Abstract
Peroxiredoxins (Prxs) are a family of antioxidant proteins that reduce peroxide levels by using reducing agents such as thioredoxin. These proteins were characterized to have a number of cellular functions, including cell proliferation and differentiation and protection of specific proteins from oxidative damage. However, the physiological roles of the peroxiredoxins have not been determined. To clarify the physiological relevance of this protein type, we generated a mouse model deficient in Prx II, which is abundantly expressed in all types of cells. The Prx II–/– mice were healthy in appearance and fertile. However, they had splenomegaly caused by the congestion of red pulp with hemosiderin accumulation. Heinz bodies were detected in their peripheral blood, and morphologically abnormal cells were elevated in the dense red blood cell (RBC) fractions, which contained markedly higher levels of reactive oxygen species (ROS). The Prx II–/– mice had significantly decreased hematocrit levels, but increased reticulocyte counts and erythropoietin levels, indicative of a compensatory action to maintain hematologic homeostasis in the mice. In addition, a labeling experiment with the thiol-modifying reagent biotinylated iodoacetamide (BIAM) in Prx II–/– mice revealed that a variety of RBC proteins were highly oxidized. Our results suggest that Prx II–/– mice have hemolytic anemia and that Prx II plays a major role in protecting RBCs from oxidative stress in mice.
Introduction
Peroxiredoxin (Prx) is a scavenger of hydrogen peroxide and alkyl hydroperoxides in living organisms.1 Six distinct mammalian Prx isozymes, types I to VI, have been detected in a wide range of tissues,2 and these have been shown to have strong antioxidant activities in vitro. All of the known mammalian Prxs, except for the type VI protein, utilize thioredoxin as an immediate electron donor, and hence they were formerly known as thioredoxin peroxidases. In addition to their antioxidant activity, Prxs have been implicated in a number of cellular functions such as cell proliferation and differentiation,3-5 enhancement of the natural killer cell activity,6 protection of radical-sensitive proteins,7 heme metabolism,8 and intracellular signaling.9 The biochemical characteristics and evidence from cultured animal cell studies indicate that Prx may be one of the main players in maintaining the cellular redox potential.
Red blood cells (RBCs) are more exposed to oxidative stress than other cell types due to an abundance of heme iron and oxygen, which can generate H2O2 and lipid peroxides. These hydroperoxides themselves are not very reactive but are readily converted in the presence of free iron and electron donor molecules to hydroxyl and alkoxyl radicals, which indiscriminately inflict damage on biomolecules. Under conditions of excessive oxidative stress, RBC membrane damage may result because of the decreased activity of membrane-bound enzymes, the inhibition of transmembrane transport systems, and the leakage of cellular constituents into plasma. Therefore, RBCs are believed to have a very effective defense mechanism against peroxides. Prx II, which is highly expressed in erythrocytes and believed to play a protective role against reactive oxygen species (ROS)–mediated damage in these cells, is induced at the early stages of erythroid differentiation prior to hemoglobin accumulation.10 Recently, Prx II was found to bind to integral membrane proteins or cell membranes via its C-terminal region.11,12 In the present study, we examined the in vivo function of Prx II in RBCs of Prx II–/– mice, and found that Prx II–/– mice have hemolytic anemia and that Prx II plays a major role in protecting RBCs against oxidative stress in mice.
Materials and methods
Generation of Prx II–/– mice
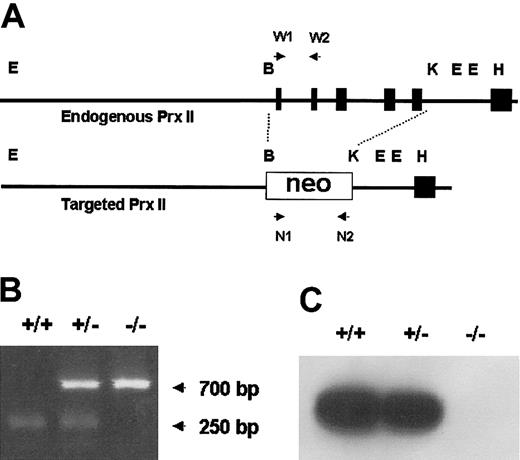
We constructed a targeting vector in which the sequence from exon 1 to exon 5 was replaced with the neo gene (Figure 1A). The vector was introduced into J1 mouse embryonic stem (ES) cells, and G-418–resistant transformants containing the mutation in one allele of the Prx II gene were established. The ES clones were injected into blastocyst embryos of C57BL/6 mice to produce chimeric mice, and the mutant allele was successfully transmitted to the next generation via their germ line. The Prx II–/– mice were produced with mendelian inheritance, and this was confirmed by polymerase chain reaction (PCR). The primers used were 5′-GCTTGGGTGGAGAGGCTATTCG-3′ and 5′-GTAAAGCACGAGGAAGCGGTCAGCC-3′ for the neo gene and 5′-GATGATCTCCGTGGGGCAAACAAAAGTGAAG-3′ and 5′-ATGGCCTCCGGCAACGCGCAAATCG-3′ for the wild type. All animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee, Korea Research Institute of Bioscience and Biotechnology (KRIBB).
Targeted disruption of the mouse Prx II gene. (A) The genomic structure of the wild-type Prx II gene (top) and the targeted locus (bottom) are shown. The recognition sites for BamHI (B), KpnI (K), EcoRI (E), and HindIII (H) restriction enzymes are indicated. Exons encoding Prx II are represented by black boxes. W1, W2, N1, and N2 indicate PCR primers. (B) PCR analysis of the genomic DNA isolated from wild-type (+/+), Prx II heterozygous (+/–), and Prx II homozygous (–/–) mice. The 700-bp and 250-bp arrows indicate the PCR products of Prx II–/– and Prx II+/+ mice, respectively. (C) Western blot analysis of Prx II expression in RBCs from wild-type, Prx II heterozygous, and Prx II–/– mice.
Targeted disruption of the mouse Prx II gene. (A) The genomic structure of the wild-type Prx II gene (top) and the targeted locus (bottom) are shown. The recognition sites for BamHI (B), KpnI (K), EcoRI (E), and HindIII (H) restriction enzymes are indicated. Exons encoding Prx II are represented by black boxes. W1, W2, N1, and N2 indicate PCR primers. (B) PCR analysis of the genomic DNA isolated from wild-type (+/+), Prx II heterozygous (+/–), and Prx II homozygous (–/–) mice. The 700-bp and 250-bp arrows indicate the PCR products of Prx II–/– and Prx II+/+ mice, respectively. (C) Western blot analysis of Prx II expression in RBCs from wild-type, Prx II heterozygous, and Prx II–/– mice.
Histologic and hematologic analyses of Prx II–/– mice
For the histologic study, livers and spleens were fixed in 10% buffered neutral formalin, embedded in paraffin, and stained with hematoxylin and eosin. To detect stainable iron in the liver and spleen, the Perls method was used as follows: 4-μm sections were incubated in potassium ferrocyanide solution for 5 minutes and then incubated in potassium–hydrochloric acid solution for 20 minutes. Sections were rinsed in distilled water and counterstained with nuclear fast red.
The hematologic parameters of the blood were determined using an automatic hematology analyzer (Sysmex 9000, Toa Medical Electronics, Kobe, Japan). The morphology of the density-fractionated RBCs was examined in smears stained with Wright solution, Heinz bodies were examined in smears stained with brilliant cresyl blue (Merck, Rahway, NJ), reticulocyte counts in smears stained with new methylene blue, and bone marrow cells on touch slides stained with Wright solution. Na+ and K+ content in the RBCs was measured by atomic absorption spectrometry after washing the RBCs 4 times with phosphate-buffered saline (PBS) (pH 7.4). The percentage of reticulocytes was calculated by counting 1000 cells on each slide.
Erythrocyte density and morphology
Density fractionation of RBCs was performed on discontinuous gradients of arabinogalactan (Larcoll; Sigma, St Louis, MO). Larcoll solutions of 10 different densities ranging from 1.135 to 1.014 g/mL were successively layered on the gradient and centrifuged for 45 minutes at 25 000g at 4°C. The ratio of erythroid to myeloid was deduced by counting 400 cells on each slide. Bone marrow cells were stained with Wright solution.
Measurement of reactive oxygen species and methemoglobin
The intracellular accumulations of ROS were determined using 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA). RBCs were washed with PBS (pH 7.0) and incubated with 20 μM DCFH-DA for 60 minutes at 37°C. After the RBCs had been washed with PBS, the fluorescence intensity of the cell suspension was read using a FACScan (Becton Dickinson, San Jose, CA).
Blood from normal and Prx II–/– mice was incubated in various concentrations of H2O2. After incubation at 37°C for 20 minutes, cells were pelleted by centrifugation at 2000 rpm for 10 minutes. The cells were passed through a syringe to mix thoroughly and then analyzed by blood gas electrolyte (Rapidlab 865, Chiron Diagnostics, East Walpole, MA). Methemoglobin concentration was expressed as a percentage of the total hemoglobin concentration.
Labeling of erythrocyte membrane proteins with BIAM
Blood of Prx II+/+ and Prx II–/– mice was centrifuged (2000 rpm, 10 minutes) at 4°C to remove plasma and buffy coats. RBC pellet was washed 3 times by centrifugation (2000 rpm, 10 minutes) at 4°C with PBS (pH 7.4). RBCs were dissolved in 8 volumes of hypotonic buffer (10 mM 2-(N-Morpholino) ethanesulfonic acid [MES, pH 6.5], 1 mM EDTA [ethylenediaminetetraacetic acid], 0.1 mM AEBSF, leupeptin [2.0 μg/mL], and aprotinin [1.9 μg/mL]) containing 40 μM N-(biotinoyl)-N′-(iodoacetyl) ethylenediamine (biotinylated iodoacetamide [BIAM]) (Molecular Probes, Eugene, OR) and incubated for 30 minutes at room temperature. The buffer was rendered free of oxygen by bubbling with nitrogen gas for 30 minutes. The labeling reaction was quenched by the addition of 4 mM dithiothreitol (DTT). The RBC membrane was collected by centrifugation (13 000 rpm, 20 minutes) at 4°C, and membrane proteins were dissolved in SDS–loading dye and subjected to 10% and 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Schleicher & Schuell, Einbeck, Germany). After blocking with 2% bovine serum albumin (BSA) in TBS (20 mM Tris [tris(hydroxymethyl)aminomethane], 150 mM NaCl, adjust the pH to 7.4 with HCl) containing 0.05% Tween 20, the membrane was incubated with horseradish peroxidase (HRP)–conjugated streptavidin (1:50 000; Pierce, Rockford, IL) and developed by the chemiluminescent Western blotting detection reagent (Pierce).
Results
The splenomegaly induced by the damaged RBC cells in Prx II–/– mice
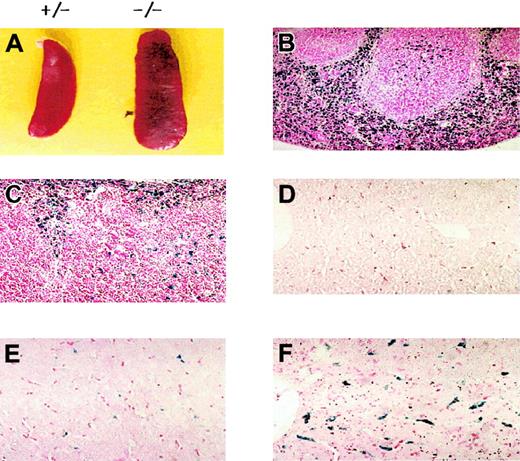
Prx II–deficient mice were generated by replacing the sequence from exon 1 to exon 5 with the neo gene. Homozygous knock-out mice were generated from the breeding of Prx II heterozygous mice, and homologous recombination was confirmed by polymerase chain reaction (Figure 1B) and Southern blot analysis (data not shown). The lack of Prx II protein was confirmed by Western blot analysis (Figure 1C). Although the Prx II–/– mice appeared apparently healthy, they were found to have splenomegaly (Figure 2A). When compared with the control littermates (wild-type and heterozygous mice), splenomegaly was observed among the Prx II–/– mice at 6 weeks of age. Spleen sizes were 1.5 times larger in the Prx II–/– mice from 8 weeks after birth than in the Prx II+/+ littermates (P < .0001, Table 1). Histologic studies showed that hemosiderin was substantially increased and red pulp was dramatically increased in the spleens of Prx II–/– mice (Figure 2B) versus the Prx II+/+ littermates (Figure 2C). To test whether hemolyzed RBCs accumulate specifically in the spleen, the Prx II–/– mice were splenectomized and analyzed histologically. Significantly, more hemosiderin was found to have accumulated within the Kupffer cells of livers of splenectomized Prx II–/– mice at 8 weeks of age (Figure 2F) than in unsplenectomized Prx II–/– mice (Figure 2E), while none was detected in the livers of the splenectomized Prx II+/+ littermates (Figure 2D).
Morphologic phenotypes of Prx II–/– mice. (A) Splenomegaly in Prx II–/– mice and heterozygous mice at 8 weeks of age. (B-C) Prussian blue–stained sections of spleen from wild-type (B) and Prx II–/– (C) mice. Red pulp was increased in Prx II–/– mice. (D-F) Prussian blue–stained sections of liver from splenectomized wild-type mice (D), unsplenectomized Prx II–/– mice (E), and splenectomized Prx II–/– mice (F). Stained iron was markedly increased in the livers of splenectomized Prx II–/– mice but scant in the livers of unsplectomized Prx II–/– mice and absent from the livers of wild-type mice. Original magnifications: panels B-C, × 200; panels D-F, × 400.
Morphologic phenotypes of Prx II–/– mice. (A) Splenomegaly in Prx II–/– mice and heterozygous mice at 8 weeks of age. (B-C) Prussian blue–stained sections of spleen from wild-type (B) and Prx II–/– (C) mice. Red pulp was increased in Prx II–/– mice. (D-F) Prussian blue–stained sections of liver from splenectomized wild-type mice (D), unsplenectomized Prx II–/– mice (E), and splenectomized Prx II–/– mice (F). Stained iron was markedly increased in the livers of splenectomized Prx II–/– mice but scant in the livers of unsplectomized Prx II–/– mice and absent from the livers of wild-type mice. Original magnifications: panels B-C, × 200; panels D-F, × 400.
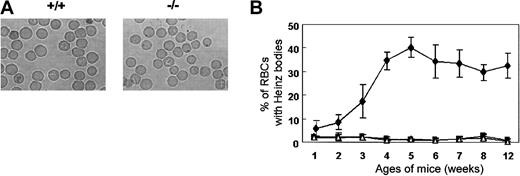
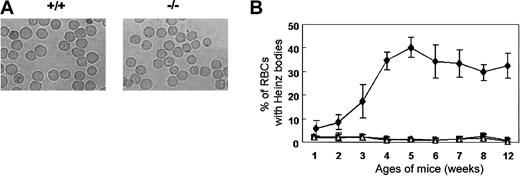
Heinz bodies, which are precipitates of denatured globin, form within red cells due to the oxidation of the globin chain sulfhydryl groups. Heinz bodies were detected in the peripheral blood of Prx II–/– mice at 2 weeks of age and peaked in number (accounting for about 30% of RBCs) at 5 weeks of age, while they were absent from the blood of the wild-type mice controls (Figure 3). The rate of Heinz body formation in RBCs of splenectomized Prx II–/– mice was similar to that of Prx II–/– mice (data not shown), indicating that the oxidative stress may be generated within the RBCs of Prx II–/– mice, and then some damaged RBCs accumulated in the spleen, even though the other cells were removed by the macrophages in the spleen.
Heinz bodies in Prx II–/– mice. (A) RBCs stained with brilliant cresyl blue. Heinz bodies (dark inclusions within the RBCs) were found only in Prx II–/– mice. Original magnification, × 1000. (B) Heinz body formation in homozygous (⋄), heterozygous (▪), and wild-type (▵) mice from 1 to 12 weeks of age. Bars represent SEMs.
Heinz bodies in Prx II–/– mice. (A) RBCs stained with brilliant cresyl blue. Heinz bodies (dark inclusions within the RBCs) were found only in Prx II–/– mice. Original magnification, × 1000. (B) Heinz body formation in homozygous (⋄), heterozygous (▪), and wild-type (▵) mice from 1 to 12 weeks of age. Bars represent SEMs.
Increased ROS levels in dense fractions having abnormal RBC cells in Prx II–/– mice
One of the typical features of damaged RBCs is the presence of dense cells. Dense fractions were increased in Prx II–/– mice compared with the wild-type mice (Figure 4A), and morphologically abnormal cells were increased in the dense RBC fractions of these mice. Spherocytes, burr cells, schistocytes, and polychromatophilic macrocytes were observed in the first to second, third to fifth, third to fifth, and the sixth to seventh fractions of Prx II–/– mice, respectively (Figure 4B). We measured intracellular ROS levels within RBCs with DCFH-DA fluorescent dye. The ROS levels of the total RBCs were similar in the Prx II–/– and wild-type mice as shown in Figure 5A. However, the ROS level in the dense fractions (from the third to the sixth) was 3- to 9-fold higher in Prx II–/– mice (Figure 5C-F). Increased DCF values in negative controls as shown in Figure 5A,F-G indicate autofluoresence, which may result from destabilization of intracellular hemoglobin of Prx II–/– mice. These data indicate that Prx II acts to decrease intracellular ROS levels in RBCs, thus preventing the generation of abnormal RBCs in the peripheral blood.
Separation of dense cells and their morphology. (A) RBCs were separated with an arabinogalactan density discontinuous gradient into 7 bands (H1 to H7) using blood from Prx II–/– mice (left) and 6 bands (W2 to W7) using blood from wild-type mice (right). (B) Abnormally shaped RBCs were found in all fractions (H1 to H7) from the Prx II–/– mice. Spherocytes (Sp), schistocytes (Sc), burr cells (B), and polychromatophilic macrocytes (P) are indicated.
Separation of dense cells and their morphology. (A) RBCs were separated with an arabinogalactan density discontinuous gradient into 7 bands (H1 to H7) using blood from Prx II–/– mice (left) and 6 bands (W2 to W7) using blood from wild-type mice (right). (B) Abnormally shaped RBCs were found in all fractions (H1 to H7) from the Prx II–/– mice. Spherocytes (Sp), schistocytes (Sc), burr cells (B), and polychromatophilic macrocytes (P) are indicated.
ROS levels in RBCs. ROS levels of the various density fractions of RBCs from Prx II–/– (shaded histograms) and wild-type (open histograms) mice were determined by DCFH-DA staining followed by fluorescence-activated cell sorter (FACS) analysis. (A) Fluorescence intensity of total RBCs from Prx II–/– and wild-type mice. The panels B-G correspond to fractions H2 to H7 from the Prx II–/– mice and equivalent fractions W2 to W7 from the wild-type mice in Figure 4. The ROS levels in fractions H3 to H6 from the Prx II–/– mice were significantly increased compared with wild type. The x- and y-axes represent the number of cells and fluorescence intensity, respectively. Inset panels indicate negative controls of panels A-G, which DCFH-DA was not preincubated to each samples.
ROS levels in RBCs. ROS levels of the various density fractions of RBCs from Prx II–/– (shaded histograms) and wild-type (open histograms) mice were determined by DCFH-DA staining followed by fluorescence-activated cell sorter (FACS) analysis. (A) Fluorescence intensity of total RBCs from Prx II–/– and wild-type mice. The panels B-G correspond to fractions H2 to H7 from the Prx II–/– mice and equivalent fractions W2 to W7 from the wild-type mice in Figure 4. The ROS levels in fractions H3 to H6 from the Prx II–/– mice were significantly increased compared with wild type. The x- and y-axes represent the number of cells and fluorescence intensity, respectively. Inset panels indicate negative controls of panels A-G, which DCFH-DA was not preincubated to each samples.
To further assess the consequences of oxidative treatment on RBCs of Prx II–/– mice, we measured oxidant of hemoglobin in the form of methemoglobin. In H2O2-treated cells, increased levels of methemoglobin were observed in Prx II–/– RBCs compared with normal cells (Figure 6). Methemoglobin was significantly 2-fold increased in Prx II–/– mice, indicating that the Prx II–/– RBCs are sensitive to oxidant stress more than normal RBCs.
Increased methemoglobin formation in Prx II–/– blood in response to H2O2 treatment. Data points represent mean values (n = 3); bars represent SEM. P < .05 for all values compared with wild-type levels.
Increased methemoglobin formation in Prx II–/– blood in response to H2O2 treatment. Data points represent mean values (n = 3); bars represent SEM. P < .05 for all values compared with wild-type levels.
Erythropoietic compensation for maintenance of hematologic homeostasis in Prx II–/– mice
We compared the hematologic parameters of Prx II–/– mice with those of the Prx II+/+ mice. Hemoglobin (P < .05) and hematocrit (P < .05) were significantly decreased and the reticulocyte count (P < .0001) was markedly increased in Prx II–/– mice (Table 1), indicating that Prx II–/– mice have hemolytic anemia. The number of RBCs (P = .224), mean cell volume (MCV) (P = .878), mean corpuscular hemoglobin (MCH) (P = .309), and WBCs (P = .307) were similar in Prx II–/– and Prx II+/+ mice. The monovalent cation contents (K+ [P = .187] and Na+ [P = .223]) of RBCs were not increased significantly in Prx II–/– mice versus wild-type mice.
We evaluated the erythropoietic response to hemolytic anemia in Prx II–/– mice. The erythropoietin (EPO) level of Prx II–/– mice was twice that of the wild-type mice at 8 weeks (Figure 7A). In addition, the number of normoblasts was higher in the bone marrow of Prx II–/– mice (Figure 7C) than in Prx II+/+ mice (Figure 7B), and the ratio of erythroid to myeloid was increased 1.4-fold in bone marrow of Prx II–/– mice, from 23% in Prx II+/+ mice to 33% in Prx II–/– mice. Our results suggest that compensation by erythropoietin may play an important role in maintaining hematologic homeostasis in Prx II–/– mice.
EPO expression in serum and the ratio of erythroid to myeloid precursors in bone marrow. (A) Western blot analysis of EPO in serum. Serum was collected at the indicated times from the peripheral blood of Prx II+/+ and Prx II–/– mice. Each fraction was separated by 12% SDS-PAGE and Western blot hybridized with antierythropoietin antibody. “W” and “H” indicate samples from the wild-type and Prx II–/– mice, respectively. (B-C) Bone marrow smears in wild-type (B) and Prx II–/– mice (C). Dark black cells indicate normoblasts. Original magnification, × 1000.
EPO expression in serum and the ratio of erythroid to myeloid precursors in bone marrow. (A) Western blot analysis of EPO in serum. Serum was collected at the indicated times from the peripheral blood of Prx II+/+ and Prx II–/– mice. Each fraction was separated by 12% SDS-PAGE and Western blot hybridized with antierythropoietin antibody. “W” and “H” indicate samples from the wild-type and Prx II–/– mice, respectively. (B-C) Bone marrow smears in wild-type (B) and Prx II–/– mice (C). Dark black cells indicate normoblasts. Original magnification, × 1000.
Biochemical mechanism of oxidative stress in red blood cells
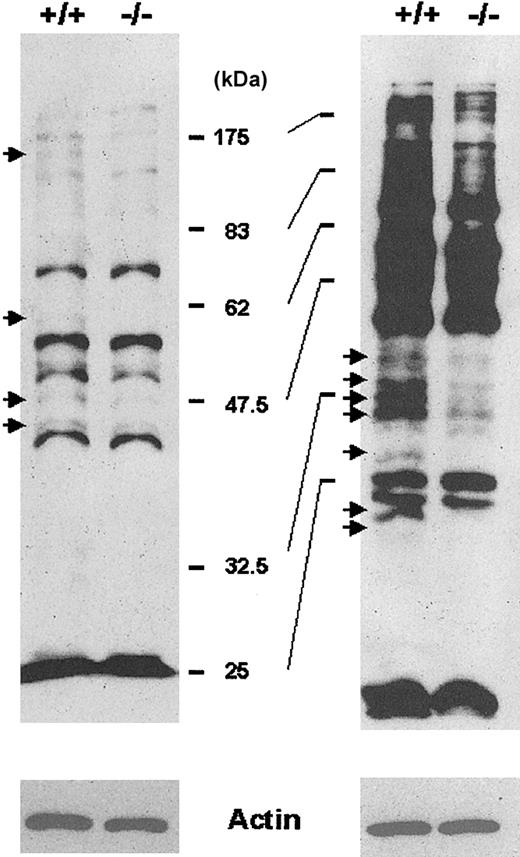
To better understand why RBCs from Prx II–/– mice are fragile, we investigated biochemical changes within these RBCs. The enzymatic activity of glucose-6-phosphate dehydrogenase (G6PDH) and pyruvate kinase, whose mutations are frequently observed in hemolytic anemia of human beings, was similar in RBCs from Prx II–/– and wild-type mice (data not shown). Lipid peroxidation and protein nitration, well-known markers of oxidative damage, were not increased in Prx II–/– mice (data not shown). We then monitored cysteine oxidation of cellular proteins by using BIAM as an alkylating agent specific for reactive thiol,13 because thiol groups are known to be easily oxidized by attack of ROS, resulting in cysteine sulfenic acid or disulfide.14 The extent of BIAM labeling of various membrane proteins from RBCs was decreased in Prx II–/– mice compared with wild-type mice (Figure 8), proving that the decreased level of antioxidant activity in the Prx II–/– mice resulted in the oxidation of various proteins containing low acid constant (pKa) cysteine residues. The oxidative inactivation of cellular proteins may result in structural damage, which puts RBCs in Prx II–/– mice at risk for hemolysis. This result also suggests that cysteine oxidation may be a more sensitive indicator of oxidative stress than lipid peroxidation or protein nitration.
Detection of proteins containing cysteine residues sensitive to ROS. The membrane proteins labeled with BIAM were subjected to either a 10% SDS-PAGE (left) or a 12% SDS-PAGE (right) and detected by enhanced chemiluminescence (ECL) after blotting with HRP-conjugated streptavidin. Arrows indicate proteins with reduced BIAM labeling in Prx II–/– mice compared with wild-type mice. Actin was used as an internal control.
Detection of proteins containing cysteine residues sensitive to ROS. The membrane proteins labeled with BIAM were subjected to either a 10% SDS-PAGE (left) or a 12% SDS-PAGE (right) and detected by enhanced chemiluminescence (ECL) after blotting with HRP-conjugated streptavidin. Arrows indicate proteins with reduced BIAM labeling in Prx II–/– mice compared with wild-type mice. Actin was used as an internal control.
Discussion
Mature RBCs are prone to oxidative damage because they are constantly exposed to a higher oxygen concentration than other tissues. Moreover, mature RBCs have a limited capacity to replace damaged proteins by de novo synthesis. Under normal circumstances, RBCs are protected against oxidative damage by antioxidant enzymes and molecules. The importance of these protective mechanisms is illustrated by the many hemolytic disorders caused by deficiencies in enzymes that maintain these intracellular reductants.15-19 For example, a deficiency in glutathione synthesis20 or an abnormal glutathione peroxidase17 or glutathione reductase21 level causes hemolytic anemia. γGCS-HStm1 (targeted disruption of the heavy subunit of γ-glutamylcysteine synthetase) also showed that glutathione synthesis is essential for mouse development.22
Here, we show that the disruption of Prx II, a thiol-specific antioxidant, results in a hemolytic disorder. Prx II deficiency causes cysteine oxidation of many proteins on RBC membranes at least, which may induce the denaturation of intracellular molecules and subsequent destruction of RBCs. We propose that Prx II may also protect RBC membrane proteins from intravascular oxidative stress. The activity and expression levels of the other enzymatic H2O2 scavengers, such as Gpx and catalase, were unchanged in Prx II–/– RBCs (data not shown). Unlike Gpx and catalase, Prx II appears to have a major role in RBC redox regulation. Gpx-1–/– mice showed a normal profile of red cells and reticulocytes and were found to be susceptible to exogenous oxidative stress.23,24 In our experiment with Gpx-1–/– mice, Heinz bodies were not seen in the absence of exogenous stress (data not shown). Moreover, homozygous mutant Gpx-1tm1Mgr mice have 20% less body weight than normal animals and an increased level of liver lipid peroxides, presumably because of reduced mitochondrial energy production due to increased oxidative stress.25
Prx II, previously named calpromotin, is known to be widely distributed in all tissues and to interact specifically with protein 7.2b. This interaction is of interest because Prx II is involved in the activation of a calcium-dependent potassium channel sensitive to charybdotoxin.12,26 However, our results showed the monomeric cation content (K+ and Na+) was unchanged in Prx II–/– RBCs.
Hemolysis is characterized by the presence of abnormally shaped RBCs in the peripheral blood. This phenotype is associated with various defects in the RBC membrane expression of skeletal proteins, such as spectrin, ankyrin, band 3, protein 4.1, protein 4.2, glycophorin A, actin, and glyceraldehyde 3-phosphate dehydrogenase (G3PDH).27,28 The posttranslational modification of membrane skeletal proteins can also contribute to hemolysis. For example, disulfide bridge formation in β-actin between Cys284 and Cys373 was identified as a cause of irreversibly sickled cells.29,30 Disruption of Prx II increased ROS levels in some RBCs and resulted in the oxidation of specific cysteine residues of some RBC proteins. This was expressed at the cellular level as morphologic abnormalities, hemolysis, and an accumulation of dense cells and, at the organ level, as splenomegaly. Thus, in this study we identified that the ROS scavenging function of Prx II is a critical factor for the full life span of RBCs. To further characterize the molecular mechanism underlying the RBC abnormalities in Prx II–/– mice, we are using a proteomics approach to identify the oxidized proteins.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-08-2548.
Supported by a grant from the Korea Ministry of Science and Technology (Critical Technology 21 [NSM0210132]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr I. P. Choi for meaningful discussions and Dr J. S. Lim for performing the FACScan analysis at KRIBB. The authors also thank researchers at the Laboratory of Experimental Animal Resources at KRIBB.