During pregnancy, physiologic and anatomic changes can complicate the diagnosis of venous thromboembolism (VTE) as well as the management of patients with a high risk of or established VTE. As in nonpregnant subjects, clinical diagnosis of VTE by itself is unreliable and accurate objective testing is essential. Few diagnostic studies of VTE have been performed in pregnant women and, therefore, approaches are largely extrapolated from those used in nonpregnant subjects with modifications to limit the radiation exposure and overcome the limitations of diagnostic testing in pregnancy. Therapy of established VTE during pregnancy consists of therapeutic doses of unfractionated heparin (UFH) or low–molecular-weight heparin (LMWH), generally given throughout pregnancy subcutaneously and for 4 to 6 weeks after childbirth. A key unresolved issue includes the optimum dosing of LMWH therapy. Maternal warfarin can be safely used after childbirth because it is safe to use in the breast-fed infant of a mother receiving warfarin. Finally, pregnant women with prior VTE (with or without a hypercoagulable state) have an increased risk of recurrent venous thrombosis. A recent study has demonstrated that for women with a single episode of prior VTE, many can be managed without anticoagulants. However, for many, anticoagulant therapy with prophylactic UFH or LMWH is a reasonable option.
Introduction
The management of venous thromboembolism (VTE) in pregnancy is challenging for several reasons. Many diagnostic tests are less accurate in pregnant than in nonpregnant patients, and some of the radiologic procedures utilized are potentially hazardous to the fetus. When anticoagulants are required, the available options are suboptimal. Coumarins can cause embryopathy and other adverse effects in the fetus. Although unfractionated heparin (UFH) and low–molecular-weight heparins (LMWHs) are safe for the fetus, they can produce maternal osteoporosis and thrombocytopenia, and because they must be given parenterally, long-term administration is inconvenient. LMWHs probably cause less maternal osteoporosis and thrombocytopenia than UFH, but the appropriate dose regimens for prevention and treatment of thrombosis during pregnancy have not been established. Finally, reliable information on the incidence of venous thrombosis and recurrent thrombosis in untreated high-risk patients, such as those with previous VTE or asymptomatic thrombophilia, is sparse.
In this review, we address the following questions: In pregnant women, how do we (1) diagnose deep vein thrombosis (DVT) and pulmonary embolism (PE), (2) treat VTE once a diagnosis is confirmed, and (3) manage those with a high risk of VTE? For each of these problems, the relevant background is briefly summarized (with emphasis on differences between pregnant and nonpregnant subjects); approaches recommended; and limitations of the available literature, along with needs for future research, highlighted.
How we diagnose DVT and PE during pregnancy
Epidemiology of VTE during pregnancy
DVT and PE are 2 different manifestations of one disease, VTE. This implies that finding DVT in a patient with suspected PE (and vice versa) provides grounds to diagnose VTE and treat the patient.1 The incidence of VTE probably increases 2- to 4-fold when a woman becomes pregnant and is higher after a cesarean delivery than after a vaginal delivery.2 Several studies have reported a striking propensity for venous thrombi during pregnancy to occur in the left leg,3 possibly due, in part, to compression of the left iliac vein by the right iliac artery as they cross.4 In addition, when pregnant women lie in the supine position, blood flow velocity decreases, particularly in the left leg, perhaps contributing to the preponderance of left leg DVT.3,5 Although leg swelling and calf pain are most common in the third trimester, results from prospective studies indicate that objectively proven VTE occurs with similar frequency in each of the 3 trimesters.3 Therefore, most women with leg swelling (with or without calf pain) in the third trimester do not have DVT, whereas when these symptoms occur in early pregnancy, particularly in the left leg, DVT is much more likely to be present.
Hemodynamic changes causing venous stasis and, possibly, hypercoagulability contribute to the increased risk of VTE during pregnancy. Increased venous distensibility and capacity, due to high estrogen levels, occur early in pregnancy and cause venous stasis.4,6 Increased plasma volume, which peaks during the second trimester,7 as well as compression of the inferior vena cava by the enlarging gravid uterus also cause venous stasis.8
Altered levels of coagulation factors have been described both during pregnancy and after childbirth. Hypercoagulability is thought to result from increased levels of coagulation factors, such as fibrinogen, von Willebrand factor, and factor VIII,9-11 as well as decreased levels of natural inhibitors of coagulation, such as protein S,12 and the development of an acquired activated protein C resistance.13 Furthermore, reduced fibrinolytic activity has been described during pregnancy,9,14 perhaps as a consequence of increased levels of plasminogen activator inhibitor 1 (PAI 1) and (PAI 2),14-16 the latter being produced by the placenta.
Risks of radiologic procedures to the fetus
The diagnosis of VTE during pregnancy presents special problems not only because hemodynamic changes can interfere with the interpretation of tests for DVT but also because some of the pivotal diagnostic tests for DVT and PE expose the fetus to ionizing radiation. The potential adverse effects of radiation on the fetus include oncogenicity and teratogenicity (congenital malformations, intrauterine growth retardation, and intrauterine death). The literature reporting adverse effects after fetal radiation exposure of up to 0.05 Gy (5 rad) has been comprehensively reviewed.24 Most published reports are case-control studies, and most of the larger studies that examined the association between childhood cancer and radiation exposure in utero reported a slight (but statistically significant) increase in the relative risk (ranging from 1.2 to 2.4). Even with those increases in relative risk, the absolute risk of childhood cancer following in utero radiation exposure is likely to be small because the incidence of cancer in the first 10 years of life in the general population is approximately 0.1% and, thus, even a doubling of the incidence would increase it to approximately 0.2%.25 The studies examining the teratogenic potential of radiation exposure in utero reported no increase in pregnancy loss, growth retardation, or mental retardation.24 A slight increase in minor congenital eye abnormalities and a small increase in the proportion of male offspring born to those exposed in utero have been associated with radiation exposure.24
In the same publication, estimates of the doses of absorbed fetal radiation with the procedures used to diagnose DVT and PE were reported (Table 1).24 Because the radiation dose absorbed for a given procedure is heavily influenced by the equipment and techniques used, the dose absorbed by the fetus will vary, sometimes markedly, among facilities. However, when appropriate precautions are taken, such as lead shielding of the fetus, minimizing fluoroscopy, and reduction of the dose of radioisotopes used to perform lung scans, the amount of radiation absorbed by the fetus and the consequent risk to the fetus are trivial.
Clinical assessment of women with suspected DVT or PE during pregnancy
By itself, the clinical diagnosis of DVT and PE is unreliable.26 In nonpregnant individuals, DVT is confirmed by objective investigations in about 20% to 30% of suspected cases.26 In symptomatic pregnant women, DVT and PE appear to be less prevalent than in nonpregnant subjects because leg symptoms, chest pain, and dyspnea due to nonthrombotic causes are common during pregnancy. Two cohort studies, one in pregnant women with suspected DVT27 and the other in pregnant women with suspected PE,28 reported a prevalence of DVT of 8% and of PE of less than 5%, respectively. Anticoagulant treatment of pregnant women with VTE is highly effective but carries significant risks (bleeding, osteoporosis, heparin-induced thrombocytopenia), whereas not treating patients with VTE can result in fatal and nonfatal PE.1Therefore, when VTE is suspected, it is essential to diagnose the disease when it is present and exclude the disease when it is not.
Testing beyond clinical assessment
Objective tests that have been evaluated for the diagnosis of DVT in nonpregnant individuals include contrast venography, compression ultrasonography (CUS), impedance plethysmography (IPG), D-dimer assays, and magnetic resonance venography (MRV). For the diagnosis of PE, the tests that have been extensively evaluated in nonpregnant individuals include pulmonary angiography, radionuclide (ventilation-perfusion [V/Q]) lung scanning, and spiral computed tomographic (CT) scanning. In addition, the tests for DVT, including D-dimer testing, are useful in patients with suspected PE.
Venography remains the “gold standard” test for the diagnosis of DVT of the lower extremities,29 and an adequately performed, normal test excludes calf and proximal DVT.30Despite this, the performance of venography during pregnancy is unpopular because it is painful and there is absorption of ionizing radiation by the fetus. CUS is sensitive and specific for the diagnosis of proximal DVT in symptomatic nonpregnant patients26,31,32and is the test of choice in these individuals. The safety of withholding therapy in nonpregnant patients with normal serial ultrasonography (repeated 1 week after presentation) has been demonstrated.32 This strategy has not been evaluated in pregnant patients with suspected DVT. This is an important limitation for 2 reasons. First, there is a clinical impression (although little reliable data) that the risk of isolated iliac vein DVT is increased during pregnancy.33,34 CUS is probably insensitive to such thrombi, and Doppler ultrasound of the iliac vessels looking for evidence of intraluminal thrombus has not been validated. Second, the accuracy of CUS for calf DVT is less than for proximal DVT.26 Isolated calf DVT rarely causes PE in nonpregnant subjects unless it extends into the proximal veins. This occurs about 20% to 30% of the time, invariably within 7 to 14 days of presentation, and therefore will be detected during the course of serial CUS. However, the natural history of calf DVT might be different in pregnant patients. For example, extension into the proximal system may occur more frequently in pregnant women, and the time course of extension may differ from that in nonpregnant individuals. If extension occurs more rapidly during pregnancy, waiting 7 days after an initial normal CUS would delay treatment of proximal DVT. Conversely, if extension occurs more slowly in pregnant women, proximal extension would occur beyond the window of serial testing and go undetected. Thus, the safety of a strategy involving serial CUS in pregnant women with suspected DVT needs evaluation.
IPG is accurate for the diagnosis of symptomatic occlusive proximal, but not calf, DVT in nonpregnant patients.32,35 Serial testing is required (over 7 to 14 days) in patients with a normal initial test to exclude clinically important, extending thrombi. In pregnant patients with suspected DVT, serial IPG is the only diagnostic approach that has been carefully evaluated in a sizable (n = 152) cohort study, which demonstrated the safety of withholding anticoagulants if the results were normal.5 However, IPG has largely been abandoned because the test is less accurate than CUS in nonpregnant patients and the machines are no longer being manufactured.
High levels of D-dimer, a specific fibrin degradation product, have been reported in most patients with DVT and PE.36 Levels of D-dimer can be measured using 3 types of assays: (1) enzyme-linked immunosorbent assays (ELISAs), (2) latex agglutination assays, and (3) a red blood cell whole blood agglutination assay (SimpliRED; Agen Biomedical, Brisbane, Australia). Each has developed a format suitable for rapid testing, albeit with differing accuracy indices. ELISAs have high sensitivities but low specificities,36 the new microparticle latex assays have high sensitivities and moderate specifities,37 whereas the SimpliRED D-dimer assay has a lower sensitivity but the highest specificity. D-dimer testing has assumed an increasingly prominent role in the exclusion of acute DVT in the nonpregnant population, but it has not yet been rigorously evaluated in pregnant patients. D-dimer levels increase with gestational age and during complicated pregnancies, such as those associated with preterm labor, abruptio placentae, or gestational hypertension.38-40 These characteristics reduce the test's specificity and are likely to somewhat limit the use of D-dimer testing in pregnancy.
MRV of the lower extremities appears to be accurate for DVT,41 although no management studies have been performed. Although the usefulness of this test may be limited by cost and lack of accessibility, it has the potential to be useful in pregnancy because it appears to be sensitive for all lower extremity DVT, including calf and ileofemoral DVT, and is not associated with radiation exposure.
Pulmonary angiography is the criterion standard for diagnosing PE.42 However, it is invasive and expensive and should be reserved for individuals in whom less invasive tests (such as V/Q scanning) are nondiagnostic. In many centers, the radionuclide lung scan is the pivotal test for the investigation of patients with suspected PE.43,44 In nonpregnant patients, a normal perfusion lung scan excludes PE, and a high-probability scan (at least one segmental perfusion defect with normal ventilation) is considered diagnostic of PE. A lung scan showing subsegmental perfusion defects or segmental perfusion defects with matching ventilation defects has a positive predictive value of 10% to 40%; further testing is required.43,44 A recent study of approximately 120 pregnant patients with suspected PE who underwent lung scanning showed marked differences in the distribution of lung scan patterns compared with nonpregnant patients.28 High-probability lung scans were seen in less than 5% (compared with 10%-15% in the nonpregnant population), whereas almost three quarters of patients had normal perfusion scans (compared with 10%-30% in the nonpregnant population) and 20% of pregnant women had nondiagnostic scans (compared with 50%-70% of nonpregnant patients).28,43 44 These findings suggest that because pregnant women are younger they tend to have fewer comorbid conditions that cause abnormal perfusion scans. Further, although the absolute incidence of PE is higher in pregnant than in nonpregnant subjects, when patients present with suspected PE the prevalence of PE is substantially lower in pregnant than nonpregnant patients. This is probably because the threshold for investigation of PE in pregnancy is low and nonthrombotic causes of symptoms that mimic PE are relatively common.
Spiral CT is a specialized form of CT that is being used increasingly for the diagnosis of PE.45 46 It is sensitive for large central pulmonary emboli but less sensitive for smaller peripheral PE. The finding of a persistent filling defect in a large branch of a pulmonary artery is diagnostic of PE because the test has high specificity for emboli in these vessels. Although the use of spiral CT as the initial test in suspected PE has become routine in many centers and patients are being discharged if the results are normal, this practice cannot be endorsed until it has been validated in large management studies. Such studies are under way.
Diagnosis of venous thrombosis during pregnancy
Diagnosis of venous thrombosis during pregnancy is depicted in Figure 1. We recommend CUS of the proximal veins as the initial test for suspected DVT during pregnancy. Although 2-point (common femoral and popliteal veins) and 3-point (common femoral and popliteal veins as well as the calf trifurcation) compression are often recommended in nonpregnant patients, we recommend compression of the entire proximal venous system to the trifurcation in symptomatic pregnant women because neither of the former has been validated in pregnancy. If the initial test shows a clear-cut abnormality in the popliteal or femoral veins, a diagnosis of proximal DVT is made. A normal CUS does not exclude calf DVT, so the test should be repeated 1 to 2 days after referral (day 2 or 3) and, if normal, again 1 week later (days 6 to 8) to exclude the possibility of extending calf vein thrombosis. If the test becomes abnormal during repeated testing, acute proximal DVT is likely to be present (see above). When compression ultrasound is used and iliac DVT is suspected (back pain, swelling of the entire leg), 3 options are available: (1) venography, (2) MRV, or (3) pulsed Doppler and/or direct visualization of the iliac vein.
Algorithm for the investigation of suspected DVT during pregnancy.
CUS indicates compression ultrasonography; MRV, magnetic resonance imaging. *IPG is a suitable substitute for CUS. **D-dimer testing can be performed and, if normal, further testing withheld; if abnormal, investigate further. ***Repeat days 2 and 3 and 6 to 8; if highly suspicious, MRV or venography.
Algorithm for the investigation of suspected DVT during pregnancy.
CUS indicates compression ultrasonography; MRV, magnetic resonance imaging. *IPG is a suitable substitute for CUS. **D-dimer testing can be performed and, if normal, further testing withheld; if abnormal, investigate further. ***Repeat days 2 and 3 and 6 to 8; if highly suspicious, MRV or venography.
If the results of CUS are equivocal (small area of noncompressibility in proximal veins or noncompressibility of calf veins), either MRV or a limited venogram, in which the fetus is shielded from radiation by the placement of a lead shield over the abdomen, should be considered. If Doppler is normal but the clinical suspicion of isolated iliac vein thormbosis is high, we recommend MRV or a complete venogram (the latter without a lead-lined apron) to make a definitive diagnosis. It is highly likely that D-dimer testing will be complementary to venous ultrasonography for excluding DVT in pregnancy when both tests are normal. The safety of withholding anticoagulant therapy in pregnant patients whose serial IPG remains normal has been demonstrated in a cohort study,5 and this test is a suitable alternative to CUS, but it is unavailable in most centers.
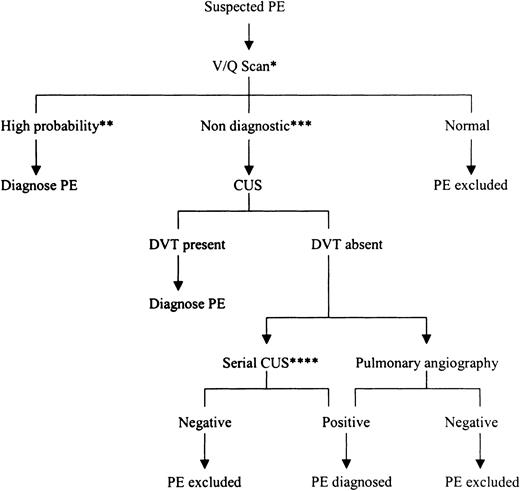
Diagnosis of PE during pregnancy
Diagnosis of PE during pregnancy is depicted in Figure2. The potential risks associated with the radiologic tests often used when PE is suspected are minimal when compared with the consequences of misdiagnosis. If untreated, patients with PE can suffer fatal recurrence, whereas treating patients with anticoagulants when the clinical symptoms and signs are not caused by PE exposes them unnecessarily to the risks of such therapy (see below). Therefore, we believe that objective testing is mandatory in pregnant patients with clinically suspected PE. The approach to the diagnosis of suspected PE in pregnancy is similar to the approach used in the nonpregnant patient. If the clinical features are compatible with PE, V/Q lung scanning is performed. If the perfusion scan is normal, the diagnosis of PE is excluded. If a segmental defect in perfusion with normal ventilation (high-probability lung scan) is seen, the diagnosis of PE is confirmed. Patients with nondiagnostic lung scans should undergo CUS; if this is abnormal, PE can be diagnosed. If CUS is normal, D-dimer,47,48pulmonary angiography, or serial CUS48 should be considered, provided that the limitations of these tests are understood. We have not included spiral CT in our algorithm because of relatively large amounts of radiation associated with its performance and the lack of validation of this test in management studies. However, it is useful if the scan shows a clear-cut abnormality in the proximal pulmonary arteries (see above); a normal result does not reliably exclude PE.
Algorithm for the investigation of suspected PE during pregnancy.
V/Q scan indicates ventilation-perfusion lung scan; CUS, compression ultrasonography. *Can substitute CUS and, if abnormal, diagnose PE; if normal, further testing is required. **At least one segmental perfusion mismatch. ***Neither normal nor high probability. ****Can substitute sensitive D-dimer test and, if negative, exclude PE.
Algorithm for the investigation of suspected PE during pregnancy.
V/Q scan indicates ventilation-perfusion lung scan; CUS, compression ultrasonography. *Can substitute CUS and, if abnormal, diagnose PE; if normal, further testing is required. **At least one segmental perfusion mismatch. ***Neither normal nor high probability. ****Can substitute sensitive D-dimer test and, if negative, exclude PE.
How we treat VTE during pregnancy
Anticoagulant therapy during pregnancy
The anticoagulants that have been evaluated for the prevention and treatment of VTE include heparin and heparinlike compounds (UFH, LMWH, heparinoids, and pentasaccharide) as well as coumarin derivatives.1 49-51 The “direct” thrombin inhibitors, such as hirudin, cross the placenta, and neither it nor pentasaccharide have been evaluated during pregnancy. Therefore, these agents are not further discussed. When determining the optimal therapeutic regimens for treatment of VTE during pregnancy, the following must be considered: (1) the safety of the drug for both the fetus and mother, (2) the efficacy of the regimen, and (3) dose regimens for acute and secondary treatment and during delivery and after childbirth. Each of these is reviewed, and a recommended approach to treatment of VTE during pregnancy is provided.
Fetal complications of anticoagulants during pregnancy
There are 2 potential fetal complications of maternal anticoagulant therapy: teratogenicity and bleeding. UFH,52LMWH,52 and danaparoid52 do not cross the placenta and therefore do not have the potential to cause fetal bleeding or teratogenicity, although bleeding at the uteroplacental junction is possible. Three studies strongly suggest that UFH and LMWH therapy are safe for the fetus.53-55
In contrast to heparin, coumarin derivatives cross the placenta and have the potential to cause both teratogenicity and bleeding in the fetus.52,56 It is probable that oral anticoagulants are safe during the first 6 weeks of gestation, but there is a risk of embryopathy, consisting of nasal hypoplasia and/or stippled epiphyses, if coumarin derivatives are taken between 6 and 12 weeks of gestation.56,57 Central nervous system abnormalities can occur after exposure to these drugs during any trimester.52 In addition, oral anticoagulants cause an anticoagulant effect in the fetus. This is a concern, particularly at the time of delivery, when the combination of the anticoagulant effect and trauma of delivery can lead to bleeding in the neonate.52
Maternal complications of anticoagulant therapy during pregnancy
The main maternal complications of anticoagulant therapy include bleeding (for all anticoagulants), osteoporosis, thrombocytopenia, and pain at injection sites for heparin-related compounds, and skin necrosis for warfarin.
Bleeding.
In a study of 100 pregnant women, the rate of major bleeding with various doses of UFH was 2%,54 which is low and not inconsistent with the reported rates of bleeding associated with heparin therapy in nonpregnant patients and with warfarin therapy when used for the treatment of DVT.58 Therapeutic doses of subcutaneous UFH (adjusted-dose UFH) can cause a persistent anticoagulant effect at the time of delivery, which can complicate its use prior to labor.59 In a small study, prolongation of the activated partial thromboplastin time (aPTT) persisted for up to 28 hours after the last injection of adjusted-dose subcutaneous heparin.59 The mechanism for this prolonged effect is unclear. Bleeding complications appear to be very uncommon with LMWH.55
Heparin-induced thrombocytopenia (HIT).
Approximately 3% of nonpregnant patients receiving UFH develop immune, immunoglobulin G (Ig G)–mediated thrombocytopenia (HIT), which is frequently complicated by extension of preexisting VTE or the development of new arterial thrombosis.60 This should be differentiated from an early, benign, transient thrombocytopenia that can occur with initiation of UFH. The incidence of HIT is much lower with LMWH than UFH.60 Diagnosing immune thrombocytopenia is often difficult because definitive platelet activation assays are not widely available and turnaround times for these assays are slow. HIT should be suspected when the platelet count falls to less than 100 × 109/L or to 50% of the baseline value 5 to 15 days after commencing heparin (sooner with recent heparin exposure).60
In pregnant women who develop HIT and require ongoing anticoagulant therapy, use of the heparinoid, danaparoid sodium, is recommended because it is an effective antithrombotic agent that does not cross the placenta and has low cross-reactivity with UFH and therefore rarely produces recurrent HIT. LMWH should not be used when HIT is caused by UFH because it has relatively high potential to produce recurrent HIT.
Heparin-induced osteoporosis.
Long-term UFH therapy has been reported to cause osteoporosis in both laboratory animals and humans.61-68 A number of studies have attempted to quantify the risk of osteoporosis when UFH is administered for 1 month or more. When the relevant studies are pooled, symptomatic vertebral fractures have been reported to occur in about 2% to 3% of heparin-treated patients, and significant reductions in bone density have been reported in up to 30% of patients receiving long-term UFH.52
Recently, Shaughnessy and associates61 have performed a series of studies in Sprague Dawley rats and other experimental models that have provided new information on the mechanism of heparin-induced osteoporosis. In one study, animals treated with UFH at doses ranging from 0.25 to 1.0 anti-Xa units per gram for 28 days showed a dose-dependent decrease in cancellous bone volume in the distal third of the femur. These investigators were also able to show that UFH causes bone loss by decreasing rates of bone formation while at the same time increasing rates of bone resorption.62
Two clinical trials now suggest that LMWHs have a lower risk of osteoporosis than UFH. In a study by Monreal and colleagues,67 significantly lower fracture rates were reported in nonpregnant individuals treated with dalteparin (5000 IU anti-Xa subcutaneously twice a day) than with UFH (10 000 IU subcutaneously twice a day). In a separate study by Pettila and colleagues,68 44 pregnant women were randomly allocated to prophylactic doses of dalteparin (n = 21) or UFH (n = 23), and the UFH group showed significantly lower bone density results.
Efficacy of UFH for treatment of VTE
Both continuous intravenous and subcutaneous UFH are effective for the initial treatment of VTE in nonpregnant subjects; the efficacy of UFH seems to be more dependent upon the use of an adequate starting dose (ie, at least 30 000-35 000 units per 24 hours) than on the route of administration.1 Recently, a problem with the therapeutic range of UFH during pregnancy has been identified.69 The relation between the aPTT and heparin levels differs in pregnant compared with nonpregnant patients. The aPTT response of the plasma of pregnant women to heparin is attenuated because of elevated levels of heparin-binding proteins as well as factor VIII. This causes a “blunting” of the aPTT response relative to the heparin level and a resultant increased requirement for UFH. Consequently, the use of an aPTT range that corresponds to therapeutic heparin levels in nonpregnant patients would be expected to result in higher dosing (and heparin levels) in pregnant women than nonpregnant patients. It is not clear whether the expected increase in heparin levels translates into excessive bleeding, because the reported rates of bleeding using the “nonpregnant aPTT range” appear to be very low.
Efficacy of LMWH and heparinoids for treatment of VTE
Based on the results of large clinical trials in nonpregnant patients, LMWH and heparinoids (eg, danaparoid sodium) are at least as effective and safe as UFH for the treatment of patients with acute proximal DVT 70-72 and for the prevention of DVT in high-risk patients.73 LMWHs have the advantage of a longer plasma half-life and a more predictable dose response than UFH,73 the latter due, at least in part, to less nonspecific binding to heparin-binding proteins. These properties render LMWHs suitable for treatment of the nonpregnant patient with VTE with once-daily, weight-adjusted subcutaneous dosing. LMWHs are more expensive than UFH, but given their advantages over UFH, the clear-cut evidence of their efficacy in nonpregnant patients, and the fact that they are safe for the fetus, we endorse them for clinical use in pregnant patients who require anticoagulant therapy.
Use of anticoagulants in the nursing mother
UFH and LMWHs are not secreted into breast milk and can be safely given to nursing mothers.52 There have been 2 small but convincing reports that warfarin does not induce an anticoagulant effect in the breast-fed infant when the drug is given to a nursing mother.74 75 Therefore, the use of warfarin in women who require postpartum anticoagulant therapy is safe.
Recommended approach to the treatment of VTE during pregnancy
Based upon the safety data for the fetus, LMWH and UFH are the preferred drugs for the treatment of VTE during pregnancy.
We recommend the use of LMWH throughout pregnancy. If one of these agents is used for acute treatment of VTE, we initiate therapy with a weight-adjusted dose (as per the manufacturer's recommendations [Table 2]). As the pregnancy progresses (and most women gain weight) the volume of distribution of LMWH changes. Therefore, 3 options are available. The first is maintenance of the initial dose regimen throughout the treatment period (usually the entire pregnancy). The second is to simply alter the dose in proportion to the weight change.76 The third is to perform periodic (eg, every 1 to 3 months) heparin (anti–factor Xa) levels 4 to 6 hours after the morning dose and adjust the dose of LMWH to achieve an anti–factor Xa level of approximately 0.5 to 1.2 U/mL if a twice-daily LMWH regimen is used. The corresponding target anti-Xa range with once-daily LMWH is expected to be somewhat higher. Until properly designed trials are performed the correct approach is unknown, but we favor the third, if anti-Xa levels are available, because it provides a more consistent anticoagulant effect. Alternatively, UFH initiated by either an intravenous bolus followed by a continuous infusion to maintain the aPTT in the therapeutic range (or adjusted-dose subcutaneous UFH) for at least 5 days, followed by adjusted-dose subcutaneous UFH for the remainder of the pregnancy, can be used. To minimize the volume of injection, concentrated UFH solutions should be used, if available. The midinterval aPTT should be monitored every 1 to 2 weeks because UFH requirements often vary as pregnancy progresses. If patients require very high doses of UFH (> 40 000 units every 24 hours) to achieve a therapeutic aPTT, this is likely a result of attenuation of the aPTT response to heparin due (at least in part) to elevated levels of factor VIII. When this “heparin resistance” occurs, 1 of 2 approaches is recommended. First, plasma heparin (anti–factor Xa) levels can be measured and the dose adjusted to attain a 6-hour postdose anti-Xa level of 0.3 to 0.7 U/mL.77 This is expected to reduce the daily heparin dose requirements because anti-Xa levels are insensitive to elevated factor VIII levels. Second, the patient can be switched to weight-adjusted LMWH.
Management of anticoagulant therapy at the time of delivery
To avoid an unwanted anticoagulant effect during delivery in women receiving therapeutic doses of LMWH or UFH, we recommend discontinuing either drug 24 hours prior to elective induction of labor. If the woman is deemed to have a very high risk of recurrent VTE (eg, proximal DVT or PE within 4 weeks), therapeutic doses of intravenous UFH can be initiated and discontinued 4 to 6 hour prior to the expected time of delivery. If spontaneous labor occurs in women receiving adjusted-dose subcutaneous UFH, careful monitoring of the aPTT is required and, if it is prolonged near delivery, protamine sulfate may be required to reduce the risk of bleeding. The approach taken if spontaneous labor occurs in women receiving therapeutic doses of LMWH depends on the proximity of the last dose to the expected time of delivery and, if available, the anti–factor Xa level. If there is a reasonable expectation that, at the expected time of delivery, a significant anticoagulant effect will be present or if an anti–factor Xa level shows an anticoagulant effect, several precautions should be taken. First, epidural analgesia should be avoided. Second, judicious use of protamine sulfate should be considered. Finally, the obstetrician should be made aware of the potential for bleeding and make efforts to minimize the risk.
Postpartum heparin therapy should be recommenced as soon as it is safe to do so, usually within 12 hours of delivery. Warfarin can be started at the same time. Heparin is continued until an international normalized ratio (INR) of 2.0 or greater is reached. Anticoagulants should be given for at least 4 weeks following delivery; however, if the DVT or PE was diagnosed late in pregnancy, anticoagulants should be continued for a minimum of 3 months in total.
Inferior vena cava filters are indicated in women who have a contraindication to anticoagulants, predominantly those who develop clinically significant bleeding while receiving anticoagulants or who have a high risk of major bleeding if given anticoagulants.1 Removable filters are a reasonable approach to women who have a transient contraindication to anticoagulants, such as the development of DVT near the time (within 1 to 2 weeks) of delivery. To minimize the risk of bleeding in such women, anticoagulants might have to be discontinued just prior to and immediately after delivery, resulting in a high-risk period for thrombus extension and embolization. Insertion of a removable filter provides protection from PE during this period and, once the risk of bleeding with resumption of full-dose anticoagulants becomes acceptable, the filter can be removed.
How we manage pregnant women who have a high risk of VTE
Patients with prior VTE or laboratory abnormalities that predispose to VTE (“hypercoagulable” states, see below) have an increased risk of pregnancy-associated VTE.52,78 The magnitude of this risk can be stratified according to the presence or absence of clinical risk factors at the time of the prior episode of VTE and the presence or absence of a hypercoagulable state. The former can be subdivided into (1) transient (within 3 months) risk factors (ie, recent major surgery, major trauma, or prolonged immobilization) or (2) permanent risk factors (ie, metastatic cancer or permanent leg paralysis). In nonpregnant patients, those who have a prior episode of “unprovoked” VTE (idiopathic, not associated with a clinical risk factor) or VTE associated with a permanent risk factor have a higher risk of recurrence than those with a single episode of VTE associated with a transient risk factor, particularly if it is a major risk factor. 78-80 Hypercoagulable states include congenital deficiencies of protein C, protein S, or antithrombin and the presence of APLA, factor V Leiden, the prothrombin gene mutation, hyperhomocysteinemia, persistently elevated levels of factor VIII, as well as dysfibrinogenia. The presence of APLA definitely increases the risk of recurrent VTE.1 Although many investigators believe that other hypercoagulable states (particularly antithrombin deficiency) are associated with an increased risk of recurrent VTE, the supporting evidence is weak.
Prior VTE
Estimates of the rate of recurrent VTE during pregnancy in women with a history of VTE have varied between 0% and 13%.81-84 The higher of these estimates has prompted some authorities to recommend routine anticoagulant prophylaxis during pregnancy and the postpartum period in all women with a history of VTE.85 However, the risk is likely to be lower than has been suggested by some of these studies because objective testing was used uncommonly to confirm the diagnosis or recurrent VTE (which would result in an overdiagnosis of recurrence). Furthermore, the higher estimates of the frequency are derived from retrospective studies of nonconsecutive patients,83,84 whereas the lower estimates come from prospective, albeit small (n = 20, n = 59), studies.81 82
To obtain a reliable estimate of the true incidence of recurrent VTE in women with prior VTE, Brill-Edwards and colleagues85 recently completed a prospective study of 125 pregnant women with a single previous episode of objectively diagnosed VTE. Women with an episode of VTE within 3 months before pregnancy or a prior documented hypercoagulable state were excluded from participating in the study. Antepartum heparin was withheld, and anticoagulants (usually warfarin with a target INR of 2.0 to 3.0 with an initial short course of UFH or LMWH) were given in the postpartum period for 4 to 6 weeks. The antepartum recurrence rate was 2.4% (95% confidence interval [CI], 0.2%-6.9%). Ninety-five patients had blood testing to identify laboratory abnormalities. There were no recurrences in the 44 patients (0%, 95% CI = 0.0%-8.0%) who did not have thrombophilia and had a previous episode of thrombosis that was associated with a temporary risk factor. Patients with abnormal test results and/or a previous episode of thrombosis that was idiopathic had an antepartum recurrence rate of 5.9% (95% CI = 1.2%-16%). Based on these results, the absolute risk of antepartum-recurrent VTE in women without known thrombophilia and whose previous episode of thrombosis was associated with a transient risk factor is sufficiently low that routine antepartum prophylaxis is not justified. Further studies are needed to determine whether antepartum prophylaxis is warranted in patients with thrombophilia by laboratory testing and/or a previous episode of idiopathic thrombosis. Unfortunately, there are insufficient data from this or other studies to provide management guidelines for women whose prior VTE occurred in the setting of a high estrogen state (ie, oral contraceptive use or prior pregnancy). We believe that these women have sufficiently high risk of antepartum recurrence to merit prophylactic anticoagulation prior to delivery as well as after childbirth.
The role of antepartum surveillance with noninvasive tests (eg, CUS) in women not receiving prophylaxis is controversial because if we postulate that the prevalence of recurrent VTE is low (5%), even with a sensitivity of 96% (which is likely to be an overestimate in asymptomatic subjects) and a specificity of 98%, the positive predictive value of CUS is only 10%. Further, the timing of screening with ultrasound is problematic. Even if performed as often as weekly, a woman could still develop clinically important recurrence 2 to 3 days after a normal ultrasound. Therefore, we do not recommend routine screening for VTE with regular noninvasive testing. Rather, pregnant women should be counseled about the importance of seeking medical attention immediately if they develop symptoms suggestive of DVT or PE. These women should be investigated aggressively if they present with suspicious symptoms.
Hypercoagulable states
Women with no prior VTE and a hypercoagulable state have an increased risk of VTE during pregnancy, but the absolute magnitude of the risk is unknown and, therefore, recommendations are empiric and include prophylaxis or clinical surveillance for VTE alone. A recent study has shown that asymptomatic women with congenital deficiencies of antithrombin, protein C, or protein S, have approximately an 8-fold increase in their risk of VTE during pregnancy compared with normal controls.20 However, in absolute terms the risk of VTE was low (7 [4.1%] of 169 pregnancies). Two of these episodes occurred during the third trimester, and the remaining 5 occurred after childbirth. In another study of 2480 pregnant women, those with activated protein C resistance due to the factor V Leiden mutation also had an 8-fold increase in the risk of VTE compared with those without this abnormality, although the absolute rates of thrombosis were lower in this study (0.1% in women with the factor V Leiden abnormality).17 The antepartum management of pregnant women with known thrombophilia and no prior VTE remains controversial because of the low rate of pregnancy-associated VTE in thrombophilic women in the studies cited above and a lack of trials of VTE prophylaxis.
Recommended approaches to pregnant patients who have an increased risk of VTE
Based on the above considerations, we suggest the following (Table 2 for definitions of dose regimens): (1) For women with a single episode of prior VTE associated with a transient risk factor (and no additional current risk factors), we recommend clinical surveillance before childbirth. (2) For women with a single episode of previous idiopathic VTE who are not receiving long-term anticoagulation therapy, we recommend surveillance, mini-dose UFH, moderate-dose UFH, or prophylactic LMWH before childbirth. (3) For women with a single episode of VTE and a confirmed hypercoagulable state who are not receiving long-term anticoagulation therapy, we recommend clinical surveillance, mini-dose UFH, moderate-dose UFH or prophylactic LMWH before childbirth. The indication for active antepartum prophylaxis is stronger in women with APLA or those with antithrombin deficiency than the other hypercoagulable states. (4) For women with no prior VTE who have a confirmed hypercoagulable state, we recommend clinical surveillance, mini-dose UFH, or prophylactic LMWH. Again, the indication for active antepartum prophylaxis is probably stronger in women with APLA or those with antithrombin deficiency than the other hypercoagulable states. For (1) through (4), we also recommend postpartum anticoagulants for all regimens. (5) For women with multiple (more than 2) episodes of VTE and/or women receiving long-term anticoagulation therapy (eg, single episode of VTE, either idiopathic or associated with a hypercoagulable state), we recommend adjusted-dose UFH, or either prophylactic or weight-adjusted LMWH before childbirth, followed by resumption of long-term anticoagulation therapy after childbirth.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-03-0965.
S.M.B. is a recipient of a New Investigator Award from the Canadian Institutes of Health Research/bioMérieux. J.S.G. is a recipient of a Career Investigator Award from the Heart and Stroke Foundation of Ontario and a Research Chair from the Canadian Institutes of Health Research/Astra Zeneca.
References
Author notes
J. S. Ginsberg, HSC 3X28, McMaster University Medical Centre, 1200 Main St West, Hamilton, ON L8N 3Z5 Canada; e-mail: ginsbrgj@mcmaster.ca.