Migration of antigen (Ag)-loaded dendritic cells (DCs) from sites of infection into draining lymphoid tissues is fundamental to the priming of T-cell immune responses. We evaluated monocyte-derived DCs (MoDCs) and peripheral blood DCs (PBDCs) to respond to proinflammatory mediators, CD40L, and intact bacteria. All classes of stimuli induced DC phenotypic maturation. However, for MoDCs, only prostaglandin E2 (PGE2)–containing stimuli induced migratory-type DCs. Thus, immature MoDCs that encountered proinflammatory cytokines or CD40L or intact bacteria in the presence of PGE2 acquired migratory capacity but secreted low levels of cytokines. Conversely, MoDCs that encountered pathogens or CD40L alone become nonmigratory cytokine-secreting cells (proinflammatory type). Interestingly, both migratory- and proinflammatory-type DCs expressed equivalent levels of chemokine receptors, suggesting that the role of PGE2 was to switch on migratory function. We demonstrate that PGE2 induces migration via the E-prostanoid 2/E-prostanoid 4 (EP2/EP4) receptors and the cAMP pathway. Finally, migratory-type MoDCs stimulated T-cell proliferation and predominantly IL-2 secretion, whereas proinflammatory-type MoDCs induced IFN-γ production. In contrast, CD1b/c+ PBDC rapidly acquired migratory capacity irrespective of the class of stimulus encountered and secreted low levels of cytokines. This suggests that not all mature stages of DCs are destined to migrate to lymphoid organs and that the sequence in which stimuli are encountered significantly affects which functions are expressed. Thus, certain immature DC subsets recruited from the resting precursor pool may have multiple functional fates that play distinct roles during the induction and effector phases of the immune response. These findings have important implications for the clinical utility of DCs in immunotherapy.
Introduction
Dendritic cells (DCs) represent a heterogeneous family of leukocytes that establishes sentinel networks throughout body tissues.1 DCs sample the microenvironment for evidence of barrier breakage, tissue damage, and pathogen entry. Such perturbations of the local microenvironment induce DC maturation.1The induction of adaptive immune responses is initiated once antigen (Ag)-bearing DC traffic from peripheral sites of inflammation into draining lymph nodes.1,2 One functional consequence of DC maturation is the up-regulation of the lymph node–directing chemokine receptor, CCR7, and acquisition of migratory capacity toward lymph node–directing chemokines CCL21 (6Ckine) and CCL19 (MIP-3β).3-6 However, migratory DCs may represent only one functional fate of these immature sentinel cells. DCs secrete high levels of interleukin-12 (IL-12) in response to either pathogen signals7-10 or CD40L (in the presence of other cofactors),11-20 which induces IFN-γ and cytolytic function in T cells. DCs also have been described as cellular bridges between the innate and adaptive immune response, a functional trait that may be less effectively achieved in noninflamed lymphoid tissues, where innate immune response effectors are rare. It is therefore unclear whether DCs can perform these various functions simultaneously or whether these functions are characteristic of specific stages of the DC life cycle that may occur in geographically distinct niches in vivo.
DCs are being evaluated as cellular vaccine adjuvants in the immunotherapy of cancer.1 In humans, DCs can be directly isolated from peripheral blood dendritic cells (PBDCs), and at least 2 subsets of immature PBDCs can be identified on the basis of HLA-DR and CD1b/c or IL-3R expression.1 However, PBDCs are not widely used as vaccine adjuvants in clinical trials due to their rarity in blood and the feasibility of procuring sufficient numbers for multiple vaccinations. The recent discovery that the systemic administration of the hematopoietic growth factor Flt-3 ligand (FL) can dramatically increase the numbers of PBDCs in humans may substantially overcome these limitations.21 22
More commonly, DC-like cells (resembling dermal or interstitial DCs of skin and lymphoid organs) can be generated in vitro from blood monocyte-derived DCs (MoDCs) when cultured in granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 for 5 to 7 days.1 Although it is unclear whether MoDCs represent physiologic DC subsets in vivo or the fate of monocytes under highly selective conditions, Randolph et al23 have shown that a subset of monocytes can rapidly (< 24 h) transform into MoDCs in the absence of GM-CSF and IL-4 simply by migrating through an endothelial monolayer and ingesting particulates in the collagen matrix below.23 However, regardless of whether MoDCs are physiologically relevant cells in vivo, they are, nonetheless, clinically relevant given that the majority of human DC-based trials use MoDCs for vaccine delivery.1 Therefore, understanding the mechanisms that regulate MoDC function and thus their ability to stimulate appropriate T-cell effector function will ultimately benefit their clinical use.
Proinflammatory mediators found at sites of infection and inflammation (eg, IL-1β, tumor necrosis factor-α [TNF-α], IL-6, interferon-α [IFN-α], prostaglandin E2[PGE2]) not only induce MoDC maturation but also down-regulate the secretion of IL-12 in response to CD40L or bacterial stimuli.24-31 Also produced at high levels are byproducts of the phospholipid and arachidonic acid pathway,32 the latter including products of the cyclooxygenase pathway such as prostaglandins (eg, PGE2) as well as products of the 5-lipoxygenase pathway such as the leukotrienes.32,33 In mouse, the cystenyl leukotrienes (leukotriene C4[LTC4] or leukotriene D4[LTD4]) are crucial to the induction of migration of DCs toward CCL19 chemokine.33 It is not clear, however, whether all mediators that induce DC maturation are required for the development of migratory function.
In this study we investigated how proinflammatory factors (TNF-α, IFN-α and PGE2), pathogen signals (intactEscherichia coli [E coli]), and CD40L affect the functional development of MoDCs and FL-expanded CD1b/c+PBDCs. We report that although all 3 classes of stimuli were equivalent at inducing MoDC phenotypic maturation, only PGE2-containing combinations induced MoDC migratory function, while CD40L or pathogen signals alone induced cytokine-secreting cells (proinflammatory-type MoDCs). In contrast, PBDCs were not as dependent on the presence of PGE2 for acquisition of migratory function and were low producers of cytokines, suggesting that this DC subset was functionally distinct to MoDCs. Finally, we show that CCR7 (and CXCR4) expression is not predictive of migration toward chemokines for MoDCs. Rather, stimuli that contain PGE2 not only up-regulate CCR7 expression but also license responsiveness to CCR7 ligands and induce migratory function. The mechanism of PGE2 action on migratory function was induced via cyclic adenosine 3′,5′-monophosphate (cAMP) mobilization and appeared to use E-prostanoid 2/E-prostanoid 4 (EP2/EP4) receptors. The present study suggests dual functional fates for immature MoDCs. Migratory-type MoDCs secrete low levels of cytokines (eg, IL-12p70) and induce T cells to secrete a wide profile of cytokines (eg, IL-2, IL-5, and IFN-γ). The proinflammatory-type MoDC (nonmigratory) secretes high levels of cytokines and predominantly induces T cells to secrete IFN-γ.
Patients, materials, and methods
Media
DCs were cultured in RPMI 1640 (Trace Biosciences, Melbourne, Australia) supplemented with 20 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid), 60 mg/L penicillin G, 12.6 mg/L streptomycin, 2 mM L-glutamine, 1% nonessential amino acids, and 10% heat-inactivated fetal calf serum (FCS; CSL Ltd., Melbourne, Australia) in a 5% CO2incubator. Mixed leukocyte reactions (MLRs) were performed in Iscoves modified Dulbecco medium (IMDM) (GIBCO/Life Technologies, Melbourne, Australia) and 5% pooled normal human serum (gift of the Victorian Tissue Typing Service, Royal Melbourne Hospital, Australia) in a 10% CO2 incubator.
Monoclonal antibodies, enzyme-linked immunosorbent assay (ELISA) kits, and cytokines
Flow cytometric analysis of cells was performed using the following monoclonal antibodies (mAbs): fluorescein isothiocyanate (FITC)–conjugated IgG1 isotype control; phycoerythrin (PE)-conjugated IgG1 isotype control; anti-CD80/B7, anti–HLA-DR (Becton Dickinson, San Jose, CA); anti–HLA-A, B, C, W6/32 (Dako, Carpenteria, CA); anti-CD86/B70/B7-2, (PharMingen, San Diego, CA); FITC-conjugated sheep anti–mouse mAb (Silenus, Miami, FL); and anti-CD83 (Immunotech, Beckman Coulter, Sydney, Australia). Cytokine ELISA-kits (Opteia brand) for IL-2 and IL-5 were purchased from PharMingen/Becton Dickinson (San Jose, CA). Capture and horse-radish peroxidase (HRP)–conjugated detection antibodies for IFN-γ ELISAs were a kind gift from CSL Ltd. The following cytokines were added to DC cultures: recombinant human (rh)TNF-α (10 ng/mL), rhIL-1β (1-2 ng/mL), IL-6 (50 ng/mL) (R&D Systems, Minneapolis, MN), rhGM-CSF (40 ng/mL) (Schering-Plough, Sydney, Australia), rhIL-4 (500 U/mL) (Schering-Plough, New Jersey City, NJ); IFN-α2a (1000 IU/mL) (Roferon-A; Roche Products PTY, Sydney, Australia), and IFN-γ (PeproTech, Rocky, NJ). CD40L-trimer (1 μg/mL final concentration) was a gift from Immunex (Seattle, WA). PGE2 (1 μM final concentration) was purchased from ICN Biomedicals (Aurora, OH). The cAMP agonist, forskolin (Sigma, St Louis, MO), was titrated and used at a final concentration of 50 μM/mL. cAMP agonists dibutyryl cAMP (db-cAMP) and 8-bromo-cAMP (8-b-cAMP) (Wako, Tokyo, Japan) were used at concentrations between 0.1 and 1.0 μM/mL. The PGE2receptor agonists 11-deoxy-PGE1(11-d-PGE1)(EP2/EP4 agonist) and Sulprostone (EP1/EP3 agonist) (Sapphire Biosciences, Crows Nest, Australia) were used at concentrations of 0.1-1.0 μM/mL.
MoDCs
Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coat preparations from healthy donors from the Red Cross Blood Bank (Melbourne, Australia) and used to produce MoDCs. CD14+ monocytes (5 × 105) were affinity purified using the MACS CD14 isolation kit (Miltenyi Biotech, Sunnyvale, CA) and cultured in 1 mL RPMI, 10% FCS, GM-CSF (40 ng/mL), and IL-4 (500 U/mL) in 24-well plates. By day 7, MoDCs represented more than 90% of cultured cells. On day 7, all wells were pooled and readjusted to a concentration of 1 × 105 DCs per mL. Maturation-inducing factors were added on day 7, and cells and supernatants were harvested on day 10 for functional assessment. All cytokines and stimuli examined for their ability to stimulate DC functional maturation in the present study (eg, IFN-α2a, IFN-α2a, CD40L, PGE2, IL-1β, and intact E coli) were thoroughly tested in dose titration analyses, and the concentrations used in the figures represent those found to be optimal.
Enrichment of PBDCs from FL-treated patients
PBDCs were enriched from frozen PBMC samples obtained from a Phase 1 randomized study performed in HLA-A2+ patients with minimal residual disease stages III and IV malignant melanoma receiving 14 consecutive days of Flt-3 ligand, FL (25 μg/kg/d) (Immunex), with or without peptide vaccines (LUD-97-012). Blood for PBDCs was taken at day 15 of FL treatment. The protocol was approved by the Ludwig Institute's Investigators Review Board and the Ethics Committee at the Austin and Repatriation Medical Centre, and informed consent was obtained from all patients. Alternatively, PBDCs were isolated from buffy packs obtained from healthy donors from the Australian Red Cross Blood Bank. After thawing, CD14+ monocytes were depleted using immunomagnetic beads (MACS; Miltenyi Biotech) according to the manufacturer's instructions. These CD14-depleted PBMCs underwent a second round of depletion using MACS beads coupled to anti-CD3, anti-CD14, and anti-CD19 (Miltenyi Biotech) in combination with rat-anti–mouse IgG MACS beads (Miltenyi Biotech). This depletion procedure yielded more than 60% CD1b/c+CD14−HLA-DR+ PBDCs as assessed by fluorescence-activated cell-sorting scanner (FACS). The enriched PBDCs were then sorted on the basis of CD1b/c and HLA-DR expression on a MoFlo cell sorter (Cytomation, Fort Collins, CO). These immature PBDCs were then cultured in 96-well plates (1 × 105/well) in RPMI-10% FCS for 3 days with various combinations of stimuli prior to examination of function.
Measurement of Ag uptake
MoDCs were harvested after culture in maturation-inducing conditions. Following incubation with 1 mg/mL FITC-dextran (44 kd and 260 kd) (Sigma) for 30 to 60 minutes at 0°C or 37°C, cells were washed 3 times in phosphate-buffered saline (PBS) 5% FCS and then incubated with PE–anti-CD11c. FITC-dextran uptake was quantified as mean fluorescence intensity (MFI) on gated CD11c+ cells. Nonspecific FITC signal was assessed by incubating MoDCs with FITC-dextran at 0°C. Phagocytosis was assessed by incubating cells with 1 mg/mL PE-latex beads (Sigma) for 90 minutes at 37°C. In some conditions, cells were pretreated with 10 uM cytochalasin D (Sigma) for 30 minutes at 37°C to depolymerize actin. To verify that the flow cytometry–based FITC signal represented internalized dextran or beads, cells were analyzed by epifluorescence and phase-contrast microscopy.
RNA isolation and cDNA synthesis
Total RNA was isolated from MoDCs using a Rneasy Mini Kit (Qiagen, Hilden, Germany) according to manufacturer's instructions. In brief, cells were lysed and homogenized in lysis buffer containing guanidine isothiocyanate (GITC) and β-mercaptoethanol. Ethanol (70%) was added to the samples, and the RNA was immobilized on spin columns and eluted in RNase-free water. Total RNA (0.16 μg) was used to synthesize cDNA using 1 μg random hexamers (Promega, Madison, WI), 1 mM deoxynucleoside triphosphate (dNTP) (Amersham Pharmacia Biotech, Piscataway, NJ), 2 U RNase inhibitor (Promega), 5 mM MgCl2(Applied Biosystems, Foster City, CA), 1x polymerase chain reaction (PCR) buffer (Applied Biosystems), and 2 U Moloney-murine leukemia virus (M-MLV) reverse transcriptase (Life Technologies, Rockville, MD) in a 20 μL reaction for 60 minutes at 42°C. The enzyme was inactivated at 95°C for 5 minutes. Of the resulting 20μLs, 1 μL cDNA was used for real-time polymerase chain reaction (PCR) quantitation.
Quantitative real-time PCR
Gene expression levels were quantitated using ABI Prism 7700 Sequence Detection System (Applied Biosystems). Predeveloped assay reagents (PDARs) for CCR7 were obtained from Applied Biosystems and used in multiplex reactions with 18S rRNA PDAR (Applied Biosystems) for normalization. PCR reactions were set up in 96-well plates (25 μL/reaction) according to the manufacturer's instructions and analyzed using the SDS program version v1.7. Relative expression was calculated using the ΔCt method and is expressed relative to a calibrator, in this case the GM-CSF/IL-4 DC control:
Cytokine ELISAs
Cytokine secretion by stimulated MoDCs or by allogeneic T cells was measured by cytokine ELISAs. IL-1β, IL-6, IL-10, and IL-12p70 ELISAs were performed on supernatants (SNs) of MoDCs and IFN-γ, IL-2, and IL-5 ELISAs were performed on SNs of MLRs according to the manufacturer's instructions using Maxisorp plates (Nunc, Melbourne, Australia). The HRP-substrate was tetramethylbenzidine (TMB) peroxidase (KPL, Gaithersburg, MD); the color reaction was terminated by adding 100 μL orthophosphoric acid (1 M). Wells were developed using a substrate solution of 548 mg/mL ABTS (2,2′azino-bis13-ethylbenz-thiazoline-6-sulfonic acid) (Sigma Aldrich Pty Ltd, Castle Hill, Australia) with 0.001% hydrogen peroxide (Ajax Chemicals, Auburn, Australia) in 0.1M citric acid, pH4.2. Plates were read in a Thermomax microplate reader (BioMediq, Melbourne, Australia).
Migration assays
MoDCs matured with the indicated stimuli for 24 to 48 hours were harvested from their wells, washed 3 times, and tested for migration toward chemokines using the transwell assay. Briefly, lower chambers of transwell plates (8.0 μm pore size; Costar, Corning, NY) were filled with 500 μL IMDM/5% HS with or without chemokines. Chemokines tested included CCL19 (MIP-3β; 3-300 ng/mL); CCL21 (6Ckine; 5-250 ng/mL); CXCL12 (SDF-1α; 100 ng/mL); CCL3 (MIP-1α; 50 ng/mL); CXCL9 (Mig; 50 ng/mL); and CCL7 (MCP-3, 50 ng/mL; PeproTech, Rocky Hill, NJ). Briefly, 1-2 × 104 DCs were added in 50 μL IMDM/5% HS into the upper chamber, and cells were incubated at 37°C for 2 hours. Cells in the lower chambers were harvested, concentrated to 50 μL volumes in Eppendorf tubes, and counted with a hemocytometer. Migration for all stimulation conditions was performed in triplicate wells.
T-cell purification and MLR
Allogeneic CD2+ T lymphocytes were obtained by rosetting PBMCs with aminoethylisothiouronium (AET)-treated sheep red blood cells (SRBCs). T cells were further fractionated using anti-CD4 and anti-CD8 MACS beads (Miltenyi Biotech) and were between 88% and 95% pure. Negatively selected CD4− and CD8− cells were further separated into CD45RA+and CD45RA− cells using MACS beads (Miltenyi Biotech). DCs were cultured in round-bottomed 96-well plates in triplicate at various cell numbers with 105 allogeneic PBMCs for 5 days in RPMI with 10% HS. After 5 days, 200 μL of SNs were harvested, and fresh medium containing 1 μCi (0.037 MBq)/well [3H]thymidine (DuPont, Sydney, MA) was added for 8 hours. Cells were transferred onto a glass fiber filter (Wallac, Turku, Finland), and [3H]thymidine incorporation was measured using an NXT TopCount Betaplate scintillation counter (Packard, Meriden, CT).
Results
Phenotypic changes of MoDCs in response to soluble proinflammatory factors
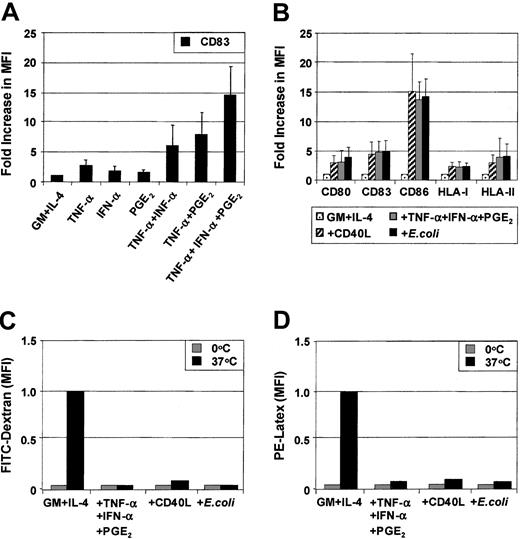
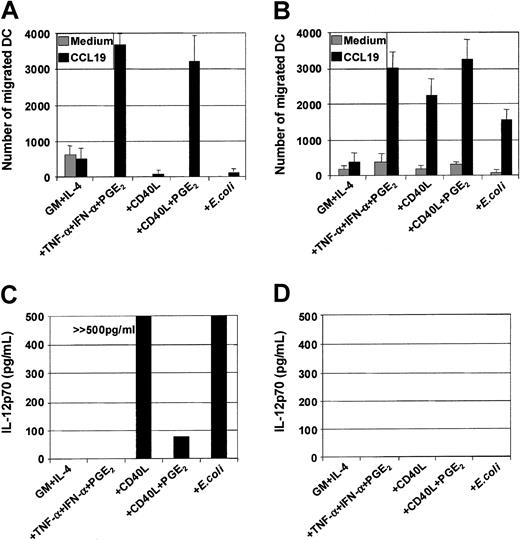
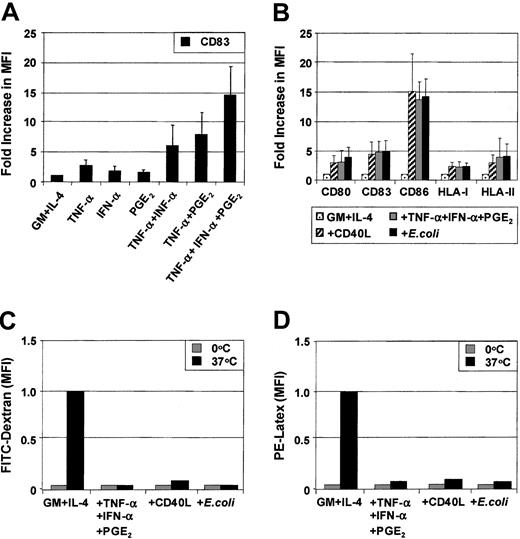
MoDCs were generated from sorted CD14+ monocytes. Phenotypic maturation of MoDCs can be induced by pathogen signals7-10 or CD40L.11-20 Alternatively, maturation also can be induced by proinflammatory cytokines.24-31 To determine the role of individual proinflammatory mediators, immature MoDCs were exposed to combinations of TNF-α (10 ng/mL), IFN-α (1000 IU/mL), and PGE2 (1 μM) for 3 days. The optimal concentrations of each of these factors in combination were previously determined by cross-titration of each soluble mediator. Figure 1A shows the fold increase in the MFI of CD83 expression above that expressed by immature MoDCs following stimulation with either TNF-α or IFN-α or PGE2 or combinations thereof. TNF-α alone weakly up-regulated CD83 expression on MoDCs. The effects of dual combinations (ie, TNF-α and PGE2 or TNF-α and IFN-α) and, in particular, the triple combination of TNF-α, IFN-α, and PGE2 substantially increased the level of expression of CD83. Similar effects were seen when examining CD80, CD86, HLA-ABC, and HLA-DR (data not shown). Up-regulation of the above surface markers was induced as early as 18 hours, reaching maximal levels by 48 hours (data not shown). These results demonstrate that phenotypic maturation of MoDCs is a multistep process that can be enhanced by combining several proinflammatory mediators.
Up-regulation of maturation markers on MoDCs in response to proinflammatory factors.
MoDCs were prepared by culturing purified CD14+ monocytes for 7 days in GM-CSF and IL-4 and stimulated for 3 days with the indicated stimuli. Surface expression of maturation markers was examined by flow cytometry on day 10. (A) Immature MoDCs were stimulated with either TNF-α (20 ng/mL) or IFN-α (1000 IU/mL) or PGE2 (1 μM) or combinations thereof as indicated for 3 days, and flow cytometric analysis of CD83 expression was performed. (B) Immature MoDCs were stimulated for 3 days with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL) or intact E coli(1 × 106), and flow cytometric analysis of CD80, CD83, CD86, HLA-I, and HLA-II expression was assessed. Results are shown as fold increase of mean fluorescence levels relative to nonstimulated control MoDCs (control, normalized to 1). Figures represent the means (SEM of 4 experiments for (C) FITC-dextran uptake or (D) PE-latex bead uptake (1 μm) at either 4°C or 37°C for 30 minutes. Cells were examined by flow-cytometry to assess internalized FITC or PE. The data are presented as the MFI of internalized FITC or PE and represent means (SEM) of 4 experiments.
Up-regulation of maturation markers on MoDCs in response to proinflammatory factors.
MoDCs were prepared by culturing purified CD14+ monocytes for 7 days in GM-CSF and IL-4 and stimulated for 3 days with the indicated stimuli. Surface expression of maturation markers was examined by flow cytometry on day 10. (A) Immature MoDCs were stimulated with either TNF-α (20 ng/mL) or IFN-α (1000 IU/mL) or PGE2 (1 μM) or combinations thereof as indicated for 3 days, and flow cytometric analysis of CD83 expression was performed. (B) Immature MoDCs were stimulated for 3 days with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL) or intact E coli(1 × 106), and flow cytometric analysis of CD80, CD83, CD86, HLA-I, and HLA-II expression was assessed. Results are shown as fold increase of mean fluorescence levels relative to nonstimulated control MoDCs (control, normalized to 1). Figures represent the means (SEM of 4 experiments for (C) FITC-dextran uptake or (D) PE-latex bead uptake (1 μm) at either 4°C or 37°C for 30 minutes. Cells were examined by flow-cytometry to assess internalized FITC or PE. The data are presented as the MFI of internalized FITC or PE and represent means (SEM) of 4 experiments.
We next compared how maturation induced by the combination of proinflammatory mediators compares to other known inducers of MoDC maturation such as soluble CD40 ligand (CD40L) or intact E coli. Proinflammatory mediators (TNF-α, IFN-α, PGE2), CD40L, or intact E coli all induced the phenotypic maturation of MoDCs to a similar degree when added for 48 hours. In this way, all 3 classes of stimuli induced mature MoDCs that were phenotypically indistinguishable (Figure 1B). Analysis of endocytosis and phagocytosis by MoDCs was next examined. As expected, immature MoDCs were maximally capable of internalizing soluble dextran (260 kDa) and phagocytosing 1-μm latex particles (Figure 1C,D). Maturation with either proinflammatory mediators or CD40L or intactE coli maximally reduced the ability of MoDCs to ingest these particulates. Once again, these 3 classes of stimuli demonstrated an equivalent capacity to down-regulate Ag uptake function in MoDCs.
Migration of immature and mature MoDCs toward CXCR4 or CCR7 ligands
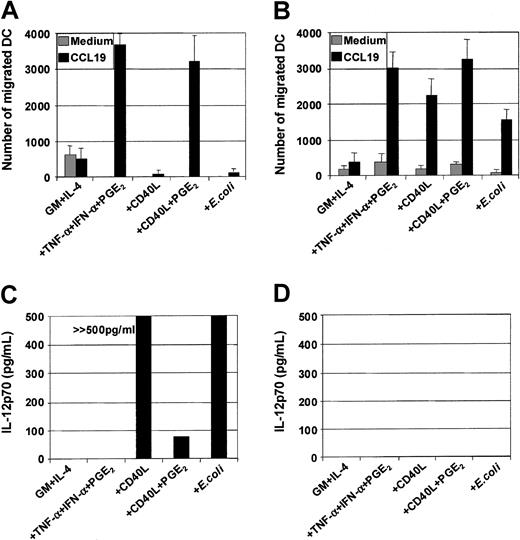
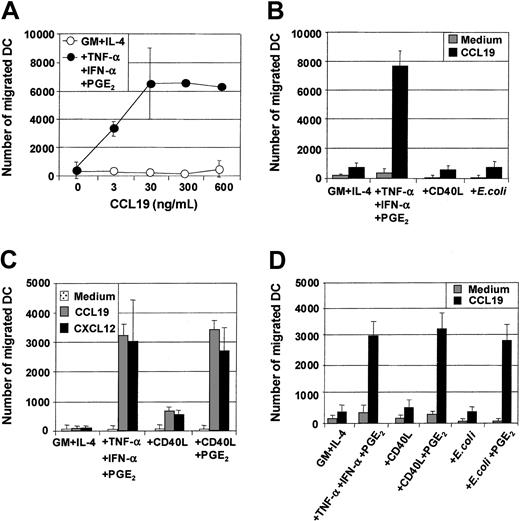
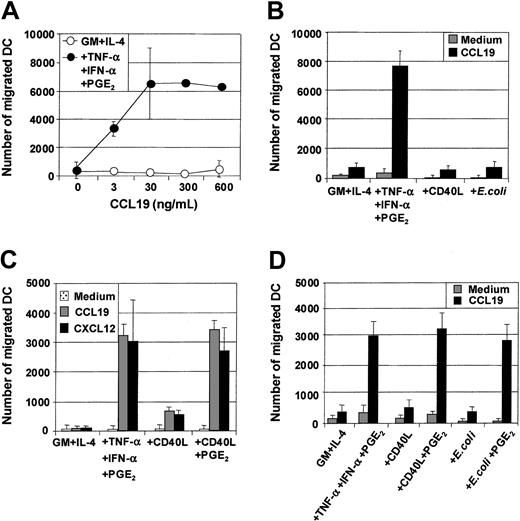
Maturation of MoDCs results not only in loss of Ag uptake capacity but also in substantial changes in the expression of chemokine receptors and the ability to migrate toward chemokine gradients. The capacity of the 3 classes of stimuli to induce MoDC migratory capacity toward chemokines was therefore evaluated, and several important points were identified. First, MoDCs matured with proinflammatory mediators (TNF-α, IFN-α, PGE2) were highly sensitive to the CCR7-ligands CCL19 (MIP-3β) (Figure2A,C) and CCL21 (6Ckine) (data not shown), migrating toward 3 to 10 ng/mL of either chemokine. Second, in contrast to previous reports,34 35 immature MoDCs did not migrate substantially to the CXCR4-binding proinflammatory chemokine CXCL12 (SDF-1α) (Figure 2C). Third, although immature MoDCs matured with either CD40L or intact E coli were phenotypically identical to those matured with TNF-α, IFN-α, PGE2, they were poor migratory cells (Figure 2B). Fourth, immature MoDCs matured with TNF-α, IFN-α, PGE2 not only migrated toward the CCR7-ligands CCL19 and CCL21 but also to proinflammatory chemokines such as CXCL12 (Figure 2C) and CCL3 (MIP-1α), CXCL9 (Mig), and CCL7 (MCP-3) (data not shown). Finally, simultaneous stimulation of immature MoDCs with either CD40L and PGE2 or intact E coli and PGE2 induced mature MoDCs that migrated toward chemokines (Figure 2C,D). These results demonstrate that in our cultures, migration of MoDCs was induced by all 3 classes of physiologic stimuli, provided PGE2 was present.
Migration of immature and mature MoDCs toward proinflammatory and CCR7-binding chemokines.
Immature MoDCs (GM + IL-4) or MoDCs stimulated with the indicated stimuli for 2 days were examined for their migratory capacity toward either CCL19 (MIP-3β) or CXCL12 (SDF-1α) in transwell assays. (A) Dose titration of CCL19 in transwell assay and comparison of migratory function of immature MoDCs and MoDCs matured with TNF-α + IFN-α + PGE2. (B) Migration toward CCL19 (30 ng/mL) by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL) or intact E coli (1 × 106). Data are representative of 5 separate experiments. (C) Migration toward either CCL19 or CXCL12 (50 ng/mL) by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL) or CD40L + PGE2. Data are representative of 5 separate experiments. (D) Migration toward CCL19 by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L or CD40L + PGE2 or intact E coli or intactE coli + PGE2. Data are representative of at least 5 separate experiments.
Migration of immature and mature MoDCs toward proinflammatory and CCR7-binding chemokines.
Immature MoDCs (GM + IL-4) or MoDCs stimulated with the indicated stimuli for 2 days were examined for their migratory capacity toward either CCL19 (MIP-3β) or CXCL12 (SDF-1α) in transwell assays. (A) Dose titration of CCL19 in transwell assay and comparison of migratory function of immature MoDCs and MoDCs matured with TNF-α + IFN-α + PGE2. (B) Migration toward CCL19 (30 ng/mL) by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL) or intact E coli (1 × 106). Data are representative of 5 separate experiments. (C) Migration toward either CCL19 or CXCL12 (50 ng/mL) by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL) or CD40L + PGE2. Data are representative of 5 separate experiments. (D) Migration toward CCL19 by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L or CD40L + PGE2 or intact E coli or intactE coli + PGE2. Data are representative of at least 5 separate experiments.
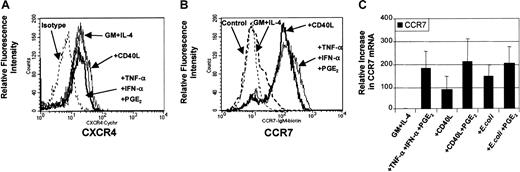
Analysis of chemokine receptor expression on mature MoDCs
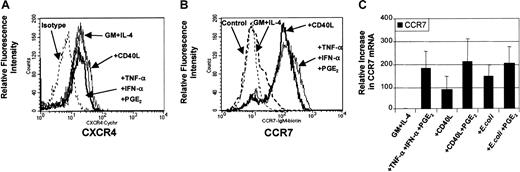
DC maturation involves the coordinated expression of specific chemokine receptors.34 35 In this way, the presence or absence of chemokine receptors has been used to predict migratory capacity toward specific chemokines. To evaluate the levels of chemokine receptor expression on DCs before and after maturation, analysis of surface expression for CXCR4 (Figure3A) and CCR7 (Figure 3B) were performed by flow cytometry. Figure 3A demonstrates that CXCR4 protein can be constitutively expressed on the surface of nonmigratory immature DCs and that this level of expression was not considerably increased following stimulation. Figure 3B demonstrates that CCR7 expression was expressed on a minority of immature MoDCs and up-regulated on most MoDCs following maturation with either proinflammatory mediators (TNF-α, IFN-α, PGE2) or CD40L. Molecular analysis of CCR7 mRNA by quantitative real-time PCR (qRT-PCR) shows that CCR7 mRNA was induced in immature MoDCs following stimulation with all 3 classes of stimuli (Figure 3C). In addition, analysis of 3 separate experiments revealed that the up-regulation of CCR7 mRNA expression between migratory-type MoDCs (ie, those matured with PGE2-containing conditions) and nonmigratory-type MoDCs (ie, CD40L or E coli alone) varied by no more than 2-fold (Figure 3C). These data highlight the fact that chemokine receptor expression is not necessarily predictive of migratory capacity.
Regulation of expression of CCR7 and CXCR4 chemokine receptors.
Immature MoDCs (GM + IL-4) or MoDCs stimulated with the indicated stimuli for 3 days were examined for expression of chemokine receptors by FACS and by quantitative real time PCR (qRT-PCR). (A) Analysis of CXCR4 expression on immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL). Data are representative of 3 separate experiments. (B) Analysis of CCR7 expression on immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL). Data are representative of 4 separate experiments. (C) Analysis of CCR7 mRNA levels by qRT-PCR on immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L or CD40L + PGE2 or intact E coli or intactE coli + PGE2. Data represent the mean ± SEM of 4 separate donors.
Regulation of expression of CCR7 and CXCR4 chemokine receptors.
Immature MoDCs (GM + IL-4) or MoDCs stimulated with the indicated stimuli for 3 days were examined for expression of chemokine receptors by FACS and by quantitative real time PCR (qRT-PCR). (A) Analysis of CXCR4 expression on immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL). Data are representative of 3 separate experiments. (B) Analysis of CCR7 expression on immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L (1 μg/mL). Data are representative of 4 separate experiments. (C) Analysis of CCR7 mRNA levels by qRT-PCR on immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α + PGE2 or CD40L or CD40L + PGE2 or intact E coli or intactE coli + PGE2. Data represent the mean ± SEM of 4 separate donors.
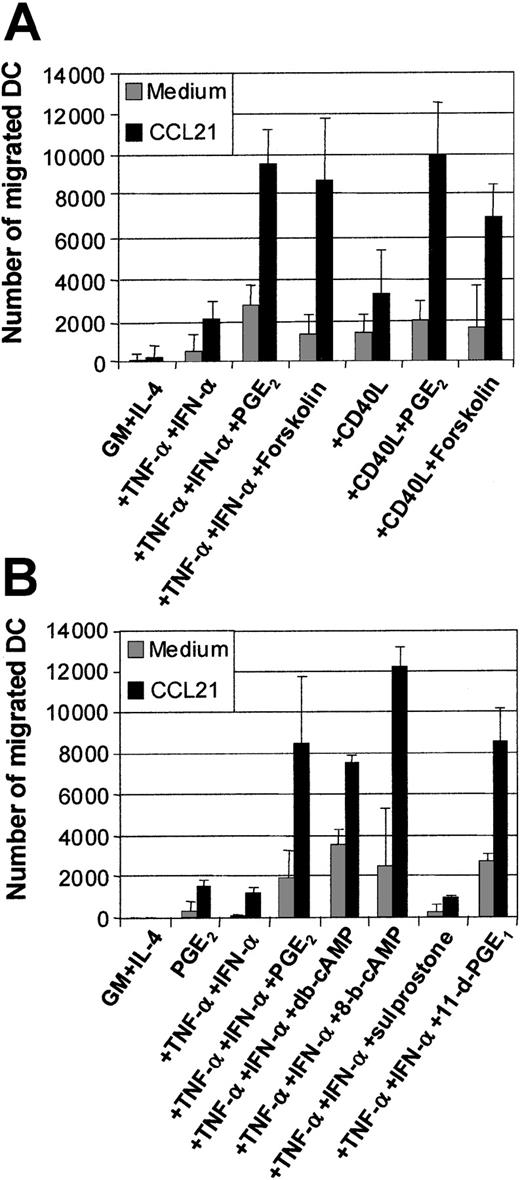
Actions of PGE2 involve cAMP pathway via EP2/EP4 receptor signaling
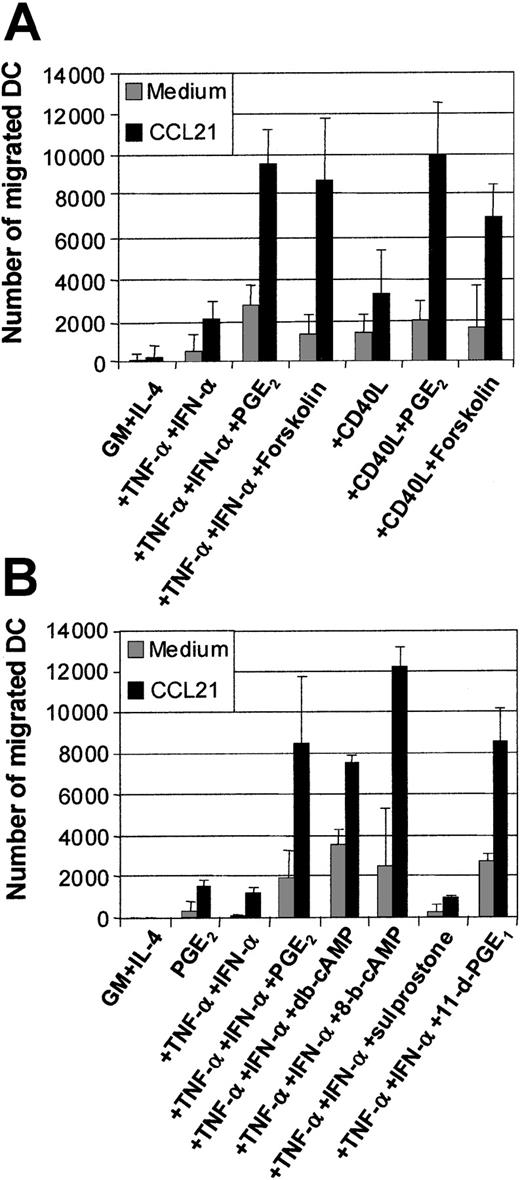
The effects of PGE2 have been shown to be mediated via the elevation of intracellular cAMP.36 The mobilization of cAMP can be synthetically induced using an activator of adenylate cyclase, such as forskolin. Figure 4A shows the means of migration assays generated from 5 separate experiments. The addition of forskolin (50 μM) to TNF-α and IFN-α or CD40L or intact E coli could significantly replace the requirement for PGE2 in the induction of MoDC migration toward CCL19 (Figure 4A). This demonstrates that a major mechanism used by PGE2 during the induction of migratory capacity is via cAMP mobilization. We next examined whether cAMP analogs, such as db-cAMP or 8-bromo-cAMP (8-b-cAMP), could also induce migratory function in MoDCs. Figure 4B demonstrates that both of these cAMP analogs, when combined with TNF-α and IFN-α, could mimic PGE2 at inducing MoDC migration toward CCL19, confirming the effects of forskolin.
Examination of the effects of the cAMP and PGE2 receptor agonists on MoDC migratory function.
Immature MoDCs (GM + IL-4) or MoDCs stimulated with the indicated stimuli for 2 days were examined for their migratory capacity toward CCL21 (6Ckine) (100 ng/mL) in transwell assays. (A) Migration toward CCL21 by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α ± PGE2 or ± forskolin (1 μM) or CD40L ± PGE2 or ± forskolin (1 μM). Data represent the mean ± SEM of triplicate wells from 5 separate donors. (B) Migration toward CCL21 by immature MoDCs or MoDCs stimulated with either PGE2, or TNF-α + IFN-α or +PGE2 or + the cAMP analogs dibutyryl-cAMP (db-cAMP) or 8-bromo-cAMP (1 μM) or + the PGE2 receptor agonists, 11-deoxy-PGE1 (11-d-PGE1) (EP2/EP4 agonist) or sulprostone (EP1/EP3 agonist) (1 μM). Data represent the mean ± SEM of triplicate wells and are representative of 3 separate experiments.
Examination of the effects of the cAMP and PGE2 receptor agonists on MoDC migratory function.
Immature MoDCs (GM + IL-4) or MoDCs stimulated with the indicated stimuli for 2 days were examined for their migratory capacity toward CCL21 (6Ckine) (100 ng/mL) in transwell assays. (A) Migration toward CCL21 by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α ± PGE2 or ± forskolin (1 μM) or CD40L ± PGE2 or ± forskolin (1 μM). Data represent the mean ± SEM of triplicate wells from 5 separate donors. (B) Migration toward CCL21 by immature MoDCs or MoDCs stimulated with either PGE2, or TNF-α + IFN-α or +PGE2 or + the cAMP analogs dibutyryl-cAMP (db-cAMP) or 8-bromo-cAMP (1 μM) or + the PGE2 receptor agonists, 11-deoxy-PGE1 (11-d-PGE1) (EP2/EP4 agonist) or sulprostone (EP1/EP3 agonist) (1 μM). Data represent the mean ± SEM of triplicate wells and are representative of 3 separate experiments.
PGE2 can bind to 4 separate receptors for signaling: EP1-4.36,37 The EP2 and EP4 receptors signal via cAMP mobilization, while the EP1/EP3 receptors do not. We examined which of the EP receptors PGE2 acts through to induce MoDC migratory function by using the EP receptor agonists 11-deoxy-PGE1(which mediates signaling via the EP2/EP4receptors) or Saprostone (which signals via the EP1/EP3 receptors).36 37 Figure 4B shows that only the EP2/EP4 receptor agonist 11-deoxy-PGE1 mimics PGE2 in inducing MoDC migratory function when combined with TNF-α and IFN-α. Given that EP2/EP4 signal via the cAMP pathway, this finding further supports the data generated using the cAMP analogs and the agonist forskolin.
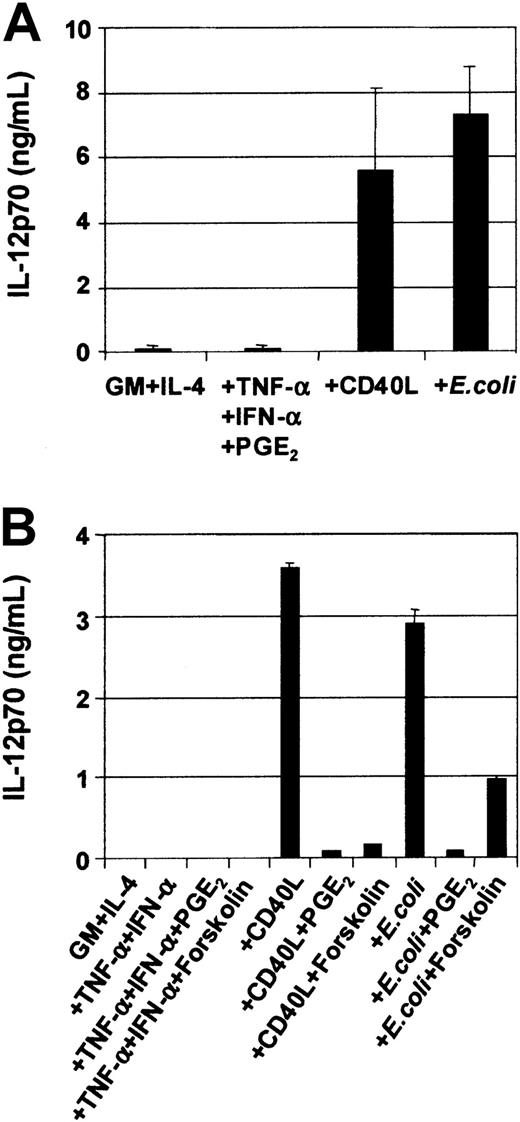
DC cytokine secretion is induced by CD40L or intact E coli and inhibited by PGE2
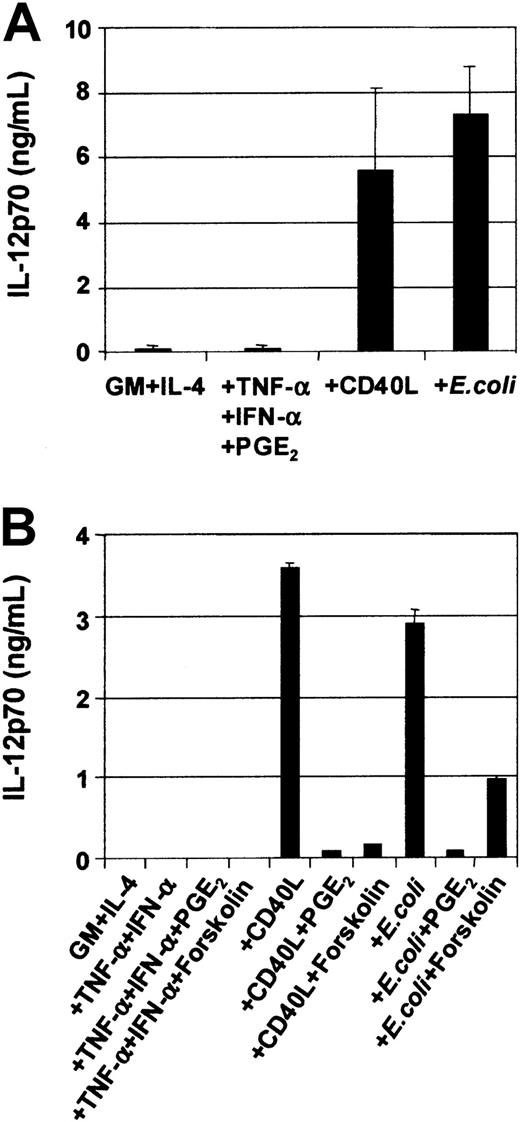
DCs secrete an array of cytokines, including IL-12, when stimulated with either infectious organisms such as intact bacteria, viruses, and protozoa7-10 or with CD40L.11-20In this way, DCs may be central to the development of IFN-γ–secreting T-cell effectors.1 Although cytokines such as TNF-α, IL-1β, and IFN-α are potent inducers of DC maturation, they are not sufficient to induce secretion of IL-12p7026-29,37 and can even inhibit IL-12 secretion in response to IL-12–inducing stimuli.26-29 Optimal CD40L-mediated IL-12p70 production requires the presence of cofactors such as IFN-γ, IL-4, and IL-1β or IFN-α.13-16,18-20Figure 5A shows that immature MoDCs stimulated with either CD40L or intact bacteria in the presence of their own conditioned media secrete high levels of IL-12p70 (Figure5A), and this was further potentiated by addition of IFN-γ (Table1). Table 1 also shows that MoDCs were induced to secrete high levels of IL-6, IL-10, and IL-12p70 in response to CD40L, CD40L + IFN-γ, or E coli. In contrast, MoDCs stimulated with the proinflammatory mediators TNF-α, IFN-α, PGE2, were poor cytokine-secreting cells (Figure 5A). Furthermore, the addition of PGE2 to CD40L or intactE coli inhibited the induction of IL-12p70 secretion by MoDCs (Figure 5B). Finally, the inhibitory effect of PGE2 could be replaced by the cAMP agonist forskolin (Figure 5B) and the cAMP analogs db-cAMP and 8-b-cAMP (data not shown), indicating that mobilization of cAMP was, in part, responsible for down-regulation of IL-12p70 production. Finally, Table2 shows that mature, migratory-type MoDCs produce substantially lower levels of cytokines following stimulation with either CD40L or intact E coli as compared with immature MoDCs, suggesting a differential developmental commitment of the 2 cell populations. This is in accordance with the reports by others showing that cytokine production by MoDCs is optimal when the stimulus is given at the onset of maturation.13-16 38
Modulation of IL-12p70 production in MoDCs by various stimuli.
On day 7, immature MoDCs were pooled and adjusted to 1 × 105 cells per mL. MoDCs were activated for 3 days with the indicated stimuli, and cytokine ELISAs were performed on culture SN. (A) Secretion of IL-12p70 by immature MoDCs and MoDCs stimulated with either TNF-α + IFN-α + PGE2or CD40L (1 μg/mL) or intact E coli(1 × 106/mL). Data represent the means ± SEM of 7 different donors. (B) PGE2 or forskolin inhibits CD40L orE coli–mediated IL-12p70 secretion. IL-12p70 secretion by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α ± PGE2 or ± forskolin (1 μM) or CD40L ± PGE2 or ± forskolin (1 μM) or E coli ± PGE2 or ± forskolin (1 μM). Data represent the mean ± SEM of triplicate wells.
Modulation of IL-12p70 production in MoDCs by various stimuli.
On day 7, immature MoDCs were pooled and adjusted to 1 × 105 cells per mL. MoDCs were activated for 3 days with the indicated stimuli, and cytokine ELISAs were performed on culture SN. (A) Secretion of IL-12p70 by immature MoDCs and MoDCs stimulated with either TNF-α + IFN-α + PGE2or CD40L (1 μg/mL) or intact E coli(1 × 106/mL). Data represent the means ± SEM of 7 different donors. (B) PGE2 or forskolin inhibits CD40L orE coli–mediated IL-12p70 secretion. IL-12p70 secretion by immature MoDCs or MoDCs stimulated with either TNF-α + IFN-α ± PGE2 or ± forskolin (1 μM) or CD40L ± PGE2 or ± forskolin (1 μM) or E coli ± PGE2 or ± forskolin (1 μM). Data represent the mean ± SEM of triplicate wells.
Effect of mature MoDCs on T-cell proliferation and cytokine secretion
Because DCs are the most potent stimulators of naive T cells, we investigated the ability of differentially matured MoDCs to stimulate cytokine secretion in alloreactive, naive, and memory CD4+T cells. Figure 6 shows that the differential capacity of MoDCs matured with proinflammatory factors or CD40L to secrete IL-12p70 correlated with their capacity to induce IFN-γ and inhibit IL-5 secretion by naive CD4+CD45RA+ T cells. Only CD40L- or CD40L + IFN-γ–activated DCs induced the highest levels of IFN-γ in both naive (Figure 6A) and memory T cells (data not shown) and significantly reduced IL-5 secretion in these cultures (Figure 6C). In contrast, both IFN-γ and IL-5 were secreted in low but significant quantities by naive (and memory) T cells stimulated with either immature DCs or DCs matured with TNF-α, IFN-α, PGE2 (Figure 6A,C). This indicates immature MoDCs matured with PGE2-containing stimuli will induce T cells to secrete both IL-5 and IFN-γ. Surprisingly, migratory-type MoDCs (ie, matured with PGE2-containing stimuli) induced significant IL-2 secretion in naive (and memory) CD4+CD45RA+ T cells (Figure 6B). These increased IL-2 levels also correlated with significantly higher proliferation of T cells in the MLR (Figure 6D). These differences between migratory-type and IL-12-producing nonmigratory-type MoDCs (proinflammatory DCs) were most prominent at the lower DC:T cell ratios (102 and 103 DCs per 105 T cells) and was less significant at higher DC concentrations (104 DCs per 105 T cells, data not shown). These results demonstrate that the different functional states of MoDCs (ie, migratory-type versus nonmigratory proinflammatory-type) stimulate different T-cell cytokine profiles. While CD40L-activated DCs induced higher IFN-γ and decreased IL-5 secretion by T cells, migratory-type DCs stimulated IL-2, IL-5, and IFN-γ secretion and augmented T-cell proliferation.
T-cell stimulation by immature and mature MoDCs.
Immature (GM + IL-4) or MoDCs (1 × 104) matured with the indicated stimuli were washed and used as stimulators of alloreactive T cells (1 × 105) in the MLR. On day 5 of the MLR, supernatants were harvested and fresh medium containing 1 μCi (0.037 MBq) [3H]thymidine was added into each well for 8 hours. Cytokines secreted by naive CD4+, CD45RA+ alloreactive T cells following stimulation with DCs were also assessed by ELISA. (A) IFN-γ production. (B) IL-2 production. (C) IL-5 production. Data represent the means ± SEM of experiments from 3 separate donors. (D) Proliferation of T cells stimulated with graded numbers of MoDCs. Data represent the means ± SEM of triplicate wells. The figure is representative of experiments from 5 separate donors.
T-cell stimulation by immature and mature MoDCs.
Immature (GM + IL-4) or MoDCs (1 × 104) matured with the indicated stimuli were washed and used as stimulators of alloreactive T cells (1 × 105) in the MLR. On day 5 of the MLR, supernatants were harvested and fresh medium containing 1 μCi (0.037 MBq) [3H]thymidine was added into each well for 8 hours. Cytokines secreted by naive CD4+, CD45RA+ alloreactive T cells following stimulation with DCs were also assessed by ELISA. (A) IFN-γ production. (B) IL-2 production. (C) IL-5 production. Data represent the means ± SEM of experiments from 3 separate donors. (D) Proliferation of T cells stimulated with graded numbers of MoDCs. Data represent the means ± SEM of triplicate wells. The figure is representative of experiments from 5 separate donors.
Fate of irreversibly differentiated MoDCs and role of CD40L as a survival factor
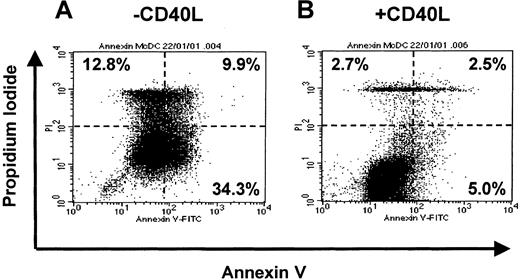
Maturation of MoDCs is a graded process involving several intermediate stages, which maintain the potential for reversion to a macrophage phenotype upon removal of cytokines.28,30,31 To further elucidate these findings, we investigated the stability of immature and mature DCs upon withdrawal of cytokines and exposure to human serum. MoDC cultures were washed on day 7 and resuspended in fresh medium with or without cytokines. After 3 days, cells were washed again and resuspended in medium containing 5% heat-inactivated human serum. Immature MoDCs reverted to adherent macrophages in the absence of cytokines and presence of HS (data not shown). This confirmed that immature MoDCs represent a transient and reversible state of development, analogous to a bipotential precursor of macrophages and MoDCs.30 31 In contrast, MoDCs matured with the proinflammatory factors TNF-α, IFN-α, and PGE2 did not revert to adherent macrophages but rather underwent cell death. Interestingly, the addition of CD40L prevented migratory-type MoDCs from undergoing cell death, inducing them instead to vigorously form cell clusters (data not shown).
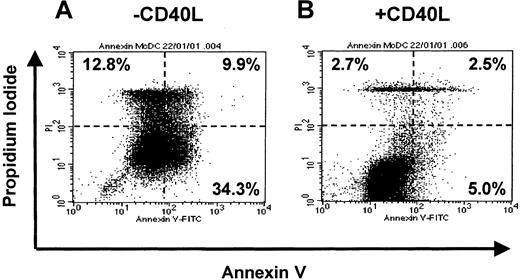
To address whether CD40L prevented migratory-type DCs from undergoing apoptosis, we assessed annexin V (apoptotic cells) versus propidium iodide (dead and necrotic cells) uptake. Figure7 demonstrates Annexin V binding to migratory-type MoDCs that were washed and exposed to HS for 3 days (Figure 7A) or exposed to HS and CD40L (Figure 7B). The presence of CD40L significantly decreased the proportion of Annexin V+, apoptotic MoDCs (5.0% vs 34.3%), or PI+, dead/dying MoDCs (5.2% vs 22.7%). This suggests that the productive interaction with antigen-specific T cells (via CD40L) may be necessary for the prolonged survival of mature migratory-type DCs once they enter the T-cell areas of lymphoid tissues. It also demonstrates the importance of the sequence of cytokine signals on the recipient cells.
Effect of soluble mediators on the maturation and cell survival of MoDCs in the absence of growth factors and presence of human serum.
On day 7, immature MoDCs cultures were washed and resuspended in fresh medium containing GM-CSF (GM, 20 ng/mL). Proinflammatory factors with TNF-α (10 ng/mL), IFN-α (2000 IU/mL), and PGE2 (1 μM) were added for 3 days. On day 10, cells were washed and recultured in either IMDM + 5% human serum alone or with CD40L (1 μg/mL). MoDCs from these conditions were assessed by Annexin V and PI labeling for death by apoptosis using FACS. Data are representative of experiments from 5 separate donors.
Effect of soluble mediators on the maturation and cell survival of MoDCs in the absence of growth factors and presence of human serum.
On day 7, immature MoDCs cultures were washed and resuspended in fresh medium containing GM-CSF (GM, 20 ng/mL). Proinflammatory factors with TNF-α (10 ng/mL), IFN-α (2000 IU/mL), and PGE2 (1 μM) were added for 3 days. On day 10, cells were washed and recultured in either IMDM + 5% human serum alone or with CD40L (1 μg/mL). MoDCs from these conditions were assessed by Annexin V and PI labeling for death by apoptosis using FACS. Data are representative of experiments from 5 separate donors.
PGE2 is not essential for the induction of migratory function of PBDCs
To evaluate whether the effect of PGE2 on the regulation of DC migratory function was specific to only MoDCs or necessary for other DC subsets, we examined how PGE2affects the migratory functions of PBDCs. Here we sorted to high purity CD1b/c+ PBDCs (> 98%) from healthy individuals and patients receiving the hematopoietic growth factor FL. We have previously shown that FL-expanded PBDCs are phenotypically immature but mature rapidly following culture in vitro.19 Figure8 compares MoDCs and autologous CD1b/c+ PBDCs generated from 4 separate donors. Unlike MoDCs, which required PGE2 to acquire migratory capacity when stimulated with CD40L or intact E coli (Figure 8A), sorted CD1b/c+ PBDCs were efficient migratory cells following stimulation with these 2 classes of stimuli even in the absence of PGE2 (Figure 8B). Although the overall trends suggested that the addition of PGE2 could enhance, to some degree, the migratory function of CD1b/c+ PBDCs, this varied substantially from donor to donor. In addition, unlike MoDCs, which required at least 24 hours for the acquisition of migratory capacity, CD1b/c+ PBDCs acquired migratory capacity within 8 hours of culture (M.J., manuscript in preparation). This suggests that CD1b/c+ PBDCs may represent a presensitized population of antigen-presenting cells (APC) that migrates following encounter with all 3 classes of stimuli, whereas immature MoDCs are temporally delayed in acquiring migratory function following stimulation and require the presence of PGE2 to do so. Finally, unlike MoDCs, which secrete high levels of IL-12p70 following stimulation with either CD40L or intact E coli (Figure 8C), CD1b/c+ PBDCs were poor producers of IL-12p70 (Figure 8D). In this regard, CD1b/c+ PBDCs produced less than 150 pg/mL of IL-12p70 even when stimulated with combinations known to induce IL-12p70 in MoDCs (eg, GM-CSF + IL-4 + CD40L + IL-1β + IFN-γ + intact E coli), suggesting that CD1b/c+ PBDCs are not major producers of this cytokine.19
Analysis of migration and cytokine secretion by MoDCs or autologous FL-generated PBDCs.
PBDCs were purified by negative depletion from the peripheral blood of melanoma patients treated with FL for 14 consecutive days, and then FACS were sorted to high purity (> 95%) on the basis of CD1b/c and HLA-DR expression. Autologous MoDCs were generated from blood isolated from pretreatment bleeds and cultured 7 days prior to the isolation of PBDCs. (A) Migration toward CCL19 by immature MoDCs or MoDCs stimulated with the indicated stimuli for 48 hours. (B) Migration toward CCL19 by sorted FL-generated PBDCs from melanoma patients with minimal residual disease stimulated with the indicated stimuli for 48 hours. (C) IL-12p70 secretion by immature MoDCs or MoDCs stimulated (1 × 105/well) with the indicated stimuli for 3 days. (D) IL-12p70 secretion by FL-generated PBDCs (1 × 105/well) from melanoma patients with minimal residual disease stimulated with the indicated stimuli for 3 days. Culture SNs were examined for cytokine production by ELISA. Data represent the means ± SEM of experiments from 7 separate donors.
Analysis of migration and cytokine secretion by MoDCs or autologous FL-generated PBDCs.
PBDCs were purified by negative depletion from the peripheral blood of melanoma patients treated with FL for 14 consecutive days, and then FACS were sorted to high purity (> 95%) on the basis of CD1b/c and HLA-DR expression. Autologous MoDCs were generated from blood isolated from pretreatment bleeds and cultured 7 days prior to the isolation of PBDCs. (A) Migration toward CCL19 by immature MoDCs or MoDCs stimulated with the indicated stimuli for 48 hours. (B) Migration toward CCL19 by sorted FL-generated PBDCs from melanoma patients with minimal residual disease stimulated with the indicated stimuli for 48 hours. (C) IL-12p70 secretion by immature MoDCs or MoDCs stimulated (1 × 105/well) with the indicated stimuli for 3 days. (D) IL-12p70 secretion by FL-generated PBDCs (1 × 105/well) from melanoma patients with minimal residual disease stimulated with the indicated stimuli for 3 days. Culture SNs were examined for cytokine production by ELISA. Data represent the means ± SEM of experiments from 7 separate donors.
Discussion
During bacterial and viral infections, high levels of TNF-α, IFN-α, PGE2, IL-1β, and GM-CSF are secreted by monocytes, macrophages, keratinocytes, and endothelial cells.39-44 This cascade of proinflammatory mediators (as well as chemokines) will directly act on immature, sentinel DCs present in or near inflamed tissues, thus facilitating the up-regulation of CCR7 on their surface and migration into draining lymphoid tissues.1-4 The present study demonstrates that the same proinflammatory mediators released at sites of inflammation synergistically induce phenotypic maturation of immature MoDCs to a similar degree as complete pathogens or cellular activation signals such as CD40L. However, for immature MoDCs, functional maturation appears to involve at least 2 developmental options resulting in APC populations of distinct functional capacity: migratory-type and proinflammatory-type DCs. Interestingly, CD1b/c+ PBDCs are functionally distinct from MoDCs in this respect, predominantly expressing a migratory-type profile.
Several novel findings have been made in the present study. First, MoDCs differentiate into mature migratory-type DCs in response to PGE2-containing stimuli (eg, TNF-α + IFN-α + PGE2 or CD40L+PGE2 or E coli + PGE2). These mature MoDCs migrated toward proinflammatory chemokines (CXCL12) and the lymph node–directing chemokines CCL19 and CCL21. PGE2 was found to be a critical switch-factor for the acquisition of migratory capacity, highlighting a novel role for PGE2 upon DC functional maturation. Furthermore, migratory-type MoDCs appear to represent a terminally differentiated DC stage that is less responsive to cytokine-inducing stimuli such as intact E coli or CD40L, and thus these DCs produce lower levels of cytokines (including IL-12p70). This confirms the findings by Kalinski and colleagues, who show that PGE2-matured DCs are refractory to IL-12–inducing stimuli.24-26 It has also been reported that MoDCs matured in the presence of PGE2bias the differentiation of naive CD4+ T cells toward IL-5–secreting Th2-cells.24-27 Although our results confirm that migratory-type MoDCs increase secretion of IL-5 by alloreactive T cells, this was not at the expense of IFN-γ or IL-2 secretion. In fact, the absolute amount of T-cell IFN-γ (∼3000 pg/mL) induced by migratory-type MoDCs was always higher than that of IL-5 (∼500 pg/mL) (Figure 6A,C). Thus, it maybe simplistic to propose that PGE2-matured MoDCs strictly promote Th2-type T-cell responses.24-27 We propose, therefore, that MoDCs matured with PGE2-containing stimuli promote Ag-specific expansion of T cells capable of producing a wider array of cytokines, rather than biasing the T-cell cytokine repertoire toward either Th1 or Th2. One difference between our results and the published reports of others25-27 is the presence of IFN-α in our maturation cocktail, a cytokine present during the early phase of most bacterial and viral infections. IFN-α has known effects on STAT-4 activation and IL-12Rβ2 chain expression by human T cells.45However, others have also reported that PGE2-containing stimuli do not preferentially bias T-cell cytokine repertoire toward Th2 (Scandella et al, personal written communications, November 15, 2001).46 Finally, human clinical studies show that MoDCs matured in the presence of PGE2-containing stimuli (eg, TNF-α, IL-1β, IL-6, and PGE2) and injected directly into lymph nodes induced optimal IFN-γ T-cell responses.47 This in vivo human data suggests that PGE2-matured MoDCs do not preferentially induce type 2 immune responses in vivo.
Second, immature MoDCs may have an alternate functional fate. Proinflammatory-type MoDCs were generated when immature MoDCs were stimulated with CD40L-trimers or intact E coli in the absence of PGE2. These cells secreted high levels of cytokines, including IL-10 and IL-12p70, and induced naive T cells to secrete high amounts of IFN-γ. Surprisingly, proinflammatory-type DCs expressed poor migratory capacity toward either CXCR4 ligand, toward CXCL12, or toward CCR7 ligands CCL19 and CCL21, despite expressing high levels of the relevant chemokine receptors. These cells were also less efficient at inducing IL-2 secretion and proliferation of alloreactive T cells, probably due to their high production of IL-10. The most striking observation was the ability of PGE2 to override the cytokine-inducing capacity of CD40L or intact E coli and instead induce migratory-type MoDCs that secreted low levels of cytokines. This emphasizes that PGE2 is a dominant regulator of MoDC function.
PGE2 has previously been shown to induce migration in a variety of different cell types, such as endothelial cells, mesangial cells, Lewis lung carcinoma cells, mammary tumor cells, and evenOesophagostomum dentatum larvae.48-52 This suggests a more universal mechanism of action of PGE2 that may go beyond our focus on DC maturation and activation. We show that mobilization of cytoplasmic cAMP is likely one of the main mediators for PGE2 activity since forskolin and the cAMP analogs, db-cAMP and 8-bromo-cAMP, could mimic the effect of PGE2. In addition, only the EP2/EP4 receptor agonist 11-deoxy-PGE1 could mimic the effects seen with PGE2, suggesting that these 2 receptors (which signal via the cAMP cascade) mediate the effects of PGE2 upon MoDC migratory capacity. This latter finding confirms the findings of Scandella and colleagues, who recently demonstrated by quantitative real-time PCR that exposure of immature MoDCs to PGE2-containing stimuli results in the down-regulation of EP2 and EP4 mRNA and induces MoDC migration (Scandella et al, personal written communications, November 15, 2001).
Interestingly, cAMP induction decreases lipopolysaccharide (LPS)– and TNF-mediated NFkB-activation in a variety of experimental systems, resulting in reduced secretion of cytokines, including IL-12.53-59 It is therefore tempting to speculate on the involvement of these 2 antagonistic pathways (both stress induced) in the regulation of DC differentiation. However, it is intriguing that the same PGE2 signal that enhances TNF-α– and IFN-α–induced MoDC maturation simultaneously inhibits CD40L/E coli–induced cytokine secretion. This suggests that PGE2 may act as a signal modulator of other stimuli analogous to a rheostat model rather than directly signaling on its own. This is supported by the observation that neither PGE2nor forskolin nor the cAMP analogs had effects on their own but depended upon the presence of other maturation factors (such as TNF-α, IFN-α, CD40L, or intact E coli). It is likely therefore that PGE2 may act by completing the engagement of signaling modules proximal to the chemokine receptor to facilitate signaling and induce migratory function. This may explain why the expression of chemokine receptors (CXCR4 or CCR7) on mature MoDCs was not predictive of their migratory capacity unless PGE2 was present during maturation. Further evidence that chemokine receptor expression is not predictive of migratory function and that appropriate coupling and signal transduction is regulated downstream of the surface receptor has been reported by others.60 61
Interestingly, purified PBDCs resembled migratory-type MoDCs rather than immature MoDCs in their response to the 3 classes of stimuli tested. This was also the case with respect to the types of T-cell cytokines induced by PBDCs (ie, higher levels of IL-2 regardless of stimulus class, with no clear bias toward either IL-5 or IFN-γ production) (data not shown). This may reflect either that PBDCs are functionally distinct to MoDCs or that PBDCs represent cells that are the product of a completely distinct stimulation history in vivo. Interestingly, cAMP is known to be a second messenger for a variety of hormones, including vasoactive intestinal peptide (VIP) and sympathomimetics present in serum.53,58 We performed preliminary studies using sympathomimetics for MoDC activation and observed similar effects as with PGE2 (T.L., unpublished data, 2001). Although speculative, it is possible that freshly isolated PBDCs already have been exposed to cAMP-inducing serum factors prior to purification and thus lost their proinflammatory potential. Similarly, MoDCs maybe the product of cells generated in the presence of inhibitors of migratory function. IL-4 is not only an inhibitor of macrophage development in vitro but also an inhibitor of metabolites and catalysts of the arachidonic acid pathway.62,63 Thus, immature nonmigratory MoDCs may be the result of inhibitory effects of culture in IL-4, revealing a dependence on exogenous PGE2to acquire migratory function upon stimulation. Further experimental studies to address these issues are ongoing. Interestingly, PGE2 is secreted by monocytes, macrophages, fibroblasts, and keratinocytes in response to inflammatory stimuli,43,44,64 and its production is increased by IL-1.43 64 This suggests that immature sentinel DCs will be exposed to PGE2 in situ and at most infectious and inflammatory sites.
Finally, migratory-type MoDCs did not revert to adherent macrophages when removed from their maturation stimuli but underwent cell death within 5 days. In contrast, immature MoDCs differentiated into macrophages as previously reported.28,30 31 Interestingly, CD40L prolonged the survival of migratory-type DCs, emphasizing the importance of the sequence of cytokine signals on recipient cells. Similar enhancement of in vitro survival was seen when CD1b/c+ PBDCs were stimulated with CD40L (data not shown). This suggests that the productive interaction with antigen-specific T cells (via induction of CD40L expression) is a positive signal for maintaining DC survival and Ag presentation, whereas absence of Ag-specific T cells will result in the death of the immigrant DC. This may provide a critical selective mechanism against inappropriate immune-response induction.
Our data suggest that DCs that acquire migratory capacity are capable of efficiently stimulating the expansion of antigen-specific T cells in lymphatic organs. Indeed, several clinical groups have now demonstrated that MoDCs matured with PGE2-containing stimuli (eg, TNF-α, IL-1β, IL-6, and PGE2) induce more potent CD8+ and CD4+ T-cell immune responses in cancer patients.65-67 These reports provide proof of principle that this type of MoDC is more efficient at reaching the lymph nodes and expanding T-cell responses as compared to immature MoDCs, where the T-cell responses generated have been disappointing and have even resulted in tolerance.68 Similar results have also been reported using enriched PBDCs from FL-treated patients.22
The data are also consistent with the proposal that the type of T-cell immune response may be initiated and antigen-specific T cells preferentially expanded by migratory-type DCs within lymph nodes, while T-cell cytokine bias and effector function may be terminally coordinated at the effector site by proinflammatory-type DCs or APC (site high in IL-12p70 and other IFN-γ inducers).69These results have led us to propose a hypothesis that emphasizes the importance of an interplay between migratory-type DCs in lymph nodes and proinflammatory-type DCs at the effector sites for the optimal expansion and differentiation of effector T lymphocytes.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2001-12-0360.
Supported by the Sylvia and Charles Viertel Foundation. Dr T. Luft is supported by a fellowship from The Anti Cancer Council of Victoria, Australia; Dr P. Leutjens and Dr H. Hochrein are supported by a Deutche Krebshilfe fellowship; and Dr M. Jefford is supported by the Stewardson Family Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eugene Maraskovsky, Ludwig Institute Oncology Unit, Austin and Repatriation Medical Centre, Studley Road, Heidelberg, Victoria 3084, Australia; e-mail: eugene.maraskovsky@ludwig.edu.au.

![Fig. 6. T-cell stimulation by immature and mature MoDCs. / Immature (GM + IL-4) or MoDCs (1 × 104) matured with the indicated stimuli were washed and used as stimulators of alloreactive T cells (1 × 105) in the MLR. On day 5 of the MLR, supernatants were harvested and fresh medium containing 1 μCi (0.037 MBq) [3H]thymidine was added into each well for 8 hours. Cytokines secreted by naive CD4+, CD45RA+ alloreactive T cells following stimulation with DCs were also assessed by ELISA. (A) IFN-γ production. (B) IL-2 production. (C) IL-5 production. Data represent the means ± SEM of experiments from 3 separate donors. (D) Proliferation of T cells stimulated with graded numbers of MoDCs. Data represent the means ± SEM of triplicate wells. The figure is representative of experiments from 5 separate donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood-2001-12-0360/6/m_h81623079006.jpeg?Expires=1770981670&Signature=yTpk9vuyi3Az7K6tJpqwUUweR7b2i-JOPnhYd1qdCm-t-dKrjOLot4XE~yhROak5LnfcaQQoAvvDFxD-1M36uhNFS4zvHxQ5CQZ~F80VmGbR29duWmjxycl~ivnGbfm9GdODQYVEr9ZI4rdP1Y9Bj5spS6MSBta0YedMZc0qJYQkJS6j3Z2Ok5wX-dyPlq5~yyqyQJldHifWRFF7NMXXT1EyyZ1XtkOmLbWXx4Dd9yQJ4CMwTjIrSkk2rGAYV2w0knJGSohUuhfUY9TjtKZQg5SAJ58yj3YFhDAc7FMYmJIb61cHD5WCm22oRIFtAf7-yNEv4LO-3~L5MkMEUX6b-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. T-cell stimulation by immature and mature MoDCs. / Immature (GM + IL-4) or MoDCs (1 × 104) matured with the indicated stimuli were washed and used as stimulators of alloreactive T cells (1 × 105) in the MLR. On day 5 of the MLR, supernatants were harvested and fresh medium containing 1 μCi (0.037 MBq) [3H]thymidine was added into each well for 8 hours. Cytokines secreted by naive CD4+, CD45RA+ alloreactive T cells following stimulation with DCs were also assessed by ELISA. (A) IFN-γ production. (B) IL-2 production. (C) IL-5 production. Data represent the means ± SEM of experiments from 3 separate donors. (D) Proliferation of T cells stimulated with graded numbers of MoDCs. Data represent the means ± SEM of triplicate wells. The figure is representative of experiments from 5 separate donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood-2001-12-0360/6/m_h81623079006.jpeg?Expires=1770981671&Signature=o3Iwc7Od566c6cDNm0sCyNnPZNJS2svP5cOqKNEfqbGn9UShCBW0SXbPeblJrLEeaTEpovXXYo7iDLGT5YJ7WHuWXYAdSS0GOCtQ2Ii7RUkfErlFBZaaJxZ8doBJrl28EyS~oiUH1e4J97NYjyPSSQx~Ue~Ap3O1Wv3naxRtvCkuKDdJbMsOLL8WUeTVHksLpsXIeWJt1buQuAFYCSSjPtYOBbdysVN8rNyqX7No4HgyvkziySnDqMd7bgUoL7KdnHHdBdXorDzCBw1zDaWO1feqSoSxdA13bVc946TdWraSV7J4QuqHNolp5Sha6zOizb0m6gi58nheOEukLhmY2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)