Abstract
Thrombin activatable fibrinolysis inhibitor (TAFI) is a carboxypeptidase B-like proenzyme that after activation down-regulates fibrinolysis. Platelets are known to contain antifibrinolytic factors that are secreted during platelet activation. Therefore, the presence of TAFI in platelets was analyzed. TAFI was identified in platelets in a concentration of about 50 ng/1 × 109 platelets and was secreted on platelet activation. Thrombin-mediated activation of platelet-derived TAFI resembled that of plasma-derived TAFI with respect to stimulation by thrombomodulin and spontaneous loss of activity at 37°C. The different glycosylation of platelet-derived TAFI compared with plasma-derived TAFI suggests that platelet-derived TAFI is synthesized in the megakaryocyte. This suggestion was substantiated by the detection of mRNA in the megakaryocytic cell lines DAMI and CHRF, representing the intermediate and late stages of megakaryocyte development. These results establish the presence of TAFI in platelets and suggest a role for platelet-derived TAFI in the protection of the clot against fibrinolysis.
Introduction
Thrombin activatable fibrinolysis inhibitor (TAFI, plasma procarboxypeptidase B, procarboxypeptidase U, EC 3.4.17.20) provides a balance between coagulation and fibrinolysis.1 Because the concentration of TAFI in plasma (70-250 nM) is 10- to 30-fold below its Michaelis constant (KM) for activation by thrombin, the amount of TAFIa formed during activation is dependent on the TAFI concentration.2,3 TAFI levels vary considerably between individuals, and individual differences in clot lysis times could be attributed to variations in TAFI levels.3 Increased TAFI levels are associated with thromboembolic disease, and polymorphic variations in the TAFI gene have been linked to plasma TAFI levels.4-8 These observations indicate an important role for the concentration of TAFI in plasma on the regulation of fibrinolysis and the occurrence of thromboembolic disease.
Because platelets are known to increase local concentrations of coagulation and fibrinolytic factors by releasing the contents of their α-granules on activation, this study was initiated to evaluate if TAFI is present in platelets and could contribute to the variations in plasma TAFI levels.
Study design
Immunofluorescence microscopy
Cytospins of gel-filtered platelets, fixed with 2% para-formaldehyde, were permeabilized with 1% saponine and subsequently incubated with monoclonal antibody (Moab) 9H10 against TAFI and a fluorescein isothiocyanate (FITC)–labeled secondary goat antimouse antibody.3
Detection of TAFI in platelets by ELISA
Determination of TAFIa activity in platelets
Gel-filtered platelets were incubated for 60 minutes with thrombin (50 nM), thrombomodulin (10 nM), CaCl2 (5 mM), and hippuryl-Arg (5 mM) after which the amount of hippuric acid was determined in the supernatant as described.10
Binding of plasminogen to minimally degraded fibrin (Desafib X; Chromogenix, Mölndal, Sweden) was performed as described.11
Detection of mRNA in megakaryocytic cell lines
Results and discussion
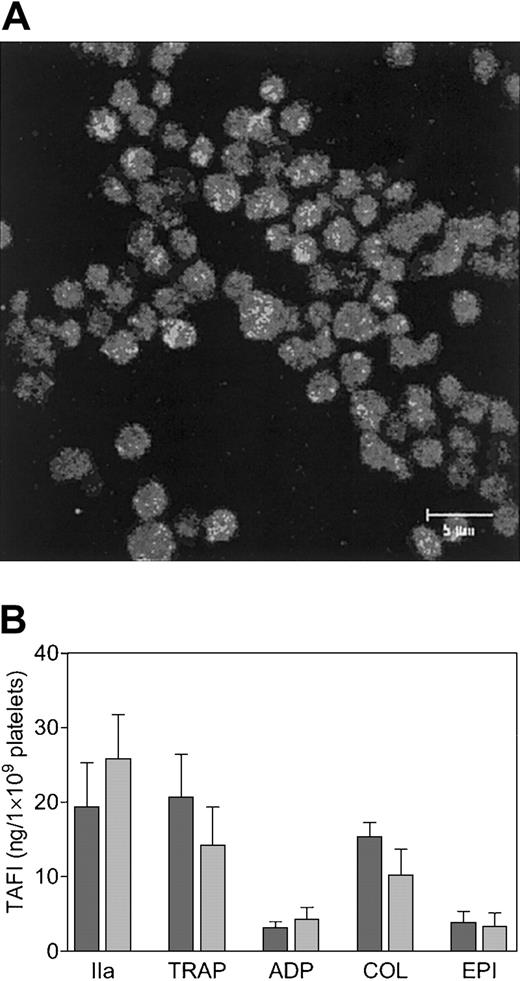
Using an ELISA specific for the zymogen form of TAFI, the concentration of TAFI in the lysate of gel-filtered platelets (mean ± SD) obtained from 3 different donors was estimated to be 48 ± 5 ng/1 ×109 platelets. The presence of TAFI in platelets was confirmed by immunofluorescence (Figure 1A). The absence of fluorescence in nonpermeabilized platelets (data not shown) indicates that TAFI was localized intracellularly and that no plasma-derived TAFI was bound to the surface of the platelets. After permeabilization, TAFI exhibited a spotted staining pattern (Figure 1A), which suggests intracellular localization of TAFI in granules. Granular localization of TAFI in platelets was substantiated by analyzing the secretion of TAFI by ELISA using different agonists of platelet activation and was found to be consistent with localization of TAFI in the α-granules (Figure 1B). Thrombin was the strongest agonist of TAFI release from platelets and induced the secretion of approximately 50% of the total amount of TAFI in platelets. The decreased secretion of TAFI at high versus low thrombin concentrations was due to the activation of TAFI by thrombin. The ELISA used in this study does not react with activated TAFI, and the formation of TAFIa at high thrombin concentrations was confirmed on Western blot using a sheep polyclonal anti-TAFI antibody (data not shown). TRAP, collagen, ADP, and epinephrine resulted in the secretion of approximately 40%, 30%, less than 10%, and less than 10% of the total amount of TAFI present in platelets, respectively. On Western blot (Figure 2A) secretion of TAFI from platelets was detected in the supernatant of thrombin-activated platelets (lane 4), whereas in the supernatant of resting platelets (lane 3) the presence of TAFI was much less pronounced and is probably due to the release of TAFI from platelets as a result of spontaneous activation and/or mechanical stress during the preparation of the supernatant.
Identification of TAFI in platelets. TAFI was identified in cytospins of gel-filtered platelets by immunofluorescence confocal microscopy (A), using a monoclonal antibody against TAFI (9H10) and an FITC-labeled secondary antibody. The scale bar represents 5 μm. TAFI secretion by stimulated platelets (B). Gel-filtered platelets (2.8 × 107) were incubated with a high (dark bars) or a low (light bars) concentration of platelet agonist for 15 minutes at 37°C, and the secretion of TAFI was determined by ELISA. The agonists used were thrombin (IIa; 5 or 0.5 U/mL), thrombin receptor–activating peptide (TRAP; 100 or 10 μM), adenosine 5′-diphosphate (ADP; 20 or 2 μM), collagen (COL; 10 or 1 μg/mL), and epinephrine (EPI; 10 or 1 μM). Each bar represents the mean ± SEM of 5 independent experiments using different donors.
Identification of TAFI in platelets. TAFI was identified in cytospins of gel-filtered platelets by immunofluorescence confocal microscopy (A), using a monoclonal antibody against TAFI (9H10) and an FITC-labeled secondary antibody. The scale bar represents 5 μm. TAFI secretion by stimulated platelets (B). Gel-filtered platelets (2.8 × 107) were incubated with a high (dark bars) or a low (light bars) concentration of platelet agonist for 15 minutes at 37°C, and the secretion of TAFI was determined by ELISA. The agonists used were thrombin (IIa; 5 or 0.5 U/mL), thrombin receptor–activating peptide (TRAP; 100 or 10 μM), adenosine 5′-diphosphate (ADP; 20 or 2 μM), collagen (COL; 10 or 1 μg/mL), and epinephrine (EPI; 10 or 1 μM). Each bar represents the mean ± SEM of 5 independent experiments using different donors.
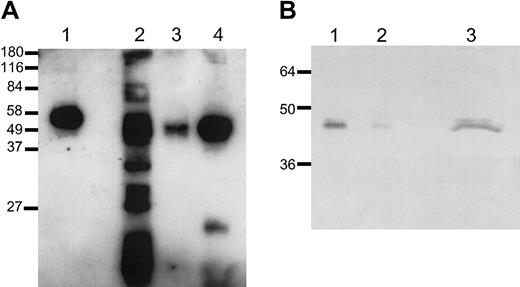
Analysis of platelet-derived TAFI. Platelet-derived TAFI and TAFI purified from plasma (lane 1; 300 pg) were analyzed on Western blot (A). Gel-filtered platelets (3 × 107 cells) were incubated with either 0.5% triton (lane 2), buffer (lane 3), or 0.5 U/mL thrombin (lane 4) 15 minutes at 37°C. Deglycosylation of platelet-derived TAFI (B). Purified TAFI (16 ng, lane 1, or 1.6 ng, lane 2) and gel-filtered platelets (7 × 107 cells) incubated with thrombin (0.5 U/mL) for 15 minutes at 37°C (lane 3) were subjected to PNGase F treatment. TAFI was detected with a monoclonal antibody against TAFI (9H10). Approximate molecular masses (kDa) are shown on the left.
Analysis of platelet-derived TAFI. Platelet-derived TAFI and TAFI purified from plasma (lane 1; 300 pg) were analyzed on Western blot (A). Gel-filtered platelets (3 × 107 cells) were incubated with either 0.5% triton (lane 2), buffer (lane 3), or 0.5 U/mL thrombin (lane 4) 15 minutes at 37°C. Deglycosylation of platelet-derived TAFI (B). Purified TAFI (16 ng, lane 1, or 1.6 ng, lane 2) and gel-filtered platelets (7 × 107 cells) incubated with thrombin (0.5 U/mL) for 15 minutes at 37°C (lane 3) were subjected to PNGase F treatment. TAFI was detected with a monoclonal antibody against TAFI (9H10). Approximate molecular masses (kDa) are shown on the left.
Platelet-derived TAFI migrated with an estimated molecular mass of 50 kDa (Figure 2A, lanes 3-4), which is slightly lower than that of plasma-derived TAFI (58 kDa; lane 1). Deglycosylation of both plasma-derived TAFI and platelet-derived TAFI, however, indicated a similar molecular mass of approximately 47 kDa (Figure 2B), suggesting that the difference in molecular mass was not due to proteolysis of the platelet-derived TAFI but rather originates from differences in glycosylation. Interestingly, a similar difference in molecular mass (50 versus 58 kDa) was reported previously for recombinant TAFI produced in insect cells compared with plasma TAFI, indicating that one or more of the glycosylation sites in TAFI are susceptible to variation in glycosylation.17 This finding implies that platelet-derived TAFI is not derived from the liver and endocytosed by the platelet from plasma but instead is synthesized in the megakaryocyte. To find evidence for this hypothesis, synthesis of TAFI by megakaryocytes was studied using reverse transcriptase (RT)–PCR on mRNA of megakaryocytic cell lines. The megakaryocytic cell lines MEG-01, DAMI, and CHRF represent the early, intermediate, and late stages of megakaryocyte development, respectively.12-14 TAFI mRNA could not be detected in MEG-01 cells and human umbilical vein endothelial cells (HUVECs) but was present in DAMI, CHRF, and HepG2 cells (data not shown). The absence of detectable TAFI mRNA in HUVECs is not in contrast to Hori et al,18 because they inappropriately attributed the expressed mRNA to TAFI, whereas it should have been attributed to pancreatic/tissue carboxypeptidase B on the basis of their published primer sequences. The detection of TAFI mRNA by RT-PCR in megakaryocytic cell lines representing the more mature stages of megakaryocytopoiesis and the different glycosylation of platelet-derived TAFI both suggest synthesis in the megakaryocyte.
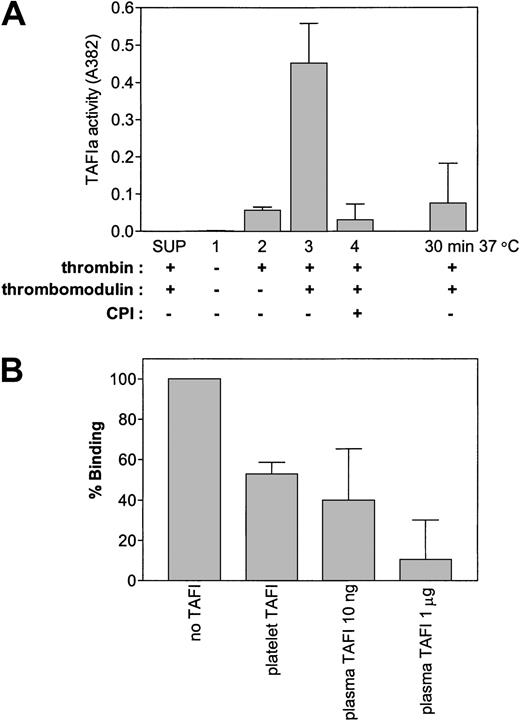
The enzymatic characteristics of platelet-derived TAFI, with respect to activation by thrombin, stimulation of activation by thrombomodulin, inhibition by carboxypeptidase inhibitors, and the spontaneous loss of activity of TAFIa at 37°C resembled that of TAFI purified from plasma (Figure 3A). This finding suggests that platelet-derived TAFI and plasma-derived TAFI appear to have similar enzymatic characteristics despite their differences in glycosylation. Similar to activated plasma TAFI, platelet TAFIa reduced binding of plasminogen to minimally degraded fibrin (Figure 3B). Binding of plasminogen to carboxyterminal lysines on partially degraded fibrin comprises an essential step for efficient activation of plasminogen and suggests that platelet TAFIa is likely to inhibit fibrinolysis in a similar manner as plasma TAFIa.
Activation of platelet-derived TAFI. (A) Gel-filtered platelets (3 × 108) were either incubated with thrombin (50 nM), thrombomodulin (10 nM), and/or CPI (10 μg/mL) as indicated. As a control, platelets were removed by centrifugation before addition of thrombin-thrombomodulin to the supernatant (SUP). The inactivation of platelet-derived TAFIa was analyzed by incubation of the activated platelet mixture for 30 minutes at 37°C after the addition of PPACK but before addition of the substrate (hippuryl-Arg). (B) Thrombin (50 nM)-thrombomodulin (10 nM), activated platelets (2 × 108) or plasma TAFI were incubated for 1 hour at room temperature with immobilized minimally degraded fibrin (Desafib X), after which binding of plasminogen was determined. In both panels, each bar represents the mean ± SEM of 3 independent experiments.
Activation of platelet-derived TAFI. (A) Gel-filtered platelets (3 × 108) were either incubated with thrombin (50 nM), thrombomodulin (10 nM), and/or CPI (10 μg/mL) as indicated. As a control, platelets were removed by centrifugation before addition of thrombin-thrombomodulin to the supernatant (SUP). The inactivation of platelet-derived TAFIa was analyzed by incubation of the activated platelet mixture for 30 minutes at 37°C after the addition of PPACK but before addition of the substrate (hippuryl-Arg). (B) Thrombin (50 nM)-thrombomodulin (10 nM), activated platelets (2 × 108) or plasma TAFI were incubated for 1 hour at room temperature with immobilized minimally degraded fibrin (Desafib X), after which binding of plasminogen was determined. In both panels, each bar represents the mean ± SEM of 3 independent experiments.
Secretion of coagulation factors during platelet activation increases the local concentration of specific coagulation factors at the site of injury. Some factors are present in relatively high concentrations in the platelet compared with plasma (eg, fibrinogen and plasminogen activator inhibitor-1)19,20 and some are not (eg, protein S and protein C inhibitor).21,22 The concentration of TAFI in platelets (48 ng/1× 109 platelets) represents only a small percentage (0.1%) of the total amount of TAFI found in plasma (TAFI plasma concentration 4-15 μg/mL),2,3 whereas the concentration of TAFI inside the platelet (± 7 μg/mL, based on a platelet volume of 7 × 10—15 L) is in the same order of magnitude compared with TAFI in plasma. The level of TAFI in platelets, however, does not necessarily preclude its physiologic importance. Because platelets are concentrated within the fibrin clot, local concentrations of TAFI could approach significant levels, and relatively small variations in the TAFI concentration were found to influence the clot lysis time.3
The results of the present study show the presence of TAFI in platelets that is presumably synthesized in the megakaryocyte during the process of megakaryocytopoiesis. Platelet-derived TAFI is secreted on activation of platelets and resembles the enzymatic characteristics of plasma-derived TAFI on activation by thrombin or thrombin/thrombomodulin, suggesting a role for platelet-derived TAFI in the protection of the clot against fibrinolysis.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2002-09-2944.
Supported in part by grants 96.088 and 98.061 from the Netherlands Heart Foundation. J.C.M.M. was an Established Investigator of the Netherlands Heart Foundation (grant D96.021).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank P. E. Litjens, I. A. Relou, G. van Willigen, and G. Gorter for their support, suggestions, and help in the preparation of gel-filtered platelet and E. den Dekker for the culturing of the megakaryocytic cell lines.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal