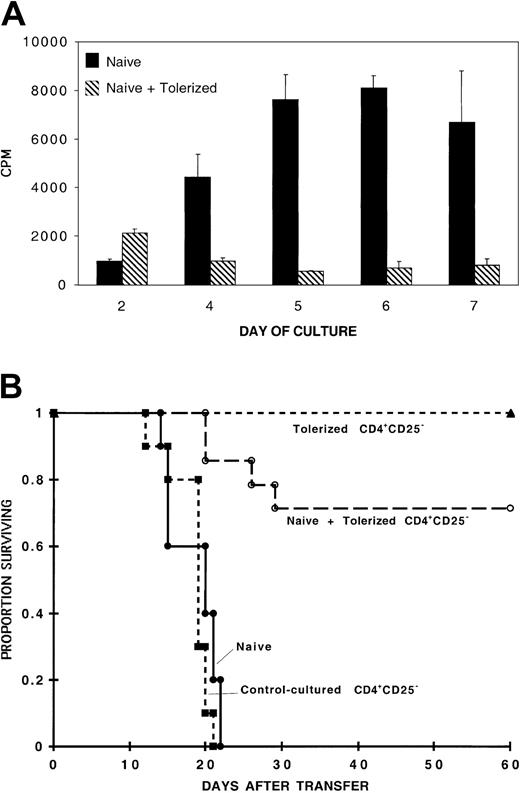
To evaluate whether the regulatory effect of IL-10/TGF-β–tolerized cells was operative in vivo, we used a murine model of GVHD induction where adoptive transfer of 105 naive B6 CD4+ T cells uniformly results in GVHD lethality in sublethally irradiated bm12 recipients.20 The infusion of 105 naive B6 CD4+ T cells resulted in GVHD-induced bone marrow aplasia and GVHD lethality within 23 days after transfer of cells in all control mice (Figure 3). As previously reported, infusion of 105 8-day MLR IL-10/TGF-β–cultured B6 CD4+ T cells resulted in long-term survival (> 60 days), which in these experiments was 100%.20 Cotransfer of 105 8-day MLR IL-10/TGF-β–cultured B6 CD4+ T cells with 105 naive CD4+ T cells resulted in long-term survival (> 60 days) of 43% of recipient mice from 3 pooled experiments. Comparable results were obtained when 105 naive CD4+ T cells and 5 × 104 tolerized CD4+ T cells were cotransferred into bm12 recipients (data not shown). HCT values were assessed in all mice on day 14 after transfer of cells to evaluate CD4+ T-cell–mediated GVHD-induced bone marrow aplasia. In one representative experiment, the HCT was 15.6% ± 2.8% in mice receiving naive CD4+ cells versus 24.5% ± 1.1% in mice receiving both naive and tolerized CD4+ cells (P < .006). Taken together, our in vitro and in vivo results demonstrate the acquisition of regulatory function in alloreactive cells cultured with IL-10 and TGF-β.
In vivo inhibition of GVHD mortality by the co-infusion of tolerized cells. Tolerized cells reduce GVHD mortality induced by naive cells. One hundred thousand naive B6 CD4+ T cells were injected with or without 105 tolerized B6 CD4+ T cells into sublethally irradiated bm12 recipients. Three separate experiments were pooled; n = 24 for recipients of naive cells and n = 23 for recipients of naive and tolerized cells (P < .001). Also shown is long-term survival of all recipients of tolerized cells alone (n = 23). On the x-axis are days after transfer of T cells. The y-axis shows the proportion of recipients surviving after transfer.
In vivo inhibition of GVHD mortality by the co-infusion of tolerized cells. Tolerized cells reduce GVHD mortality induced by naive cells. One hundred thousand naive B6 CD4+ T cells were injected with or without 105 tolerized B6 CD4+ T cells into sublethally irradiated bm12 recipients. Three separate experiments were pooled; n = 24 for recipients of naive cells and n = 23 for recipients of naive and tolerized cells (P < .001). Also shown is long-term survival of all recipients of tolerized cells alone (n = 23). On the x-axis are days after transfer of T cells. The y-axis shows the proportion of recipients surviving after transfer.
IL-10/TGF-β–tolerized CD4+ T cells have a reduced capacity to down-regulate nonspecific responses
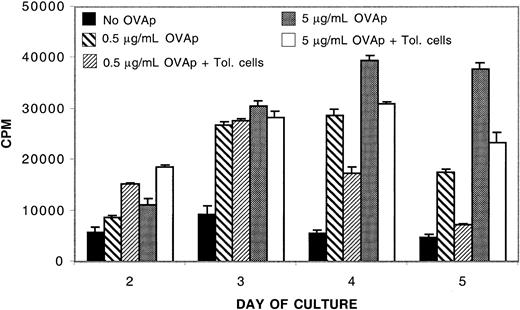
To determine the specificity of immune suppression mediated by IL-10/TGF-β–tolerized alloreactive T cells, we measured their ability to modify the proliferative response of naive OT-II transgenic CD4+ T cells to chicken OVAp. Suboptimal (0.5 μg/mL) and optimal (5.0 μg/mL) concentrations of OVAp were added to naive OT-II CD4+ cells in the presence or absence of an equal number of IL-10/TGF-β–tolerized alloreactive cells. Nonspecific suppression of OT-II responses was not apparent at early time points, whereas the addition of tolerized cells reduced optimal OT-II responses by 21% and suboptimal OT-II responses by 40% at the time of peak response on day 4 (Figure 4). Moreover, no suppression was observed at any time point in a repeat experiment done with optimal antigen concentration (data not shown). Thus, most of the nonspecific response was preserved, especially at optimal antigen concentrations. Under these conditions, it appeared that cells tolerized to alloantigen by IL-10/TGF-β had the capacity to modestly down-regulate nonspecific responses, which diminished as antigen concentrations increased. IL-10/TGF-β–tolerized cells suppressed naive alloresponses in vitro by more than 90% in both experiments (data not shown). These data indicate that there is a substantial degree of specificity to the suppression mediated by IL-10/TGF-β–tolerized alloreactive cells.
IL-10/TGF-β–tolerized CD4+ T cells modestly down-regulate nonspecific responses. Tolerized cells exert a modest down-regulatory effect on naive OT-II responders. 104 OT-II CD4+ T cells were cultured with or without 104 tolerized B6 CD4+ T cells in the presence of B6 antigen-presenting cells. 0.5 μg/mL or 5 μg/mL OVAp was added to some wells as indicated. Tolerized B6 CD4+ T cells suppressed naive alloresponses by more than 90%. Shown is 1 of 2 replicate experiments with similar results.
IL-10/TGF-β–tolerized CD4+ T cells modestly down-regulate nonspecific responses. Tolerized cells exert a modest down-regulatory effect on naive OT-II responders. 104 OT-II CD4+ T cells were cultured with or without 104 tolerized B6 CD4+ T cells in the presence of B6 antigen-presenting cells. 0.5 μg/mL or 5 μg/mL OVAp was added to some wells as indicated. Tolerized B6 CD4+ T cells suppressed naive alloresponses by more than 90%. Shown is 1 of 2 replicate experiments with similar results.
CD4+CD25+ cells are not required for the induction of tolerance and generation of regulatory capacity
We have previously reported that the presence of immune regulatory CD4+CD25+ T cells is required for tolerance induction and the generation of regulatory activity via ex vivo costimulatory blockade.5,25 Specifically, depletion of CD4+CD25+ T cells from the CD4+ responder population completely prevented ex vivo tolerance induction to alloantigen and the simultaneous generation of cells with immune regulatory capacity by costimulatory blockade. To determine whether CD4+CD25+ cells also were required for tolerance induction and the acquisition of regulatory capacity by MLR culture with IL-10 and TGF-β, B6 CD4+CD25– T cells and bm12 splenic stimulators were cultured in the presence of IL-10 and TGF-β. This treatment suppressed a primary MLR with CD25-depleted responders (≥ 99.6% purity) by more than 95% by day 5 (Figure 5A). After 8 days, the cells from this primary culture were washed free of cytokines and replated with fresh bm12 splenic stimulators. Secondary hyporesponsiveness to alloantigen stimulation was profound; the secondary response of IL-10/TGF-β–treated CD4+CD25– cells was more than 90% suppressed at all time points (Figure 5B). These data demonstrate that CD4+CD25+ T cells are not required for induction of tolerance to alloantigen via the immune suppressive cytokines IL-10 and TGF-β.
CD4+CD25+ cells are not required for the induction of tolerance to alloantigen via 8-day MLR culture with IL-10 and TGF-β (A) Primary MLR consisted of 0.5 × 106/mL B6 CD4+CD25– T-cell responders (≥ 99.6% pure) and 0.5 × 106/mL bm12 splenic stimulators cultured for 8 days with IL-10 and TGF-β. Control MLR was not treated with cytokines. (B) To measure tolerance induction of CD4+CD25– cells by IL-10/TGF-β, the CD4+ T cells were recovered after an 8-day primary MLR culture period, washed, and restimulated with fresh bm12 splenic stimulators. Shown is 1 of 4 representative experiments with similar results.
CD4+CD25+ cells are not required for the induction of tolerance to alloantigen via 8-day MLR culture with IL-10 and TGF-β (A) Primary MLR consisted of 0.5 × 106/mL B6 CD4+CD25– T-cell responders (≥ 99.6% pure) and 0.5 × 106/mL bm12 splenic stimulators cultured for 8 days with IL-10 and TGF-β. Control MLR was not treated with cytokines. (B) To measure tolerance induction of CD4+CD25– cells by IL-10/TGF-β, the CD4+ T cells were recovered after an 8-day primary MLR culture period, washed, and restimulated with fresh bm12 splenic stimulators. Shown is 1 of 4 representative experiments with similar results.
To determine whether CD4+CD25+ cells were required for the generation of regulatory cells by IL-10/TGF-β treatment, cells from a CD25-depleted population were tolerized to bm12 alloantigen by 8-day MLR culture with IL-10 and TGF-β, washed free of cytokines, and added to a naive B6 CD4+ MLR culture containing bm12 splenic stimulators. By day 4 of the culture, the presence of 1 tolerized cell to 4 naive CD4+ T cells reduced the naive alloresponse by more than 75%, and by the time of peak naive alloresponse on day 6, the tolerized cells inhibited the response by more than 90% (Figure 6A). Similar down-regulation of the peak naive alloresponse was present when cultures included up to 16 naive CD4+ T cells to 1 tolerized cell (data not shown). The kinetics and amplitude of the inhibition was very similar to the inhibition mediated by tolerized whole B6 CD4+ T cells (Figure 1).
CD4+CD25– cells acquire regulatory function that inhibits naive alloresponses via 8-day MLR culture with IL-10 and TGF-β CD4+CD25+ T cells are not required for acquisition of in vitro (A) or in vivo (B) inhibitory function. (A) MLR culture contained 105 naive B6 CD4+ T-cell responders per well and 105 bm12 splenic stimulators per well plated with or without CD4+ T cells (2.5 × 104 per well), derived from a CD4+CD25– culture that had been previously tolerized to bm12 alloantigen by an 8-day culture with IL-10 and TGF-β. Shown is 1 of 2 replicate experiments with similar results. (B) 105 naive B6 CD4+ T cells were injected alone (n = 10) or with 105 8-day MLR IL-10/TGF-β–cultured CD25-depleted CD4+ T cells (n = 14) (P < .001). Also shown is long-term survival of all recipients of 105 8-day MLR IL-10/TGF-β– cultured CD25-depleted CD4+ T cells (n = 14) and uniform lethality by day 21 of all recipients (n = 10) of 105 8-day MLR control-cultured CD4+CD25– T cells. Data represent 2 pooled experiments with similar results.
CD4+CD25– cells acquire regulatory function that inhibits naive alloresponses via 8-day MLR culture with IL-10 and TGF-β CD4+CD25+ T cells are not required for acquisition of in vitro (A) or in vivo (B) inhibitory function. (A) MLR culture contained 105 naive B6 CD4+ T-cell responders per well and 105 bm12 splenic stimulators per well plated with or without CD4+ T cells (2.5 × 104 per well), derived from a CD4+CD25– culture that had been previously tolerized to bm12 alloantigen by an 8-day culture with IL-10 and TGF-β. Shown is 1 of 2 replicate experiments with similar results. (B) 105 naive B6 CD4+ T cells were injected alone (n = 10) or with 105 8-day MLR IL-10/TGF-β–cultured CD25-depleted CD4+ T cells (n = 14) (P < .001). Also shown is long-term survival of all recipients of 105 8-day MLR IL-10/TGF-β– cultured CD25-depleted CD4+ T cells (n = 14) and uniform lethality by day 21 of all recipients (n = 10) of 105 8-day MLR control-cultured CD4+CD25– T cells. Data represent 2 pooled experiments with similar results.
Next, we examined whether B6 CD4+CD25– T cells tolerized by culture with bm12 splenic stimulators in the presence of IL-10 and TGF-β for 8 days would result in the generation of cells that could confer protection from GVHD in vivo. Figure 6B shows that cotransfer of 105 8-day MLR-tolerized CD25-depleted cells with 105 naive whole B6 CD4+ T cells prevented GVHD lethality in approximately 70% of bm12 recipients, whereas transfer of 105 naive B6 CD4+ T cells alone resulted in uniform lethality by day 22 after transfer of cells. Collectively, these data indicate that CD4+CD25+ immune regulatory cells are not required for either tolerance induction or the acquisition of regulatory capacity during culture with IL-10 and TGF-β in primary MLR cultures.
The suppressive mechanism of IL-10/TGF-β–treated cells is mediated independently of endogenous production of both IL-10 and TGF-β
Upon alloantigen restimulation, IL-10/TGF-β–tolerized cells produce no IL-2, little IL-4, decreased IFN-γ, and increased IL-10 compared to control-primed cultures (data not shown). These data are consistent with others who have reported that regulatory cells induced in vitro by immune regulatory cytokines results in their production of IL-10 or TGF-β, but neither a Th1 nor Th2 pattern of cytokines.13,19 Therefore, we sought to determine whether IL-10 or TGF-β production by these cells was required for their inhibitory function.
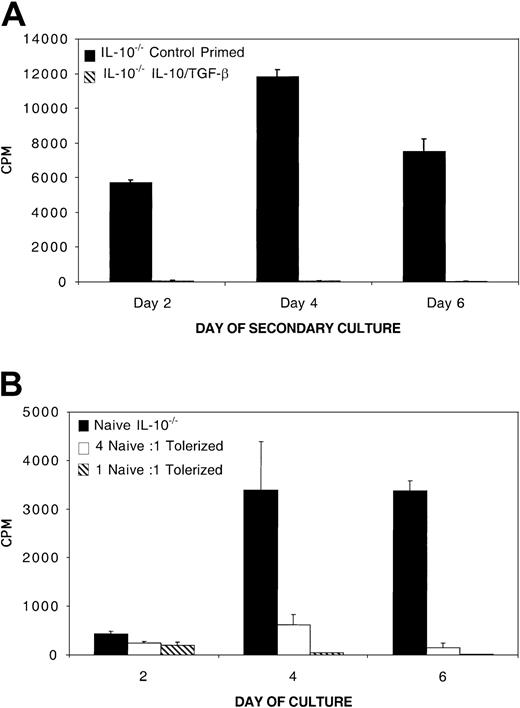
To determine whether IL-10 production by tolerized cells was responsible for suppression, we adapted our model to use B6 mice with a deletional mutation in the IL-10 gene. CD4+ T cells were purified from B6 IL-10–/– mice and cultured in an 8-day MLR with bm12 splenic stimulators and IL-10 and TGF-β. After 8 days, the cells were washed and restimulated with bm12 alloantigen. Figure 7A illustrates that IL-10/TGFβ–treated IL-10–/–CD4+ T cells were profoundly hyporesponsive to restimulation with alloantigen. Secondary hyporesponsiveness to alloantigen by B6 IL-10–/–CD4+ T cells was comparable to hyporesponsiveness of tolerized wild-type B6 CD4+ T cells (data not shown). Furthermore, IL-10–/–CD4+ T cells also were able to suppress a naive alloresponse by 80% at a culture ratio of 1 tolerized B6 IL-10–/–CD4+ T cell to 4 naive B6 CD4+ T cells (Figure 7B). The naive alloresponse was suppressed by IL-10/TGF-β–tolerized B6 IL-10–/–CD4+ T cells by more than 95% on day 4 when cultured at a ratio of 1 tolerized to 1 naive cell. IL-10/TGF-β–tolerized IL-10–/–CD4+ T cells were able to suppress naive alloresponses from B6 CD4+ T cells and B6 IL-10–/ –CD4+ T cells with equal potency (data not shown).
IL-10–/–CD4+ T cells become tolerant and develop in vitro regulatory function during MLR culture with IL-10 and TGF-β (A) IL-10–/–CD4+ cells become tolerant after MLR culture with IL-10 and TGF-β. MLR consisted of B6 IL-10–/–CD4+ responders (0.5 × 105/mL) and bm12 splenic stimulators (0.5 × 105/mL) cultured with and without IL-10 and TGF-β for 8 days. Eight-day MLR cultured B6 IL-10–/–CD4+ were washed free of cytokines and restimulated with bm12 splenic stimulators. Shown is 1 of 3 experiments with similar results. (B) IL-10/TGF-β–treated IL-10–/–CD4+ T cells inhibit naive alloresponses in vitro. 105 naive B6 IL-10–/–CD4+ T-cell responders per well and 105 bm12 splenic stimulators per well were plated alone or with 2.5 × 104 or 105 8-day B6 IL-10–/–CD4+ cells that had been previously tolerized to bm12 alloantigen by an 8-day culture with IL-10 and TGF-β. Shown is 1 of 3 representative experiments.
IL-10–/–CD4+ T cells become tolerant and develop in vitro regulatory function during MLR culture with IL-10 and TGF-β (A) IL-10–/–CD4+ cells become tolerant after MLR culture with IL-10 and TGF-β. MLR consisted of B6 IL-10–/–CD4+ responders (0.5 × 105/mL) and bm12 splenic stimulators (0.5 × 105/mL) cultured with and without IL-10 and TGF-β for 8 days. Eight-day MLR cultured B6 IL-10–/–CD4+ were washed free of cytokines and restimulated with bm12 splenic stimulators. Shown is 1 of 3 experiments with similar results. (B) IL-10/TGF-β–treated IL-10–/–CD4+ T cells inhibit naive alloresponses in vitro. 105 naive B6 IL-10–/–CD4+ T-cell responders per well and 105 bm12 splenic stimulators per well were plated alone or with 2.5 × 104 or 105 8-day B6 IL-10–/–CD4+ cells that had been previously tolerized to bm12 alloantigen by an 8-day culture with IL-10 and TGF-β. Shown is 1 of 3 representative experiments.
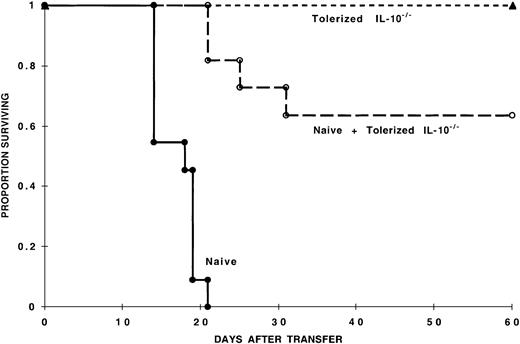
To assess the capacity of IL-10/TGF-β–tolerized cells for in vivo suppression of naive alloresponses, 105 8-day IL-10/TGF-β–tolerized B6 IL-10–/–CD4+ T cells were cotransferred with 105 GVHD-causing naive B6 CD4+ T cells into sublethally irradiated bm12 recipients. Approximately 60% of mice receiving tolerized and naive cells survived the observation period (60 days), in contrast to mice that received naive cells alone that uniformly died of GVHD within 3 weeks of naive cell infusion (Figure 8), similar to studies using wild-type tolerized cells (Figure 3). Collectively, these data show that CD4+ T cells tolerized by MLR culture with IL-10 and TGF-β can significantly inhibit naive alloresponses in vitro and in vivo independently of IL-10 production.
IL-10 production by IL-10/TGF-β–tolerized cells is not required for in vivo inhibition of GVHD mortality. Tolerized IL-10–/–CD4+ T cells reduce mortality induced by naive cells. One hundred thousand naive B6 CD4+ T cells were injected with or without 105 tolerized IL-10–/–B6 CD4+ T cells into sublethally irradiated bm12 recipients (n = 11 per group) (P < .001). Also shown is long-term survival of all recipients of 105 8-day MLR IL-10/TGF-β–cultured B6 IL-10–/–CD4+ T cells (n = 10). Data represent 2 pooled experiments with similar results.
IL-10 production by IL-10/TGF-β–tolerized cells is not required for in vivo inhibition of GVHD mortality. Tolerized IL-10–/–CD4+ T cells reduce mortality induced by naive cells. One hundred thousand naive B6 CD4+ T cells were injected with or without 105 tolerized IL-10–/–B6 CD4+ T cells into sublethally irradiated bm12 recipients (n = 11 per group) (P < .001). Also shown is long-term survival of all recipients of 105 8-day MLR IL-10/TGF-β–cultured B6 IL-10–/–CD4+ T cells (n = 10). Data represent 2 pooled experiments with similar results.
After determining that IL-10 was not required for regulatory function, we investigated whether TGF-β was required for the suppressive action of the IL-10/TGF-β–tolerized cells. B6 CD4+ T cells, tolerized to bm12 alloantigen by an 8-day MLR culture with IL-10 and TGF-β, were added to naive B6 CD4+ T cells and bm12 splenic stimulators at a ratio of 1 tolerized cell to 4 naive cells in the presence or absence of monoclonal antibodies that neutralize IL-10, IL-10 receptor, and TGF-β (Figure 9).
Suppression of naive alloresponses by IL-10/TGF-β–treated cells is not dependent on IL-10 and TGF-β MLR culture consisted of 105 naive B6 CD4+ T-cell responders per well and 105 bm12 splenic stimulators per well plated with and without B6 CD4+ T cells (2.5 × 104 per well) that had been previously tolerized to bm12 alloantigen by an 8-day culture with IL-10 and TGF-β. Cultures were treated or not with IL-10, IL-10 receptor, and TGF-β neutralizing monoclonal antibodies (mAb). Shown is 1 of 5 representative experiments with similar results.
Suppression of naive alloresponses by IL-10/TGF-β–treated cells is not dependent on IL-10 and TGF-β MLR culture consisted of 105 naive B6 CD4+ T-cell responders per well and 105 bm12 splenic stimulators per well plated with and without B6 CD4+ T cells (2.5 × 104 per well) that had been previously tolerized to bm12 alloantigen by an 8-day culture with IL-10 and TGF-β. Cultures were treated or not with IL-10, IL-10 receptor, and TGF-β neutralizing monoclonal antibodies (mAb). Shown is 1 of 5 representative experiments with similar results.
Even in the presence of IL-10 and TGF-β neutralizing antibodies, IL-10/TGF-β–tolerized cells significantly inhibited proliferation of naive alloreactive B6 CD45.2 CD4+ T cells (Figure 9) and recovery of naive B6 CD45.2 CD4+ T cells at the end of primary culture (138% for naive + mAb vs 35% for coculture + mAb) (Table 2). Based on these data, the in vitro suppressive effect of IL-10/TGF-β–tolerized cells is clearly not reversed by neutralizing IL-10 and TGF-β with antibodies.
Analysis of the division profile of naive CFSE-labeled B6 CD45.2 CD4+ T cells in a coculture with tolerized cells indicated similar results (Table 2). Neutralizing antibodies increased responder frequency, proliferative capacity, and recovery of naive cells whether cocultured with tolerized cells or not. Despite increases in these measures of proliferation, naive + tolerized cocultures were significantly inhibited compared to naive only cultures, regardless of mAb treatment. In the presence of blocking mAbs, cocultured naive cells have a responder frequency 44% lower than naive cells cultured without tolerized cells (0.105 vs 0.046), and each responding cell produced 51% fewer daughter cells (14.9 vs 7.3). Thus, addition of IL-10 and TGF-β neutralizing antibodies increased the responder frequency of naive cells in all cultures but was unable to fully reverse suppression.
Discussion
In this study, we present the finding that ex vivo alloantigen-specific tolerance induction by IL-10 and TGF-β treatment of an MLR results in the generation of regulatory T cells that function to inhibit a naive alloresponse both in vitro and in vivo. It was determined that CD4+CD25+ cells are not required for either tolerance induction or generation of regulatory cells that suppress naive alloresponses. Furthermore, neither IL-10 nor TGF-β production by ex vivo–generated regulatory T cells is required for their in vitro regulatory effect. Moreover, IL-10 production is clearly not required for an in vivo regulatory effect. These data provide formal evidence that combined IL-10/TGF-β treatment of alloreactive murine CD4+ T cells can be exploited for generation of potent regulatory cells with the capacity to inhibit alloreactive responses in vivo. In combination with anergy induction, the generation of regulatory cells may contribute to tolerance induced in murine CD4+ T cells by IL-10 and TGF-β.
IL-10 is well known to be an immunoregulatory cytokine with the ability to facilitate the generation of murine and human regulatory T cells. Short-term treatment of human CD4+ T cells with IL-10 in an MLR can inhibit primary responsiveness and induce anergy,21 but this population of cells does not have the capacity to inhibit a naive alloresponse.26 Furthermore, additional supplementation with TGF-β does not contribute to the generation of regulatory activity.26 In contrast, chronic activation of murine and human CD4+ T cells in the presence of IL-10 results in the generation of the Tr1 cells.13 When cloned, these cells have the capacity to inhibit in vitro and in vivo alloresponses.13 Interestingly, this subset of cells appears to be functionally distinct from human CD4+CD25+ regulatory cells with different cytokine production profiles.27 We do not know if the mechanism responsible for generation of Tr1 cells by chronic IL-10 exposure is similar to the mechanism for induction of murine regulatory cells by combined short-term IL-10 and TGF-β treatment. However, it appears that the functional mechanism of IL-10/TGF-β–tolerized cells is distinct from that of Tr1 cells, which are dependent on the production of IL-10 and TGF-β.13
The role of TGF-β in the induction of regulatory T cells is less well defined. TGF-β1 deletional mutant mice rapidly develop an autoimmune phenotype and die within several weeks of birth,28-30 indicating that TGF-β1 likely has an important anti-inflammatory role in vivo. Like other animal models of autoimmune disease, it was a formal possibility that deficiency or impaired function of regulatory cells was responsible for the phenotype of these mice. A recent report by Piccirillo et al has discounted this theory, however, as CD4+CD25+ regulatory cells purified from TGF-β1–/– mice are functionally similar to CD4+CD25+ regulatory cells from wild-type mice when used for in vitro assays.31 While it remains undetermined whether TGF-β1–/–CD4+CD25+ regulatory cells would be functional if transferred to wild-type hosts, they are clearly not sufficient to prevent autoimmunity in TGF-β1 deletional mutant mice. Although murine CD4+CD25+ cells can develop in the absence of TGF-β1, Yamagiwa and coworkers have recently demonstrated that in vitro activation of naive human CD4+ T cells in the presence of TGF-β results in the expansion of the CD4+CD25+ subset, and these cells can potently inhibit CD8+ responses.19
It is not known whether the actions of TGF-β toward the generation of human regulatory cells are similar to the actions of TGF-β in the generation of murine regulatory cells by combination treatment with IL-10 and TGF-β. However, the combination of IL-10 and TGF-β are known to be cooperative in several systems during CD4+ T-cell activation. We have shown previously that MLR cultures treated with only the combination of IL-10 and TGF-β result in prolonged secondary hyporesponsiveness of murine CD4+ T cells to alloantigen and inhibition of GVHD lethality, whereas MLR cultures treated with TGF-β alone result in minimal protection from GVHD.20 Recently, it has been reported that during activation, IL-10 modulates the response of CD4+ T cells to TGF-β by enhancement of TGF-β receptor type II expression32 and facilitation of TGF-β production.33 Thus, because the induction of tolerance and regulatory capacity in our system requires both IL-10 and TGF-β, it is likely that IL-10 and TGF-β act cooperatively to maximize the contribution of each cytokine on murine CD4+ T cells to induce potent regulatory capacity.
With respect to the role of TGF-β in mediating the function of regulatory cells, several investigators have reported that TGF-β does play a critical role in suppression mediated by regulatory cells.12,34,35 In contrast, other investigators have found that TGF-β does not have a role in suppression mediated by either murine or human CD4+CD25+ regulatory cells.6,31,36,37 The role for TGF-β in CD4+CD25+ regulatory cell immunosuppression may be dependent on the experimental system. Although TGF-β is intimately related to generation of the regulatory cell population that we have presently described, we show that TGF-β is unlikely to contribute significantly to their in vitro immunosuppressive function.
Although the mechanism by which TGF-β induces naive human CD4+ T cells to become regulatory cells is unknown, the presence of a small frequency (0.9%) of CD4+CD25+ cells appears to be required in the initiating population.19 Because cultures initiated with 0.4% CD4+CD25+ cells did not acquire regulatory cell function, these investigators concluded that TGF-β induced the expansion of thymus-derived CD4+CD25+ regulatory cells. In separate experiments, however, this group showed that human CD4+CD25– cells stimulated with low-dose staphylococcal enterotoxin and TGF-β developed a regulatory phenotype characterized by TGF-β secretion.24 Previous work from our group has described the generation of a potent regulatory cell population by ex vivo blockade of the CD40:CD40L costimulatory pathway in murine CD4+ T cells, which is also dependent on the presence of CD4+CD25+ T cells in the starting population.25 Furthermore, depletion of CD4+CD25+ cells also precludes tolerance induction via costimulatory blockade.5 In the present manuscript, using a distinct tolerance-induction strategy, we report the generation of regulatory CD4+ cells with a similar ability to down-regulate naive alloresponses. However, we report that maximal depletion of CD4+CD25+ T cells from the starting population does not affect tolerance induction or the generation of regulatory cells by the combined treatment of IL-10 and TGF-β. It remains a formal possibility that there is a minimum requirement for CD4+CD25+ T cells in the starting population for the generation of regulatory cells by IL-10 and TGF-β, however, if this were the case this threshold level would be less than 0.4% CD4+CD25+ cells.
Phenotypic analysis indicates that an average of 40% of whole B6 CD4+ cells cultured with IL-10/TGF-β expressed CD25 on day 8 of culture.20 In contrast, 35% of IL-10/TGF-β–cultured CD4+CD25– cells acquired CD25 expression (n = 4 experiments, data not shown). Because CD4+CD25– cells can gain CD25 expression with activation, the expression of CD25 at the end of culture could be derived from either CD4+CD25– activation or expansion of CD4+CD25+ cells in the starting population. For the latter possibility, CD25-depleted cultures starting with less than 0.4% residual CD4+CD25+ cells on day 0, which on day 8 contain 35% CD4+CD25+ cells, implies a significant (> 87-fold) enrichment of the CD4+CD25+ population during the 8-day culture interval. Because this would require such a dramatic enrichment, we favor the possibility that the IL-10/TGF-β tolerization strategy provides a substitute for CD4+CD25+ cells in the starting population by direct induction of regulatory cells. Thus, IL-10 and TGF-β combined treatment appears to result in the preferential expansion and/or activation of CD4+CD25– cells to become potent regulatory cells that facilitate tolerance induction.
In summary, we have shown that treatment of an MLR culture with IL-10 and TGF-β results in the generation of potent regulatory cells, in addition to secondary hyporesponsiveness to alloantigen.20 The generation of regulatory cells represents a mechanism that can control alloreactivity of cells that may escape ex vivo tolerance induction. We hypothesize that ex vivo tolerization strategies that simultaneously facilitate generation of regulatory cells during tolerance induction may have clinical potential for prevention and therapy of T-cell–mediated immune disorders including GVHD.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-09-2798.
Supported by National Institutes of Health (NIH) grants RO1 AI 34495, 2R37HL56067, RO1 HL63452, and PO1 AI-35225 to B.R.B. M.J.O. was supported by the NIH Medical Scientist Training grant T32 GM08244-15. Z.C. is a recipient of the National Research Service Award.
Z.C. and M.J.O. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Kevin Moore for providing anti–IL-10 and anti–IL-10R antibodies, Dr Marc Jenkins and Dr William Heath for providing OT-II mice, and Dr Patricia Taylor for helpful manuscript review.