Abstract
Oxidized low-density lipoprotein (oxLDL) and macrophages play a central role in atherosclerosis. Here, we obtained evidence that oxLDL induced hypoxia-inducible factor-1α (HIF-1α) protein accumulation in human macrophages (Mono-Mac-6) under normoxia. HIF-1α accumulation was attenuated by pretreatment with the antioxidant N-acetyl-L-cysteine (NAC), the nitric oxide (NO) donor S-nitrosoglutathione (GSNO), and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitors such as diphenyleniodonium (DPI) or 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), thus implicating the contribution of oxLDL-generated reactive oxygen species (ROS). Whereas oxLDL did not modulate HIF-1α mRNA levels, experiments with cycloheximide pointed to a translational mechanism in oxLDL action. HIF-1–dependent luciferase reporter gene analysis underscored HIF-1 transactivation. Our results indicate that oxLDL induced HIF-1α accumulation and HIF-1–dependent reporter gene activation in human macrophages via a redox-mediated pathway. This finding may suggest a role of HIF-1 in atherosclerosis and oxLDL-induced pathogenesis.
Introduction
Oxidatively modified low-density lipoprotein (oxLDL) emerged as a pathogenetic factor in atherosclerosis.1 There is accumulating evidence for oxidative stress in vascular dysfunction/atherogenesis and a significant contribution of macrophages in oxLDL uptake, foam cell formation, and disease progression.1 Considering the role of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases in generating (reactive oxygen species) ROS, we recently demonstrated that NADPH oxidase is a major source of ROS in response to oxLDL with the further notion that diphenyleniodonium (DPI), a NADPH oxidase inhibitor, eliminated ROS generation in macrophages.2
Hypoxia-inducible factor-1 (HIF-1) is a heterodimeric transcription factor composed of α and β subunits.3 Although HIF-1β is constitutively expressed in many cell types, HIF-1α is present at undetectable amounts under normoxia because of rapid proteasomeal degradation and is stabilized on hypoxia.4,5 Following HIF-1α/HIF-1β heterodimerization and translocation to the nucleus, binding to promoter-specific HRE (hypoxia response element) sites drives classical hypoxia responsive genes.6,7 Studies suggested that ROS provides a redox signal for hypoxic HIF-1 induction.8 Moreover, ROS appears to regulate HIF-1 under normoxia. In some cell types, increased ROS production has been associated with HIF-1 activation by hormones, growth factors, and transition metals.8,9 In addition, direct exposure of cells to ROS may stabilize HIF-1α and promote vascular endothelial growth factor (VEGF) expression.8,10 Along that line cytokine-mediated regulation of HIF-1 may require a nonhypoxic but ROS-sensitive pathway.11
Here, we showed that oxLDL provoked HIF-1α accumulation and HIF-1–dependent transcriptional activation in human Mono-Mac-6 (MM6) macrophages through a ROS-dependent pathway.
Study design
Cell cultures
MM6 cells were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 1% nonessential amino acids, 1 mM sodium pyruvate, 9 μg/mL bovine insulin and antibiotics in a humidified 5% CO2 atmosphere at 37°C. Cell dishes at 80% to 90% confluence were exposed to native low-density lipoprotein (nLDL), oxLDL, and/or inhibitors. RCC4 cells, which constitutively express HIF-1α were kindly provided by Prof Ratcliffe, Wellcome Trust Centre for Human Genetics, University of Oxford, United Kingdom.12,13 nLDL was purchased from Sigma (Taufkirchen, Germany) and oxidized as described.2
Western blot analysis
HIF-1α (antibody from Becton Dickinson, Heidelberg, Germany) as well as actin (to confirm equal protein loading) was determined by Western analysis as described.14
Transient transfection
MM6 cells (5 × 105) were seeded in 6-cm dishes 1 day before transfection with 3 μg plasmid pGL3-EPO-HRE-SV40-LUC, which harbors 3 copies of the erythropoietin hypoxia responsive element in front of the SV 40 promoter or a VEGF reporter plasmid (pGL3-1.5kbVEGFprom, obtained from Prof Maity, University of Pennsylvania Medical Center, Philadelphia), for 8 hours, using dioleoyl-1,2-diacyl-3-trimethylammonium-propane (DOTAP) for transfection according to company instructions. Thereafter, medium was changed and cells were stimulated.
RT-PCR
MM6 cells (5 × 106) were incubated with or without 200 μg/mL oxLDL for 5 hours. Total RNA was isolated using the peqGOLD RNAPure kit, followed by HIF-1α mRNA reverse transcriptase–polymerase chain reaction (RT-PCR). Primer selection was as follows: HIF-1α,5′-CTCAAAGTCGGACAGCCTCA-3′,5′-CCCTGCAGTAGGTTTCTGCT-3′; actin, 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′, 5′-CTAGAAGCATTTGCGGTCGACGATGGAGGG-3′. Amplification program was as follows: 95°C, 30 seconds; 56°C, 30 seconds; 72°C, 1 minute; 20 cycles; 72°C, 10 minutes. Products were separated on 2% agarose gels and visualized with ethidium bromide.
Statistical analysis
Each experiment was performed at least 3 times, and representative data are shown. Data are presented as means ± SD. The unpaired Student t test was used to evaluate the significance of differences between groups, accepting P < .05 as the level of significance.
Results and discussion
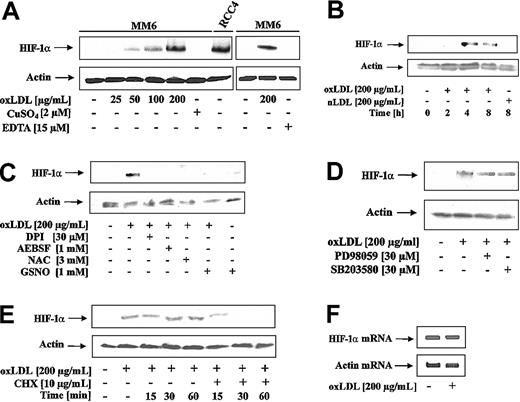
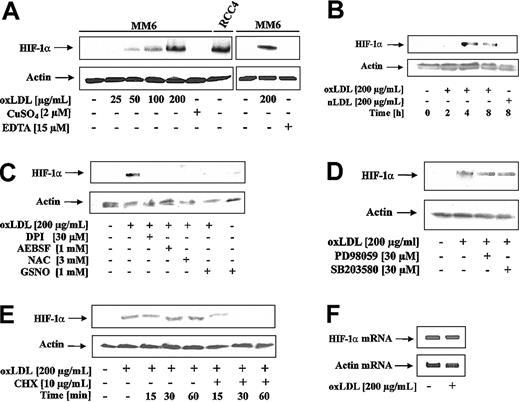
To examine the effect of oxLDL on HIF-1α accumulation, MM6 cells were treated with oxLDL or nLDL. As shown in Figure 1A, oxLDL dose-dependently (25-200 μg/mL) induced HIF-1α accumulation. oxLDL was obtained by the CuSO4/EDTA oxidation protocol. Therefore, we ruled out that neither CuSO4 nor EDTA at the relevant concentrations promoted HIF-1α accumulation (Figure 1A). As a positive control, to unequivocally localize HIF-1α, we used an RCC4 protein lysate with constitutive HIF-1α expression (Figure 1A). Furthermore, we established that oxLDL but not nLDL, induced HIF-1α accumulation (Figure 1B). The most efficient response was noticed between 4 and 8 hours. Under these experimental conditions oxLDL was nontoxic (data not shown).
HIF-1α accumulation in response to oxLDL. (A) MM6 cells were treated with increasing concentrations of oxLDL for 8 hours. For control reasons, 2 μM CuSO4, 15 μM EDTA (ethylenediaminetetraacetic acid), and a lysate of RCC4 cells were processed in parallel. (B) MM6 cells were time-dependently stimulated with 200 μg/mL oxLDL or nLDL. (C) MM6 cells were pretreated with 3 mM NAC, 30 μM DPI, and 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF) for 2 hours, followed by stimulation with 200 μg/mL oxLDL for 8 hours. S-nitrosoglutathione (GSNO; 1 mM) was supplied together with oxLDL for 8 hours. (D) Cells were pretreated with 30 μM PD98059 or 30 μM SB203580 for 2 hours, following by stimulation with 200 μM oxLDL for 8 hours. (E) HIF-1α expression was induced by exposing MM6 cells to 200 μg/mL oxLDL for 8 hours. Cycloheximide (CHX) at a final concentration of 10 μg/mL was added for the times indicated (15-60 minutes). (F) Cells were treated with 200 μg/mL oxLDL for 5 hours, and HIF-1α mRNA was followed by RT-PCR analysis as described. All experiments were performed at least 3 times and representative data are shown. HIF-1α protein was detected by Western blot analysis as outlined in “Study design.”
HIF-1α accumulation in response to oxLDL. (A) MM6 cells were treated with increasing concentrations of oxLDL for 8 hours. For control reasons, 2 μM CuSO4, 15 μM EDTA (ethylenediaminetetraacetic acid), and a lysate of RCC4 cells were processed in parallel. (B) MM6 cells were time-dependently stimulated with 200 μg/mL oxLDL or nLDL. (C) MM6 cells were pretreated with 3 mM NAC, 30 μM DPI, and 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF) for 2 hours, followed by stimulation with 200 μg/mL oxLDL for 8 hours. S-nitrosoglutathione (GSNO; 1 mM) was supplied together with oxLDL for 8 hours. (D) Cells were pretreated with 30 μM PD98059 or 30 μM SB203580 for 2 hours, following by stimulation with 200 μM oxLDL for 8 hours. (E) HIF-1α expression was induced by exposing MM6 cells to 200 μg/mL oxLDL for 8 hours. Cycloheximide (CHX) at a final concentration of 10 μg/mL was added for the times indicated (15-60 minutes). (F) Cells were treated with 200 μg/mL oxLDL for 5 hours, and HIF-1α mRNA was followed by RT-PCR analysis as described. All experiments were performed at least 3 times and representative data are shown. HIF-1α protein was detected by Western blot analysis as outlined in “Study design.”
Reports from several groups suggested that ROS stabilizes HIF-1α and activates HIF-1.8-10 Therefore, we investigated whether ROS, produced in response to oxLDL, contributed to HIF-1α accumulation (Figure 1C). N-acetyl-L-cysteine (NAC) is an antioxidant, known to decrease ROS. The addition of 3 mM NAC to MM6 cells for 2 hours prior to the stimulation with 200 μg/mL oxLDL for 8 hours completely blocked HIF-1α accumulation. DPI inhibits oxLDL-mediated ROS formation in macrophages by blocking NADPH oxidase.2 When cells were pretreated with 30 μM DPI for 2 hours prior to oxLDL stimulation, HIF-1α accumulation was suppressed. Because DPI may inhibit mitochondrial complex I, we studied the effect of the structurally unrelated NADPH oxidase inhibitor AEBSF in parallel, which again prevented HIF-1α accumulation. The effect of nitric oxide (NO) derived from the most physiologic donor GSNO may be rationalized by the diffusion controlled interaction of
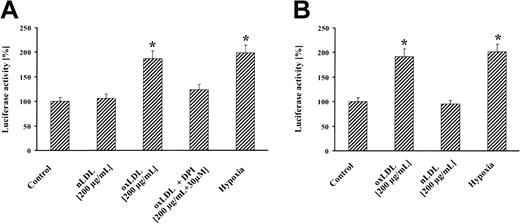
Treatment of MM6 cells with oxLDL resulted in significant reporter gene activation that was largely abrogated by DPI (Figure 2A). nLDL showed no reporter signal, whereas hypoxia elicited a luciferase activity comparable to oxLDL. Similarly, treatment of cells with oxLDL led to an increase in VEGF promoter activity (Figure 2B). Our results are consistent with previous studies, showing a 2- to 3-fold increase in reporter activity by hypoxia14,15 and further studies reporting hypoxic VEGF production in macrophages.16 Atherosclerosis is a progressive disease and a common cause of morbidity and mortality in the Western world. Therefore, our results on HIF-1α accumulation by oxLDL appear of clinical relevance. It is well established that HIF-1α plays a major role in VEGF expression and angiogenesis9 with the notion that VEGF mediates important alterations associated with atherogenesis and the angiogenic activity of macrophages.16 Recent findings suggest that neovascularization within atherosclerotic plaques is a sign of advanced atherosclerosis/restenosis.17 It is tempting to speculate that oxLDL-induced HIF-1α accumulation and VEGF up-regulation are important in these pathophysiologic settings. A series of molecules and molecular pathways emerged that contribute to the pathogenesis and progression of both atherosclerosis and cancer.18,19 HIF-1 may be added to that list, considering the important tumor angiogenic role of HIF-1α. Our observations propose a new signaling quality of oxLDL in HIF-1 accumulation and demonstrate ROS involvement. This appears of considerable importance to fully understand progression of atherosclerosis and remodeling of the arterial wall by oxLDL.
HIF-1–dependent reporter gene activation by oxLDL. MM6 cells were transiently transfected with a luciferase reporter gene construct, containing (A) 3 copies of the HRE of the erythropoietin gene (pGL3-EPO-HRE-SV40-LUC) or (B) pGL3-1.5kbVEGFprom. Transfected cells were stimulated for 8 hours with 200 μg/mL oxLDL or nLDL, in the absence or presence of 30 μM DPI, or exposed for 8 hours to hypoxia (1% oxygen) followed by luciferase activity determination. Luciferase activity is expressed relative to controls set as 100%. Values represent the fold induction of 4 independent experiments.
HIF-1–dependent reporter gene activation by oxLDL. MM6 cells were transiently transfected with a luciferase reporter gene construct, containing (A) 3 copies of the HRE of the erythropoietin gene (pGL3-EPO-HRE-SV40-LUC) or (B) pGL3-1.5kbVEGFprom. Transfected cells were stimulated for 8 hours with 200 μg/mL oxLDL or nLDL, in the absence or presence of 30 μM DPI, or exposed for 8 hours to hypoxia (1% oxygen) followed by luciferase activity determination. Luciferase activity is expressed relative to controls set as 100%. Values represent the fold induction of 4 independent experiments.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-09-2711.
Supported by funds of the Deutsche Forschungsgemeinschaft (DFG) (BR 999), European Commission (EC) (QLK 6-GT-2000-64), and VerUm Foundation. V.A.S. is a research fellow of the Alexander von Humbolt Foundation.
V.A.S. and V.V.S. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.