FLT3 is a receptor tyrosine kinase expressed by immature hematopoietic cells and is important for the normal development of stem cells and the immune system. The ligand for FLT3 is expressed by marrow stromal cells and other cells and synergizes with other growth factors to stimulate proliferation of stem cells, progenitor cells, dendritic cells, and natural killer cells. Mutations of FLT3 have been detected in about 30% of patients with acute myelogenous leukemia and a small number of patients with acute lymphocytic leukemia or myelodysplastic syndrome. Patients with FLT3 mutations tend to have a poor prognosis. The mutations most often involve small tandem duplications of amino acids within the juxtamembrane domain of the receptor and result in constitutive tyrosine kinase activity. Expression of a mutant FLT3 receptor in murine marrow cells results in a lethal myeloproliferative syndrome and preliminary studies suggest that mutant FLT3 cooperates with other leukemia oncogenes to confer a more aggressive phenotype. Taken together, these results suggest that FLT3 is an attractive therapeutic target for kinase inhibitors or other approaches for patients with mutations of this gene.
FLT3 is a member of the class III receptor tyrosine kinase family
FLT3 (Fms-like tyrosine kinase 3), also known as FLK-2 (fetal liver kinase-2) and STK-1 (human stem cell kinase-11), was cloned independently by 2 groups in 1991.2-4FLT3 has strong sequence similarities with other members of the class III receptor tyrosine kinase (RTKIII) receptor family. A subset of RTKIII family members that includes FLT3, FMS, platelet-derived growth factor receptor (PDGFR), and KIT are characterized by an extracellular domain comprised of 5 immunoglobulinlike (Ig-like) domains and by a cytoplasmic domain with a split tyrosine kinase motif.5 6
The FLT3 gene encodes a 1000– and 993–amino acid protein in the mouse and human, respectively,3,7 and is expressed in immature hematopoietic cells, placenta, gonads, and brain.8,9 Immunoprecipitation studies of FLT3 expressed in COS-7, and in other cell types, demonstrate a major band at about 140 kDa, and a less abundant, more diffuse band of about 160 kDa. Pulse-chase experiments demonstrate that the larger band is derived from the smaller band by posttranslational N-linked glycosylation and is localized to the plasma membrane.9 10
FLT3 expression and function in normal hematopoietic cells
FLT3 is expressed in a variety of human and murine cell lines of both myeloid and B-lymphoid lineage.11,12 In normal bone marrow, expression appears to be restricted to early progenitors, including CD34+ cells with high levels of expression of CD117 (c-KIT).13,14 FLT3 is also expressed at high levels in a spectrum of hematologic malignancies including 70% to 100% of acute myelogenous leukemia (AML) of all French-American-British (FAB) subtypes, B-precursor cell acute lymphoblastic leukemia (ALL), a fraction of T-cell ALL, and chronic myelogenous leukemia (CML) in lymphoid blast crisis.13 15
Targeted disruption of FLT3 results in healthy adult mice with normal mature hematopoietic populations.16 However, there are deficiencies in primitive B-lymphoid progenitors, and bone marrow transplantation (BMT) experiments show a reduced ability of stem cells lacking FLT3 to reconstitute both T cells and myeloid cells.16 These data demonstrate an important role for FLT3 in development of multipotent stem cells and B cells.
FLT3 ligand
The ligand for FLT3 (FLT3 ligand or FL) was cloned in 1993.17 FL is a type I transmembrane (TM) protein that can be released as a soluble homodimeric protein,18-20 and is expressed in cells of the hematopoietic bone marrow microenvironment, including bone marrow fibroblasts,21 as well as in hematopoietic cell lines of myeloid, and B- and T-cell lineages.11 Both the membrane-bound and soluble forms can activate the tyrosine kinase activity of the receptor and stimulate growth of progenitor cells in the marrow and blood. However, like Steel factor, the ligand for c-KIT, FL does not efficiently induce proliferation of normal myeloid and lymphoid progenitors by itself, but strongly synergizes with other hematopoietic growth factors and interleukins.22-26 FL is a growth factor for immature myeloid cells and stem cells and can expand CD34+ cells in vitro and in vivo.14 27-43 The ability of FL, in combination with other growth factors, to stimulate expansion of CD34+ hematopoietic progenitors may be useful in marrow recovery after cytotoxic chemotherapy and in expansions of hematopoietic progenitors for various clinical applications.
The FLT3 ligand also plays an important role in the immune response. Targeted disruption of the FL gene in mice is associated with significant impairment of the immune system, as well as a reduction in myeloid progenitor cells. The number of B-cell progenitors, dendritic cells (DCs), and natural killer (NK) cells is significantly reduced in vivo.44 The basis for the apparent disparity in the hematopoietic phenotype between the FL and FLT3 knockouts is not known, but may be due to experimental approach, strain differences, or second ligand or receptors in this system. DCs are the most efficient antigen-presenting cells for T cells and have been investigated as cellular immunotherapeutic agents in cancer. Daily injection of FL into mice results in a dramatic increase in cells expressing the characteristic DC markers such as class II major histocompatibility complex (MHC), CD11c and CD86. This effect is not observed with either colony-stimulating factor 1 (CSF-1) or Steel factor. FL synergizes with granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin 4 (IL-4) in induction of DC differentiation in vitro and in vivo.45 It has also been demonstrated that FL also promotes the expansion of a CD34+ NK cell progenitor that responds to IL-15 through induction of IL-2/15Rβ expression, indicating a role for FL in early NK cell development.46
The FLT3 ligand stimulation of proliferation of DCs and NK cells has suggested that FL might potentially be useful as a broad-spectrum antitumor agent.47-50 In support of this hypothesis, FL has been reported to induce tumor regression and antitumor response in vivo in a methyl cholanthrene–induced fibrosarcoma model in mice.51 In another model system, 80% of mice receiving 500 μg/kg/d human FL for 10 days were transiently protected from tumors caused by C3L5 breast cancer cells, and transduction of the C3L5 cells with FL also provided protection.52 FL also inhibits tumor growth in murine melanoma (B16 and CL8-1) and lymphoma (EL-4) models.53 In addition, FL has antileukemia activity in a murine model of leukemia induced by FDCP1 cells transfected with BCR/ABL and injected into syngeneic hosts.54
In acute leukemia, however, administration of FL may be disadvantageous, because abundant data indicate that FL stimulation of FLT3 enhances the proliferation and survival of leukemia blasts.55 FL stimulates the proliferation of FLT3-expressing primary AML cells and myeloid and monocytoid leukemic cell lines, and synergizes with other growth factors to stimulate proliferation of primary AML and ALL cells.15,56-60 A majority of myeloid leukemia cells lines show expression of either FL or FLT3, or in some cases both. Furthermore, FL has significant antiapoptotic and survival-promoting effects on both primary AML cells and on myeloid cell lines in serum-free conditions.61 FL may exert its proliferative and antiapoptotic signal in leukemia cells in part through regulation of BCL2 and BAX.62
Taken together, these data suggest that inhibition or augmentation of the FL-FLT3 ligand receptor system in therapy of leukemia will need to be undertaken with caution and circumspection. Administration of FL may enhance antitumor immunity, but may also enhance proliferation and survival of leukemia blasts. In addition, chronic expression of FL in a murine retroviral transduction model induced leukocytosis, severe anemia, marked splenomegaly, and fibrosis, in addition to expansion of DCs and activated T lymphocytes.63 As indicated below, FLT3 inhibition may also be a valid therapeutic approach for AML, but inhibition of FLT3 may also impair endogenous antileukemia immunity.
FLT3 signal transduction
Initial analysis of the signal transduction properties of FLT3 was performed prior to the cloning of FL, using a chimeric receptor composed of the extracellular domain of human FMS and the transmembrane and cytoplasmic domains of FLT3. Stimulation of chimeric receptor with macrophage colony-stimulating factor (M-CSF) transformed NIH3T3 fibroblasts, and conferred IL-3–independent growth to the murine hematopoietic cell line Ba/F3.64 Phospholipase C-γ 1 (PLCγ), Ras guanosine triphosphatase (GTPase)–activating protein, the p85 subunit of phosphatidylinositol 3′-kinase (PI3K), SHC, GRB2, VAV, FYN, and SRC pathways were all activated by the stimulated chimeric receptor. Of these, PLCγ, the p85 subunit of PI3K, SHC, GRB2, and the SRC family kinases directly associated with the FLT3 cytoplasmic domain.64
These findings with chimeric receptors have been for the most part confirmed65,66 using FL stimulation of the native receptor after the cloning of the murine and human FL in 1993 and 1994, respectively.17,67 FL stimulation of the native receptor also results in tyrosine phosphorylation of SHC, SHP-2, and SHIP, which are associated with GRB2 and SHC in Ba/F3 cells stably transfected with FLT3.66,68 p85 had been reported to associate with FLT3 residue 958 in the chimeric receptors.69-71 However, subsequent analysis indicates that the p85 subunit of PI3K does not bind to FLT3, but rather associates with 100-kDa and 120-kDa proteins, respectively, in a complex with SHP2 and SHIP in Ba/F3 cells stably transfected with FLT3.65 In retrospect, the 100-kDa and 120-kDa proteins represent GAB2 and CBL, respectively. Stimulation of the native receptor with FL has been reported to result in phosphorylation of CBL and complex formation between CBL and the p85 subunit of PI3K,72 and it has subsequently been reported that FL induces tyrosine phosphorylation of GAB1 and GAB2 and their association with SHP2, GRB2, and PI3K.73
FL stimulation of FLT3 also results in activation of Stat5a in Ba/F3 cells, but not Stat 1 through 4, 5b, or 6. FL does not activate any JAK family members, indicating that FLT3 activates Stat5a through a JAK-independent mechanism. Furthermore, FL could not induce proliferation of hematopoietic progenitors in Stat5a−/−cells, but could both stimulate and costimulate proliferation of Stat5a+/+, Stat5b−/−, or Stat5b+/+ hematopoietic cells. These data indicate distinctive functions for Stat5a and Stat5b, and that Stat5a is required for at least some of the proliferative effects of FL stimulation of FLT3.74
The role of FLT3 in human leukemias
FLT3 expression in human leukemias
FLT3 is expressed at high levels in 70% to 100% of cases of AML and in a high percentage of ALL cases.75-78 For example, Rosnet and colleagues reported expression of FLT3 on leukemia blasts in 18 of 22 AML and 3 of 5 ALL cases.13 Carow and coworkers found FLT3 RNA expressed at higher levels than in normal bone marrow in 33 of 33 B-lineage ALL, 11 of 12 AML, and 3 of 11 T-cell ALL cases. Western blotting did not detect FLT3 expression in normal bone marrow, but identified FLT3 protein in 14 of 14 B-cell ALL, 36 of 41 AML, and 1 of 4 T-cell ALL cases.77 These data indicate that FLT3 expression may play a role in the survival or proliferation of leukemic blasts. In support of this hypothesis, as noted above, FL induced dose-dependent proliferation of leukemic blasts in 36 of 45 patients with AML and was synergistic when used in combination with G-CSF, GM-CSF, IL-3, or Steel factor.56 FLT3 is also expressed at high levels in leukemia and lymphoma cell lines,79,80including pre-B, myeloid, and monocytic cell lines, and FL expression can be detected in a majority of cell lines. In one series, 40 of 110 cell lines examined expressed both FL and FLT3, suggesting that autocrine stimulation may also play a role in proliferation of leukemia blasts.80
FLT3 mutations in human leukemias
FLT3 internal tandem duplications.
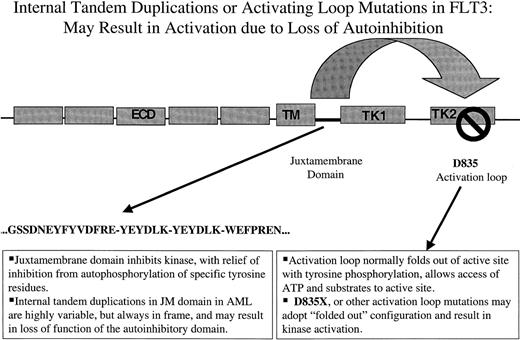
Nakao and colleagues first reported the presence of internal tandem duplications (ITDs) in the juxtamembrane (JM) domain of FLT3 in AML in 1996.81 They noted that 17% (5 of 30) of patients with AML, there were length polymorphisms in the JM domain. Sequence analysis of gDNA demonstrated that each of the 5 patients harbored in-frame ITD mutations in the JM domain. Each patient had a normal residual FLT3 allele, and each of the duplication mutants were predicted to produce mutant FLT3 protein (Figure1). Nakao et al suggested that these mutations might play an important role in pathogenesis of AML.81
Two types of activating mutations in FLT3 are associated with AML.
The first type consists of ITDs of amino acids in the JM domain. These are variable in length from patient to patient, but are always in-frame. These repeat sequences may serve to disrupt autoinhibitory activity of the JM domain resulting in constitutive tyrosine kinase activation. The second type of mutation are point mutations in the so-called activation loop of the second tyrosine kinase domain. Mutation at a specific aspartic acid residue, Asp835, which is highly conserved among tyrosine kinases, also results in constitutive FLT3 activation. In the context of other tyrosine kinases, activation loops are thought also to exert autoinhibitory function by limited access of ATP and substrate to the catalytic domain. Mutations at this site are thought to alter the configuration of the activation loop in a manner similar to that of ligand-induced conformational changes, resulting in increased access of ATP and substrate. ECD indicates extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain; TK, tyrosine kinase domain; KI, kinase insert.
Two types of activating mutations in FLT3 are associated with AML.
The first type consists of ITDs of amino acids in the JM domain. These are variable in length from patient to patient, but are always in-frame. These repeat sequences may serve to disrupt autoinhibitory activity of the JM domain resulting in constitutive tyrosine kinase activation. The second type of mutation are point mutations in the so-called activation loop of the second tyrosine kinase domain. Mutation at a specific aspartic acid residue, Asp835, which is highly conserved among tyrosine kinases, also results in constitutive FLT3 activation. In the context of other tyrosine kinases, activation loops are thought also to exert autoinhibitory function by limited access of ATP and substrate to the catalytic domain. Mutations at this site are thought to alter the configuration of the activation loop in a manner similar to that of ligand-induced conformational changes, resulting in increased access of ATP and substrate. ECD indicates extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain; TK, tyrosine kinase domain; KI, kinase insert.
These observations have been subsequently confirmed by many groups82-89 (Table 1). Combining data from all studies reported to date, the overall frequency of FLT3-ITD in adult AML is 385 of 1595 (24%) of patients (combined data from Table 1 and Table 2). FLT3-ITDs have also been detected at lower frequency in myelodysplastic syndrome (MDS82,84,90,91; Table 1), but are rarely detected in ALL.81,84,87,90 The frequency of FLT3-ITD in pediatric AML appears to be somewhat lower than in adults with AML, occurring in about 10% to 15% of pediatric patients.87-89,92,93 In addition, the frequency of FLT3-ITD appears to be higher in elderly patients with AML.86 FLT3-ITDs have been detected in all FAB subtypes of AML, with the highest reported frequency in the M3 subtype, and less frequently in the M2 subtype.81,83,84,88,92,94,95 FLT3-ITDs have also been reported at a frequency of 15% in secondary AML82 and may be associated with disease progression or relapse of AML.82,96 FLT3-ITDs have not been detected in limited numbers of patients with CML,84 CML blast crisis,85 juvenile myelomonocytic leukemia (JMML),87 non-Hodgkin lymphoma, adult T-cell ALL, chronic lymphocytic leukemia, or multiple myeloma84 (Table 1). Recent data presented in abstract form indicate that FLT3-ITD mutations are not present in systemic mast cell disease97 nor in a spectrum of solid tumors.98 FLT3-ITDs have not been detected in normal hematopoietic cells, including cord blood and bone marrow cells in which there are high levels of expression of FLT3.99 In addition to length mutations in one allele of FLT3, several studies have demonstrated bialleleic mutations in FLT3,100 as well as patients in whom the residual wild-type allele is lost.100 101 These data suggest that the wild-type allele may interfere with the activity of the FLT3-ITD mutants, and the possible functional implications of these findings are discussed below.
FLT3 “activation loop” mutations in human leukemias.
It has recently been reported that an additional cohort of AML patients contains mutations in the so-called activation loop of FLT3 (Figure 1). The activation loop is a general component of tyrosine kinases and when the kinase is in the “inactive” state, it functions to block access of adenosine triphosphate (ATP) and substrate to the kinase domain. After activation, which would normally be through ligand binding in the case of RTKIII family members, a specific tyrosine residue within the loop is typically phosphorylated, causing the loop to adopt an “activated” configuration allowing access to the kinase. Activation loop mutations have been reported at position Asp816 of c-KIT and at the corresponding position in other RTKs, including MET and RET. The analogous aspartic acid residue in FLT3 is at amino acid position 835. Asp835 substitutions have been reported in 30 of 429 (7%) AML, in 1 of 29 (3%) MDS, and 1 of 36 (3%) of ALL patients.90,102 A similar frequency was reported in 7 of 35 AML patients (7%).103 In both series, Asp825Tyr was the most common substitution, but other substitutions included Asp835Val, Asp835His, Asp835Glu, and Asp835Asn.
Taken together these data indicate that approximately 30% of AML patients have acquired mutations in FLT3 comprised of either FLT3-ITD mutations (24%) or FLT3 activation loop mutations (7%). FLT3 is thus the single most commonly mutated gene in AML.
General mechanisms of RTK activation: relevance to FLT3 mutations in human leukemia
The FLT3 JM length polymorphisms and activation loop substitutions in FLT3 are frequent in AML, surpassing the incidence of any known chromosomal translocations or point mutations. What are the consequences of these mutations on the function of FLT3 and pathophysiology of AML? Although the mechanism of activation of FLT3 has not been extensively studied, insights into the role of these mutations can be gleaned from analysis of other nonreceptor TK and receptor TK, including the Eph,104-107ERBB2,108,109 insulinlike growth factor 1 receptor (IGF-1R),110 fibroblast growth factor receptor 1 (FGFR1),111,112 and epidermal growth factor (EGF) receptors.113
The RTKs have autoinhibitory domains that maintain the kinase in an inactive conformation. In most tyrosine kinases, a so-called activation loop in the kinase domain folds into the active site, blocking access of ATP and substrates. Phosphorylation or substitution mutation of critical residues in the activation loop allows the loop to fold out of the active site, allowing access. For example, intermolecular autophosphorylation of 3 specified residues in the activation loop of IGF-1R stabilizes the loop in a conformation that facilitates catalysis.110 In addition, substitution mutations at a critical conserved aspartic acid residue in the activation loop of the insulin receptor results in relief of autoinhibition and increases the ability of the unphosphorylated kinase to bind ATP.114 The FGFR1 structure indicates that residues in the activation loop appear to interfere with substrate peptide binding but not with ATP, suggesting a second and perhaps more general autoinhibitory mechanism for RTKs.111
Another autoinhibitory domain is the JM domain of a subset of RTKs, such as the Eph receptor.105 This group of RTKs has conserved JM domains, and includes FLT3 and other members of the type III RTK family. Biochemical data had indicated that, in addition to the autoinhibitory activity of the activation loop of Eph, there was a second autoinhibitory domain in the JM domain of Eph that was regulated by tyrosine phosphorylation.105 Structural analysis of an autoinhibited unphosphorylated form of EphB2 comprised of the JM and kinase domains has provided insight into the mechanisms of autoinhibition.104 The JM domain adopts a helical conformation that distorts the small lobe of the kinase domains and blocks the activation segment from attaining an activated conformation. Phosphorylation of conserved JM tyrosine residues relieve this repression by disturbing the association of the JM segment with the kinase domain and liberates phosphotyrosine sites for binding SH2 domains of target proteins.
Repression of autoinhibitory domains is achieved in part by binding of RTKs to their cognate ligands, such as FL. Many RTK ligands, including FL, exist as homodimers, and are thought to activate their respective target RTKs through induction of dimerization or oligomerization.113 In addition, activating point mutations have been identified in the extracellular and TM domains of RTKs that may serve to dimerize or induce conformational changes. Ligand may also serve to induce conformational changes that result in relief of autoinhibition, in that dimerization is necessary but not sufficient to activate RTK receptors. For example, substitution of the TM of the HER2/NEU RTK with the TM of glycophorin induces dimerization but not activation,108,109 and some receptors including Eph have intrinsic dimerization modules called SAM (sterile α motif) domains.106
To date, there are no published structural studies explaining how mutations within JM domains lead to constitutive receptor activation. However, based on the model from x-ray crystal structures of EphR2, it is quite possible that JM domain mutations in an RTK like FLT3 may cause the JM domain to fall away from the kinase domain, facilitating kinase activation, transphosphorylation, and initiation of signaling. This model is an attractive one to explain how such a diverse set of mutations within the JM domains of multiple RTKs all lead to kinase activation. Specifically, the mutations would each be predicted to interfere with the normal kinase-inhibitory function of the JM domain, leading to ligand-independent kinase activation.
Taken together, these data indicate that several classes of mutations could result in constitutive activation of the FLT3 kinase. In particular, the mutations in the FLT3 JM domain and activation loop might be predicted to result in loss of autoinhibitory function and subsequent constitutive activation of the FLT3 kinase and its downstream proliferative signals.
Biologic activity of FLT3-ITD and activation loop mutations
The available evidence indicates that either length mutations in the JM domain or activation loop mutations result in constitutive activation of the FLT3 kinase. Kiyoi et al reported in 1998 that the ITD mutations of FLT3 result in ligand-independent dimerization and tyrosine autophosphorylation when expressed in COS7 cells.115 Several groups have subsequently documented constitutive activation of FLT3-ITDs and activation of downstream targets known to be activated by the native FLT3 in response to FL, including the STAT5 and RAS/MAPK pathways.116,117 FLT3-ITD confers factor-independent growth to the murine hematopoietic cell lines 32D116,117and Ba/F3116 that are dependent on IL-3 for growth. In contrast, wild-type FLT3, even in the presence of FL, does not confer factor-independent growth to these cells. One group has reported that FLT3-ITD expression results in activation of STAT5 and RAS/MAPK pathways in stably transfected cell lines as well as in primary AML cells harboring FLT3-ITD mutations.116 Another group has reported strong activation of the STAT5 pathway in 32D cells stably transfected with FLT3-ITD mutants, but weak activation of the RAS/MAPK and the PI3K/AKT pathway that could be further stimulated by FL.117 Correlative data have also been obtained using a TEL-FLT3 construct that is constitutively activated and results in factor-independent growth of Ba/F3 cells and activation of the JAK/STAT pathway.118
Injection of 32D or Ba/F3 cells stably transfected with constitutively activated FLT3 into syngeneic recipient mice results in the development of a leukemia phenotype. Furthermore, retroviral transduction of FLT3-ITD mutations into primary murine bone marrow cells results in a myeloproliferative phenotype in a BMT assay.119 The BMT assay demonstrated that although FLT3-ITDs have been associated primarily with AML in humans, FLT3-ITD alone is not sufficient to induce AML in primary hematopoietic cells. Furthermore, point mutations that inactivated the FLT3 kinase in the context of the FLT3-ITD abrogates disease, indicating an absolute requirement of FLT3 kinase activity for development of myeloproliferative disease in this model. No difference in biologic activity of FLT3-ITD alleles has been ascertained in studies in cell culture or in the murine models of disease, despite considerable variation in repeat length that ranges from several to more than 50 amino acids.115-117 119
Similar data have been obtained demonstrating that the Asp835 substitution mutations in FLT3 result in constitutive kinase activation.90 120 Thus, both JM length repeat and activation loop mutations result in constitutive activation of the FLT3 kinase and downstream targets including STAT5, RAS/MAPK, and PI3K/AKT. Expression of FLT3 activating mutations in primary hematopoietic cells results in a myeloproliferative phenotype, and may indicate, as discussed below, that additional mutations are required for development of AML.
Prognostic significance of FLT3 mutations in leukemia
The majority of retrospective data indicate that FLT3 mutations are an independent variable that confer a poor prognosis in AML (Table2). In 3 pediatric series, a poor prognosis was reported.88,89 95 The largest of these studies reported on 91 pediatric patients treated in the Children's Cooperative Group (CCG). Fifteen of 91 (16.5%) had FLT3-ITD mutations. There was a 40% versus 73% remission induction rate in FLT3-ITD patients compared with non–FLT3-ITD patients (P = .005), and the 8-year event-free survival (EFS) was 7% versus 44%, respectively (P = .002).
Mutations in FLT3-ITD also confer a poor prognosis in adult AML. All studies have shown a worse prognosis in patients under the age of 60.47,94,100,101,121-123 In the largest series of 854 AML patients treated on the United Kingdom Medical Research Council AML 10 and 12 Trials, 231 of 854 (27%) of patients were FLT3-ITD+.100 FLT3-ITD correlated with higher leukocyte and blast counts, with decreased remission induction rates (P = .005), and decreased disease-free survival (DFS), EFS, and overall survival (OS; P < .001 for each).100 Similar results were reported in a smaller analysis of 23 of 82 AML patients with FLT3-ITD, with significance values for DFS and OS of .0017 and .0014, respectively.101Furthermore, in this study evidence supported an even worse prognosis for the 8 of 23 (35%) of FLT3-ITD patients with loss of the residual allele of FLT3 (P = .008). In elderly patients with AML, 2 studies have reported that FLT3-ITDs do not confer an appreciably worse prognosis. The largest study of 140 elderly patients with AML reported a higher frequency of FLT3-ITDs in this cohort (47 of 140 = 34%), but FLT3-ITDs did not confer a worse prognosis.86 In a smaller study stratified for age less than or more than 60 years, patients over age 60 with FLT3-ITDs had a trend toward worse prognosis (P = .03).124 It is plausible that because the prognosis overall is significantly worse in elderly AML patients, FLT3-ITD is not as important a prognostic indicator as in younger individuals with AML.
FLT3 selective inhibitors
There has been intense focus on development of FLT3 inhibitors because of the high frequency and poor prognosis of AML patients with mutant FLT3. This approach has been fueled in part by recent reports of activity of the ABL kinase inhibitor imatinib mesylate in CML and CML blast crisis. Proof of principle for this strategy has been reported using cell culture and murine models of leukemia mediated by FLT3-ITD.125-127 For example, AG1296, a tryphostin that is a selective inhibitor of FLT3, KIT, and PDGFβR, inhibits the growth of Ba/F3 transformed by FLT3-ITD.125 AG1296 inhibits FLT3-ITD autophosphorylation and activation of target molecules including STAT5A/B. A related compound AG1295 also inhibits FLT3 and has specific toxicity for primary AML blasts harboring FLT3-ITD compared with cytosine arabinoside.126 It has also been reported that Herbimycin A is a submicromolar inhibitor of FLT3 and could prevent leukemia progression in mice injected with 32D cells expressing FLT3-ITD. Although none of these compounds would be suitable for consideration in clinical trials in humans, these experiments provide evidence that FLT3 inhibition may be an effective approach to at least a subset of leukemias.
Several promising compounds have recently been reported in abstract form that are likely to move forward into phase I clinical trials. CEP-701, an orally bioavailable inhibitor of FLT3-ITD and activation loop mutants, has activity in a murine model based on injection of FLT3-ITD–transfected Ba/F3 cells,128 as well as in primary AML cells containing FLT3-ITD.129 CT53518 is also a potent inhibitor of FLT3, as well as PDGFβR and c-KIT. CT53518 has activity both in cell culture and in animal models, but does not inhibit the Asp835Tyr activation loop mutant of FLT3.130SU5614, SU5416, and SU11248 developed by Sugen (South San Francisco, CA), have also been reported to have activity in inhibition of FLT3-ITD–transformed cells in vitro and in vivo.131,132 PKC412, a benzoylstaurosporine from Novartis Pharmaceuticals (Basel, Switzerland) was developed as a vascular endothelial growth factor receptor (VEGFR) inhibitor and has been tested with minimal toxicity in a phase I trial in patients with solid tumors. PKC412 is an inhibitor of several kinases including PKC and SYK in addition to VEGFR and is also a potent submicromolar inhibitor of FLT3-ITDs in cell culture and murine models of leukemia.133 Each of these has pharmacokinetic and toxicity profiles suitable for phase I/II testing. There are several points to be considered in study design for FLT3 inhibition in AML. First, there is evidence that autocrine stimulation of the wild-type receptor may confer a proliferative advantage to AML cells, although it is not yet clear whether inhibition of overexpressed wild-type FLT3 would have cytocidal effects. It may therefore be most prudent in initial testing to study only those patients with activating mutations in FLT3. Second, it is not clear what response rate might be anticipated with FLT3 as a single agent in AML. However, a response rate of 30% may be a reasonable starting point based on the experience with imatinib mesylate in BCR/ABL+ AMLs (ie, CML in myeloid blast crisis). Third, it would seem most appropriate to test FLT3 inhibitors as single agents in a patient population for whom there are no better alternatives. These could include older patients who are not candidates for either BMT or induction chemotherapy, patients with relapsed AML, or patients with advanced stage MDS for whom there are no better treatment alternatives. Based on these considerations, relatively small samples sizes on the order of 12 to 16 patients would provide reasonable power to detect a response rate of at least 30% with statistical significance. There is a high likelihood that several FLT3 selective inhibitors will be tested in clinical trials within the next year, with hope and promise for improving therapy in AML.
FLT3 mutations are probably not sufficient to cause AML: a “2-hit” model of leukemogenesis
Several lines of evidence indicate that additional mutations are required for the development of an AML phenotype. First, FLT3 expression in primary hematopoietic progenitors is not sufficient to cause AML in a murine BMT assay. Instead, mutant FLT3 appears to have the transforming activity that is similar to other constitutively activated tyrosine kinases such as BCR/ABL, TEL/ABL, TEL/PDGFβR, and TEL/JAK2. Each of these fusions is associated with a chronic myeloproliferative phenotype in humans and results in a myeloproliferative disorder when expressed in primary hematopoietic cells. As for FLT3 mutants, the myeloproliferative phenotype is dependent on kinase activity. Thus, the constitutively activated tyrosine kinases are necessary and sufficient for the myeloproliferative phenotype, but do not in themselves cause an AML characterized by impaired differentiation of hematopoietic progenitors. Progression of CML phenotypes mediated by tyrosine kinases to AML (blast crisis) requires additional mutations in humans and in murine models. It therefore appears likely that second mutations are required in addition to activating mutations in FLT3 for the development of AML.
In support of this hypothesis, FLT3 mutations frequently occur in conjunction with other gene rearrangements and point mutations. For example, FLT3-ITDs have been reported in patients with t(8;21), inv(16), t(15;17), 11q23 gene rearrangements involving mixed lineage leukemia (MLL), and MLL internal tandem repeat mutations.83,100,122,134,135 These translocations result in expression of fusion genes including theAML1/ETO, CBFB/SMMHC, andPML/RARA, respectively. There are convincing data that these fusion genes are not sufficient to cause leukemia,136 but do contribute to impaired differentiation of hematopoietic cells. For example, the AML1/ETO and CBFβ/SMMHC fusion proteins are dominant-negative inhibitors of core binding factor, which is required for normal hematopoietic development.137-140
Thus, cooperation between FLT3 mutations and the gene rearrangements listed above may be required for the AML phenotype. In this model, FLT3-ITDs confer proliferative or survival advantage to cells through activation of the STAT, RAS/MAPK, and PI3K/AKT pathways, and are complemented by expression of proteins such as AML1/ETO that result in impaired differentiation and the AML phenotype141 142(Figure 2).
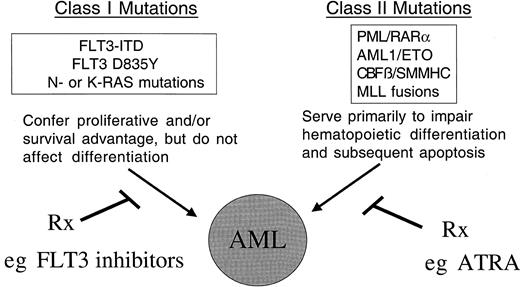
Model of cooperativity of activating mutations in FLT3 and gene rearrangements involving hematopoietic transcription factors.
The model of cooperativity is based in part on the observation that FLT3-ITD mutations have been associated with all FAB subtypes of AML, and with the majority of known chromosomal translocations associated with AML, including the t(8;21), inv(16), t(15;17), and MLL gene rearrangements. We hypothesize that there are 2 broad classes of mutations that contribute to AML: class I and class II mutations. FLT3-ITD would be exemplary of a class I mutations that, alone, confers a proliferative and survival advantage to hematopoietic progenitors but does not affect differentiation. Consistent with this hypothesis, expression of FLT3-ITD alone in a murine BMT assay results in a myeloproliferative phenotype characterized by leukocytosis and normal differentiation. Another example of class I mutations would be activating mutations in N-RAS or K-RAS in AML. In contrast, class II mutations would be exemplified by AML1/ETO, CBFβ/SMMHC, PML/RARα, and MLL-related fusion genes appear to impair hematopoietic differentiation, but are not sufficient to cause leukemia when expressed alone. We hypothesize that expression of both classes of mutations results in the AML phenotype characterized by enhanced proliferation and survival capacity of progenitor cells, and by impaired differentiation. The hypothesis has important implications in approach to novel therapies of AML, in that molecular targeting of both FLT3-ITD and fusion proteins involving transcription factors may improve outcome in AML.
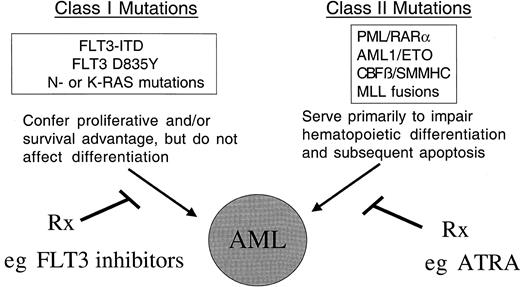
Model of cooperativity of activating mutations in FLT3 and gene rearrangements involving hematopoietic transcription factors.
The model of cooperativity is based in part on the observation that FLT3-ITD mutations have been associated with all FAB subtypes of AML, and with the majority of known chromosomal translocations associated with AML, including the t(8;21), inv(16), t(15;17), and MLL gene rearrangements. We hypothesize that there are 2 broad classes of mutations that contribute to AML: class I and class II mutations. FLT3-ITD would be exemplary of a class I mutations that, alone, confers a proliferative and survival advantage to hematopoietic progenitors but does not affect differentiation. Consistent with this hypothesis, expression of FLT3-ITD alone in a murine BMT assay results in a myeloproliferative phenotype characterized by leukocytosis and normal differentiation. Another example of class I mutations would be activating mutations in N-RAS or K-RAS in AML. In contrast, class II mutations would be exemplified by AML1/ETO, CBFβ/SMMHC, PML/RARα, and MLL-related fusion genes appear to impair hematopoietic differentiation, but are not sufficient to cause leukemia when expressed alone. We hypothesize that expression of both classes of mutations results in the AML phenotype characterized by enhanced proliferation and survival capacity of progenitor cells, and by impaired differentiation. The hypothesis has important implications in approach to novel therapies of AML, in that molecular targeting of both FLT3-ITD and fusion proteins involving transcription factors may improve outcome in AML.
Additional indirect evidence supports a “2-hit” model of leukemogenesis in which one class of mutations (class I) confers a proliferative or survival advantage to cells, and a second class of mutations (class II) serves primarily to interfere with hematopoietic differentiation and subsequent apoptosis of cells. This model would predict that only one mutation in each class would be required for development of AML. In support of this, FLT3-ITD, FLT3 activation loop, and N-RAS mutations, each of which confers proliferative and survival signals, do not coexist in the same patient. The very rare examples in which, for example, FLT3-ITDs have been found in patients with N-RAS mutations86 may suggest that there is an additional selective advantage in cells that harbor both mutations, or alternatively that there are 2 separate clones of AML cells, one with FLT3-ITD and one with N-RAS mutation. Similarly, the t(8;21), inv(16), t(15;17), and 11q23 gene rearrangements are mutually exclusive. However, FLT3-ITDs occur in approximately 30% to 40% of t(15;17) patients,83,122,123 143 which provides epidemiologic support for cooperative effects of these mutations in development of leukemia. These data do not preclude the possibility that additional mutations may be required for the acute leukemia phenotypes in humans, but strongly suggest that at a minimum, these 2 classes of mutations are required. In addition, the model allows for the possibility that acquired or germ line mutations in DNA repair or p53-Rb pathway enzymes could contribute to acquisition of these 2 classes of mutation.
FLT3 is the most commonly mutated gene in AML, accounting for about 30% of cases overall. N-RAS and K-RAS mutations account for another 20% of AML cases. Thus, another implication of the “2-hit” model of leukemogenesis is that there may be activating point mutations in other hematopoietic tyrosine kinases, or their downstream effectors, that contribute to the pathogenesis of the remaining 50% of AML cases. In support of this hypothesis, activation loop mutations have been reported at position Asp816Tyr in c-KIT in less than 5% of cases.144 In addition, exon 8 in-frame deletions and insertions in c-KIT have been reported in AML,145 and internal deletions of the JM domain of c-KIT confer constitutive activation in gastrointestinal stromal cell tumors, and render these tumors susceptible to inhibition by imatinib mesylate. Strategies to screen for additional activating mutations in tyrosine kinases or their downstream effectors in acute leukemias is warranted. Although FLT3-ITD may be associated with any of the major classes of cytogenetic abnormalities, including those targeting core binding factor (CBF), retinoic acid receptor (RAR), and MLL, FLT3-ITDs are most often associated with normal cytogenetics. In these cases the 2-hit model would predict that there are loss of function point mutations in genes required for normal hematopoietic development, such as AML1, C/EBPA, PU.1, and others. These testable hypotheses will have important implications in molecular targeting of therapy in AML, and in prognosis.
Promise and challenges for FLT3 inhibition in AML
FLT3 is a promising molecular target for therapy of AML. However, enthusiasm should be tempered by several considerations. First, as noted above, it is likely that, as for CML in blast crisis, AML associated with activating mutations in FLT3 will have additional mutations that may not be sensitive to FLT3 inhibition. One might therefore predict a response rate to FLT3 inhibition in AML comparable to that of imatinib mesylate in CML blast crisis of approximately 30%.146 147 It is likely that additional agents will be required for effective therapy of AML.
Second, 70% to 100% of AML and most ALL cases overexpress the FLT3, but only a fraction of these have activating mutations. It is not yet known what effect FLT3 inhibition might have on AML associated with overexpression of the wild-type FLT3 allele.
Third, none of the inhibitors currently under investigation is truly specific for FLT3. Although these agents are selective, they also target other kinases including PDGFβR, c-FMS, SYK, c-KIT, VEGFR, and PKC to name a few. The toxicities of these drugs will need to be carefully evaluated. However, as has been suggested for imatinib mesylate, the additional targets may in some cases prove beneficial. Thus, although a number of different inhibitors may be found to effectively inhibit FLT3 in vivo, therapeutic efficacy may vary considerably depending on the other targets.
Fourth, although most data indicate that FLT3 inhibitors targeted to the ATP binding site will inhibit all FLT3-ITD alleles, it will be important to evaluate effects on all alleles, and to determine whether there are any differences in the biologic properties or response to therapy for different ITD alleles. In addition, not all inhibitors are effective at targeting activation loop mutations. It is known that imatinib mesylate, for example, is quite effective at inhibition of BCR/ABL,146 and the JM deletion mutations in c-KIT associated with gastrointestinal stromal cell tumors.148However, imatinib mesylate has minimal activity for the most common c-KIT activating mutation in AML, Asp816Tyr. Similarly, not all of the FLT3 inhibitors under evaluation have activity against the FLT3 Asp835Tyr mutations, and it is not yet known which compounds are active for the other Asp835 substitution mutations.
Last, we can almost certainly expect resistance to develop to FLT3 inhibitors, as has been observed with STI571 therapy of CML blast crisis.149 In some cases, resistance may be due to enhanced degradation or cellular export of drug, but it is also clear that point mutations near the ATP binding site can also effect resistance. It will be important to anticipate this problem and to characterize molecular mechanisms of resistance to each inhibitor. This approach may allow for selection of combinations of FLT3 inhibitors that will preclude the development of resistance, analogous to the use of combinations of reverse transcription inhibitors in treating HIV infection. In this light, the completion of clinical trials to identify efficacious and nontoxic FLT3 inhibitors, among the many that are currently under evaluation, may provide one or more active agents that could be used in combination.
In summary, FLT3 is an important new molecular target for therapy of AML. Although there are challenges in the implementation of FLT3 inhibitors in AML, there is promise that these new therapies will improve outcome without increasing toxicity in treatment of AML. It is also plausible that there will be enhanced efficacy in the use of molecularly targeted therapies for both class I mutations and class II mutations. For example, drugs that inhibit FLT3 may be highly effective in combination with ATRA for treating acute promyelocytic leukemia (APL) associated with the PML/RARA gene rearrangement, an approach that can be tested in recently reported murine models.152
We gratefully acknowledge administrative and secretarial support from Lindsay Seaton and Marcie Cabral, and members of the Griffin and Gilliland laboratory for helpful discussions of this work.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-02-0492.
Supported in part by National Institutes of Health (NIH) PO1 CA66996 (D.G.G. and J.D.G.), a Leukemia and Lymphoma Society Specialized Center of Research Grant 7059 (D.G.G. and J.D.G.), and NIH PO1 DK5654 (D.G.G. and J.D.G.). D.G.G. is an Associate Investigator of the Howard Hughes Medical Institute.
J.D.G. has declared a financial interest in a company whose potential product was described in the present work.
References
Author notes
D. Gary Gilliland, Harvard Institutes of Medicine, 4 Blackfan Circle, Room 418, Boston, MA 02115; e-mail:gilliland@calvin.bwh.harvard.edu.