Reactivation of fetal hemoglobin genes has been proposed as a potential therapeutic procedure in patients with β-thalassemia, sickle cell disease, or other β-hemoglobinopathies. In vitro model systems based on small plasmid globin gene constructs have previously been used in human and mouse erythroleukemic cell lines to study the molecular mechanisms regulating the expression of the fetal human globin genes and their reactivation by a variety of pharmacologic agents. These studies have led to great insights in globin gene regulation and the identification of a number of potential inducers of fetal hemoglobin. In this study we describe the development of enhanced green fluorescence protein (EGFP) reporter systems based on bacterial artificial chromosomes (EBACs) to monitor the activity of the ε-, Gγ-, Aγ-, δ-, and β-globin genes in the β-globin locus. Additionally, we demonstrate that transfection of erythroleukemia cells with our EBACs is greatly enhanced by expression of EBNA1, which also facilitates episomal maintenance of our constructs in human cells. Our studies in human cells have shown physiologically relevant differences in the expression of each of the globin genes and also demonstrate that hemin is a potent inducer of EGFP expression from EGFP-modified ε-, Gγ-, andAγ-globin constructs. In contrast, the EGFP-modified δ- and β-globin constructs consistently produced much lower levels of EGFP expression on hemin induction, mirroring the in vivo ontogeny. The EGFP-modified β-globin eukaryotic BAC (EBAC) vector system can thus be used in erythroleukemia cells to evaluate induction of the ε- and γ-globin genes from the intact human β-globin locus.
Introduction
β-Thalassemia and sickle cell disease are among the most common inherited genetic disorders in the world. In β-thalassemia, unpaired α-globin chains accumulate and precipitate within erythroid cells, resulting in red cell damage, hemolysis, ineffective erythropoiesis, and anemia. In sickle cell disease, intracellular accumulation of polymerized hemoglobin causes sickling of red blood cells and vascular occlusion. Amelioration of clinical symptoms associated with these hemoglobinopathies has been reported in patients with elevated levels of γ-globin chain synthesis. In β-thalassemia, the presence of γ-globin inhibits the precipitation of unpaired α-globin through the formation of fetal hemoglobin (HbF, α2γ2,), whereas in sickle cell disease the presence of γ-globin inhibits the polymerization of sickle hemoglobin (α2β) via the formation of mixed hemoglobin tetramers (α2βsγ) and the maintenance of a higher oxygen tension in the capillaries.1
A diverse group of genetic mutations termed hereditary persistence of fetal hemoglobin (HPFH) are associated with high levels of HbF in adult life. Coexistence of HPFH with homozygous β-thalassemia or sickle cell disease often results in complete phenotypic complementation of the disease.2 Many of the HPFH mutations are the result of deletions or point mutations in the β-globin locus.3However, a small number of HPFH cases have been identified that are not linked to the β-globin locus, implicating the presence of trans-acting factor(s).4-6 Other genetic conditions that contribute to elevated levels of HbF include metabolic disorders such as propionic acidemia7 and β-ketothiolase deficiency,8 implicating short chain fatty acids in the induction of HbF. In addition, many acquired conditions have also been reported to reactivate HbF, including pregnancy and starvation ketosis.9 Pharmacologic reactivation of HbF has therefore been proposed as a potential therapeutic strategy for the treatment of hemoglobinopathies (for review see Olivieri and Weatherall10). Drugs such as 5-azacytidine (5-AzaC),11 hydroxyurea (HU),12 and butyrate analogues13,14 have been shown to increase HbF synthesis in patients. However, many of these drugs have low efficacy and specificity, while some are potentially carcinogenic. There is therefore an urgent need to identify new types of pharmacologic agents that can induce HbF with greater efficacy and less toxicity.15-18
A number of published studies have focused on the development of sensitive assays for HbF inducers using various reporters in small plasmid constructs, in combination with some of the regulatory elements from the β-globin locus. While some of these assays have yielded interesting results,19-21 it is questionable whether the use of globin regulatory elements out of their natural context can recapitulate the requirements for therapeutic HbF reactivation in the erythropoietic compartment.
The usefulness of an assay system for HbF reactivation is critically dependent on the choice of cell line to be used. The human erythroleukemia K562 cell line has been shown to undergo erythroid differentiation and γ-globin gene expression after treatment with a variety of chemical compounds.10-18 Importantly, many of these compounds have also been reported to stimulate HbF production in human erythroid precursor cells from healthy subjects and in human patients. Murine erythroleukemia cells (MEL) with an adult type of hemoglobin expression have also been used extensively in globin gene expression studies. Studies on mice transgenic for the human β-globin locus indicate that the pattern of developmental regulation is generally well retained, although the γ- to β-globin switch appears to operate earlier in mice than in humans.22-26Furthermore, the mouse transcriptional machinery does not appear to transcribe the human β-globin locus as efficiently as the mouse locus, presumably as a result of variations in the regulatory sequences and the amino acid sequences of the various factors needed for transcription. It has also been reported that the direct transfer of a β-globin yeast artificial chromosome (YAC) into MEL cells resulted in loss of position-independent expression of the globin genes,27 suggesting that the human locus control region (LCR) may not function properly when the locus is directly transferred into a murine erythroid cell.
In this study, we have developed novel assay systems for the assessment of globin gene expression using a bacterial artificial chromosome (BAC) containing the intact human β-globin locus. One of the main advantages of BACs is that they carry genomic fragments in the 100 kb to 300 kb range, which are large enough to contain most genes intact together with their long-range regulatory elements that are essential for regulated gene expression in a tissue-specific manner.28,29 The development of genetic modification techniques for BACs, such as recE/recT-based homologous recombination,30-32 and site-specific mutagenesis,33 has also largely overcome the technical limitations in manipulating large genomic BAC clones. Recently, the development of eukaryotic-BAC vectors (EBACs) has further simplified functional gene expression studies in eukaryotic cells.30,34 EBACs contain the bacterial elements of BACs and a number of other eukaryotic elements to facilitate extrachromosomal replication, episomal maintenance, and functional studies in eukaryotic cells. Such elements include the Epstein-Barr virus (EBV) nuclear antigen-1 (EBNA1),35 the latent origin of EBV replication (oriP), and positive and negative selectable markers.36 These vectors can readily be “shuttled” between bacterial and human cells, thus allowing for the rapid modification of genomic fragments in bacteria and their functional analysis in eukaryotic cells.
We report here the development of enhanced green fluorescence protein (EGFP) reporter systems to monitor the activity of the ε-,Gγ-, Aγ-, δ-, and β-globin genes in the human erythroleukemic K562 cell line, in the context of the human β-globin locus, cloned in an EBAC vector. The EGFP insertions were designed to replace each of the ε-, Gγ-,Aγ-, δ-, and β-globin coding regions with the EGFP gene, such that EGFP expression is driven by the upstream regulatory elements. In this context, expression of the EGFP reporter construct should reflect globin chain synthesis, and may therefore be used to evaluate globin gene expression in human erythroid cells. We demonstrate physiologically relevant expression of EGFP from each globin promoter in response to hemin-induced erythroid differentiation. Our results show that the EGFP-modified β-globin locus EBAC reporter system represents a novel assay system, which may be used to detect and evaluate potentially therapeutic compounds that can alter globin chain synthesis, thus enabling a rational approach to drug design and evaluation of globin gene inducers.
Materials and methods
Construction of EBAC vectors
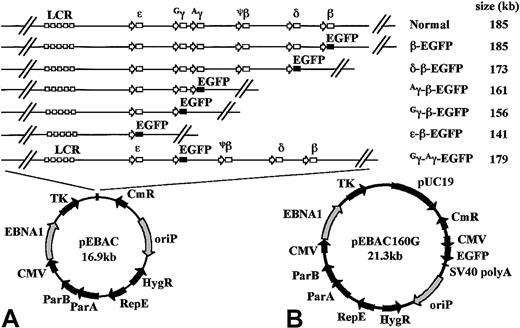
The P1-derived artificial chromosome (PAC) clone (148O22) was identified from the RPCI I PAC library to contain the β-globin locus in a 185-kb genomic fragment (http://www.chori.org/bacpac/).29 Further detailed mapping revealed that the 73-kb sequence of the β-globin locus (accession no.U10317) is flanked by 92 kb of sequence at the 5′ end and 20 kb of sequence at the 3′ end. The 185-kb genomic fragment was retrofitted into the pEBAC140 cloning vector as a single NotI fragment to generate pEBAC/148β (Figure 1, also referred to as pBH148β34). The pEBAC140 vector is a hybrid system based on the bacterial F1 origin of replication of BACs and the EBNA1/oriP elements of EBV-based vectors. The pEBAC160G vector was generated from pEBAC140 by the insertion of a modified pUC19 sequence into the multicloning site, and by the blunt end cloning of an AflIII-AflII fragment from pEGFP-N22 (see next paragraph) into the uniqueBst1107I site (Figure 1). Last, the 185-kb globin genomic fragment was retrofitted into the pEBAC160G vector to produce pEBAC/148βG, a 205-kb clone in which EGFP expression from the backbone of the vector may be used to monitor transfection efficiency of large BACs.
Schematic representation of the EGFP-modified β-globin EBACs used in this study.
(A) An EGFP-Neo/Kan expression cassette starting with the first codon of the EGFP gene and finishing with the last codon of the Neo/Kan gene was inserted by GET Recombination between the start codon of either the β-, δ-, Aγ-, Gγ-, or ε-globin genes and the termination codon of the β-globin gene, in the 185-kb genomic insert of pEBAC/148β which contains the intact β-globin locus. This created a series of deletions ranging in size from 1.4 kb to 44 kb, while placing the EGFP gene under the regulatory elements of the corresponding gene in the context of the β-globin locus. In the Gγ-Aγ-EGFP construct, the EGFP-expression cassette was inserted between the start codon of theGγ- and the stop codon of the Aγ-globin gene, with the deletion of all intervening genomic sequences. The approximate size of the resulting genomic insert in each construct is indicated. The genomic insert in each construct was maintained as a single NotI fragment in the rare multicloning site of the pEBAC vector. (B) The complete pEBAC160G cloning vector containing a modified pUC19 in the multicloning site and the EGFP reporter gene driven by the cytomegalovirus early promoter on the backbone of the vector.
Schematic representation of the EGFP-modified β-globin EBACs used in this study.
(A) An EGFP-Neo/Kan expression cassette starting with the first codon of the EGFP gene and finishing with the last codon of the Neo/Kan gene was inserted by GET Recombination between the start codon of either the β-, δ-, Aγ-, Gγ-, or ε-globin genes and the termination codon of the β-globin gene, in the 185-kb genomic insert of pEBAC/148β which contains the intact β-globin locus. This created a series of deletions ranging in size from 1.4 kb to 44 kb, while placing the EGFP gene under the regulatory elements of the corresponding gene in the context of the β-globin locus. In the Gγ-Aγ-EGFP construct, the EGFP-expression cassette was inserted between the start codon of theGγ- and the stop codon of the Aγ-globin gene, with the deletion of all intervening genomic sequences. The approximate size of the resulting genomic insert in each construct is indicated. The genomic insert in each construct was maintained as a single NotI fragment in the rare multicloning site of the pEBAC vector. (B) The complete pEBAC160G cloning vector containing a modified pUC19 in the multicloning site and the EGFP reporter gene driven by the cytomegalovirus early promoter on the backbone of the vector.
The construction of the EGFP-modified pEBAC/148β clones has previously been described.37 In brief, pEGFP-N2 vector (Clontech, Palo Alto, CA) was modified by removing the multicloning site located at the 5′ end of the EGFP gene using BglII andBamHI double digest, and by blunting the NotI site located downstream of the EGFP gene, resulting in the pEGFP-N22 vector. The pEGFP-N22 vector was then used as a template to amplify by polymerase chain reaction (PCR) the 2.7-kb EGFP-Neo/Kan cassette. The EGFP-Neo/Kan PCR product, designed to be flanked by 50 nucleotides of pEBAC/148β homology, was introduced into the pEBAC/148β via homologous recombination using the pGETrec plasmid. The plasmid pGETrec is a 6.5-kb plasmid that contains the Escherichia coli recEand recT genes and bacteriophage λ gam gene in a polycistronic operon.30 The 5′ end of the EGFP-Neo/Kan expression cassette was placed in frame at the start codon of either the ε-, Gγ-, Aγ-, δ-, or β-globin genes, while the 3′ end of the EGFP-Neo/Kan expression cassette was inserted at the 3′ end of the β-globin gene, resulting in a series of deletions ranging from 1.4 kb to 44 kb (Figure 1). The Gγ-Aγ–EGFP construct was similarly created by targeting the EGFP-Neo/Kan cassette between the start codon of the Gγ gene and the termination codon of theAγ gene. The EGFP-Neo/Kan cassette was amplified with primers γEGFP-F, 5′-CTCGCTTCTGGAACGTCTGAGATTATCAATAAGCTCCTAGTCCAGACGCCATGGTGAGCAAGGGCGAGGAGC-3′ and γNEO-R, 5′-CTTGCAGAATAA AGCCTATCCTTGAAAGCTCTGAATCATGGGCAAGAGGCCCAGAGTCCCGCTCAGAAG-3′. Due to the high homology between the 2 γ-globin genes, these primers can target to the start and end of each γ-globin gene, thus yielding recombinants with replacement of either of the 2 γ-globin genes with the EGFP-Neo/Kan cassette, or with the simultaneous replacement of both γ-globin genes by the cassette. TheGγ-Aγ–EGFP construct was distinguished from the Gγ-EGFP and Aγ-EGFP constructs using the screening primers γF, 5′-CCTCACTGGAGCTACAG ACAAGA-3′,GγR, 5′-GCACATGACACAAACACACATAG-3′ and AγR, 5′-ACTTGCAGAACTCCCGTGTA-3′.
Preparation of EBAC vectors
The EBAC vectors were propagated in the E coli strain DH10B (Invitrogen, Carlsbad, CA). Bacterial cultures were routinely grown in Luria broth (LB) liquid culture, or on LB agar plates (15 g/L) containing the following antibiotics 12.5 μg/mL chloramphenicol (Cm) or 25 μg/mL kanamycin (Km). Large-scale preparation of EBAC constructs for transfection studies was performed using CsCl–ethidium bromide density gradient centrifugation.
Construction of K562-EBNA1 cell lines
Stable K562 cell lines that constitutively express EBNA1 were generated by transfection with the pEB vector (kindly provided by Dr Burt, Division of Molecular Biology, Roslin Institute, Edinburgh, Scotland, United Kingdom).38 The pEB vector contains a picornaviral internal ribosome entry site that allows the EBNA1 gene and neomycin resistance gene to be transcribed as a dicistronic mRNA under the control of the phosphoglucokinase (PGK) promoter. The pEB vector (10 μg) was linearized with MluI restriction endonuclease and transfected into K562 cells (2 × 106) by lipofection using DMRIE-C (Invitrogen), at a 4:1 lipid-to-DNA ratio, according to the manufacturer's recommended protocol. Cells were seeded 2 days following transfection in 96-well flat-bottom plates and selected in Dulbecco modified Eagle medium (Sigma, Castle Hill, NSW, Australia) supplemented with G418 (400 μg/mL). G418-resistant colonies were isolated via limiting cell dilution. The presence of the EBNA1 gene was confirmed by EBNA1-specific PCR38 and the relative levels of EBNA1 protein expression were measured by flow cytometry using an EBNA1-specific monoclonal antibody (Mab) 1H439 (kindly provided by Dr Grasser, Institut fur Medizinische Mikrobiologie und Hygiene, Abteilung Virologie, Universitatskliniken, Homburg/Saar, Germany). Individual clones were evaluated for their transfectability with the pEBAC160G vector and the pEBAC/148β/EGFP construct. A single clone (denoted clone 8.13) with moderate levels of EBNA1 expression and high transfectability was selected for subsequent studies.
Cell cultures
The human erythroleukemia cell line K562 and the K562-EBNA1 derivatives were maintained in continuous culture in Dulbecco modified Eagle medium (Sigma) supplemented with 10% fetal calf serum (FCS) for K562 cells and K562-EBNA1 cells, 100 U/mL penicillin, and 100 μg/mL streptomycin. K562-EBNA1 cells transfected with EBACs were grown in media containing 20% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 400 μg/mL hygromycin and supplemented with an antioxidant mix (1 mM sodium pyruvate, 50 μM α-thioglycerol, and 20 nm bathocuprionedisulfonate).40 The cell density was maintained between 1 × 105 cells/mL and 8 × 105 cells/mL and cultures were incubated at 37°C in a humidified 5% CO2 incubator.
Transfection of EBAC vectors into K562 and K562-EBNA1 cells
Transient transfection of K562 and K562-EBNA1 cells with pEBAC160G was carried out for 5 hours using DMRIE-C (Invitrogen) at a 4:1 lipid-to-DNA ratio, according to the manufacturer's recommended procedure optimized in the absence of phorbol myristate acetate (PMA), since PMA is used to enhance the differentiation of K562 cells toward the megakaryocytic lineage.41 42 Transfection of pEBAC/148β EGFP-modified β-globin constructs was similarly carried out at a 2:1 lipid-to-DNA ratio. For the establishment of episomal cultures, hygromycin was added after 48 hours (400 μg/mL) and maintained throughout the growth of the cultures.
Green fluorescent protein expression analysis
The percentage of EGFP-positive cells and relative levels of EGFP expression were measured by flow cytometry using a FACScan flow cytometer (Becton Dickinson, Los Angeles, CA). K562 or K562-EBNA1 cells (2 × 106) transfected with EGFP-modified EBAC constructs were assayed 2 days following transfection and grown for 40 days in media containing hygromycin (400 μg/mL) before further analysis. Data acquisition and analysis were performed using WinMDI software (http://pingu.salk.edu /software.html). Propidium iodide (0.25 μg/mL) was added to transfected cells to exclude dead cells from analysis.
Hemin induction
Hemin (Sigma) (5 mM stock solution) was prepared as previously described.43 K562-EBNA1 cells transfected with EBAC vectors were cultured in media containing hygromycin (400 μg/mL) for 40 days prior to hemin induction. Transfected cells (3 × 105) were induced with 0 μM to 100 μM of hemin in the absence of hygromycin, and analyzed by flow cytometry on days 3 and 5. The effects of hemin on each globin promoter were examined by measuring the percentage of EGFP-expressing cells and median peak fluorescence (MPF).
Fluorescence in situ hybridization
Cell cultures grown under hygromycin selection for 40 days were treated with colcemid for 4 to 12 hours before harvesting. Chromosome preparations were obtained using standard techniques. Cells were spread onto slides and subsequently denatured by immersion in 70% formamide in 2x sodium chloride/sodium citrate (SSC) at 70°C for 3 minutes. EBAC vector and genomic globin DNA were labeled with digoxigenin and biotin, respectively, using nick translation according to the manufacturer's recommended method (Roche, Indianapolis, IN). The labeled DNA was alcohol precipitated together with COT-1 DNA (30 μg) and resuspended in 50% formamide, 10% dextran sulfate, and 2x SSC to give a concentration for each labeled DNA of 40 ng DNA/μL. The probe was denatured by heating to 75°C for 8 minutes, followed by preannealing at 37°C for 20 minutes. Hybridization was at 37°C for 16 hours followed by washing in 1x SSC at 70°C for 5 minutes. The digoxigenin-labeled vector DNA was detected with antidigoxigenin conjugated to rhodamine (Roche) and the biotin-labeled insert DNA was detected with avidin conjugated to fluorescein (Vector Laboratories, Burlingham, CA). The slides were mounted in Vectashield (Vector Laboratories) containing 4′,6-diamidino-2-phenylindole (DAPI) counterstain. The cells were examined and analyzed using a Zeiss (Göttingen, Germany) epifluorescence microscope with appropriate filters, and images were captured using Cytovision imaging equipment and software (Applied Imaging, Santa Clara, CA). We analyzed 10 metaphases and 20 interphases for individual and colocalized signals.
Results
Construction and characterization of K562 cells stably expressing EBNA1
Transient transfection studies in K562 cells showed that approximately 4% of the K562 cells expressed EGFP with the 21-kb pEBAC160G vector, while transfection studies with pEBAC/148βG yielded much lower levels of EGFP-positive cells (≤ 0.1%). In contrast, transfection studies of K562 cells with fluorescently labeled DNA showed cytoplasmic uptake in most of the cells (data not shown). Thus, while DNA appeared to enter the cells readily under our transfection conditions, only a very small percentage of cells appeared able to take the DNA into the nucleus and show detectable levels of EGFP expression by flow cytometry.
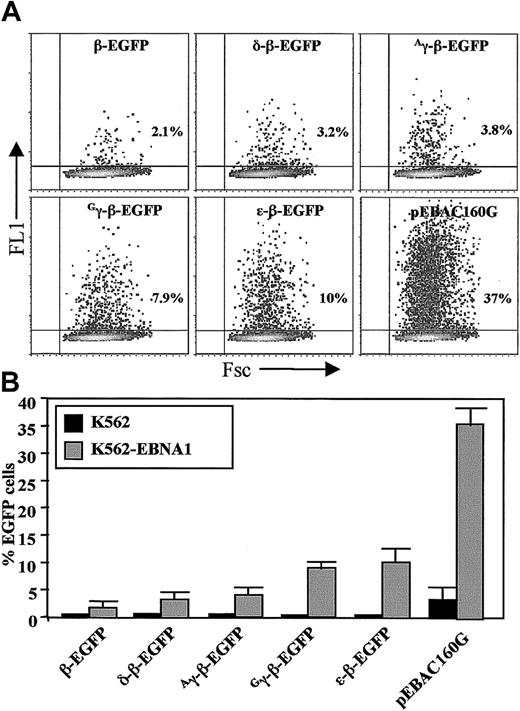
Several studies have previously reported that human cells either transformed with human EBV or stably expressing EBNA1 support extrachromosomal replication and episomal maintenance of oriP-containing plasmids.35,36 To assist in our study, K562 cell lines stably expressing EBNA1 were therefore generated by transfecting an EBNA1/Neo expression cassette.38 A quantity of 30 G418 resistant clones were isolated via limiting cell dilution. All clones tested positive for the presence of the EBNA1 gene and showed EBNA1 protein expression by flow cytometry (data not shown). Although our studies showed an absolute dependence on EBNA1 expression for an increase in transfection efficiency with pEBAC160G, there was no clear correlation between the efficiency of transfection and the level of EBNA1 expression among different K562-EBNA1 clones (data not shown). A sharp reduction in transfection efficiency with clone size was also observed in K562 cells, which was significantly relieved by expression of EBNA1 in K562-EBNA1 cells. A single K562-EBNA1 clone (clone 8.13) with moderate levels of EBNA1 expression and high transfectability (Figure 2) was selected for subsequent studies.
Analysis of EGFP expression by transiently transfected K562-EBNA1 cells.
(A) Dot plot analysis of K562-EBNA1 cells transiently transfected with EGFP-modified globin EBACs and the pEBAC160G vector, as indicated. Flow cytometric analysis was carried out 2 days following transfection. The total number of EGFP-positive cells was expressed as a percentage of the total number of viable cells. (B) Comparison of K562 and K562-EBNA1 cells after transient transfection with EGFP-modified β-globin EBACs and the pEBAC160G vector as in panel A. The data represent the means ± SD of 3 independent experiments.
Analysis of EGFP expression by transiently transfected K562-EBNA1 cells.
(A) Dot plot analysis of K562-EBNA1 cells transiently transfected with EGFP-modified globin EBACs and the pEBAC160G vector, as indicated. Flow cytometric analysis was carried out 2 days following transfection. The total number of EGFP-positive cells was expressed as a percentage of the total number of viable cells. (B) Comparison of K562 and K562-EBNA1 cells after transient transfection with EGFP-modified β-globin EBACs and the pEBAC160G vector as in panel A. The data represent the means ± SD of 3 independent experiments.
Transient transfection studies with EGFP-modified globin EBACs in erythroid cells
The percentage of EGFP-positive cells was determined 48 hours following transfection (Figure 2A). We found the transfection efficiency of clone 8.13 cells with the pEBAC160G vector to be significantly higher (35% ± 4%) when compared with normal K562 cells (4% ± 2%, P < .05). The transfection efficiency of all EGFP-modified β-globin EBACs was also significantly higher in K562-EBNA1 cells when compared with the transfection efficiency of K562 cells (Figure 2B). It was noted that the expression levels of EGFP differed according to the specific globin promoter driving EGFP expression, with the intensity of EGFP expression varying over 2 orders of magnitude between the different constructs (Figure2A). The β- and δ-β-EGFP EBAC constructs produced not only the lowest levels of transfection but also the lowest level of EGFP expression, whereas the Gγ-, Aγ-, and ε-EGFP β-globin EBACs resulted in the highest levels of transfection and EGFP expression, which is in line with the embryonic phenotype of globin gene expression of K562 cells.44 45
We have previously demonstrated that transient transfection of MEL cells with pEBAC/148β-EGFP enabled the identification of clones expressing EGFP under the regulatory elements of the β-globin gene.44 However, the efficiency of transfection was very low, making it difficult to perform functional studies. In this study, we have generated stable clones of MEL cells expressing EBNA-1 (MEL-EBNA1) and demonstrated a similar increase in transfection efficiency with our EBAC constructs, as with K562-EBNA1 cells (data not shown). Stable expression of EBNA1 protein in K562 and MEL cells therefore facilitates increased transfection efficiency with EBAC DNA, enabling for the first time functional studies with globin BACs in both cell types. We also found that EBACs could be maintained episomally for at least 3 months in the K562-EBNA1 cell lines under hygromycin selection, while the episomes were lost quickly in MEL-EBNA1 cell lines (data not shown), presumably due to less specific interaction between mouse chromatin and the EBNA1 protein.
Episomal maintenance and expression of EGFP-modified β-globin EBACs
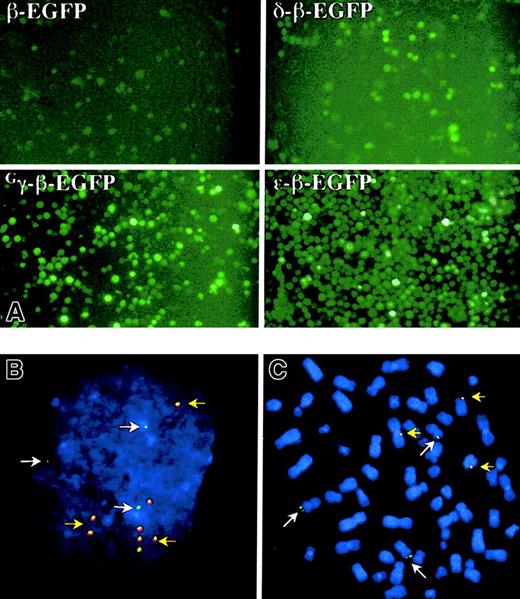
The K562-EBNA1 cell line (clone no. 8.13) was transfected with the ε-, Gγ-, Aγ-,Gγ-Aγ-, δ-, or β-EGFP globin EBACs (Figure 1) and grown for 40 days in continuous culture under hygromycin (400 μg/mL) selection. A gradual increase in the percentage of EGFP expressing cells was observed under antibiotic selection in all cultures, reaching 40% to 60% of the cells transfected with the ε-β-, Gγ-β-, and Aγ-β-EGFP globin EBACs (Figure 3A). In contrast, the β- and δ-β-EGFP globin EBACs showed only a small rise in the proportion of EGFP-expressing cells during hygromycin selection. On the other hand, removal of antibiotic selection from the growth media resulted in a gradual reduction in the proportion of EGFP-expressing cells (data not shown), indicating an episomal pattern of maintenance of the β-globin locus EGFP constructs. This was further demonstrated by fluorescence in situ hybridization (FISH) analysis of interphase (Figure 3B) and metaphase spreads (Figure 3C) of K562-EBNA1 cells episomally transfected with the Gγ-β-EGFP construct, and simultaneously probing for the vector (red) and globin locus sequences (green). Most cells contained 3 green signals, corresponding to the 3 copies of chromosome 11 that are known to exist in K562 cells.46 Additionally, both interphase and metaphase cells were found to contain 5 ± 3 colocalized signals for red and green. The signals in metaphase cells appeared tethered to single chromatids, as has been previously observed for the unmodified pEBAC/148β clone.34 EBNA1 has been shown to tether oriP-containing vectors to condensed chromosomes during mitosis, thereby facilitating segregation of EBV-based vectors.47
Fluorescent microscopy of K562-EBNA1 cells episomally transfected with the EGFP-modified globin EBACs.
Pools of transfected K562-ENBA-1 cells were cultured for 40 days in the presence of hygromycin before examination. (A) Direct examination of EGFP expression after transfection with 4 different EGFP constructs, as indicated. Original magnification × 200. (B-C) Fluorescent in situ hybridization analysis of K562-EBNA1 cells episomally transfected with the Gγ-β-EGFP globin EBAC. Cell spreads were probed with the β-globin EBAC (green) and pEBAC160 vector (red). DNA was counterstained with DAPI. Most cells contained 3 single green signals (white arrows), which correspond to the 3 copies of chromosome 11. Typical interphases (B) and metaphases (C) were found to contain 5 ± 3 colocalized signals for red and green (yellow arrows), that were intimately associated with single chromatids in metaphases. Original magnification B-C, × 400.
Fluorescent microscopy of K562-EBNA1 cells episomally transfected with the EGFP-modified globin EBACs.
Pools of transfected K562-ENBA-1 cells were cultured for 40 days in the presence of hygromycin before examination. (A) Direct examination of EGFP expression after transfection with 4 different EGFP constructs, as indicated. Original magnification × 200. (B-C) Fluorescent in situ hybridization analysis of K562-EBNA1 cells episomally transfected with the Gγ-β-EGFP globin EBAC. Cell spreads were probed with the β-globin EBAC (green) and pEBAC160 vector (red). DNA was counterstained with DAPI. Most cells contained 3 single green signals (white arrows), which correspond to the 3 copies of chromosome 11. Typical interphases (B) and metaphases (C) were found to contain 5 ± 3 colocalized signals for red and green (yellow arrows), that were intimately associated with single chromatids in metaphases. Original magnification B-C, × 400.
The activity of each globin promoter in episomal format was assessed by measuring the level of EGFP expression by flow cytometry (Figure4A, no hemin induction). As with transient transfection (Figure 2A), the β- and δ-β-EGFP EBACs showed the lowest percentage of EGFP-expressing cells and the lowest MPF values. In contrast, the ε-β-, Gγ-β-,Aγ-β-, and Gγ-Aγ-EGFP globin EBACs all showed a much higher proportion of EGFP-expressing cells, as well as 5- to 10-fold higher MPF values.
Hemin induction of K562-EBNA1 cells episomally transfected with EBACs carrying EGFP modifications in the β-globin locus.
Pools of transfected cells were grown continuously in the presence of hygromycin for 40 days before flow cytometry (shaded, no hemin induction). Transfected cells were induced for 5 days with hemin. (A) Comparison of EGFP expression without (shaded) and with hemin (100μM) induction (unshaded) in the various EGFP constructs, as indicated. The percent of cells expressing EGFP and the median peak fluorescence (MPF) without and with hemin is shown for each construct. (B) The effect of hemin induction on EGFP gene expression was determined by measuring MPF on day 3 (filled symbols) and day 5 (empty symbols) at different hemin doses, for the same constructs as in panel A. The data represent the means ± SD of 3 independent experiments.
Hemin induction of K562-EBNA1 cells episomally transfected with EBACs carrying EGFP modifications in the β-globin locus.
Pools of transfected cells were grown continuously in the presence of hygromycin for 40 days before flow cytometry (shaded, no hemin induction). Transfected cells were induced for 5 days with hemin. (A) Comparison of EGFP expression without (shaded) and with hemin (100μM) induction (unshaded) in the various EGFP constructs, as indicated. The percent of cells expressing EGFP and the median peak fluorescence (MPF) without and with hemin is shown for each construct. (B) The effect of hemin induction on EGFP gene expression was determined by measuring MPF on day 3 (filled symbols) and day 5 (empty symbols) at different hemin doses, for the same constructs as in panel A. The data represent the means ± SD of 3 independent experiments.
Hemin induction of K562-EBNA1 cells carrying episomally maintained EGFP-modified β-globin EBACs
Under normal growth conditions K562 cells appear to express mainly the γ-globin gene, small amounts of the ε-globin gene, and very little if any of the β-globin gene. Hemin can induce K562 cells to undergo erythroid differentiation, which is associated with a sharp increase in embryonic and fetal globin gene expression.44,45 48 We therefore investigated whether hemin could enhance EGFP expression from the EGFP-modified β-globin EBAC constructs. K562-EBNA1 cells transfected with the EGFP-modified globin EBACs and cultured for 40 days were treated with various doses of hemin (0-100 μM) for up to 5 days. To minimize the effect of clonal differences in the measurement of gene expression, pools of hygromycin-resistant cells were used throughout this study.
While there was little if any increase in the fraction of cells expressing EGFP for each of the constructs during the period of hemin induction, a significant dose-dependent shift in MPF was observed for all constructs (Figure 4). Moreover, a continuing increase in EGFP expression between day 3 and day 5 of induction was obvious for the ε-β-, Gγ-β-, andGγ-Aγ-EGFP constructs (Figure 4B). Interestingly, although the Aγ-β construct also showed a dose-dependent response to hemin, it seemed to reach a maximal response within 3 days. This may relate to the fact that theAγ-β construct showed a high level of EGFP expression prior to induction (Figure 4A). The largest increase in the level of EGFP expression was seen with the ε-β-EGFP globin EBAC, resulting in a 14.1-fold increase in MPF when induced with hemin at 100 μM for 5 days (day 0 MPF = 115, and day 5 MPF = 1620) (Figure 4A-B). Somewhat lower MPF values were obtained for the Gγ-β-,Aγ-β-, and Gγ-Aγ-EGFP globin EBACs (MPF = 1260, 980, and 741, respectively, following hemin induction), while the β- and δ-β-EGFP globin EBACs showed much lower MPF values (MPF = 50 and 42, respectively), with only a marginal increase after hemin induction. Thus, our findings are quantitatively and qualitatively in general accordance with the sharp increase in the expression of the ε- and γ-globin genes that occurs in K562 cells after induction with hemin.44,45,48 However, the expression of EGFP under each globin promoter in the different constructs may be influenced by the deletion of downstream regulatory elements during the creation of these constructs. This was directly examined by comparing the expression from the Gγ-β- andGγ-Aγ-EGFP EBAC constructs (Figure 1). In both of these constructs EGFP expression is driven by theGγ promoter, but the Gγ-β-EGFP construct has a larger downstream deletion than theGγ-Aγ- EGFP construct. Thus, it is interesting to note a 2-fold higher EGFP expression following hemin induction in the Gγ-β-EGFP construct when compared with the Gγ-Aγ-EGFP construct, (MPF 1260 vs 741), a situation analogous to the increase in Gγ levels in some human β-thalassemia deletions.22
Discussion
The sequence of the β-globin locus has been known for almost 15 years, but progress in understanding the factors that underlie the developmental and tissue-specific regulation of globin gene expression has been very slow. The identification and analysis of the role of various cis-acting elements and trans-acting factors has traditionally depended on the use of small plasmid constructs in human and mouse erythroleukemic cells. However, it is difficult from such studies to extrapolate to the coordinated control of the different regulatory elements in the context of the whole β-globin locus in erythropoietic cells, under various physiologically relevant conditions. This limitation has been partly overcome by the use of transgenic mice carrying the intact human β-globin locus in YACs22-24 or BACs.25,26 The transgenic model approach is indeed yielding many insights but it is very cumbersome, while the regulation of the human β-globin locus in transgenic mice may differ in many subtle ways from the regulation in human erythropoietic cells. Thus, there is still the need for convenient cellular model systems that recapitulate as precisely as possible the regulation of the human β-globin locus in human erythropoietic cells and are readily amenable to experimental manipulation. The development of techniques for the cloning, targeted modification, and functional analysis of large genomic fragments in BACs30-32 provides an opportunity to create cellular assays that overcome most, if not all, of these limitations.
In this study, we report the delivery and functional analysis of EBACs containing EGFP-modified β-globin locus in human and mouse erythroleukemic cells. The EBAC system incorporates the EBNA1 gene and oriP elements of EBV and thus facilitates the maintenance of large genomic fragments as episomes in human cells under hygromycin selection. However, we have found that expression of EBNA1 is not only essential for the episomal maintenance of EBACs, but it also significantly enhances transfection efficiency (Figure 2). This effect is particularly marked for larger molecules and is manifested only when EBNA1 is present in the recipient cells prior to transfection. The exact mechanism by which EBNA1 facilitates preferential transfection of large EBACs is not entirely clear, but it may be related to its known binding to karyopherins.49-51 Based on the known role of karyopherins in nuclear import, it is proposed that the nuclear import of EBNA1 protein also facilitates the uptake of large EBACs by their attachment to the nuclear import machinery through the oriPsequence. Interestingly, although MEL cells stably expressing EBNA1 showed an enhancement of transient transfection efficiency similar to K562-EBNA1 cells, episomes could not be maintained in long-term cultures in these cells. The stable expression of EBNA1 in MEL cells therefore facilitates their use with our globin EBACs in transient transfection studies and in the generation of stable clones through hygromycin selection, but not for episomal studies.
In order to monitor expression of each of the globin genes in the context of the β-globin locus, we have used the GET Recombination system30,37 to create a series of EGFP-modified β-globin EBACs (Figure 1). Transient transfection studies in K562-EBNA1 cells (Figure 2) have shown significant differences in the fraction of EGFP-expressing cells between the different constructs. These differences do not appear to arise from the relatively small differences in the size of the various globin constructs, but are generally in line with the known pattern of expression of the endogenous globin genes in K562 cells.44 45 Furthermore, initiation of selection with hygromycin 48 hours after transfection leads to cultures in which the EGFP-modified β-globin EBACs are episomally maintained (Figure 3). The in-frame use of EGFP as a reporter allows the study of the expression of each globin gene of interest in the context of the whole locus. We show that globin gene expression in K562-EBNA1 cells containing EGFP-modified β-globin EBACs can be quantitatively assayed in single cells by flow cytometry.
Although the globin-EGFP gene fusion constructs reported in this paper retain all upstream regulatory elements intact, each has a deletion of the genomic sequences extending from the start codon of the corresponding globin gene to the stop codon of the β-globin gene (or from the start codon of the Gγ- to the stop codon of theAγ-globin gene in the case of theGγ-Aγ-EGFP construct), thus disrupting the role of intragenic and 3′ end regulatory sequences. The absence of any competition from downstream promoter sequences may account for the very high level of EGFP expression observed with the ε-β-EGFP construct, and the higher level of expression from the Gγ-β-EGFP construct than from the Gγ-Aγ-EGFP construct. Since other key regulatory elements may be located in the deleted regions, work is in progress to develop additional EGFP constructs, which will faithfully retain not only the upstream elements, but also the intragenic and downstream elements as well.
The human erythroleukemic cell line K562 has been used extensively to study the molecular mechanisms involved in regulating ε- and γ-globin gene expression, although it is not certain that it can accurately recapitulate the conditions necessary for the reactivation of fetal globin genes in adult patients. Hemin-induced erythroid differentiation of K562 cells has previously been reported to result in a sharp increase in ε-globin and γ-globin gene expression that is associated with cytoplasmic accumulation of embryonic Gower (ζ2ε2) and fetal Portland (ζ2γ2) hemoglobin molecules.44,45,48 In this study, we show that hemin-induced erythroid differentiation of episomally transfected K562-EBNA1 cells resulted in a significant increase in EGFP expression driven by the ε-, Gγ- and Aγ-globin promoters, while EGFP expression from the β- and δ-globin promoters consistently produced low levels of EGFP. Importantly, we were able to demonstrate quantitatively the dependence of EGFP expression in K562-EBNA1 cells on hemin concentration. Given that the half-life of EGFP in mammalian cells is about 26 hours,52 the increase in EGFP expression with increasing hemin concentration over 5 days must primarily reflect increased differentiation of K562-EBNA1 cells down the erythropoietic pathway. Thus, K562-EBNA1 cells episomally transfected with the EGFP-modified globin EBACs mirrored the expected developmental, stage-specific, globin gene expression profile of normal K562 cells.44,45 48
In conclusion, this study describes the use of the well-characterized EBNA1/oriP elements of human EBV to facilitate for the first time transient transfection studies with the EGFP-modified globin BACs in human and murine erythroleukemia cells. The same approach can be used to generate stable clones with such constructs in both cell types, while the human K562 cells can additionally maintain the constructs in episomal format, thus avoiding variations in expression that might arise from positional integration effects. We propose that this novel system provides a convenient approach to study the molecular mechanisms regulating globin gene expression and ε- and γ-globin gene induction. The further development of a dual fluorescent reporter system in an adult erythropoietic environment should allow the simultaneous monitoring of expression from embryonic/fetal and adult globin genes, thus enabling the rapid screening and identification of pharmacologic agents with the highest activity to reactivate the embryonic or fetal genes. Similar approaches may also be applied to the study of other genes that are cloned intact in PAC/BAC clones, thereby facilitating the identification of distal regulatory elements and the development of targeted approaches for the modification of gene expression as a potential approach to the therapy of various diseases.
We would like to thank Dr D. Burt for providing the pEB plasmid and Dr F. Grasser for providing the EBNA1-specific MAb 1H4. We would also like to acknowledge the support of Dr Simon Bol for the use of the FACScan flow cytometer.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2001-12-0365.
Supported by grants from the National Health and Medical Research Council (NHMRC) of Australia, the Brockhoff Foundation, the Ronald Geoffrey Arnott Foundation, and the Thalassaemia Society of New South Wales.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Panos Ioannou, CAGT Research Group, The Murdoch Children's Research Institute, Royal Children's Hospital, Flemington Rd, Parkville 3052, Melbourne, Australia; e-mail:ioannoup@cryptic.rch.unimelb.edu.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal