Abstract
We constructed chimeric receptors to dissect the role of the transmembrane (TM) domain in cell surface expression of and phagocytosis by the γ chain–dependent Fcγ receptors FcγRIIIA and FcγRI. FcγR chimeras containing the TM and cytoplasmic (CY) domains of the γ chain were expressed on the cell surface and mediated an efficient phagocytic signal. In contrast, chimeras containing the FcγRIIIA TM were poorly expressed. Receptors containing the FcγRI TM and the γ chain CY but lacking the γ chain TM also were expressed efficiently and mediated phagocytosis, suggesting that a γ chain dimer induced by the γ chain TM is not required for efficient phagocytosis. Cotransfection of FcγRI or FcγRIIIA with the chimera CD8-γ-γ (EC-TM-CY) resulted in FcγR cell surface expression and phagocytosis, whereas CD8-CD8-γ, whose TM does not associate with FcγR, allowed cell surface expression of (but not phagocytosis by) FcγRI. CD8-CD8-γ also did not allow surface expression of FcγRIIIA. Exchanging FcγRI and CD8 TMs indicated that the C-terminal 11 amino acids of the FcγRI TM are essential for association of FcγRI with the γ chain and phagocytosis. The data indicate that specific sequences in the FcγRIIIA and FcγRI TMs govern their different interactions with the γ chain in cell surface expression and phagocytosis and that γ chain TM sequences are not required for γ chain–mediated phagocytosis. The data identify a specific region of the FcγRI TM and its asparagine as important for FcγRI cell surface expression in the absence of the γ chain and for distinguishing the FcγRI and FcγRIIIA phenotypes.
Introduction
Receptors for the constant region of immunoglobin G (IgG) are expressed on many cells of hematopoietic lineage. These Fcγ receptors are important in several cell functions, including endocytosis, phagocytosis, and the release of inflammatory mediators. There are 3 classes of human Fcγ receptors: FcγRI, FcγRII, and FcγRIII (for reviews, see references 1, 2, 3, 4, 5, 6). The cytoplasmic domains (CY) of FcγRIIIA (α chain) and FcγRI (α chain) contain no known signaling motifs. However, FcγRIIIA and FcγRI are associated with a γ chain7,8, 9, 10, 11 that contains an immunoreceptor tyrosine-based activation motif (ITAM sequence) through which signal transduction can proceed.
The FcγRIIIA and FcγRI α chains interact with the γ chain through sequences within their homologous transmembrane (TM) regions.12, 13, 14 The γ chain plays a dual role in FcγRIIIA and FcγRI signaling. In addition to providing ITAM tyrosines for initiation of the Fcγ receptor signaling cascades, association with the γ chain protects the α chain of these Fcγ receptors from degradation in the endoplasmic reticulum, thus facilitating their cell surface expression in monocytes/macrophages.15, 16, 17 Coexpression of the γ chain also is required for cell surface expression of FcγRIIIA in transfected epithelial cells.9, 10 Although signaling by FcγRI has been observed to be dependent upon the ITAM of an associated γ chain, cell surface expression of FcγRI in transfected COS-1 cells occurs in the absence of the γ chain.11 The difference in the requirement of the γ chain for cell surface expression of FcγRIIIA and FcγRI provides an approach to further investigate the interaction between the γ chain and these Fcγ receptors. To this end, we have constructed chimeric receptors and receptor TM domain mutants in order to (1) examine the interaction of the γ chain with FcγRIIIA and FcγRI, and (2) identify TM sequences that contribute to the differences in the FcγRIIIA and FcγRI phenotypes.
Materials and methods
Construction of recombinant plasmids
The exchange and substitution mutants of FcγRIIIA, FcγRI, CD8, and the human γ subunit of FcγRIIIA and FcγRI were constructed by 2-step overlap extension polymerase chain reaction. The mutants and chimeric receptors as well as wild-type (WT) receptors were cloned into the HindIII and XbaI cloning sites of the eucaryotic expression vector pCDNA 3.1/Myc-His B (Invitrogen, San Diego, CA).
Cell culture and transfection of COS-1 cells
COS-1 cells were maintained in high-glucose Dulbecco modified Eagle medium (DMEM; Gibco BRL, Grand Island, NY) containing glutamine (2 mM), streptomycin (100 U/mL), penicillin (100 μg/mL), and 10% heat-inactivated fetal calf serum at 37°C with 5% CO2/95% air. Transient transfections with cDNA were carried out by a modified diethylamino ethyl (DEAE)–Dextran method. Briefly, 5 × 105 COS-1 cells were seeded onto 35-mm well plates 24 hours prior to transfection. Plates were washed twice and incubated for 30 minutes with DMEM (GIBCO BRL, Grand Island, NY) without serum before transfection. Following addition of transfection buffer (1 mL) containing 4 μg plasmid DNA (0.5 μg/mL) and incubation for 4 hours at 37°C, cells were treated with 10% dimethyl sulfoxide in phosphate-buffered saline (PBS) for 1 1/2 minutes and washed twice with DMEM. Cells were studied 48 hours after transfection.
Flow cytometry
Cell surface expression of FcγR protein 48 hours after transfection was determined by flow cytometry using anti-FcγRIIIA (3G8) or anti-FcγRI (32.2) mAbs as the primary antibodies and fluorescein isothiocyanate (FITC)–labeled F(ab)′2 goat anti–mouse IgG antibody (TAGO, Burlingame, CA) as the cross-linking secondary antibody.10 , 11 Anti-CD8 mAb was obtained from PharMingen (San Diego, CA).
Phagocytosis of IgG-sensitized RBCs
IgG-coated sheep erythrocytes (EA) were prepared as previously described.10 , 11 COS-1 cell transfectants were incubated with washed EA at 37°C for 30 minutes. Unbound EA were removed by extensive washing and stained with Wright-Giemsa to determine the number of cells with bound EA. For assessment of phagocytosis, hypotonic PBS was applied briefly (30 seconds) to remove surface-bound EA. Phagocytosed cells were stained with Wright-Giemsa, and ingested erythrocytes were counted by light microscopy (× 1000). Results were presented as phagocytic index (PI), the number of ingested erythrocytes per 100 COS-1 cells. In indicated experiments, the phagocytic index was corrected for variations in cell surface receptor expression (mean fluorescence intensity, MFI) as determined by flow cytometry.
Western blotting
COS-1 cell transfectants were lysed on plates with NP-40 buffer (1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 158 mM NaCl, 10 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.2, 5 mM NaEDTA (sodium ethylenediaminetetraacetic acid), 1 mM phenylmethylsulphonyl fluoride, 1 mM Na3VO4) at 4°C for 30 minutes. Myc-tagged Fcγ receptors and coimmunoprecipitating proteins were immunoprecipitated from cell lysates with anti-Myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and proteins were resolved on 15% SDS polyacrylamide. Following electrophoretic transfer to nitrocellulose, proteins were immuno-blotted with anti-His antibody (R&D Systems, Minneapolis, MN) to identify the His-tagged γ chain. Blots were developed with horseradish peroxidase–conjugated goat antimouse (Bio-Rad, Richmond, CA) and specific bands were detected by enhanced chemiluminescence (ECL, Amersham, Arlington Heights, IL).
Results
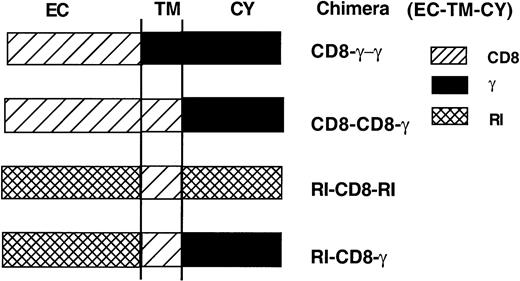
Only trace amounts of FcγRIIIA are detected on the surface of transiently transfected COS-1 cells in the absence of γ chain expression.9,10 In contrast, cell surface expression of FcγRI is evident both in the absence and presence of the γ chain (references 1, 11, 18, and 19; Table 1). FcγRI- and FcγRIIIA-mediated phagocytosis also depend on coexpression of the γ chain.9,11 The association of FcγRI and FcγRIIIA with the γ chain occurs through interaction of the individual receptor transmembrane (TM) domains.12, 13, 14 To determine which sequences in the TM regions of FcγRI and FcγRIIA are required for cell surface expression and phagocytosis, we first used single chain chimeras in which the extracellular domains (EC) and TM regions of these receptors were exchanged (Figure 1).
Schematic diagram of FcγRI, FcγRIIIA, the γ chain, and chimeric Fcγreceptors. EC indicates extracellular domain; TM, transmembrane domain; and CY, cytoplasmic domain.
Schematic diagram of FcγRI, FcγRIIIA, the γ chain, and chimeric Fcγreceptors. EC indicates extracellular domain; TM, transmembrane domain; and CY, cytoplasmic domain.
The feasibility of using Fcγ receptor/γ chain chimeric receptors for functional studies was based on our observation that appending the γ chain CY and TM to the EC of FcγRIIIA or FcγRI produced chimeric receptors RIIIA-γ-γ and RI-γ-γ, EC-TM-CY, which are well expressed in COS-1 cell transfectants.1,19 These chimeric receptors are also functional, as evidenced by their ability to phagocytose EAs with efficiencies comparable to cotransfectants of FcγRI or FcγRIIIA with the γ chain (Indik et al1,19 ; and Table 2).
Fcγ receptor chimeras containing the TM of FcγRI (RIIIA-RI-γ and RI-RI-γ, Figure 1) were also expressed efficiently in transfected COS-1 cells (Table 3). In contrast, the Fcγ receptor chimera RIIIA-RIIIA-γ was essentially undetectable on the surface of transfected COS-1 cells (Table 3). Cell surface expression of RI-RIIIA-γ, which also contains the TM of FcγRIIIA, was dramatically reduced compared to that of RI-RI-γ (Table 3). As expected, phagocytosis mediated by the chimeric FcγRs reflect their cell surface expression; that is, chimeras containing the FcγRI TM allow surface expression and induce the phagocytosis of EA, whereas those containing the FcγRIIIA TM are not expressed (Table 4). We are aware that construction of chimeras may bring the risk of artificial processing of the protein in cellular systems. We therefore also examined expression of other FcγRIIIA and FcγRI TM chimeras to determine whether profound depression of FcγR expression is specific for the TM of FcγRIIIA or a function of chimera construction (Table 3).
Expression of the FcγRI EC was consistently most depressed by the FcγRIIIA TM (Table 3). The expression of RI-γ-γ was 72% ± 25% compared with RI-RI-γ; the expression of RI-RIIA-RIIA was 87% of RI-RI-γ (not shown), but the expression of RI-RIIIA-γ was 14% of RI-RI-γ. Further, the cell surface expression of the chimera RI-CD8-γ was similar to that of WT FcγRI (Table 3). In contrast, the cell surface expression of RI-RIIIA-γ was only 11% of WT FcγRI. We also observed that the cell surface expression of RIIIA-γ-γ was 91% ± 8% of RIIIA-RI-γ, while expression of RIIIA-RIIIA-γ was only 7% of RIIIA-RI-γ. Our observation that the cell surface expression of the CD8-RI-RI chimera is 3- to 5-fold greater than that of the CD8-RIIIA-RIIIA chimera (Table 3) is also consistent with the thesis that the TM of FcγRIIIA decreases the potential for receptor cell surface expression. These experiments demonstrate that the differences in cell surface expression between FcγRI and FcγRIIIA reside largely in the sequences of their TM domains.
The data also suggest that the cytoplasmic domain may contribute to Fcγ receptor surface expression. For example, the cell surface expression of RIIIA-RI-γ is consistently greater than the cell surface expression of RIIIA-RI-RIIIA (P = .01), and the cell surface expression of RI-RIIIA-RI is consistently greater than the cell surface expression of RI-RIIIA-γ (P = .005) or RI-RIIIA-RIIIA. These data further indicate that the γ chain TM is not required for FcγRI and FcγRIIIA phagocytic signaling. Thus, dimerization of the γ chain, which occurs through formation of disulfide bridges between the TM Cys7 cysteines of the γ chains,7 is not a requirement for Fcγ receptor–mediated phagocytosis.
We used another series of Fcγ receptor chimeras to further study the role of the TM domain in Fcγ receptor expression and function and to identify the sequences of the FcγRIIIA and FcγRI TMs necessary for interaction with the γ chain. We constructed chimeric Fcγ receptors containing the EC and/or TM of CD8, (RI-CD8-γ [EC-TM-CY], RI-CD8-RI, CD8-CD8-γ, and CD8-γ-γ, Figure 2). The inability of chimeras bearing the TM of CD8 to associate with the γ chain is illustrated in Figure 3. In stable transfectants of FcγRI or of RI-CD8-RI in the mouse macrophage cell line P388 (which endogenously expresses the γ chain), the murine γ chain coimmunoprecipitated with transfected WT human (h) FcγRI but not with transfected hRI-CD8-hRI.
Schematic diagram of the Fcγ receptor/CD8 chimeras. EC indicates extracellular domain; TM, transmembrane domain; and CY, cytoplasmic domain.
Schematic diagram of the Fcγ receptor/CD8 chimeras. EC indicates extracellular domain; TM, transmembrane domain; and CY, cytoplasmic domain.
Association of the γ chain with transfected WT hFcγRI but not with the hFcγRI chimera hFcγRI-CD8-hFcγRI (EC-TM-CY). COS-1 cells were cotransfected with His-tagged human γ chain and either FcγRI-CD8-FcγRI/Myc (lane 1) or FcγRI/Myc (lane 2). Sham-transfected cells are shown in lane 3. Cell lysates were immunoprecipitated with anti-Myc antibody and blotted with anti-His antibody. Both flow cytometry and Western blot demonstrated that WT hFcγRI and hFcγRI-CD8-hFcγRI were similarly expressed, and Western blot showed that WT hFcγRI and hFcγRI-CD8-hFcγRI were similarly immunoprecipitated by anti-Myc antibody.
Association of the γ chain with transfected WT hFcγRI but not with the hFcγRI chimera hFcγRI-CD8-hFcγRI (EC-TM-CY). COS-1 cells were cotransfected with His-tagged human γ chain and either FcγRI-CD8-FcγRI/Myc (lane 1) or FcγRI/Myc (lane 2). Sham-transfected cells are shown in lane 3. Cell lysates were immunoprecipitated with anti-Myc antibody and blotted with anti-His antibody. Both flow cytometry and Western blot demonstrated that WT hFcγRI and hFcγRI-CD8-hFcγRI were similarly expressed, and Western blot showed that WT hFcγRI and hFcγRI-CD8-hFcγRI were similarly immunoprecipitated by anti-Myc antibody.
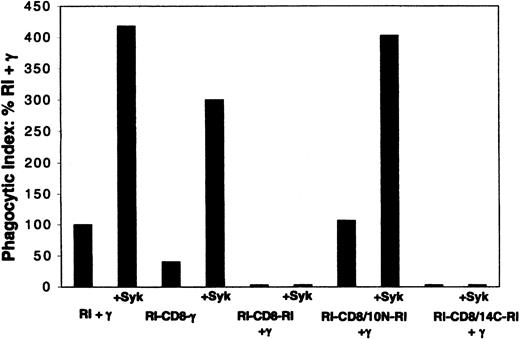
Both RI-CD8-RI and RI-CD8-γ were expressed efficiently on the surface of COS-1 cell transfectants. RI-CD8-RI, however, did not mediate phagocytosis either in the absence or presence of cotransfected γ (Figure 4), due to the inability of the CD8 TM to associate with the γ chain (Figure 3) and the inability of the FcγRI CY to mediate phagocytosis in the absence of the γ chain.9 Thus, although independent of the γ chain for surface expression, FcγRI does not function as a phagocytic receptor under conditions that do not allow γ chain/FcγRI interaction. In contrast, the chimeric receptor RI-CD8-γ mediated phagocytosis in the absence of cotransfected γ (Figure 4), demonstrating again that γ chain transmembrane sequences and dimerization of the γ chain are not required for phagocytosis mediated by the γ chain cytoplasmic domain.
Phagocytosis mediated by FcγRI/CD8 chimeras. The chimeric receptors RI-CD8-RI (EC-TM-CY) and RI-CD8-γ contain the TM of CD8. The RI-CD8/10N-RI chimera contains the first 10 aminoterminal amino acids of the CD8 TM and the 11 carboxyterminal amino acids of the FcγRI TM. The RI-CD8/14C-RI chimera contains the first 10 aminoterminal amino acids of the FcγRI TM and the 14 carboxyterminal amino acids of the CD8 TM (Figure 2). The phagocytic index (PI) values are expressed as % PI of FcγRI(RI) + γ. Receptor expression was monitored by flow cytometry. For RI + γ and each chimeric receptor, 3-5 experiments were performed. Of note, the cell surface expression of the extracellular domain of FcγRI in the chimera RI-CD8/14C-RI, which does not mediate phagocytosis with coexpression of γ, was equal to or greater than the surface expression of WT FcγRI + γ, RI-CD8-γ, or RI-CD8/10N-RI + γ, all of which mediate phagocytosis efficiently.
Phagocytosis mediated by FcγRI/CD8 chimeras. The chimeric receptors RI-CD8-RI (EC-TM-CY) and RI-CD8-γ contain the TM of CD8. The RI-CD8/10N-RI chimera contains the first 10 aminoterminal amino acids of the CD8 TM and the 11 carboxyterminal amino acids of the FcγRI TM. The RI-CD8/14C-RI chimera contains the first 10 aminoterminal amino acids of the FcγRI TM and the 14 carboxyterminal amino acids of the CD8 TM (Figure 2). The phagocytic index (PI) values are expressed as % PI of FcγRI(RI) + γ. Receptor expression was monitored by flow cytometry. For RI + γ and each chimeric receptor, 3-5 experiments were performed. Of note, the cell surface expression of the extracellular domain of FcγRI in the chimera RI-CD8/14C-RI, which does not mediate phagocytosis with coexpression of γ, was equal to or greater than the surface expression of WT FcγRI + γ, RI-CD8-γ, or RI-CD8/10N-RI + γ, all of which mediate phagocytosis efficiently.
To further study the requirements of FcγRIIIA and FcγRI interaction with the γ chain TM domain, we cotransfected FcγRI or FcγRIIIA with the CD8(EC)/γ chimeras CD8-CD8-γ or CD8-γ-γ (Table 5 and Table 6). In addition to their usefulness for examining TM function, an advantage of CD8(EC)/γ chimeras is that CD8 expression, as monitored by flow cytometry using antibody directed to the EC of CD8, serves as an indicator of γ chain expression. As anticipated, FcγRI, but not FcγRIIIA, is expressed on the surface of COS-1 cells cotransfected with CD8-CD8-γ (Tables 5 and 6). Since the FcγRI TM does not associate with the CD8 TM, cotransfection of FcγRI with CD8-CD8-γ does not support phagocytic signaling (Table 6). In contrast, cotransfection of either FcγRI or FcγRIIIA with CD8-γ-γ, which supplies the γ chain TM necessary for interaction with these Fcγ receptors, resulted in cell surface expression of and phagocytosis by both FcγRI and FcγRIIIA (Tables 5 and 6). Similar results were observed in the presence of Syk kinase (not shown).
To determine which region(s) of the FcγRI TM domain is important for FcγRI cell surface expression and its interaction with the γ chain, we constructed FcγRI (EC and CY) chimeras whose first 10 (amino-terminal) TM amino acids were replaced by the first 10 amino acids of the CD8 TM (Figure 2). Transfectants of this chimera were expressed efficiently (not shown), and phagocytosis mediated by cotransfectants with the γ chain, both in the absence and presence of Syk kinase, testifies to a productive interaction of this chimera with the γ chain (Figure 4). In contrast, replacement of the 11 carboxyterminal amino acids of the FcγRI TM by the 14 carboxyterminal amino acids of the CD8 TM produced a chimera that did not mediate phagocytosis in spite of efficient cell surface expression.
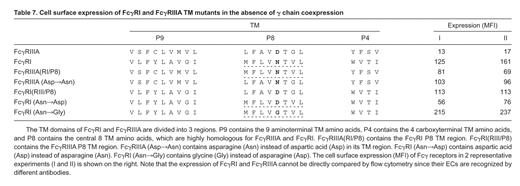
The TM domains of FcγRIIIA and FcγRI share extensive but incomplete sequence homology (Figure 5). For purposes of this study, we next divided the TM domain into 3 regions, P9, P8, and P4 (Table 7). Mutants were constructed in which the TM P8 region of FcγRI was substituted for the TM P8 region of FcγRIIIA, FcγRIIIA(RI/P8), and vice versa, FcγRI(RIII/P8) (Table 7). Transfection with FcγRIIIA(RI/P8) demonstrated that the TM P8 region of FcγRI increases cell surface expression of FcγRIIIA in the absence of the γ chain 4- to 6-fold (Table 7, lines 1 and 3). In the reciprocal experiment, in which FcγRI(RIII/P8) was transfected, surface expression decreased to 60%-80% of WT FcγRI (Table 7, lines 2 and 5). There was little or no effect on surface expression when the P9 or P4 regions of the TM domains were exchanged (not shown).
The transmembrane domains of the γ chain and the α chains of FcγRI, FcγRIIIA, FcϵRI, and FcαRI. Indicated in bold are the following TM amino acids: cysteine Cys7 and aspartic acid Asp11 of the γ chain, aspartic acid Asp203 of FcγRIIIA, aspartic acid Asp195 of FcϵRI, asparagine Asn306 of FcγRI, and arginine Arg209 of FcαRI. The TMs of FcγRI, FcγRIIIA, and FcϵRI are aligned to display amino acid homologies.
The transmembrane domains of the γ chain and the α chains of FcγRI, FcγRIIIA, FcϵRI, and FcαRI. Indicated in bold are the following TM amino acids: cysteine Cys7 and aspartic acid Asp11 of the γ chain, aspartic acid Asp203 of FcγRIIIA, aspartic acid Asp195 of FcϵRI, asparagine Asn306 of FcγRI, and arginine Arg209 of FcαRI. The TMs of FcγRI, FcγRIIIA, and FcϵRI are aligned to display amino acid homologies.
The most striking difference between the TM P8 regions of FcγRI and FcγRIIIA is the presence of asparagine in FcγRI and aspartic acid in FcγRIIIA (Table 7; Figure 5). Replacing aspartic acid with asparagine in FcγRIIIA (Asp→Asn) dramatically increased surface expression of FcγRIIIA (5- to 8-fold, Table 7, lines 1 and 4). In the reciprocal experiment, replacement of the FcγRI TM asparagine with aspartic acid (Asn→Asp) decreased surface expression of FcγRI by about 50% (Table 7, lines 2 and 6). Interestingly, replacement of asparagine in the FcγRI TM with glycine increased expression of FcγRI (Table 7, line 7).
We also examined whether there were differences in γ chain sequences required for the productive interaction of FcγRI and FcγRIIIA with the γ chain. Previous studies indicated that the association of the γ chain with some γ chain–dependent Fc receptors requires cysteine Cys7 and aspartic acid Asp11 of the γ chain TM.13 FcγRIIIA cotransfected with the mutant γ chain chimera CD8-γ(Cys7/Asp11)-γ, in which Cys7 and Asp11 of the γ chain TM are replaced by glycines (Figure 5), was not expressed on the cell surface (Table 5), consistent with the observation that elimination of γ chain/FcγRIIIA interaction eliminates surface expression of FcγRIIIA. In contrast, FcγRI cotransfected with CD8-γ(Cys7/Asp11)-γ not only was expressed on the cell surface but also was able to mediate phagocytosis, albeit at a reduced level (Table 6). These data are a further indication that FcγRI and FcγRIIIA differ in their requirements for cell surface expression and for functional interaction with the γ chain.
Discussion
The interaction of FcγRI and FcγRIIIA with the γ chain allows these Fcγ receptors, which lack cytoplasmic domain tyrosines crucial for the initiation of signaling cascades, to transmit signals from external stimuli to intracellular molecules. Interaction with the γ chain also plays a role in facilitating cell surface expression of some Fc receptors.9,13,16,17 Cell surface expression of FcγRIIIA is not observed in cells obtained from γ chain knockout mice,16 but expression of FcγRI, while greatly diminished, is detectable in a subset of monocytes/macrophages from γ chain knockout mice (van Vugt et al17 and P. M. Hogarth, oral communication, August 2002).
Differences in FcγRI and FcγRIIIA cell surface expression in the absence of the γ chain are more evident in transfectants of COS-1 cells. Only trace amounts of FcγRIIIA reach the cell surface of FcγRIIIA-transfected COS-1 cells in the absence of γ chain expression,9,10 while FcγRI is efficiently expressed in COS-1 cell transfectants in the absence or presence of the γ chain (Table 1). We and others have demonstrated that association of the γ chain with FcγRI and FcγRIIIA occurs through interactions between the individual transmembrane domains.11,12,14 The TM of FcγRI consists of 21 amino acids, 7 identical to those of the FcγRIIIA TM (Figure 5). One goal of our studies was to identify FcγRI and FcγRIIIA TM sequences that contribute to the differences in cell surface expression of these receptors.
Substitution of the FcγRI TM P8 region for that of FcγRIIIA dramatically increases cell surface expression of FcγRIIIA in the absence of the γ chain. More particularly, substitution of the FcγRI P8 asparagine for the FcγRIIIA P8 aspartic acid allows a high level of cell surface expression by FcγRIIIA in the absence of the γ chain (Table 7). In the reciprocal experiment, in which aspartic acid is substituted for asparagine in the FcγRI TM, FcγRI cell surface expression in the absence of the γ chain was reduced. Our data are consistent with the thesis that negatively charged residues in the TM are determinants for retention and rapid breakdown of some proteins in the endoplasmic reticulum20 and identify the FcγRI TM asparagine as important for γ chain–independent cell surface expression by these Fcγ receptors.
Expression of FcγRI was determined with mAb 32.2 and expression of FcγRIIIA with mAb 3G8. Therefore, direct comparison of FcγRI and FcγRIIIA expression by flow cytometry is not appropriate. We noted, however, that mutating asparagine to aspartic acid does not produce as profound a change in FcγRI expression as that induced in FcγRIIIA expression by mutating aspartic acid to asparagine in the FcγRIIIA TM. In mutating asparagine to aspartic acid, we observed a 2-fold decrease in γ chain–independent FcγRI expression; in mutating aspartic acid to asparagine, we observed a 6- to 8-fold increase in γ chain–independent FcγRIIIA expression (Table 7). One possibility is that the protein degradation apparatus in the endoplasmic reticulum does not interact efficiently with the FcγRI TM, even in the presence of a charged amino acid residue in the P8 region. Alternatively, the P4 and/or P9 regions of FcγRI may also contribute somewhat in resisting Fcγ receptor degradation.
We also noted that there is increased γ-chain independent FcγRI expression when the P8 asparagine is changed to glycine (Table 7, line 7). FcγRI expression thus appears to increase as the critical P8 residue changes from aspartic acid (negative charge) to asparagine (polar) to glycine. The increase in surface expression of the FcγRI glycine mutant may be due to the lack of charge of the substituted amino acid or to its size, each capable of resisting interaction with the protein degradation machinery.
Another goal was to further identify the FcγR TM amino acids important for productive interaction of FcγRI and FcγRIIIA with the γ chain. Previous studies suggested that charged residues in TM domains are important for protein-protein interaction in the cell membrane.12,13,20, 21, 22 FcϵRI, like FcγRIIIA, requires the γ chain for both surface expression and function in transfected cells.13 The TM of FcϵRI, which is highly homologous to the TM of FcγRIIIA, includes an aspartic acid at the TM position homologous to the aspartic acid in the FcγRIIIA TM (Figure 5). Mutation of aspartic acid to alanine in the TM of rat FcϵRI interfered with γ chain/FcϵRI interaction and reduced expression of FcϵRI,13 and cotransfection with the WT γ chain, which contains a TM aspartic acid, provided optimal expression levels of FcγRIIIA.13 Further, the TM of the ζ chain of the T-cell antigen receptor, which is highly homologous to the γ chain, requires the aspartic acid of the transmembrane domain for optimal association with FcγRIIIA.12,21 Our studies of Fcγ receptor expression and function in the presence of the γ chain demonstrate that the FcγRI TM region containing asparagine is required for productive interaction of FcγRI with the γ chain (Figure 4) and are consistent with a role for polar residues in protein-protein associations in cell membranes.
The γ chain also interacts with the receptor for IgA.23, 24, 25, 26 Cell surface expression of FcαRI is independent of γ chain association in stable transfectants of a B-cell line, although signaling functions depend upon association with the γ chain.24 Association between FcαRI and γ is also dependent upon charged residues located within the TM of both FcαRI and the γ chain.24 The 19 amino acid TM region of FcαRI displays no obvious homology to FcγRI but does contain a positively charged TM residue (arginine 209, Figure 5), which is required for its interaction with the γ chain.24 Also of note, in FcαRI/γ chain cotransfectants, γ chain protein survives in transfectants coexpressing wild-type FcαRI (which possesses TM arginine) but does not survive in cells cotransfected with FcαRI mutants, which possess a negatively charged TM residue.
Taken together, these studies further identify parameters for Fcγ receptor cell surface expression and FcγRI/FcγRIIIA γ chain association. Our studies confirm the requirement for specific charged or polar TM residues for association of the γ chain with Fc receptors using the ITAM sequence of the γ chain for signal transduction. The aspartic acid residue in the TMs of FcγRIIIA and FcϵRI fulfills this function as does the FcγRI TM polar residue asparagine. These amino acids also determine the phenotype of γ chain dependence for Fc receptor cell surface expression. We have now demonstrated that asparagine at this specific TM site allows γ chain–independent cell surface expression of FcγRI and FcγRIIIA, while the negatively charged TM residue aspartic acid plays a negative role in survival of unchaperoned Fc receptor molecules traversing the endoplasmic reticulum in transfected cell lines. Having also observed that replacement of asparagine with glycine in the FcγRI TM further increases the efficiency of FcγRI cell surface expression (Table 7), we suggest that the ability of Fc receptors to survive the action of proteolytic enzymes in the endoplasmic reticulum is related to the charge or polarity of specific TM residues.
In addition, our data indicating that cysteine Cys7 and aspartic acid Asp11 of the γ chain are essential for the cell surface expression of FcγRIIIA but not essential for the cell surface expression of FcγRI (Figure 5; Tables 5 and 6) are a further indication that FcγRI and FcγRIIIA differ in their requirements for cell surface expression and for functional interaction with the γ chain. Furthermore, since both FcγRIIIA and FcγRI are associated with γ chain dimers in vivo7,8 and signaling by these Fc receptors via the γ chain has been viewed to be contingent upon both γ chain dimerization and this specific association, our studies also indicate that neither dimerization of an associated γ chain nor the γ chain TM region itself is required for Fcγ receptor–mediated phagocytosis.
In summary, using FcγRI and FcγRIIIA, which display different phenotypes for surface expression in transfected cells, we have identified a specific region, the C-terminal region, of the FcγRI TM and its asparagine as important for FcγRI cell surface expression in the absence of the γ chain. Our data also demonstrate that a γ chain dimer induced by the γ chain TM is not required for efficient phagocytosis. These studies further define differences in phagocytic signaling between FcγRI and FcγRIIIA and extend our understanding of how transmembrane domain sequences influence the function of Fcγ receptors.
Supported by National Institutes of Health grants AI-22193 and HL-69498.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.