The inverse relationship between expression and methylation of β-type globin genes is well established. However, little is known about the relationship between expression and methylation of avian α-type globin genes. The embryonicαπ-globin promoter was unmethylated, andαπ-globin RNA was easily detected in 5-day chicken erythroid cells. A progressive methylation of the CpG dinucleotides in the απ promoter associated with loss of expression of απ-globin gene was seen during development in primary erythroid cells. A 315-bpαπ-globin promoter region was cloned in an expression construct (απpGL3E) containing a luciferase reporter gene and SV40 enhancer. The απpGL3E construct was transfected into primary erythroid cells derived from 5-day-old chicken embryos. Methylation of απpGL3E plasmid andαπ-globin promoter alone resulted in a 20-fold and 7-fold inhibition of expression, respectively. The fully methylated but not the unmethylated 315-bpαπ-globin gene promoter fragment formed amethyl cytosine-binding proteincomplex (MeCPC). Chromatin immunoprecipitation assays were combined with quantitative real-time polymerase chain reaction to assess histone acetylation associated with theαπ-globin gene promoter. Slight hyperacetylation of histone H3 but a marked hyperacetylation of histone H4 was seen in 5-day when compared with 14-day erythroid cells. These results demonstrate that methylation can silence transcription of an avian α-type embryonic globin gene in homologous primary erythroid cells, possibly by interacting with an MeCPC and histone deacetylase complex.
Introduction
The developmental regulation of globin gene expression is an intensely studied problem because of its central importance in the understanding of many common inherited diseases caused by defects in globin gene expression such as thalassemia. The chicken globin families are a well-characterized set of developmentally regulated genes. The avian β-type globin cluster has 4 functional genes (5′-ρ, βH, βΑ, ε-3′) while the α-type globin cluster has 3 functional genes (5′-απ, αΑ, αD-3′). Primitive erythroid cells express embryonic β-globin genes, ρ and ε, and the α-globin genes, απ, αΑ, and αD. Definitive cells express βH and βΑ as well as αΑand αD.1,2 Tissue-specific and developmental stage–specific regulation of both α- and β-globin gene families primarily involves the locus control regions (LCR), which are erythroid-specific enhancers located many kilobases upstream of each gene cluster.3 The inverse relationship between expression and methylation of β-type globin genes is well established.4-6 We have previously shown that methylation of the promoter or proximal transcribed region of the chicken β-type embryonic globin gene represses transcription in primary erythroid cells.6,7 However, little is known about the relationship between expression and methylation of avian α-type globin genes. A previous study examined certain specific sites of the chicken α-globin cluster in DNA from embryonic and adult erythroid cells using a methylation-sensitive restriction enzyme–based technique.8 Even though the methylation pattern atHpaII sites 5′ to the απ-globingene correlated to some extent with expression, a detailed study of the methylation pattern was not possible because of technical limitations inherent in that technique. Another study has recently shown that the domain of the chicken α-globin genes is preceded by a CpG island that is heavily methylated in lymphoid cells and is either nonmethylated or undermethylated in erythroid cells.9
Direct binding of specific transcriptional repressors to methylated DNA appears to be a major mechanism of transcriptional repression.10,11 Of the 5 proteins that have themethyl-CpG-binding domain, 4 (MBD1, MBD2, MBD3, and MeCP2) are implicated in transcriptional repression.10 MBD2 is a component of themethyl cytosine-binding protein 1 (MeCP1) complex, together with histone deacetylases HDAC1 and HDAC2.12 MBD3 is a component of the Mi2/NuRD deacetylase complex.13,14 MBD1 binds selectively to methylated DNA and represses transcription from a naked methylated promoter in vitro.10 MBD4 is a thymidine glycosylase repair enzyme and is not associated with transcriptional inactivation.15 We have shown that chicken MeCPC, which contains MBD2 and HDAC1, binds to the methylated but not the unmethylated ρ-globin gene in vitro.7 Globin gene clusters offer an interesting system to study the interaction between methylation and histone deacetylation. Despite varying methylation patterns of individual globin genes, the α- and β-globin loci are in an open chromatin configuration. At theβ-globin locus, even though methylation correlates inversely with expression, both embryonic and adult genes were highly acetylated at histones H3 and H4 in 5-day and 15-day erythrocytes.16 A recent study showed that an erythroid-specific domain of histone acetylation encompassing theα-globin genes has been conserved across several species.17
In this study, we show that methylation represses the expression of theαπ-globin gene in chicken primary erythroid cells, possibly through the formation of an MeCPC. We also show that the απ-globin gene is hyperacetylated for histones H3 and H4 in 5-day cells where it is expressed, in comparison to 14-day erythroid cells where it is transcriptionally silent.
Materials and methods
Blood collection
Fertilized chicken eggs were obtained from Truslow Farms (Chestertown, MD) and incubated to the desired degree of development in a Lyon Roll-X Automatic Incubator (Lyon Electric, Chula Vista, CA) according to the manufacturer's instructions. Blood was collected with a sterile Pasteur pipette into room temperature phosphate-buffered saline (PBS), washed twice with PBS, and spun at 320g for 5 minutes. Red blood cells (RBCs) were then resuspended in PBS and spun for 5 minutes at 720g to pellet cells.
RNA/DNA purification
Cells were resuspended in 10 volumes of RNA Stat-60 or DNA Stat-60 (Tel-Test, Friendswood, TX), and RNA or DNA was extracted following the manufacturer's protocol. Briefly, the cells were homogenized, the lysates were extracted with chloroform, and the nucleic acids were precipitated with isopropanol. Nucleic acids were resuspended in nuclease-free water (Ambion, Austin, TX) and quantitated on a Beckman DU series 64 spectrophotometer (Beckman Instruments, Fullerton, CA).
RT-PCR
Primers for απ-globin were designed so as to flank the intron-exon boundaries (accession no. V00408). PiRTF1 (5′-TCACTGGAGAGGCTTTTTGCC-3′) corresponded to positions 466 to 477 and 1055 to 1063. PiRTR1 (5′GTGGGAAAGCAGCTTGAAGTT-3′) corresponded to positions 1565 to 1554 and 1259 to 1251. Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed using the Reverse Transcription Systems from Promega (Madison, WI) following the manufacturer's protocol except that 5 μg RNA was used to make cDNA. απ-Globin cDNA was made using the specific primer PiRTR1; for β-actin, cDNA was made with the random primers included in the kit. The απ-globin cDNA was then amplified using PiRTF1 and PiRTR1; β-actin was amplified using the primers BactinRT1 (5′-CGCTCGTTGTTGACAATGGCTC-3′) and BactinRT2 (5′CCAGTTGGTGACAATACCGTGTTC-3′).
Bisulfite conversion and methylation analysis
RBCs were collected from 4-, 5-, 6-, 8-, 11-, and 14-day embryos and bisulfite treated as previously described.6,18 Primers for the amplification of the απ-globinpromoter region were designed with the help of a Microsoft Word macro program.19 Bisulfite-treated DNA was amplified using the primers PiBisulfF (5′-TTTAGTTTGTTTAAAATTTATTGAAAGG-3′, corresponding to positions −322 to −304 relative to the transcription start site) and PiBisulfR (5′-AATAAACACCCAAAACAAATTATAC-3′, corresponding to positions +22 to −3 relative to the transcription start site). Sequencing of the PCR-amplified product was performed using the forward and reverse primers. The α-33P–labeled dideoxynucleotide triphosphate (ddNTP) terminator kit (USB, Cleveland, OH) was used for sequencing. The sequencing gel was dried and exposed to a phosphorimager screen (Packard Instrument, Meriden, CT).
Plasmids
A PCR product corresponding to a 327-bp fragment of theαπ-globin promoter was amplified with the primers PiClonF1 (5′GTGAGCTCAAAATCCATTGAAAGGC-3′), which contains aSacI restriction site, and PiClonR1 (5′-GAGAAGCTTGTACTGAGTGCCCTC-3′), which contains a HindIII site. The amplified fragment was digested with restriction enzymes to create sticky ends and cloned into pGL3-Basic and pGL3-Enhancer vectors (Promega) to yield απpGL3 and απpGL3E, respectively. Methylation was accomplished with SssI methylase from New England Biolabs (Beverly, MA); the unmethylated control plasmid was treated similarly but without the addition of S-adenosylmethionine. Completion of the reaction was determined by digestion with methylation-sensitive restriction enzymes. For the probes used in electrophoretic mobility shift assays (EMSAs) the entire plasmid was methylated, and then the fragment was excised and labeled. For transient transfections, in certain experiments, the fragment was excised, methylated, and religated into the reporter plasmid.
Transient transfections
RBCs were resuspended to a final volume of 100 μL per transfection and then spun down. The pellets were resuspended in 1 mL filter-sterilized NH4Cl (pH 7.2-7.3), freshly made, and incubated at room temperature for 2 hours with periodic mixing, spun down, and resuspended in 0.5 mL transfection medium (66.6% [vol/vol] L-15 [Sigma Chemical, St Louis, MO]; 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.45; 300 μg/mL diethylaminoethyl [DEAE]–Dextran) containing the 2 μg of the DNA construct to be transfected and 200 ng of the plasmid pRL-TK as a control for transfection efficiency. All transfections were done in triplicate. The transfections were incubated at room temperature for 10 minutes, then at 37°C for 10 minutes, and then spun down. Pellets were washed with 800 μL chicken culture medium (74% L-15, 22% chicken serum [Gibco, Grand Island, NY], 3.5% fetal bovine serum [FBS] [Gibco], 0.5% penicillin-streptomycin solution [Sigma]) and then resuspended in 500 μL culture medium and added to culture flasks containing 5 mL medium. Cells were grown for 48 hours and then harvested.
Luciferase assays
Luciferase assays were performed using the Dual Luciferase Reporter Assay system from Promega following the manufacturer's protocol for single sample luminometers. The cell suspensions were spun down, and the RBC pellets were washed twice with PBS and then resuspended in 500 μL 1 × passive lysis buffer (Promega). The cell lysates were cleared by centrifugation for 2 minutes at 4°C and then transferred to fresh 1.5 mL tubes and stored at −80°C. Luciferase and Renilla activity were measured on a TD-20/20 Luminometer from Turner Designs (Sunnyvale, CA). Results were reported in terms of the ratio of luciferase to Renilla activity and expressed as the percentage of the value of unmethylatedαπ-globin promoter-driven activity.
Electrophoretic mobility shift assays
RBC nuclear extracts from 14-day embryos were prepared according to a modified Dignam procedure as described previously.6,7The probes used were in vitro–methylated and mock-methylatedαπ-globin promoter excised from απpGL3 and labeled with [α-32P]deoxycytidine triphosphate (dCTP) (New England Nuclear, Boston, MA) using the Klenow fragment of DNA polymerase I. Assay conditions were as previously described.6 7 Two micrograms of sonicatedMicrococcus lysodeikticus DNA was used as a nonspecific competitor in all assays. Assays were performed on 2% agarose gels, run approximately 3 hours at 150 V in 0.5 × TBE (Tris-borate-ethylenediaminetetraacetic acid [EDTA]), and then dried and analyzed using a Cyclone phosphorimager (Packard Instrument).
For antibody/supershift ablation experiments, EMSA was performed with or without the addition of one of the following antisera (1.5 μL) or antibodies (800 ng): anti-MBD2 antisera, anti-MBD2 IgG (Upstate Biotechnology, Lake Placid, NY), anti-MBD1, anti-MBD3, and anti-MBD4 IgG (Santa Cruz Biotechnology, Santa Cruz, CA).
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were carried out with a kit from Upstate Biotechnology using the manufacturer's protocol and reagents except that the reactions were scaled down 10-fold. Briefly, 2 × 107 cells were incubated in 1% formaldehyde for 10 minutes to cross-link bound proteins, washed, lysed in sodium dodecyl sulfate (SDS) lysis buffer, and sonicated to 100- to 500-bp lengths; 10 μL chromatin was mixed with 90 μL dilution buffer and precleared with protein A–agarose, and then the chromatin was incubated with antibody overnight at 4°C. Thirty μL protein A–agarose beads was added, and the chromatin was immunoprecipitated 2 hours at 4°C. The supernatant (unbound chromatin) and beads (bound chromatin) were separated. The beads were washed 5 times with the buffers provided, and then the chromatin was eluted twice in 1% SDS in 0.1 M NaHCO3. Both bound and unbound chromatin fractions were de–cross-linked by the addition of 5 M NaCl and incubation at 65°C for at least 4 hours. Proteins were digested by proteinase K, and then chromatin was extracted with phenol/chloroform. DNA was ethanol precipitated and dissolved in 100 μL water. The experiment was repeated on 3 separate occasions.
Quantitative real-time PCR
Primers for the analysis of the απ-globin promoter were designed with the Primer Express software package that accompanies the Applied Biosystems model 7700 sequence detector (Applied Biosystems, Foster City, CA) and are as follows (positions indicated are relative to the transcription start site): PiChipF (5′-CCAATACGTGTTCAGAAGCAAGAA-3′), corresponding to positions −210 to −187; PiChipR (5′-AGCTTGGGTCAGTGCCATT-3′), corresponding to positions +72 to +54.
The real-time PCR was performed in triplicate using 10 μL bound DNA and 5 μL unbound DNA template obtained after chromatin immunoprecipitation, the SYBR Green PCR Master Mix (Applied Biosystems), and 400 nM of each primer. The cycling conditions were 50°C for 2 minutes, 95°C for 10 minutes, followed by 50 cycles of 95°C for 15 seconds and 60°C for 1 minute each. A dissociation curve was created using software from Applied Biosystems to confirm the presence of a single PCR product. Relative quantitation of template DNA was performed as described in the User Bulletin no. 2, ABI Prism 7700 Sequence Detection System (Applied Biosystems).
Results
Expression of the chicken απ-globingene during development in primary erythroid cells
We examined the expression of avian α-type embryonicαπ-globin during development in primary erythroid cells using RT-PCR. απ-Globin mRNA is easily detected in 4- to 5-day primitive embryonic erythroid cells but is barely detectable by day 11 of embryonic development (Figure1). These results are consistent with the earlier studies that examined the expression of avian α-type globin genes during development in erythroid cells.20
Expression of the απ-globin gene during development in chicken erythroid cells.
Ten nanograms of RNA was used for RT-PCR analysis. Lane C indicates “control” lane with no template. To control for loading and RNA integrity, RT-PCR analysis was carried out with β-actin primers.
Expression of the απ-globin gene during development in chicken erythroid cells.
Ten nanograms of RNA was used for RT-PCR analysis. Lane C indicates “control” lane with no template. To control for loading and RNA integrity, RT-PCR analysis was carried out with β-actin primers.
Methylation analysis of the chicken απ-globin gene during development in primary erythroid cells
We have previously shown that the chicken β-type embryonic globin gene (ρ-globin) promoter is completely unmethylated in primitive erythroid cells and completely methylated in erythroid cells from adult chickens.6 To elucidate the methylation pattern of the chicken α-type embryonic globin gene(απ-globin) during development, we employed the bisulfite genomic sequencing method.11,21 This technique is based on bisulfite-induced oxidative deamination of genomic DNA under conditions in which cytosine is converted to uracil and 5-methylcytosine remains unchanged. The target sequence is amplified by PCR using strand-specific primers. Upon sequencing of the amplified DNA, all uracil and thymine residues become detectable as thymine and only 5-methylcytosine residues amplify as cytosines. Our laboratory has developed a Microsoft Word macro to facilitate primer design for bisulfite genomic sequencing.19 We determined the methylation pattern of απ-globin gene promoter during development in primary erythroid cells. The CpG dinucleotides in the απ-globin gene promoter are completely unmethylated in day 5 erythroid cells (Figure2). Methylation progresses during development and is complete in DNA from adult erythroid cells. The genomic DNA from the brain tissue of 5-day-old chicken embryos and oviduct from adult chicken also demonstrated complete methylation (data not shown).
Methylation pattern of CpG dinucleotides in the chicken απ-globin gene promoter.
(A) The chicken απ-globin gene promoter sequence. The bent arrow indicates the transcription start site. CpG dinucleotides are in bold and underlined. (B) Methylation analysis of the chicken απ-globin gene promoter in primary erythroid cells during development. Arrows indicate cytosine residues at CpG dinucleotides. These cytosines have been completely converted to thymidines (indicating unmethylated cytosines) in DNA from day 5 embryonic erythroid cells. Progressive failure of conversion to thymidines (indicative of methylation) is seen during development, and DNA derived from adult erythroid cells shows methylated cytosines.
Methylation pattern of CpG dinucleotides in the chicken απ-globin gene promoter.
(A) The chicken απ-globin gene promoter sequence. The bent arrow indicates the transcription start site. CpG dinucleotides are in bold and underlined. (B) Methylation analysis of the chicken απ-globin gene promoter in primary erythroid cells during development. Arrows indicate cytosine residues at CpG dinucleotides. These cytosines have been completely converted to thymidines (indicating unmethylated cytosines) in DNA from day 5 embryonic erythroid cells. Progressive failure of conversion to thymidines (indicative of methylation) is seen during development, and DNA derived from adult erythroid cells shows methylated cytosines.
Methylation of a luciferase reporter construct driven by απ-globin promoter represses expression in transient transfection assays
To determine if methylation ofαπ-globin represses transcription in primary erythroid cells, a 315-bp απ-globin promoter region was cloned in an expression construct (απpGL3E) containing a luciferase reporter gene and SV40 enhancer. The απpGL3E construct was transfected into primary erythroid cells derived from 5-day-old chicken embryos. Methylation of απpGL3E resulted in a 20-fold inhibition of expression (Figure 3A). Because methylation of the transcribed region and/or enhancer can inhibit transcription, it is possible that the inhibition of repression could be due entirely to the methylation of the luciferase gene and SV40 enhancer. To test whether methylation of the απ-globin promoter alone can inhibit transcription, απ-globinpromoter was excised, either methylated or mock-methylated withSssI methylase, religated back into the reporter plasmid, and transfected into primary erythroid cells. As shown in Figure 3B, methylation of the απ-globin promoter sequences resulted in a 7-fold inhibition of expression.
Methylation of απ-promoted reporter vector (απpGL3E) represses transcription.
Primary erythroid cells were transfected with the (A) unmethylated and fully methylated απpGL3E vectors or (B) απpGL3E vector in which only the απpromoter has been methylated or mock methylated. Cells were transfected with 2 μg expression vector. After 48 hours' growth in complete medium, cell lysates were prepared and assayed for firefly luciferase activity as described in “Materials and methods.” The figure shows the relative luciferase activity of the unmethylated and methylated vectors. The values shown are normalized to pRL-TK as a control for transfection efficiency. The error bars represent the SEM for measurements of 3 different samples.
Methylation of απ-promoted reporter vector (απpGL3E) represses transcription.
Primary erythroid cells were transfected with the (A) unmethylated and fully methylated απpGL3E vectors or (B) απpGL3E vector in which only the απpromoter has been methylated or mock methylated. Cells were transfected with 2 μg expression vector. After 48 hours' growth in complete medium, cell lysates were prepared and assayed for firefly luciferase activity as described in “Materials and methods.” The figure shows the relative luciferase activity of the unmethylated and methylated vectors. The values shown are normalized to pRL-TK as a control for transfection efficiency. The error bars represent the SEM for measurements of 3 different samples.
Methylated απ-globin promoter sequences form a methylcytosine binding protein complex
Studies from our laboratory have shown previously that methylated ρ-gene promoter sequences can bind to an MeCP1-like complex, MeCPC.6 We have also shown that a methylated 248-bp proximal transcribed region of the ρ-globin gene forms a cell type–specific MeCPC that contained MBD2 and HDAC1 proteins but differs from MeCP1.7 We examined whether the transcriptional repression conferred by methylatedαπ-globin promoter sequences correlates with their affinity for MeCPC, which would imply a role for MeCPC in mediating transcriptional repression. End-labeled methylated and mock-methylated απ-globin probes were incubated with 14-day erythroid cell nuclear extract and then subjected to EMSA to detect an MeCP1-like complex as described.6 7On autoradiography, a complex was observed with methylatedαπ-globin probe, as shown in Figure4A, and this complex was effectively competed by a 25-fold molar excess of cold methylatedαπ-globin but not by a 200-fold excess of cold unmethylated απ-globin fragment (Figure4B). However, unlike the complex observed with ρ-globingene proximal transcribed sequence, this complex could not be depleted with MBD2 antibodies or MBD2 antiserum (data not shown). We also performed EMSA using antibodies against other known methylcytosine binding proteins (MBD1, MBD3, MBD4, and MeCP2). No depletion or supershift of the complex was observed. This could be due to either failure of the antibodies (raised against human proteins) to recognize chicken homologs or due to the presence of yet unknown proteins present in the complex. Further efforts to determine the composition of this complex are underway. Interestingly, this complex was also seen with 5-day chicken red cell nuclear extract (data not shown), suggesting that the proteins that bind to methylatedαπ-globin promoter are present throughout development.
Methylated απ-globinpromoter interacts with MeCPC.
(A) A 32P-labeled mock-methylated or methylatedαπ-globin probe was incubated with increasing amounts of primary erythroid cell nuclear extract, followed by agarose gel electrophoresis and autoradiography. (B) EMSA analysis of MeCPC competition in erythroid cell nuclear extract. A32P-labeled methylated απ-globinprobe was incubated with nuclear extract from 11-day avian erythroid cells with increasing molar excess of mock-methylated or methylatedαπ-globin fragment, as indicated above each lane, followed by agarose gel electrophoresis and autoradiography.
Methylated απ-globinpromoter interacts with MeCPC.
(A) A 32P-labeled mock-methylated or methylatedαπ-globin probe was incubated with increasing amounts of primary erythroid cell nuclear extract, followed by agarose gel electrophoresis and autoradiography. (B) EMSA analysis of MeCPC competition in erythroid cell nuclear extract. A32P-labeled methylated απ-globinprobe was incubated with nuclear extract from 11-day avian erythroid cells with increasing molar excess of mock-methylated or methylatedαπ-globin fragment, as indicated above each lane, followed by agarose gel electrophoresis and autoradiography.
Histone H3 and H4 acetylation pattern of the απ-globin promoter in day 5 and day 15 chicken primary erythroid cells
Histone acetylation and deacetylation play important roles in transcriptional regulation.22 Methylated cytosines are important in guiding histone deacetylases to specific DNA sequences.23,24 This suggests that methylation represses transcription by recruiting HDAC activity, resulting in hypoacetylation of histones in methylated DNA. We measured the relative level of histone acetylation of the απ-globinpromoter in day 5 and 14 erythroid cells using a chromatin immunoprecipitation assay. Formaldehyde cross-linked chromatin was immunoprecipitated with antibodies against acetylated histones H3 and H4. The antibody-bound DNA was analyzed for theαπ-globin gene using a quantitative real-time PCR approach.25 There are many advantages to quantifying gene sequences using this technology, the foremost being sensitivity and precision. ChIP assays have previously been based on qualitative or semiquantitative analysis usually involving the examination of PCR products at a fixed cycle number, followed by densitometry of the radioactive band or ethidium bromide–stained DNA.26-28
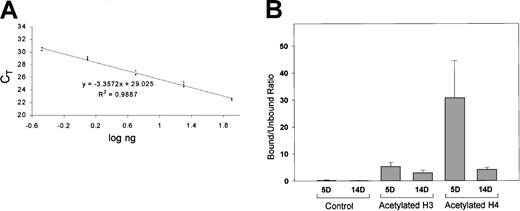
Slight hyperacetylation of histone H3 but a marked hyperacetylation of histone H4 was seen in 5-day when compared with 14-day erythroid cells (Figure 5B). These results are consistent with the recruitment of histone deacetylase–containing complexes by methylated DNA, resulting in a localized deacetylation.
ChIP/quantitative real-time PCR for analyses of the acetylated histones H3 and H4 at the απ-globin promoter in day 5 and day 14 embryonic chicken erythroid cells.
(A) Standard curve generated using varying amounts of chicken genomic DNA. X-axis shows log ng of chicken genomic DNA used as template, and y-axis shows the threshold cycle (CT) value. Based on the standard curve, a linear regression equation was determined. This equation was used to calculate the amount of input DNA from CT values. (B) Bound-unbound ratio for control (chromatin undergoing all the steps in ChIP assay but without addition of an antibody), acetylated histone H3, and acetylated histone H4. Results for acetylated histones H3 and H4 have been normalized for control. Error bars indicate results obtained with 3 independent experiments. For each experiment, real-time PCR was performed in triplicate, and a mean of CT values was used for calculation of the input DNA.
ChIP/quantitative real-time PCR for analyses of the acetylated histones H3 and H4 at the απ-globin promoter in day 5 and day 14 embryonic chicken erythroid cells.
(A) Standard curve generated using varying amounts of chicken genomic DNA. X-axis shows log ng of chicken genomic DNA used as template, and y-axis shows the threshold cycle (CT) value. Based on the standard curve, a linear regression equation was determined. This equation was used to calculate the amount of input DNA from CT values. (B) Bound-unbound ratio for control (chromatin undergoing all the steps in ChIP assay but without addition of an antibody), acetylated histone H3, and acetylated histone H4. Results for acetylated histones H3 and H4 have been normalized for control. Error bars indicate results obtained with 3 independent experiments. For each experiment, real-time PCR was performed in triplicate, and a mean of CT values was used for calculation of the input DNA.
Discussion
The distribution of methylated and unmethylated CpG dinucleotides in vertebrates conforms to a generalized pattern. About 70% to 80% of CpG sites contain methylated cytosines.29Promoter region CpG islands are usually unmethylated in all normal tissues regardless of the transcriptional activity of the gene.11,30 The inverse relationship between expression and methylation of β-type globin genes is well established.4-6 We have shown that, in the case of the developmentally regulated ρ-globin gene, methylation of both the CpG-dense (promoter and proximal transcribed region) and CpG-poor (distal transcribed region) regions correlates inversely with the stage-specific expression in avian erythroid cells.6,7In this study we have shown that methylation of the chicken embryonic α-type globin gene correlates inversely with the stage-specific expression in primary erythroid cells. Further, methylation of the απ promoter alone at the exact CpGs that are methylated in vivo resulted in transcriptional repression in a transient transfection assay. The repressive effect was more pronounced when the whole plasmid was methylated. This is likely to be related to the methylation of the luciferase gene and enhancer, because previous studies have shown that methylation of the transcribed region alone can mediate transcriptional repression.7 31
Direct binding of specific transcriptional repressor complexes to methylated DNA appears to be a major mechanism of transcriptional repression.10 MeCP1 binds to DNA containing multiple symmetrically methylated CpGs and migrates as a large complex on EMSA.32 MeCP1 has been shown to repress transcription from densely methylated genes, and cells deficient in MeCP1 show much reduced repression of methylated genes.33 We have recently shown that a novel tissue- and/or sequence-specific methyl CpG binding protein complex MeCPC exists in chicken erythrocytes.7This complex contains MBD2 and binds to sequences in the methylated but not the unmethylated ρ-globin gene proximal transcribed region. In the present study we have shown that in chicken erythroid cell nuclear extracts MeCPC binds to methylated but not to unmethylatedαπ-globin gene promoter sequences. Interestingly, in contrast to the MeCPC observed withρ-globin gene proximal transcribed region, the one observed with απ promoter sequences could not be supershifted or ablated with MBD2 antibodies, suggesting that different methylated sequences may interact with different components of MeCPC. MBD2, but not MeCP2, has been shown to bind to methylated p16 or p14 promoters in colon carcinoma cell lines.34 Interestingly, no significant binding of MBD2 to methylated Alu elements that are located between p16 and p14 was detected.
Recent evidence suggests that acetylation of the amino-terminal tails of H3 and H4 may be a principal regulator of transcription factor access to nucleosomal DNA.35 The underlying patterns of methylated cytosines are important in guiding histone deacetylases to specific DNA sequences.23,24 This suggests that methylation represses transcription by recruiting HDAC activity, resulting in hypoacetylation of histones in methylated DNA. Consistent with this hypothesis, we found the απ-globinpromoter to be enriched in acetylated H3 and H4 in chromatin derived from day 5 erythroid cells when compared with day 14 erythroid cells. Even though a domain of histone acetylation exists across theα-globin gene cluster, our results indicate that the acetylation pattern of the απ-globin varies with developmental stages.17 Similarly, the acetylation pattern of murine β-globin genes was found to vary at different developmental stages.28 Interestingly, the degree of deacetylation at the απ-globinpromoter was more prominent for histone H4 than for H3. For the β-type globin genes, other studies have shown a more prominent acetylation for H3 for the active ε-globin promoter or β-promoter.26,27 Individual activators or repressors may confer distinct patterns of histone acetylation on target promoters.36 In a recent study, mice were produced with transgene-induced methylation at the paternal allele of an imprinted gene, U2af1-rs1. In these mice, H3 was underacetylated across both the parental U2af1-rs1 alleles, whereas H4 acetylation was unaltered.37 In contrast, Rett syndrome mutations involving MeCP2 in clonal cell cultures resulted in no intact MeCP2 protein and hyperacetylated H4, but not H3.38 It is likely that methylated sequences repress transcription by interacting with distinct MBD proteins and HDAC complexes, resulting in a differential histone acetylation pattern. This may also account for the reactivation of some methylated genes, but not others, with histone deacetylase inhibitors.10,39 40
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-02-0457.
Supported by a grant from the Department of Veterans Affairs and by funding from the Feist-Weiller Cancer Center (R.S.). R.S. is a recipient of the “Advanced Research Career Development Award” from the Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rakesh Singal, Associate Professor of Medicine, Section of Hematology/Oncology, Overton Brooks VA Medical Center & LSU Health Sciences Center, 510 East Stoner Ave, 111-H, Shreveport, LA 71101-4295; e-mail:rakeshsingal@hotmail.com.