Phosphoinositide 3-kinase (PI3-kinase)–dependent phosphorylation of the proapoptotic Bcl-2 family member Bad has been proposed as an important regulator of apoptotic cell death. To understand the importance of this pathway in nontransformed hematopoietic cells, we have examined the effect of survival cytokines on PI3-kinase activity and Bad expression and phosphorylation status in human neutrophils. Granulocyte macrophage–colony-stimulating factor (GM-CSF) and tumor necrosis factor-α (TNF-α) both reduced the rate of apoptosis in neutrophils cultured in vitro for 20 hours. Coincubation with the PI3-kinase inhibitor LY294002, which in parallel experiments abolished GM-CSF–primed, fMLP-stimulated superoxide anion production and GM-CSF–stimulated PtdIns(3,4,5)P3accumulation, inhibited the GM-CSF and TNF-α survival effect. In contrast, the MAP kinase kinase (MEK1/2) inhibitor PD98059 and the protein kinase A inhibitor H-89 had only a marginal effect on GM-CSF–mediated neutrophil survival. GM-CSF substantially increased Bad phosphorylation at Ser112 and Ser136 and increased the cytosolic accumulation of Bad. GM-CSF also regulated Bad at a transcription level with a marked decrease in mRNA levels at 4 hours. TNF-α caused a biphasic effect on the rate of morphologic apoptosis, which corresponded to an early increase, and a late inhibition, of Bad mRNA levels. LY294002 inhibited GM-CSF– and TNF-α–mediated changes in Bad phosphorylation and mRNA levels. These data suggest that the survival effect of GM-CSF and TNF-α in neutrophils is caused by a PI3-kinase–dependent phosphorylation and cytosolic translocation of Bad, together with an inhibition of Bad mRNA levels. This has important implications for the regulation of neutrophil apoptosis in vivo.
Introduction
Human neutrophils undergo spontaneous apoptosis in vitro and in vivo, and this process has been proposed as a pivotal mechanism underlying the resolution of granulocytic inflammation. The process of apoptosis involves the promotion of controlled cell death while it maintains cellular integrity; this prevents the release of proinflammatory mediators and promotes the recognition and phagocytic elimination of effete cells from the site of inflammation.1-3 Wide-ranging proinflammatory cytokines and mediators that serve as neutrophil-priming or -activating agonists also inhibit the progression of apoptosis in vitro, and both events are thought to be important in the establishment of the chronic inflammatory response.4 Granulocyte macrophage–colony-stimulating factor (GM-CSF), interleukin-8 (IL-8), lipopolysaccharide (LPS), C5a, leukotriene B4(LTB4), and hypoxia have been documented to delay neutrophil apoptosis,5-10 whereas tumor necrosis factor-α (TNF-α), Fas-L, and UV radiation promote apoptosis.11-14 Several intracellular signaling pathways have been proposed to be involved in cytokine-mediated inhibition of neutrophil apoptosis, including the phosphoinositide 3-kinase (PI3-kinase)/protein kinase B (PKB), mitogen-activated protein kinase (MAPK), and cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling cascades.6 15-18 However, the relative contributions of these various pathways and their principal intracellular targets have yet to be defined.
The differential expression and phosphorylation status of Bcl-2 and Bcl-2–Like proteins, which serve as a common checkpoint for several distinct signaling pathways, appear to be important in controlling the apoptotic threshold of inflammatory cells. Proapoptotic and antiapoptotic members of the Bcl-2 family appear to regulate death signaling through their ability to form complex homodimers and heterodimers that ultimately influence the insertion of Bax and Bax-like proteins into the outer mitochondrial membrane. This event plays a key role in triggering neutrophil apoptosis and results in the release of cytochrome C and the activation of caspase 9/Apaf-1 apoptosome.19,20 Bcl-2 family members contain 4 Bcl-2 homology (BH) domains,21 designated BH1 to BH4, which are conserved in antiapoptotic members (Bcl-2, Bcl-XL, Bcl-W, Mcl-1, and A1), whereas proapoptotic members (Bax, Bak, and Bok) show less conservation for the BH4 region.22 Deletion and mutagenesis analysis of the BH3 domain has revealed this to be the minimal death domain required for heterodimerization and the promotion of apoptosis.23,24 These reports are supported by structural data relating to a subgroup of Bcl-2–Like proteins with only BH3 domain conservation (Bad, Bid, Bik, Bim, Blk, Bmf, and Hrk).23,25,26 These BH3-only proteins show no intrinsic or independent cell destructive properties; instead, they appear to function as dominant inhibitors of the antiapoptotic Bcl-2–Like proteins. One such complex interaction that may be of particular importance in mature neutrophils that lack Bcl-227,28 involves heterodimerization between Bcl-XL and Bad. In overexpression models, Bad phosphorylation at serine residues 112, 136, and 1556 29-32 has been shown to promote the association of Bad with the cytoplasmic protein 14-3-3, allowing Bcl-XL to associate with proapoptotic proteins. Substitution of Ser112 or Ser136 eliminates 14-3-3 binding and enhances the death-promoting activity of Bad. However, this proposed sequence of events is based on in vitro analysis of purified components in overexpression systems, and its relevance to primary cells has been questioned.33, 34
In this study we have examined the signal transduction pathways involved in cytokine-mediated survival in human peripheral blood neutrophils with reference to the influence of GM-CSF and TNF-α on the expression of Bcl-XL/S, Bad, and Bax. We confirm previous reports that GM-CSF inhibits the rate of spontaneous neutrophil apoptosis in vitro and have shown that this survival effect, together with the ability of GM-CSF to stimulate phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3) accumulation, is largely abolished by the selective PI3-kinase inhibitors wortmannin and LY294002. This contrasts with the effects of the MEK1/2 inhibitor, PD98059, and the PKA inhibitor, H-89, which cause only marginal inhibition of the GM-CSF–mediated survival effect. We show that GM-CSF—in addition to causing Bad phosphorylation at Ser112 and Ser136—releases Bad into the cytosolic compartment and significantly decreases the transcription rate of Bad by a PI3-kinase–dependent mechanism. TNF-α, which we have previously shown to enhance the rate of neutrophil apoptosis at early time points, caused an increase in Bad transcription rates, again through a PI3-kinase–dependent mechanism. These data provide important validation for the role of PI3-kinase–dependent Bad phosphorylation in the control of apoptotic thresholds in nontransformed cells and point to an additional and novel mechanism of action of these cytokines in regulating Bad at a transcriptional level. Although Bax has an important functional role in neutrophil apoptosis, the dual regulation of Bad at a protein phosphorylation and transcriptional level appears to be a key factor controlling the proapoptotic properties of this molecule.
Materials and methods
Neutrophil preparation and culture conditions
Human neutrophils were purified from the peripheral blood of healthy nonatopic volunteers as previously described.35 Cell purity was assessed using air-dried cytocentrifuge preparations fixed in methanol and stained with May-Grünwald-Giemsa (Merck Ltd, Lutterworth, Leicestershire, United Kingdom) and routinely identified more than 97% neutrophils with less than 0.1% mononuclear cell contamination. Freshly isolated neutrophils were routinely suspended at 5 × 106/mL in Iscove modified Dulbecco medium supplemented with 10% autologous serum, 100 U/mL penicillin, and 100 μg/mL streptomycin, and they were cultured in a humidified 5% CO2 atmosphere at 37°C. To examine the effect of various cytokines on the rate of constitutive neutrophil apoptosis, cells were incubated in the presence or absence of predetermined optimal concentrations of GM-CSF (10 ng/mL) and TNF-α (100 U/mL) for the time periods indicated (4 to 20 hours). In experiments in which kinase inhibitors were used, neutrophils were preincubated with the inhibitors (LY294002, 10 μM; PD98059, 50 μM; H-89, 1 μM) or appropriate vehicle for 10 minutes at 37°C before addition of the cytokine. Approval was obtained from the Cambridge research ethics committee for these studies. Informed consent was provided according to the Declaration of Helsinki.
Superoxide anion generation
The release of superoxide anions (O) was determined by means of the superoxide dismutase-inhibitable reduction of cytochrome C. Freshly isolated neutrophils (11.1 × 106cells/mL) were resuspended in phosphate-buffered saline (PBS) with Ca++/Mg2+and aliquoted (90 μL) into 2-mL centrifuge tubes containing 10 μL TNF-α (final concentration, 200 U/mL), GM-CSF (final concentration, 100 ng/mL), or PBS. All incubations were performed in quadruplicate. Samples were incubated at 37°C in the presence or absence of the cytokines for 30 minutes before the addition of 750 μL freshly prepared and prewarmed cytochrome C (final concentration, 1.2 mg/mL). One sample from each quadruplicate set was used as a reference control through the addition of superoxide dismutase (375 U/sample). After 10-minute incubation with N-formyl-methionyl-leucyl-phenylalanine (fMLP, 100 nM) or PBS, samples were placed on ice and were centrifuged (10 000g for 5 minutes). Optical density was determined using a scanning spectrophotometer measuring peak height between 535 and 565 nm. O release was calculated using the extinction coefficient 21 × 103M−1cm−1 and was expressed in nanomoles per 106 cells.
Measurement of PtdIns(3,4,5)P3 mass
The effect of GM-CSF on neutrophil PI3-kinase activity was determined by measuring the accumulation of PtdIns(3,4,5)P3 mass in acidified chloroform–methanol cell extracts. This was performed by quantitative conversion of PtdIns(3,4,5)P3 to Ins(1,3,4,5)P4 and measurement of this water-soluble product using a specific radioreceptor assay. Recombinant Ins1,3,4,5P4binding protein (GAPIP4BP) was obtained from Dr P. Cullen (University of Bristol, United Kingdom) and was purified as described.36,37 The binding characteristics of each batch of GAPIP4BP was confirmed using 0.1 nM InsP6(phytate), and a maximum [3H]Ins1,3,4,5P4 binding (Bmax) value of 20% was used in subsequent assays. Isolated neutrophils (8 × 106 assay point) were incubated with GM-CSF (100 ng/mL) or buffer for 30 minutes, and the reactions were stopped by the addition of methanol–chloroform (2:1, vol/vol). Lipid extractions were then performed as previously described.38 After drying, the samples were boiled in 1 M KOH for 30 minutes, which converts PtdIns(3,4,5)P3 to Ins(1,3,4,5)P4 with an efficiency of 62%. Samples were neutralized with 1 M acetic acid and washed with water-saturated butan-1-ol, petroleum ether, and ethyl acetate (20:4:1, vol/vol/vol) to remove the cleaved fatty acids. The lower aqueous phase was dried in a vacuum concentrator and stored at −20°C until assayed. Samples were resuspended in acetic acid to pH 5.0 before assay. The Ins(1,3,4,5)P4 radioreceptor assay was performed as described37 using 0.005 μCi (0.000185 MBq) [3H]Ins(1,3,4,5)P4 per sample. A standard displacement curve was constructed for each experiment using 0.001 to 120 pmol unlabeled authentic Ins(1,3,4,5)P4.
Morphologic analysis of neutrophil apoptosis
Neutrophils were cultured in 96-well, ultralow attachment plates (Costar, Hycombe, Bucks, United Kingdom) at 7.5 × 105cells/well. Following resuspension the cells were aspirated, cytocentrifuged, fixed, and stained as described above. Morphologic analysis of neutrophil apoptosis was assessed under oil immersion light microscopy with the observer masked to the experimental conditions. Apoptotic neutrophils were defined as cells with darkly stained, condensed nuclei. For each of the conditions investigated, triplicate slides were prepared, and at least 400 neutrophils were counted per slide.
Annexin V analysis of neutrophil apoptosis
Neutrophils were cultured under identical conditions in the presence or absence of GM-CSF or TNF-α as detailed above. Neutrophils were then aspirated and centrifuged (275g, 5 minutes at 4°C), and the cell pellet was resuspended in 200 μL HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) buffer (10 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2) containing Annexin-V–fluorescein isothiocyanate (FITC) (1 μg/mL) and propidium iodide (10 μg/mL). Samples were incubated for 15 minutes at 4°C in the dark, and the volume was increased to 500 μL with HEPES buffer immediately before analysis by flow cytometry.
Immunoprecipitation of Bad
Neutrophils were incubated for varying times with or without GM-CSF (10 ng/mL) or TNF-α (200 U/mL) in the presence or absence of kinase inhibitors (as detailed in the figure legends) in 6-well, ultralow attachment plates at 2.5 × 107 cells/well. Cells were then pelleted and resuspended in lysis buffer (10 mM Tris-HCl, pH 7.8), 1.5 mM EDTA (ethylenediaminetetraacetic acid), 10 mM KCl, 0.5 mM dithiothreitol, 1 mM sodium orthovanadate, 2 mM levamisole, 0.5 mM benzamidine, 0.05% NP-40, and a proteinase inhibitor cocktail (Boehringer Mannheim, Germany) and were incubated on ice for 10 minutes before and after sonication (Soniprep 150, setting 13 for 30 seconds). Cellular debris was pelleted by centrifugation for 5 minutes at 1850g (4°C), and membrane and cytosolic fractions were prepared by further centrifugation at 22 000g (20 minutes, 4°C) or 104 000g (30 minutes, 4°C) as indicated in the figure legends. Membrane fractions were resuspended in the above buffer containing 0.1% Triton-X100. Whole-cell lysates were prepared in an identical manner, except that the cells were originally lysed in the presence of 0.1% Triton-X100. After normalization for protein content, whole-cell, membrane, and cytosolic samples were incubated with a rabbit polyclonal antibody directed against Bad (H-168; Santa Cruz Biotechnology, CA). Samples were rotated for 2 hours at 4°C before the addition of immunoglobulin G (IgG) beads (Amersham Biosciences, United Kingdom) and subsequently were incubated overnight. Immunoprecipitates were washed 3 times, initially with 0.1 M Tris-HCl (pH 7.4), followed by 0.01 M Tris-HCl (pH 7.4), and finally with dH2O.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting
Immunoprecipitates were solubilized with 160 μL Laemmli buffer and were heated for 10 minutes at 100°C. Proteins were separated on 12% (wt/vol) polyacrylamide gels and electrophoretically transferred to polyvinylidene difluoride membranes. These were washed overnight in blocking solution containing 5% (wt/vol) dried-milk powder in PBS with 0.1% Tween-20 (PBS-Tw20) at 4°C. The membranes were incubated for 2 hours in PBS-Tw20 containing 0.25% dried-milk powder with polyclonal antibodies to Ser112 or Ser136 phosphorylated-Bad (New England Biolabs, Beverly, MA) or Bcl-X (Oncogene Science, Cambridge, MA) or a monoclonal antibody to Bad (36420; Transduction Laboratories, Lexington, KY), at dilutions of 1:500, 1:1000, 1:40, and 1:1000, respectively. After 3 washes in PBS-Tw20, the membranes were incubated with peroxidase-conjugated secondary antibodies (final dilution, 1:2000) in PBS-Tw20 supplemented with 0.25% dried-milk powder for 1 hour and subsequently were washed as described above. Detection was performed by chemiluminescence using an ECL kit (Amersham Biosciences) and subsequent exposure to x-ray film (XOMAT-AR; Amersham Biosciences).
RT-PCR procedures
Total RNA was isolated using TRIzol (Life Technologies). RNA (10 μg) was transcribed to cDNA using oligo(dt) primers (Life Technologies) and 50 U reverse transcriptase (Promega). Polymerase chain reaction (PCR) amplification was performed using specific primer sets for Bad (sense, 5′-TCC-CAG-AGT-TTG-AGC-CGA-GT; antisense, 3′-ATG-TGG-AGC-GAA-GGT-CAC-TG; 471-bp product), Bcl-X (sense, 5′-GAA-TCT-TAT-CTT-GGC-TTT-GGA; antisense, 3′-GTA-GAG-TGG-ATG-GTC-AGT-GT; 799-bp product), and BAX (sense, 5′-TGC-TTC-AGG-GTT-TCC-AGG; antisense, 3′-ACC-ACT-GTG-ACC-TGC-TCC-AGA-A; 476-bp product). For control reactions, a specific primer set for β-actin (sense, 5′-GTG-GGG-CGC-CCC-AGG-CAC-CA; antisense, 3′-CTC-CTT-AAT-GTC-ACG-CAG-CAC-GAT-TTC; 548-bp product) was used. PCR (35 cycles) was performed using 2 U ampliTaq DNA polymerase (Bioline). PCR products were analyzed by agarose gel electrophoresis and imaged with ethidium bromide under UV light.
Results
Functional assessment of neutrophil priming–activation status following cell isolation
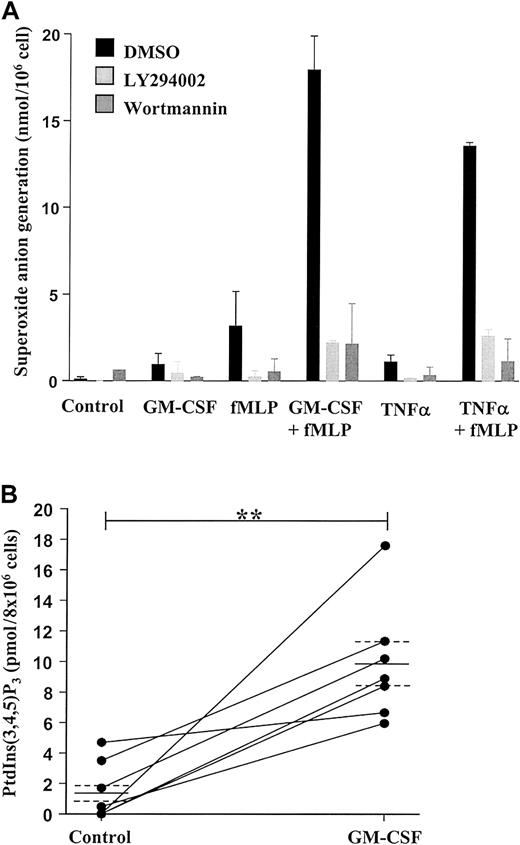
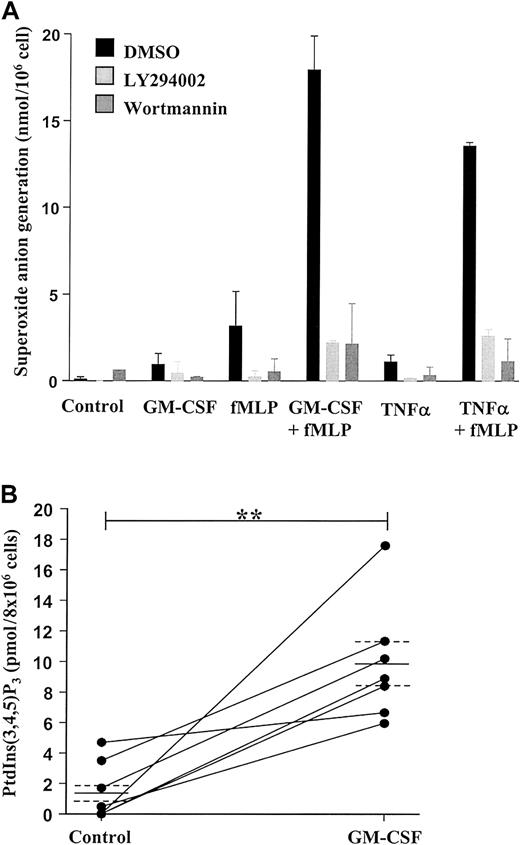
We have previously demonstrated that neutrophil priming, for example, by inadvertent exposure of cells to trace amounts of LPS during the isolation procedure, has a significant impact on the rate of constitutive neutrophil apoptosis in vitro and the modulation of this event by cytokines such as TNF-α and GM-CSF.4,8 39 To ensure that our neutrophil isolation technique did not prime cells, we examined the effect of fMLP on O generation. Exposure of neutrophils to GM-CSF (100 ng/mL), TNF-α (200 U/mL), or fMLP (100 nM) alone had little or no effect on Ogeneration; this was in contrast to the substantial effect of fMLP on O generation observed in GM-CSF– or TNF-α–primed cells (Figure 1A).
GM-CSF enhances superoxide anion production and PtdIns(3,4,5)P3 accumulation in peripheral blood neutrophils by a PI3-kinase–dependent mechanism.
(A) Freshly isolated neutrophils (1 × 106 cells) were incubated in the presence or absence of 100 ng/mL GM-CSF or 200 U/mL TNF-α for 30 minutes at 37°C before stimulation with 100 nM fMLP or buffer for 10 minutes, as indicated. Superoxide anion release was determined as detailed in the “Materials and methods” section. GM-CSF and TNF-α significantly enhanced fMLP-stimulated superoxide anion generation. The PI3-kinase inhibitors LY294002 (10 μM) and wortmannin (100 nM) abolished superoxide anion generation under all conditions studied. Data represent the mean ± SD of 2 independent experiments, each performed in triplicate. (B) GM-CSF (100 ng/mL)–stimulated PI3-kinase activity was determined by measuring PtdIns(3,4,5)P3 accumulation at 30 minutes following cytokine addition, as described in the “Materials and methods” section. GM-CSF significantly enhanced PtdIns(3,4,5)P3accumulation compared with time-matched controls. Data represent values from 7 independent experiments, each performed in duplicate, together with mean ± SEM values. **P > .005.
GM-CSF enhances superoxide anion production and PtdIns(3,4,5)P3 accumulation in peripheral blood neutrophils by a PI3-kinase–dependent mechanism.
(A) Freshly isolated neutrophils (1 × 106 cells) were incubated in the presence or absence of 100 ng/mL GM-CSF or 200 U/mL TNF-α for 30 minutes at 37°C before stimulation with 100 nM fMLP or buffer for 10 minutes, as indicated. Superoxide anion release was determined as detailed in the “Materials and methods” section. GM-CSF and TNF-α significantly enhanced fMLP-stimulated superoxide anion generation. The PI3-kinase inhibitors LY294002 (10 μM) and wortmannin (100 nM) abolished superoxide anion generation under all conditions studied. Data represent the mean ± SD of 2 independent experiments, each performed in triplicate. (B) GM-CSF (100 ng/mL)–stimulated PI3-kinase activity was determined by measuring PtdIns(3,4,5)P3 accumulation at 30 minutes following cytokine addition, as described in the “Materials and methods” section. GM-CSF significantly enhanced PtdIns(3,4,5)P3accumulation compared with time-matched controls. Data represent values from 7 independent experiments, each performed in duplicate, together with mean ± SEM values. **P > .005.
These data confirm that the isolated neutrophils were in an unprimed, nonactivated state. As previously reported, the PI3-kinase inhibitors wortmannin (100 nM) and LY294002 (50 μM) completely blocked fMLP-stimulated O generation in GM-CSF– and TNF-α–primed cells, substantiating the importance of the PI3-kinase pathway in activating the respiratory burst in human neutrophils.40
GM-CSF–stimulated PtdIns(3,4,5)P3 accumulation in human neutrophils
To investigate the potential importance of the PI3-kinase signaling pathway to cytokine-mediated neutrophil survival, we examined the effect of GM-CSF on the accumulation of PtdIns(3,4,5)P3mass, the intermediate metabolic product of PI3-kinase activity, which reflects the action of the class 1a (p85:p110α, β, δ) and the class 1b (p101:p110γ) PI3-kinase isoforms in neutrophils.
Stimulation of neutrophils with GM-CSF (100 ng/mL) for 30 minutes caused a significant (approximately 6-fold) accumulation of PtdIns(3,4,5)P3 compared with control (Figure 1B), and this response was completely blocked by preincubation with LY294002 (10 μM) (data not shown). These data signify the ability of GM-CSF to stimulate PI3-kinase in human neutrophils.
Effect of PI3-kinase inhibitors on the rate of neutrophil apoptosis in vitro
Having demonstrated that GM-CSF induces a PI3-kinase–dependent increase in PtdIns(3,4,5)P3accumulation and agonist-stimulated O generation, we next examined the role of this enzyme in GM-CSF–mediated neutrophil survival.
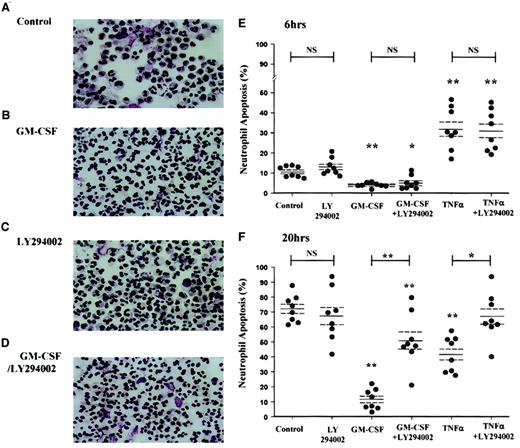
Apoptotic neutrophils have a distinct morphologic appearance with cell shrinkage, cytoplasmic vacuolation, and condensation of chromatin, leading to the loss of the normal multilobed nucleus. This allows accurate quantification of the extent of apoptosis by visual examination of cytospin preparations. The photomicrographs in Figure2 illustrate the ability of GM-CSF to inhibit neutrophil apoptosis over a 20-hour incubation period (Figure2B) and the inhibition of this survival effect by LY294002 (Figure 2C).
GM-CSF and TNF-α promote neutrophil survival through a PI3-kinase–dependent mechanism, as assessed by cell morphology.
Peripheral blood neutrophils were cultured in the presence or absence of GM-CSF (10 ng/mL) or TNF-α (200 U/mL) with or without LY294002 (10 μM) for the time periods indicated. Cells were harvested at 6 and 20 hours, and cytospins were fixed and stained as detailed in the “Materials and methods” section. (A-D) Representative photomicrographs (original magnification, × 400) of neutrophils incubated with or without GM-CSF or LY294002 for 20 hours. (E-F) TNF-α caused an early (6-hour) increase and a late (20-hour) inhibition of neutrophil apoptosis, whereas GM-CSF caused a significant survival effect at both time points. LY294002 had no effect on the constitutive rate of neutrophil apoptosis or the early proapoptotic effect of TNF-α, but it inhibited the survival effect of GM-CSF and TNF-α at 20 hours. Data represent percentage apoptosis from 8 independent experiments, each performed in triplicate, together with mean ± SEM values. *P < .05 and **P < 0.005 compared with control values, unless otherwise indicated.
GM-CSF and TNF-α promote neutrophil survival through a PI3-kinase–dependent mechanism, as assessed by cell morphology.
Peripheral blood neutrophils were cultured in the presence or absence of GM-CSF (10 ng/mL) or TNF-α (200 U/mL) with or without LY294002 (10 μM) for the time periods indicated. Cells were harvested at 6 and 20 hours, and cytospins were fixed and stained as detailed in the “Materials and methods” section. (A-D) Representative photomicrographs (original magnification, × 400) of neutrophils incubated with or without GM-CSF or LY294002 for 20 hours. (E-F) TNF-α caused an early (6-hour) increase and a late (20-hour) inhibition of neutrophil apoptosis, whereas GM-CSF caused a significant survival effect at both time points. LY294002 had no effect on the constitutive rate of neutrophil apoptosis or the early proapoptotic effect of TNF-α, but it inhibited the survival effect of GM-CSF and TNF-α at 20 hours. Data represent percentage apoptosis from 8 independent experiments, each performed in triplicate, together with mean ± SEM values. *P < .05 and **P < 0.005 compared with control values, unless otherwise indicated.
The extreme instability of wortmannin in aqueous media precludes the use of this inhibitor in experiments requiring such prolonged incubation periods. Morphologic assessment revealed that GM-CSF (10 ng/mL) inhibited apoptosis at 6 hours and 20 hours (percentage apoptosis: control 6 hours, 10.7% ± 0.9%; GM-CSF 6 hours, 4.1% ± 0.4%, P < .005; control 20 hours, 72.3% ± 3.2%; GM-CSF 20 hours, 11.9% ± 2.3%,P < .005, n = 8) (Figure 2E, F). The cytoprotective effect of GM-CSF at 20 hours was significantly attenuated (64.1% ± 8.4%, P < .005) by preincubation with LY294002 (10 μM). Of note, LY294002 alone had no effect on the rate of neutrophil apoptosis at 6 or 20 hours (Figure 2). In contrast, TNF-α (200 U/mL) was shown to have a biphasic effect on neutrophil apoptosis with an early (6-hour) enhancement in the rate of apoptosis (percentage apoptosis: control 6 hours, 10.7% ± 0.9%; TNF-α 6 hours, 32.3% ± 3.7%, P < .005, n = 8) and a later (20-hour) inhibition (percentage apoptosis: control 20 hours, 72.3% ± 3.2%; TNF-α 20 hours, 42.1% ± 4.2%,P < .005, n = 8). Although LY294002 did not influence the early proapoptotic effect of TNF-α, the 20-hour survival effect was attenuated by 98.5% ± 13.8% (P < .05, n = 8).
Annexin-V–FITC assessment of neutrophil apoptosis
Double labeling of neutrophils with Annexin-V–FITC (AnV), which binds to PS on the surfaces of apoptotic cells, and the nuclear stain propidium iodide (PI), which allows assessment of membrane integrity, enables differentiation between viable nonapoptotic cells (AnV−, PI−), viable apoptotic cells (AnV+, PI−), and late apoptotic–necrotic cells (AnV+, PI+). Previous studies have underscored the importance of using complementary but distinct methods for quantifying neutrophil apoptosis, not least because of the improved sensitivity of AnV binding over morphology as a marker of early apoptosis.41 In addition, certain experimental and pharmacologic interventions can lead to dissociation between the extent of morphologic apoptosis and the proportion of AnV+cells.20 41 To address these issues, we examined the influence of GM-CSF, TNF-α, and LY294002 on neutrophil apoptosis using dual AnV and PI staining.
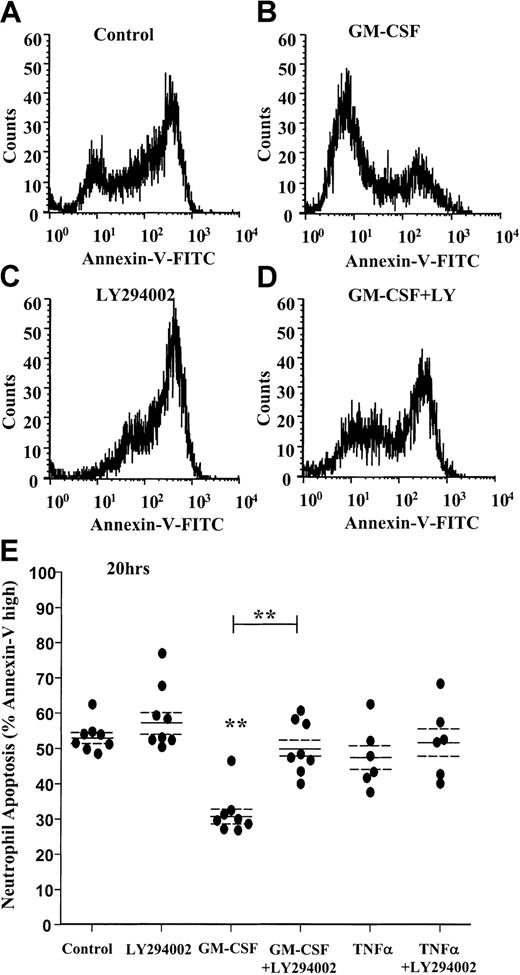
AnV analysis of neutrophil apoptosis confirmed the cytoprotective effect of GM-CSF at 20 hours (percentage apoptosis: control 20 hours, 53.2% ± 1.5%; GM-CSF 20 hours, 31.6% ± 2.2%,P < .005, n = 8) and the near complete inhibition of this survival effect with LY294002 (Figure3A-D). Of note, AnV staining (unlike the morphologic quantification above) failed to demonstrate any significant cytoprotective effect of TNF-α at 20 hours (Figure 3E).
GM-CSF prevents the externalization of phosphatidylserine through a PI3-kinase–dependent mechanism.
Freshly isolated human neutrophils were cultured in the presence or absence of GM-CSF (10 ng/mL) or TNF-α (200 U/mL) with or without LY294002 (10 μM) for 20 hours. Cells were harvested, and PS exposure was quantified by flow cytometry as described in the “Materials and methods” section. (A-D) Representative fluorescent histograms of 10 000 events showing the level of AnV binding at 20 hours for (A) control, (B) GM-CSF–, (C) LY294002-, and (D) GM-CSF + LY294002–treated cells. AnV staining is a marker of PS exposure on the cell outer plasma membrane. GM-CSF inhibited AnV staining, indicative of an inhibition of neutrophil apoptosis. The survival effect was abolished by coincubation with LY294002. (E) Quantitation of the percentage AnV high-staining cells at 20 hours following incubation with GM-CSF, TNF-α, or LY294002, or all of them, as indicated. Data represent percentage AnV high cells from 8 independent experiments, each performed in triplicate, together with mean ± SEM values. **P < .005 compared with control, unless otherwise indicated.
GM-CSF prevents the externalization of phosphatidylserine through a PI3-kinase–dependent mechanism.
Freshly isolated human neutrophils were cultured in the presence or absence of GM-CSF (10 ng/mL) or TNF-α (200 U/mL) with or without LY294002 (10 μM) for 20 hours. Cells were harvested, and PS exposure was quantified by flow cytometry as described in the “Materials and methods” section. (A-D) Representative fluorescent histograms of 10 000 events showing the level of AnV binding at 20 hours for (A) control, (B) GM-CSF–, (C) LY294002-, and (D) GM-CSF + LY294002–treated cells. AnV staining is a marker of PS exposure on the cell outer plasma membrane. GM-CSF inhibited AnV staining, indicative of an inhibition of neutrophil apoptosis. The survival effect was abolished by coincubation with LY294002. (E) Quantitation of the percentage AnV high-staining cells at 20 hours following incubation with GM-CSF, TNF-α, or LY294002, or all of them, as indicated. Data represent percentage AnV high cells from 8 independent experiments, each performed in triplicate, together with mean ± SEM values. **P < .005 compared with control, unless otherwise indicated.
Such a discrepancy, though unexplained, has been widely observed in other studies of neutrophil apoptosis20 42 and underscores the importance of using complementary techniques to assess cell death. Further analysis of the subpopulation of AnV+ cells indicated that GM-CSF did not influence the proportion of apoptotic (AnV+) cells that were PI+ at 20 hours (Table1). In contrast, TNF-α alone and LY294002 alone, though not influencing the overall extent of AnV+ cells at 20 hours, did increase the ratio of PI+/PI− cells within the apoptotic pool (Table1).
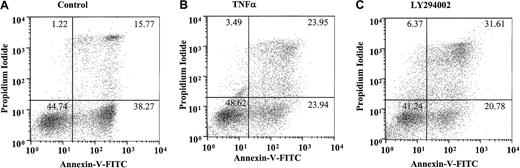
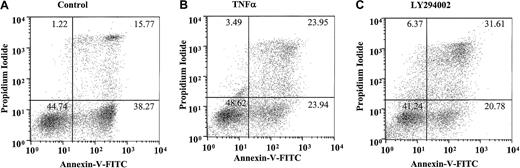
This most likely reflects the early induction of neutrophil apoptosis by TNF-α, leading to a higher percentage of late (AnV+and PI+) apoptotic cells at 20 hours and a potential role of PI3-kinase in maintaining the integrity of the cell membrane in cells that have already undergone apoptosis. Figure4A-C depicts a single representative experiment demonstrating the quadrant distribution of AnV+/− and PI+/− cells following incubation with or without TNF-α or LY294002 for 20 hours.
Effect of TNF-α and LY294002 on AnV and propidium iodide staining in human neutrophils.
Human neutrophils were incubated in the absence (A) or presence (B) of TNF-α (200 U/mL) or LY294002 (10 μM; C) for 20 hours. Cells were harvested and stained with AnV (a marker of PS exposure/apoptosis) and PI (a marker of plasma membrane integrity/postapoptotic necrosis), as detailed in “Materials and methods.” TNF-α and LY294002, while not influencing the ratio of AnV high to low staining, increased the proportion of PI-positive cells within the AnV high pool. Representative data are shown from a single experiment with identical results obtained in 7 independent experiments.
Effect of TNF-α and LY294002 on AnV and propidium iodide staining in human neutrophils.
Human neutrophils were incubated in the absence (A) or presence (B) of TNF-α (200 U/mL) or LY294002 (10 μM; C) for 20 hours. Cells were harvested and stained with AnV (a marker of PS exposure/apoptosis) and PI (a marker of plasma membrane integrity/postapoptotic necrosis), as detailed in “Materials and methods.” TNF-α and LY294002, while not influencing the ratio of AnV high to low staining, increased the proportion of PI-positive cells within the AnV high pool. Representative data are shown from a single experiment with identical results obtained in 7 independent experiments.
Effect of the MAPK inhibitor PD98059 on the rate of neutrophil apoptosis
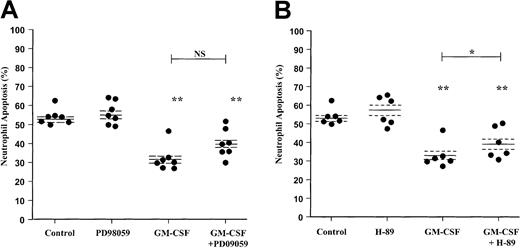
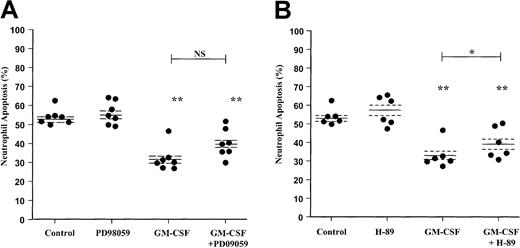
Previous studies have suggested that activation of the ERK1/2 pathway may be involved in the survival effect of GM-CSF in human neutrophils.6 To compare the relative importance and contribution of ERK1/2 and the PI3-kinase pathways to neutrophil apoptosis, we performed comparative experiments using the ERK1/2 inhibitor PD98059. In contrast to the marked effect of LY294002 on GM-CSF–mediated neutrophil survival, analysis of neutrophil apoptosis using AnV–FITC binding indicated that PD98059 did not attenuate the survival effect of GM-CSF (P = .055) (Figure5A).
Effect of the MEK1/2 inhibitor PD98059 and PKA inhibitor H-89 on GM-CSF–stimulated neutrophil survival.
Human neutrophils were cultured for 20 hours in the presence or absence of GM-CSF (10 ng/mL) with or without (A) PD98059 (50 μM) or (B) H-89 (1 μM). Apoptosis was assessed by AnV binding, as detailed in the “Materials and methods” section. Although H-89 caused a significant inhibition of GM-CSF–mediated neutrophil survival, the magnitude of this effect was substantially less than that observed with LY294002. Data represent values from 6 to 7 independent experiments, each performed in triplicate, together with mean ± SEM values. NS indicates not significant. *P < .05 and **P < .005 compared with control, unless otherwise indicated.
Effect of the MEK1/2 inhibitor PD98059 and PKA inhibitor H-89 on GM-CSF–stimulated neutrophil survival.
Human neutrophils were cultured for 20 hours in the presence or absence of GM-CSF (10 ng/mL) with or without (A) PD98059 (50 μM) or (B) H-89 (1 μM). Apoptosis was assessed by AnV binding, as detailed in the “Materials and methods” section. Although H-89 caused a significant inhibition of GM-CSF–mediated neutrophil survival, the magnitude of this effect was substantially less than that observed with LY294002. Data represent values from 6 to 7 independent experiments, each performed in triplicate, together with mean ± SEM values. NS indicates not significant. *P < .05 and **P < .005 compared with control, unless otherwise indicated.
These data were confirmed by independent assessment of cell morphology; PD98059 again failed to influence the extent of neutrophil apoptosis under control or GM-CSF–stimulated conditions at 6 and 20 hours (Table2). PD98059 also had no effect on the early proapoptotic or the late survival effect of TNF-α as judged by morphologic criteria (Table 2).
To determine whether the simultaneous inhibition of the ERK1/2 and PI3-kinase pathways induced additive inhibition of GM-CSF–mediated survival, neutrophils were pretreated with both inhibitors (LY294002 10 μM, PD98059 50 μM) before incubation with GM-CSF or TNF-α. Annexin-V–FITC analysis of apoptosis revealed inhibition of neutrophil survival to an extent similar to that seen with LY294002 treatment alone, indicating a nonadditive effect (data not shown).
Effect of the PKA inhibitor H-89 on the rate of neutrophil apoptosis
Several papers have recently reported an additional Bad phosphorylation site at Ser155, potentially phosphorylated by PKA rather than PI3-kinase/PKB. In view of these observations and our previous demonstration that PGE2 and cell-permeable cAMP analogues inhibit neutrophil apoptosis through a PKA-dependent pathway,43 we extended the above studies to examine the role of the PKA pathway in GM-CSF–induced neutrophil survival. Neutrophils were pretreated with H-89 (1 μM) before incubation with GM-CSF (10 ng/mL) for 6 and 20 hours. AnV analysis of neutrophil apoptosis indicated that H-89 induced a significant inhibition of the GM-CSF survival effect; however, the magnitude of this effect was smaller than that observed with LY294002 (Figure 5B). Together, these data support a dominant role for the PI3-kinase signaling in mediating GM-CSF– and TNF-α–mediated neutrophil survival in vitro.
PI3-kinase–dependent effect of GM-CSF on Bad localization and Bad phosphorylation
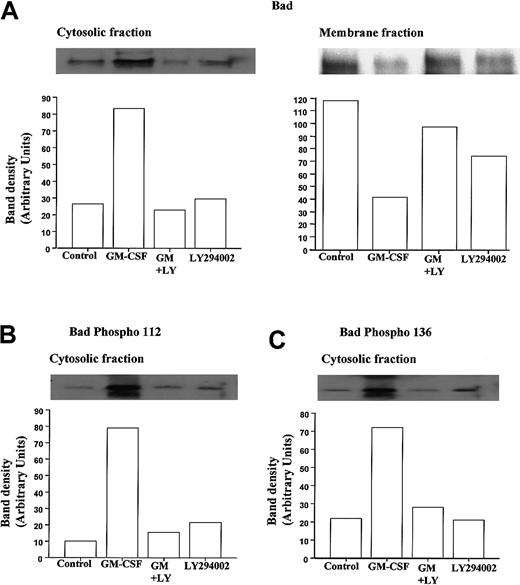
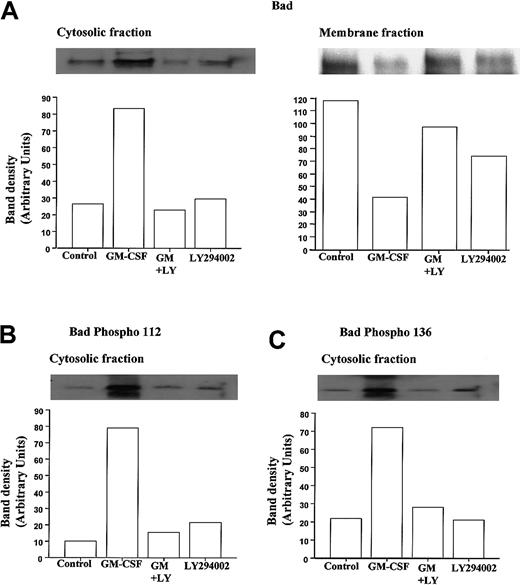
To examine the phosphorylation status and cytosolic localization of Bad following GM-CSF or TNF-α stimulation in neutrophils, we used a hypotonic lysis buffer containing a low percentage of detergent to differentiate cytosolic from membrane-bound Bad and performed Western blot analysis using anti-Bad and selective anti–phospho-Bad antibodies. Figure 6A shows representative immunoblots demonstrating the ability of GM-CSF to cause a marked increase in the amount of Bad protein present in neutrophil cytosolic fractions and a corresponding decrease in membrane-associated Bad at 30 minutes.
GM-CSF increases the cytosolic accumulation and phosphorylation of Bad in neutrophils.
Human neutrophils were incubated for 30 minutes in the presence or absence of GM-CSF (10 ng/mL) with or without LY294002 (10 μM). Neutrophil lysates were prepared, and total Bad and phospho Ser-112 and Ser-136 Bad were quantified by Western blotting of cytosolic and membrane fractions, as detailed in the “Materials and methods” section. Cytosolic immunoprecipitates and blots were performed using cell lysates prepared with hypotonic lysis buffer containing 0.05% NP-40 and centrifuged at 22 000g (20 minutes, 4°C); detergent was omitted from the initial lysis buffer used to prepare the membrane fractions that were subsequently pelleted at 104 000g (30 minutes, 4°C) and resuspended in buffer containing 0.1% Triton-X100. (A) Representative immunoblots (with corresponding densitometry) demonstrating that GM-CSF increased the amount of total immunoreactive Bad within the cytosolic compartment of neutrophils and caused a reciprocal loss in membrane associated Bad. Both effects were inhibited by LY294002. (B, C) Phosphorylation status of cytosolic Bad was assessed using phosphospecific antibodies to Ser112 and Ser136. Representative immunoblots and densitometry analyses indicate that GM-CSF substantially increased the phosphorylation of cytosolic Bad at Ser112 and Ser136 through a PI3-kinase–dependent mechanism. Identical data were obtained in 3 further independent experiments.
GM-CSF increases the cytosolic accumulation and phosphorylation of Bad in neutrophils.
Human neutrophils were incubated for 30 minutes in the presence or absence of GM-CSF (10 ng/mL) with or without LY294002 (10 μM). Neutrophil lysates were prepared, and total Bad and phospho Ser-112 and Ser-136 Bad were quantified by Western blotting of cytosolic and membrane fractions, as detailed in the “Materials and methods” section. Cytosolic immunoprecipitates and blots were performed using cell lysates prepared with hypotonic lysis buffer containing 0.05% NP-40 and centrifuged at 22 000g (20 minutes, 4°C); detergent was omitted from the initial lysis buffer used to prepare the membrane fractions that were subsequently pelleted at 104 000g (30 minutes, 4°C) and resuspended in buffer containing 0.1% Triton-X100. (A) Representative immunoblots (with corresponding densitometry) demonstrating that GM-CSF increased the amount of total immunoreactive Bad within the cytosolic compartment of neutrophils and caused a reciprocal loss in membrane associated Bad. Both effects were inhibited by LY294002. (B, C) Phosphorylation status of cytosolic Bad was assessed using phosphospecific antibodies to Ser112 and Ser136. Representative immunoblots and densitometry analyses indicate that GM-CSF substantially increased the phosphorylation of cytosolic Bad at Ser112 and Ser136 through a PI3-kinase–dependent mechanism. Identical data were obtained in 3 further independent experiments.
Moreover, though LY294002 had no effect on the levels of cytosolic or membrane-bound Bad under control conditions, it completely abolished the GM-CSF–stimulated changes in Bad distribution (Figure 6). These data suggest that under control conditions, Bad is largely sequestered to a membrane compartment, possibly in association with Bcl-XL bound to the outer mitochondria membrane. Although this conclusion is supported by a recent report indicating that GM-CSF does not alter Bcl-XL expression in neutrophils,44 we and others45 46 have been unable to identify Bcl-XL protein in neutrophils (data not shown). Because GM-CSF–induced Bad phosphorylation has been speculated to release Bad from its mitochondria-bound partner, the phosphorylation status of Bad was determined using phosphospecific antibodies directed against phosphorylated Bad at Ser112 and Ser136. Figure 6B and C show representative anti–phospho-Bad immunoblots from cytosolic extracts prepared from cells incubated with GM-CSF for 30 minutes. Following GM-CSF stimulation, a marked increase in the phosphorylation status of Bad at Ser112 and Ser136 was observed. As shown in Figure 6B and C, LY294002 significantly attenuated the GM-CSF–stimulated phosphorylation of Bad at Ser112 and Ser136, supporting the view that PI3-kinase–dependent dual phosphorylation of Bad underlies the release of Bad from its membrane-bound partner.
Regulation of Bax, Bad, and Bcl-XL transcription in human neutrophils
In addition to examining the effects of GM-CSF on Bad phosphorylation, we determined the effects of prolonged cytokine stimulation on Bax, Bad, and Bcl-XL mRNA levels. We confirmed previous reports identifying Bad and Bcl-XL mRNA in freshly isolated human neutrophils and the ability of GM-CSF (10 ng/mL) to reduce Bax mRNA levels (data not shown). The PCR primers used for Bcl-X bind to sequences shared by Bcl-XL and Bcl-XS, thus allowing simultaneous identification of both Bcl-X mRNA isoforms. Of note, we were unable to identify mRNA for the Bcl-XS isoform in freshly isolated neutrophils (data not shown).
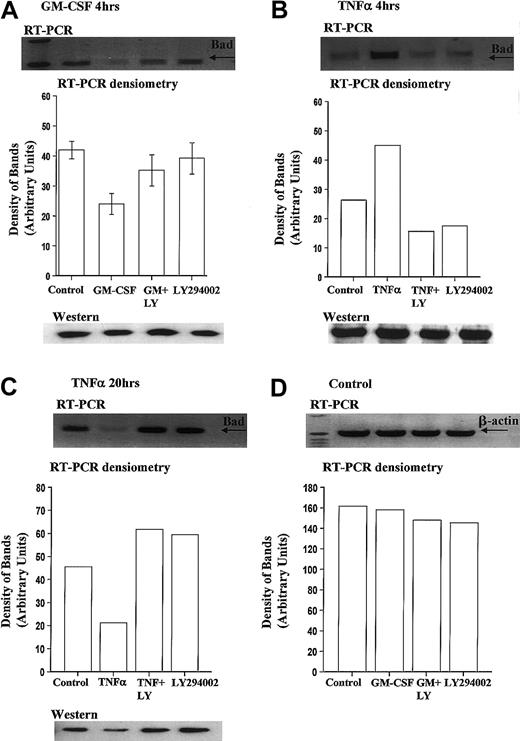
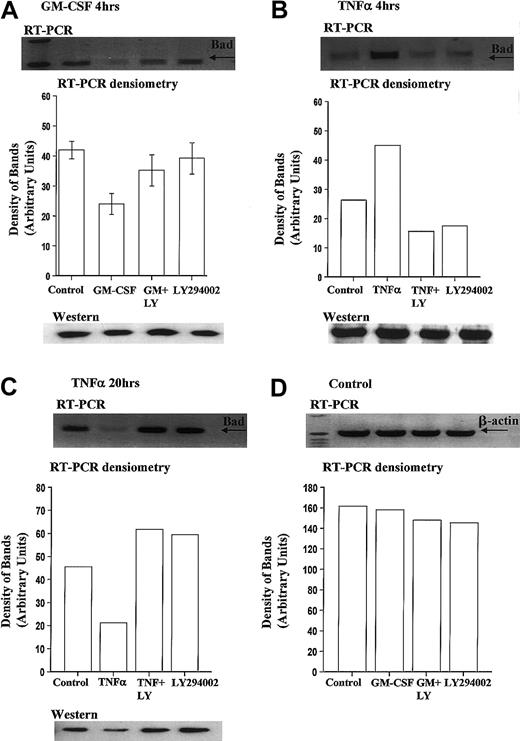
We report for the first time that levels of Bad mRNA decrease following GM-CSF stimulation. Densitometry analysis of 6 independent experiments indicated that GM-CSF reduced Bad mRNA expression by 43.8% ± 5.3% at 4 hours, compared with time-matched controls (Figure7A).
GM-CSF significantly reduces Bad transcription in human neutrophils.
Human neutrophils were incubated in the presence or absence of GM-CSF (10 ng/mL) or TNF-α (200 U/mL) with or without LY294002 (10 μM). Total RNA was isolated using TRIzol, and RT-PCR was carried out as described in the “Materials and methods” section. (A) Neutrophils incubated for 4 hours with GM-CSF showed a PI3-kinase–dependent decrease in the amount of Bad mRNA. Incubation with TNF-α led to a PI3-kinase–dependent increase in Bad mRNA at 4 hours (B) and a decrease in Bad mRNA at 20 hours (C). (D) Equal amplification of β-actin transcripts confirmed identical total RNA content of the samples at 4 hours (20-hour data not shown for clarity). Single representative immunoblots and corresponding densitometric analysis are shown. Corresponding immunoblots demonstrate the amount of total Bad present in neutrophil whole-cell lysates at these times. The data in panel A represent the mean ± SEM of 6 independent experiments.
GM-CSF significantly reduces Bad transcription in human neutrophils.
Human neutrophils were incubated in the presence or absence of GM-CSF (10 ng/mL) or TNF-α (200 U/mL) with or without LY294002 (10 μM). Total RNA was isolated using TRIzol, and RT-PCR was carried out as described in the “Materials and methods” section. (A) Neutrophils incubated for 4 hours with GM-CSF showed a PI3-kinase–dependent decrease in the amount of Bad mRNA. Incubation with TNF-α led to a PI3-kinase–dependent increase in Bad mRNA at 4 hours (B) and a decrease in Bad mRNA at 20 hours (C). (D) Equal amplification of β-actin transcripts confirmed identical total RNA content of the samples at 4 hours (20-hour data not shown for clarity). Single representative immunoblots and corresponding densitometric analysis are shown. Corresponding immunoblots demonstrate the amount of total Bad present in neutrophil whole-cell lysates at these times. The data in panel A represent the mean ± SEM of 6 independent experiments.
In contrast, TNF-α, which induces an early increase and a late inhibition of neutrophil apoptosis (Figure 2E), increased Bad mRNA expression at 4 hours (Figure 7B) and decreased Bad mRNA expression at 20 hours (Figure 7C). Of note, the expression of Bcl-XL did not alter following incubation with GM-CSF (4 hours) or TNF-α (4 and 20 hours) (data not shown). Corresponding Western blot analysis of Bad expression at these times indicated that only prolonged incubation (20 hours) with TNF-α (Figure 7C) or GM-CSF (data not shown) was associated with a reduction in Bad expression.
To determine whether the PI3-kinase pathway was involved in the differential regulation of Bad mRNA expression, neutrophils were pretreated with LY294002 before incubation with GM-CSF or TNF-α. All cytokine modulations of Bad transcription were inhibited by LY294002.
Discussion
The resolution of granulocyte inflammation is controlled by a number of factors, including the neutralization or removal of the inciting stimulus, cessation of neutrophil influx, and active removal of effete cells by the process of programmed cell death. Many of the inflammatory cytokines involved in initiating the primary inflammatory response also have major effects on the rate of which the cells involved in this response undergo apoptosis. Elucidation of the cellular mechanisms underlying such effects has important implications for understanding whether inflammation resolves or persists. This study has investigated the signal transduction pathways used by GM-CSF and TNF-α to regulate the rate of neutrophil apoptosis and the expression of the Bcl-2 family members Bcl-XL/S, Bad, and Bax. We have identified a potential dual-phase effect of GM-CSF in regulating the proapoptotic tendencies of Bad, and we report for the first time a PI3-kinase–dependent decrease in the rate of Bad transcription following GM-CSF stimulation. We have confirmed previous reports in neutrophils of a rapid PI3-kinase–dependent phosphorylation of Bad at Ser136, following GM-CSF or TNF-α stimulation of human neutrophils,6 and have identified a similar effect with regard to the phosphorylation of Bad at Ser112. These data suggest that GM-CSF–mediated phosphorylation of Bad may play a pivotal role in regulating granulocyte longevity.
Our initial observation that GM-CSF stimulation of unprimed neutrophils caused a significant increase in the cellular accumulation of PtdIns(3,4,5)P3 highlights the fact that GM-CSF has the capability to stimulate PI3-kinase activity. Al-Shami et al47 have shown that GM-CSF activates the JAK/STAT pathway, more specifically JAK2, STAT3, and STAT5B,48,49and they have demonstrated that GM-CSF stimulates PI3-kinase in a tyrosine kinase- and a JAK2-dependent, but a Lyn-independent, manner.47,50 However, the role of Lyn remains controversial. Lyn activation can induce neutrophil survival through stimulation of the ERK1/2 pathway,51 and antisense depletion of Lyn kinase inhibits GM-CSF–induced neutrophil survival.5
Contrary to previous reports, inclusion of the ERK1/2 inhibitor PD98059 caused only a marginal attenuation of the survival effect of either GM-CSF or TNF-α at 20 hours, which over even a large number of experiments failed to reach statistical significance (P = .07, n = 6, morphologic analysis, Table 2;P = .055, n = 7, Annexin-V analysis, Figure 5). The reason for this discrepancy is uncertain. In contrast, the PI3-kinase inhibitor LY294002 fully blocked the survival effect of GM-CSF and TNF-α. It should be noted that the effects of TNF-α on neutrophil survival are distinct from those of GM-CSF. TNF-α has the unique ability to enhance the initial rate of apoptosis (0-6 hours) and thereafter to induce a modest late cytoprotective effect.12 Analysis of the PI content of neutrophils following prolonged TNF-α stimulation (20 hours) identified a higher proportion of PI+ cells within the AnV+(apoptotic) pool, suggesting that such cells have undergone secondary necrosis. With phagocyte clearance of cells not possible in our assay, the above finding most likely reflects the natural fate of cells that undergo apoptosis at early times in response to TNF-α stimulation. Taking into account the early killing effect of TNF-α, AnV/PI analysis confirmed the PI3-kinase–dependent nature of the 20-hour survival effect of TNF-α.
The PI3-kinase/PKB signaling pathway has been implicated in cell survival in a wide range of cells. However, most of these studies, in particular those proposing Bad as a downstream target, have used manipulated cell lines, overexpression models, or artificial protein deletion strategies. We have used primary nontransformed cells to confirm the ability of survival cytokines to activate PI3-kinase and subsequently to enhance Bad phosphorylation at Ser136. Of interest, we also show PI3-kinase–dependent phosphorylation of Bad at Ser112. Previous studies using PKB overexpression systems report Bad phosphorylation of Ser136 only and report that coexpression and activation of ERK1/2 is required to phosphorylate Ser112. Phosphorylation of Ser112 has been suggested not to correlate with PKB activation; therefore, an additional downstream target of PI3-kinase may contribute to Bad phosphorylation at Ser112.
We have also identified the ability of GM-CSF to reduce Bad mRNA levels through a PI3-kinase–dependent mechanism; this could be explained by decreased transcription or increased transcript instability. In contrast, TNF-α increased the amount of Bad mRNA in neutrophils at the early time point (4 hours) and decreased the levels at later time points (20 hours). It should be noted that changes in protein expression were only observed at the later time points (20 hours). We have also confirmed reports of GM-CSF–mediated depletion of Bax mRNA in neutrophils.19 Although the significance of GM-CSF–mediated depletion of Bad mRNA remains to be determined, a plausible hypothesis would incorporate the ability of GM-CSF to initially stimulate the phosphorylation of Bad through the PI3-kinase–dependent pathway, followed by a secondary regulation of Bad transcription.
Bad, though showing no intrinsic or independent cell destructive properties, is believed to promote apoptosis through heterodimerization with antiapoptotic bcl-2 family proteins. We have identified in human neutrophils the ability of GM-CSF and TNF-α to stimulate phospho-dependent movement of Bad from a membrane to a cytosolic fraction. Phosphorylation of Bad has been shown to affect its subcellular distribution, from a Bcl-XL bound mitochondrial membrane associated form to a cytosolic location reflecting phospho-dependent association with 14-3-3.29,31,32However, the true binding partner for Bad in neutrophils remains to be determined. In cells overexpressing Bad, IL-3 promotes PKA-dependent Bad phosphorylation at Ser112 and Ser155, but this has yet to be confirmed in neutrophils. Although we have previously shown that a receptor-mediated or pharmacologic increase in cAMP levels in neutrophils delays apoptosis,43 the PKA inhibitor H-89 only resulted in a marginal attenuation of GM-CSF–stimulated neutrophil survival. This observation concurs with the findings of a recent paper demonstrating that cAMP-mediated neutrophil survival occurs through a PKA-independent mechanism.52 Moreover, GM-CSF has been shown to decrease rather than increase cAMP levels in neutrophils.53 Such data provide further support for our proposal that any PKA-dependent phosphorylation of Bad that may occur is not critical for the cytosolic location of Bad in GM-CSF-stimulated neutrophils.
Although the expression of other Bcl-2–like proteins in neutrophils, including Mcl-1, A1, Bcl-W, Bid, Bim, and Bax and their involvement in regulating neutrophil apoptosis, has been investigated by a number of groups, several important issues remain unsolved. In particular, the expression and role of Bcl-2 and Bcl-XL in granulocyte apoptosis remains controversial. Bcl-2 protein expression has been reported by Van Der Vliet et al54 in relatively pure populations of mature neutrophils. Most groups have been unable to identify Bcl-2 in neutrophils at either a protein or an mRNA level.10,44 The extent of Bcl-X expression is also uncertain with several groups, including our own, reporting little or no expression of Bcl-X in mature neutrophils.46 In contrast, Weinmann et al44 detected Bcl-X in neutrophils by reverse transcription–PCR (RT-PCR) and Western blot analysis; Bcl-X expression diminished slowly over 22 hours and was proposed as a potential mechanism driving the natural onset of constitutive apoptosis in vitro. In the present study, the PCR primers for Bcl-X were designed to bind sequences shared by Bcl-XL and Bcl-XSand clearly demonstrated the presence of Bcl-XL in freshly isolated neutrophils. Moreover, cytokine stimulation (4 hours) or PI3-kinase inhibition did not alter the expression of either Bcl-X isoforms, suggesting regulation through a PI3-kinase–independent mechanism.
Bax mRNA levels in neutrophils have also been reported to decline when these cells are aged in vitro.44 We have shown that the rate of Bax mRNA degradation appears to be enhanced following GM-CSF stimulation, which may further contribute to neutrophil survival. In contrast, the ability of TNF-α to increase Bax mRNA levels at the early times (4 hours) but thereafter decrease Bax expression may underlie the bimodal early killing and late survival effect observed with this cytokine.
In summary we have shown that GM-CSF– and TNF-α–mediated survival of human neutrophils is associated with a PI3-kinase–dependent phosphorylation of Bad at Ser112 and Ser136. The ERK1/2 and PKA signaling pathways appear to play lesser roles in this process. The phosphorylation of Bad is associated with its redistribution to the cytosolic compartment and a major inhibition of Bad mRNA levels. The discrete early proapoptotic effect of TNF-α is associated with a corresponding increase in Bad and Bax mRNA expression. These data, together with the evidence that GM-CSF causes a decrease in Bax expression and the maintenance of Bcl-XL expression, suggests that the PI3-kinase pathway plays a key role in regulating the expression and function of Bad and other Bcl-2 family members in neutrophils and may contribute to the inhibition of neutrophil apoptosis by inflammatory cytokines.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2001-11-0122.
Supported by Wellcome Trust, British Lung Foundation, Biotechnology and Biological Sciences Research Council (BBSRC), and Papworth Hospital National Health Service (NHS) Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Edwin R. Chilvers, Respiratory Medicine Division, Department of Medicine, Level 5, Box 157, Addenbrooke's Hospital, Hills Road, Cambridge, CB2 2QQ, United Kingdom; e-mail:erc24@cam.ac.uk.