Thrombocytopenia is a common medical problem for which the main treatment is platelet transfusion. Given the increasing use of platelets and the declining donor population, identification of a safe and effective platelet growth factor could improve the management of thrombocytopenia. Thrombopoietin (TPO), the c-Mpl ligand, is the primary physiologic regulator of megakaryocyte and platelet development. Since the purification of TPO in 1994, 2 recombinant forms of the c-Mpl ligand—recombinant human thrombopoietin (rhTPO) and pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF)—have undergone extensive clinical investigation. Both have been shown to be potent stimulators of megakaryocyte growth and platelet production and are biologically active in reducing the thrombocytopenia of nonmyeloablative chemotherapy. However, neither TPO has demonstrated benefit in stem cell transplantation or leukemia chemotherapy. Other clinical studies have investigated the use of TPO in treating chronic nonchemotherapy-induced thrombocytopenia associated with myelodysplastic syndromes, idiopathic thrombocytopenic purpura, thrombocytopenia due to human immunodeficiency virus, and liver disease. Based solely on animal studies, TPO may be effective in reducing surgical thrombocytopenia and bleeding, ex vivo expansion of pluripotent stem cells, and as a radioprotectant. Ongoing and future studies will help define the clinical role of recombinant TPO and TPO mimetics in the treatment of chemotherapy- and nonchemotherapy-induced thrombocytopenia.
Introduction
Thrombocytopenia is a common problem in the management of patients with cancer and other conditions that affect hematopoietic cells. Thrombocytopenia may be occasionally encountered with conventional chemotherapy regimens used to treat solid tumors but can be a major clinical problem in the management of patients receiving dose-intensive chemotherapy, induction and consolidation therapy for leukemia, palliative chemotherapy following multiple previous regimens, and multiple cycles of certain chemotherapeutic regimens.1Multiagent regimens such as MAID (mesna, adriamycin, ifosfamide, and dacarbazine) and ICE (ifosfamide, carboplatin, and etoposide) used in the treatment of lymphoma, sarcoma, breast, ovarian, and germ cell tumors often produce thrombocytopenia that requires dose modifications, platelet transfusions, or both to prevent bleeding complications.1 2 Thrombocytopenia associated with the use of newer chemotherapy agents such as gemcitabine may limit their use in patients with lung, breast, or ovarian cancer. Additionally, patients with associated bone marrow failure have a higher risk of severe thrombocytopenia and bleeding complications with any chemotherapy regimen.
Thrombocytopenia is also a frequent problem in the management of nonchemotherapy patients with myelodysplastic syndrome (MDS), idiopathic thrombocytopenic purpura (ITP), chronic liver disease, and acquired immunodeficiency syndrome (AIDS).3-6The chronic thrombocytopenia observed in these conditions results from defective or diminished platelet production or enhanced immunologic and nonimmunologic platelet destruction and may be associated with abnormal platelet function.3-6 Furthermore, patients undergoing liver transplantation, cardiovascular surgery, requiring intra-aortic balloon counterpulsation, or receiving supportive intensive care often experience severe, acute thrombocytopenia that is associated with increased mortality.7 8
Platelet transfusion therapy is currently the only acute treatment for severe thrombocytopenia. Although temporarily effective in controlling severe thrombocytopenia, platelet transfusion therapy is associated with several problems, including refractoriness and alloimmunization, transmission of infectious agents, and transfusion reactions.9-15 The limited supply of blood products can also be problematic. The use of dose-intensive chemotherapy regimens and hematopoietic progenitor cell transplantation, as well as intensive support for the medical and surgical patient, has resulted in an increasing demand for platelet products; this demand is likely to escalate in an attempt to improve clinical outcomes for oncology and nononcology patients. The limitations of platelet transfusions and the increased costs associated with the complications of such transfusions have prompted a search for growth factors that stimulate platelet production, thereby reducing or eliminating the need for platelet transfusions.1 2
Thrombopoietic growth factors
Over the past 2 decades, a number of hematopoietic growth factors with thrombopoietic activity have been identified, including recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF); stem cell factor (c-kit ligand or steel factor); interleukin 1 (IL-1), IL-3, IL-6, and IL-11; and thrombopoietin (TPO).16-26Early clinical studies of many of these cytokines, including IL-1, IL-3, IL-6, and IL-11, showed their ability to stimulate platelet production directly or indirectly in patients with chemotherapy-induced thrombocytopenia.27-32 In phase 1 studies, administration of IL-1α before or after carboplatin therapy increased platelet counts and was effective at attenuating thrombocytopenia associated with chemotherapy.27,32 Similarly, both IL-6 and IL-11 have been shown to produce an increase in platelet counts and accelerate platelet recovery after chemotherapy.29,30Despite its relatively modest effect on megakaryocyte and platelet production, IL-11 has been shown to reduce the need for platelet transfusions in patients with chemotherapy-induced thrombocytopenia.31 33
Although ILs stimulate thrombopoiesis, their action on platelets is not their principal physiologic function. Recently, gene-targeting studies have shown that the primary physiologic function of IL-11 is to maintain female fertility; it is not essential for hematopoiesis either in normal physiology or in response to hematopoietic stress.34,35 Furthermore, the pleiotropic effect of ILs often results in unwanted or unacceptable toxic effects, including hyperbilirubinemia, rapid induction of anemia, fever, fatigue, chills, hypotension, and headache.27,32,36-38 Although administration of IL-11 reduces the need for platelet transfusions by approximately a third in patients with severe chemotherapy-induced thrombocytopenia, it is associated with mild peripheral edema, dyspnea, conjunctival redness, and a low incidence of atrial arrhythmias and syncope.30 33 Thus, despite the ability of ILs to ameliorate thrombocytopenia in a subset of patients treated with conventional chemotherapy, the moderate toxicity encountered with IL treatment may interfere with its therapeutic effect and potential use as a thrombopoietic agent.
In contrast to ILs, TPO, also known as c-Mpl ligand, is a relatively lineage-specific cytokine that stimulates megakaryocyte growth and maturation in vitro and is a potent in vivo thrombopoietic growth factor. Gene-targeting studies have established that TPO is the most important physiologic regulator of steady-state megakaryocyte and platelet production.39-41 Cloning of the c-Mpl ligand led to the clinical development of various preparations of TPO, including recombinant human thrombopoietin (rhTPO) and pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF). This article presents an overview of the biology of the recombinant thrombopoietins, the results of all phase 1 and 2 clinical studies of different preparations of TPO, and the problems encountered in their development.
TPO and its biology
Isolation of c-Mpl ligand
The identification of TPO as the ligand for the c-Mpl receptor was heralded with much enthusiasm, as almost 40 years had elapsed since the proposal of the existence of a factor that regulates megakaryocytopoiesis and generation of platelets.42 As is often the case in research, several independent laboratories simultaneously reported the identification and molecular cloning of TPO.17-21 A sentinel discovery that preceded the purification and molecular cloning of TPO was the cloning of the retroviral oncogene, v-mpl, from the murine myeloproliferative leukemia virus.43 Subsequently, the cellular homologue, c-mpl, was cloned and shown to encode a membrane protein that possessed substantial homology with receptors for ILs and colony-stimulating factors, indicating that it might function as a hematopoietic receptor.44 Further support for the role of c-Mpl as the putative thrombopoietin receptor is provided by the presence of c-Mpl mRNA and protein primarily in platelets, megakaryocytes, and a subpopulation of CD34+ cells and the absolute requirement for the presence of a functional c-Mpl to stimulate progenitor cells of the megakaryocyte lineage in bone marrow cultures.45 Several groups were able to isolate, purify, and clone its ligand, which was initially referred to as the c-Mpl ligand, megakaryocyte growth and development factor, megapoietin, or thrombopoietin.17-21
Structure and biologic properties of TPO
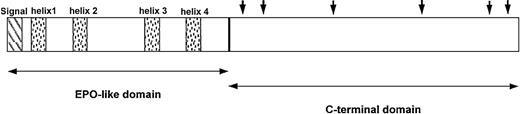
TPO is synthesized primarily in the liver as a single 353-amino acid precursor protein. On removal of the 21-amino acid signal peptide, the mature molecule consists of 2 domains: a receptor-binding domain that shows considerable homology to erythropoietin and a carbohydrate-rich carboxy-terminus of the protein that is highly glycosylated and important in maintaining protein stability (Figure1).18,19 46-48
Domain structure shows features unique to endogenous TPO.
The amino terminus (first 153 amino acids) of TPO contains 4 conserved cysteine residues and is 17% identical to erythropoietin (EPO; ∼50% identical if neutral substitutions are taken into account). It contains the entire receptor-binding region. The shaded boxes show the predicted α-helical regions of TPO. The carboxyl terminus (amino acids 154 to 332) of the molecule appears to be unique to TPO and contains theN-linked glycosylation sites indicated by solid arrows. Adapted from Foster and Hunt47 with permission.
Domain structure shows features unique to endogenous TPO.
The amino terminus (first 153 amino acids) of TPO contains 4 conserved cysteine residues and is 17% identical to erythropoietin (EPO; ∼50% identical if neutral substitutions are taken into account). It contains the entire receptor-binding region. The shaded boxes show the predicted α-helical regions of TPO. The carboxyl terminus (amino acids 154 to 332) of the molecule appears to be unique to TPO and contains theN-linked glycosylation sites indicated by solid arrows. Adapted from Foster and Hunt47 with permission.
TPO levels usually increase in response to the decline in platelet mass and remain elevated during persistent thrombocytopenia. Although hepatic transcription and translation of the TPO gene appears to be constant,49,50 most studies indicate that the circulating platelet mass directly determines the circulating level of TPO.20,51-56 Transfusion of platelets into thrombocytopenic animals or humans has resulted in a decrease in plasma TPO levels20,52-54,57 and similar results have been observed when normal platelets are transfused into c-Mpl–deficient mice.51 These findings indicate that TPO is constitutively synthesized in the liver and removed from circulation by binding to the c-Mpl receptor on platelets and possibly bone marrow megakaryocytes.
Some investigators have alternatively suggested that local production of TPO by bone marrow stromal cells is increased during thrombocytopenia and stimulates megakaryocyte growth.58Direct evidence to support the relative contribution of this mechanism to platelet production is lacking; but in experiments in which livers from TPO−/− mice were transplanted into normal mice, at least 60% of the platelet production could be accounted for by hepatic TPO production.59 Furthermore, in patients with hepatic failure undergoing liver transplantation, the low platelet counts and undetectable TPO levels before transplantation became normal after transplantation, suggesting that the liver is responsible for virtually all TPO production.60 61
As predicted, TPO increases the size, ploidy, and number of megakaryocytes and stimulates the expression of platelet-specific markers.20,62,63 In addition to acting as a potent megakaryocyte colony-stimulating factor, TPO has a synergistic effect on the growth of myeloid and erythroid precursors when combined with other hematopoietic growth factors such as erythropoietin or stem cell factor.64-66 The role of TPO as the principal physiologic regulator of platelet production has been confirmed in studies of mutant mice lacking the ability to produce either TPO (TPO−/−) or its receptor (c-Mpl−/−).67-71 Genetic elimination of TPO or c-Mpl results in an 85% to 95% reduction in the number of circulating platelets, megakaryocytes, and megakaryocyte progenitor cells.39,70,71 Although TPO-deficient mice are severely thrombocytopenic, they are healthy and show no signs of spontaneous hemorrhage, implying that TPO-independent mechanisms for platelet production exist.70 Recently, “double knock-out” mice that lack the genes for c-Mpl and one other growth factor or its receptor (GM-CSF, IL-3, IL-11, IL-6, or leukemia inhibitory factor) have been created to investigate this observation. The double knock-out mice had no additional defect in platelets or their precursors, indicating that GM-CSF, IL-3, IL-11, IL-6, or leukemia inhibitory factor alone are not responsible for the basal platelet production seen in the absence of TPO signaling.40,41 72
TPO and its receptor also have a major effect on production of primitive pluripotent stem cells and progenitor cells from other lineages. Despite normal red and white blood cell numbers, mice deficient in TPO (TPO−/−) or c-Mpl (c-Mpl−/−) exhibit a 60% to 70% reduction in the number of erythroid and myeloid progenitor cells compared with control animals.39,70 In addition, the ability of hematopoietic cells from these animals to reconstitute the hematopoietic organs of irradiated normal mice is also substantially reduced.73Administration of TPO to TPO−/− mice expanded the bone marrow and spleen progenitor pools of all hematopoietic lineages and increased the number of circulating platelets.39
Despite its activity on hematopoietic stem cells and early megakaryocytopoiesis, TPO has little effect on platelets and on the late stages of megakaryocyte development.74-77 This contrasts with granulocyte colony-stimulating factor (G-CSF) and GM-CSF, for which an action on late myeloid precursor cells and mature myeloid cells is well established.78,79 Perhaps the most significant consequence of TPO's minimal effect on late-stage megakaryocytes is the inability of TPO to hasten platelet shedding from megakaryocytes. In fact, if anything, TPO inhibits platelet shedding.74
Although TPO does not directly cause platelet activation, at pharmacologically high doses it does have a modest effect on mature platelets by increasing their reactivity to some aggregation stimuli; TPO-treated platelets require half as much adenosine diphosphate for a response.80,81 Other hematopoietic growth factors also reduce the threshold for platelet activation but the clinical relevance is uncertain. This effect may be mediated by TPO-dependent activation of phosphatidylinositide 3-kinase, which in turn phosphorylates Thr306 and Ser473 of platelet protein kinase Bα, an important antiapoptotic protein.82 However, TPO does not prevent apoptosis of platelets during ex vivo storage.83 84
Recombinant TPO
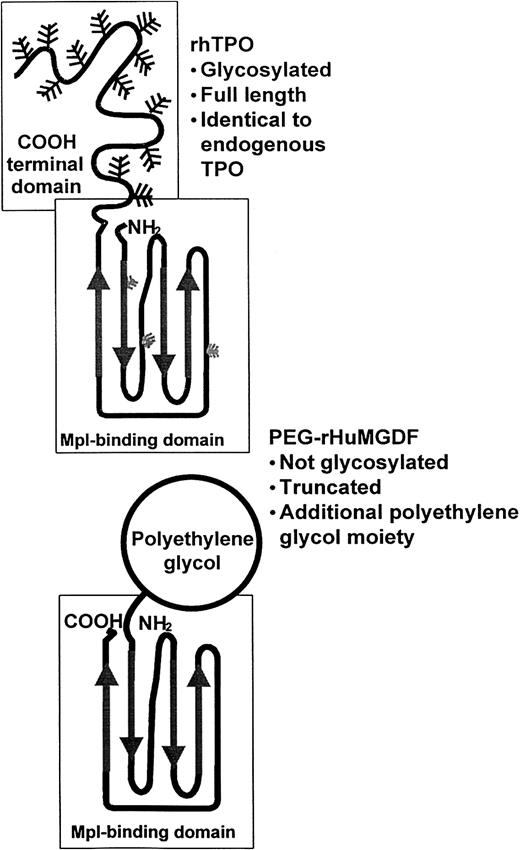
Since TPO was first cloned, several recombinant TPOs have been developed for clinical evaluation. There are 2 of these preparations, rhTPO and PEG-rHuMGDF (Figure 2), that have undergone considerable preclinical and clinical evaluation. The amino acid sequence of rhTPO (Pharmacia, Peapack, NJ; formerly developed by Genentech, South San Francisco, CA) is identical to that of endogenous TPO. rhTPO is produced in mammalian cells and is glycosylated. Nonetheless, its molecular weight is 90 kd, less than the 95 kd of the native molecule.85 PEG-rHuMGDF (Amgen, Thousand Oaks, CA), is produced in Escherichia coli and consists of the receptor-binding, 163 amino-terminal amino acids of native TPO. It is conjugated to a 20-kd polyethylene glycol moiety to increase its circulatory half-life and possesses all the biologic activity of native TPO.86 These 2 recombinant thrombopoietins have similar pharmacologic characteristics and show profound in vitro and in vivo effects on megakaryocyte development and platelet production (Table1).87 88
Molecular structures of rhTPO and PEG-rHuMGDF exhibit specific differences.
rhTPO is a glycosylated full-length TPO molecule, whereas PEG-rHuMGDF is a truncated molecule consisting of the receptor-binding portion of native TPO conjugated to a 20-kd polyethylene glycol moiety. Adapted from Begley and Basser88 with permission.
Molecular structures of rhTPO and PEG-rHuMGDF exhibit specific differences.
rhTPO is a glycosylated full-length TPO molecule, whereas PEG-rHuMGDF is a truncated molecule consisting of the receptor-binding portion of native TPO conjugated to a 20-kd polyethylene glycol moiety. Adapted from Begley and Basser88 with permission.
Pharmacologic characteristics of rhTPO and PEG-rHuMGDF
| Characteristic . | rhTPO . | PEG-rHuMGDF . |
|---|---|---|
| Terminalt1/2(h) | 24-40 | 31 |
| Kd for c-Mp1 (pM) | 150 | 150 |
| Platelet clearance (mL/h/109) | 1.28 | 1.28 |
| Time to onset of effect in mice (d) | 3 | 3 |
| Time to peak effect in mice (d) | 4-6 | 4-6 |
| Truncation | No | Yes |
| Glycosylation | Yes | No |
| Pegylation | No | Yes |
| Human route tested | Intravenous | Subcutaneous |
| Characteristic . | rhTPO . | PEG-rHuMGDF . |
|---|---|---|
| Terminalt1/2(h) | 24-40 | 31 |
| Kd for c-Mp1 (pM) | 150 | 150 |
| Platelet clearance (mL/h/109) | 1.28 | 1.28 |
| Time to onset of effect in mice (d) | 3 | 3 |
| Time to peak effect in mice (d) | 4-6 | 4-6 |
| Truncation | No | Yes |
| Glycosylation | Yes | No |
| Pegylation | No | Yes |
| Human route tested | Intravenous | Subcutaneous |
PEG-rHuMGDF, pegylated recombinant human megakaryocyte growth and development factor; rhTPO, recombinant human thrombopoietin.
In healthy animals, TPO exerts its peripheral blood effects exclusively on platelets; with no increase in white or red blood cells. Administration of either form of recombinant TPO to healthy nonhuman primates results in a dose-dependent increase in megakaryocyte number, size, and ploidy and up to a 5-fold increase in circulating platelet counts.89 There is a requisite lag time of 4 to 5 days before the platelet count rises; this reflects the finding that TPO acts primarily on early, not late, precursor cells. In murine models of severe chemotherapy- or radiation-induced thrombocytopenia or both, daily administration of recombinant TPO increases megakaryocyte numbers in the bone marrow, ameliorates the depth and duration of thrombocytopenia, and reduces the severity of leukopenia and anemia.86,90 Similar results have been observed with recombinant TPO in nonhuman primate models of chemotherapy- and radiation-induced thrombocytopenia.91-93
In addition to these recombinant forms of TPO, several other molecules that bind and activate c-Mpl are being tested. One of these molecules is a fusion protein of TPO and IL-3. Administration of this molecule has been shown to increase platelet count in animals, but it has been found to be immunogenic and is no longer under development.94 Recently, great interest has been focused on the development of TPO peptide95 and nonpeptide96 mimetics. These mimetics are designed to bind to the TPO receptor but have no sequence homology with endogenous TPO.
Finally, a TPO receptor “potentiating” peptide has been developed that binds to c-Mpl at a location distant from the TPO binding area and prevents receptor internalization after TPO binding, thereby increasing TPO action. An analogous peptide that binds the erythropoietin receptor and potentiates erythropoietin has also recently been described.97
Clinical studies of recombinant TPO
Nonmyeloablative treatments
The stimulatory effects of PEG-rHuMGDF and rhTPO on megakaryocyte and platelet production have been demonstrated in several clinical trials (Table 2).98-107PEG-rHuMGDF, the most widely studied recombinant TPO, has produced dose-dependent increases in platelet counts in patients with advanced malignancies and attenuated chemotherapy-induced thrombocytopenia in randomized, placebo-controlled clinical trials.98,99,105-107 When administered before chemotherapy as a daily subcutaneous injection, PEG-rHuMGDF produced a dose-dependent increase in peripheral blood platelet counts and a modest increase in megakaryocyte, erythroid, and myeloid progenitor cell levels in patients with advanced cancer.99 No evidence of platelet activation or altered platelet function was observed with PEG-rHuMGDF administration.108
Clinical studies of 2 forms of recombinant thrombopoietin in chemotherapy-induced thrombocytopenia
| Form . | Investigator . | Disease . | No. of patients . | Chemotherapy . | Dose (μg/kg) . | Route . | Prechemotherapy phase . | Postchemotherapy phase . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Dosing . | Outcome . | Dosing . | Outcome . | |||||||
| PEG-rHuMGDF | Fanucchi et al98 | Non-small-cell lung cancer | PEG-rHuMGDF = 41 | Carboplatin, paclitaxel | 0.03-5.0 | SC | Daily up to 10 d | Increase in platelets in 2 of 3 patients treated* | Daily up to 16 d | Increased recovery of platelets to baseline level |
| Placebo = 12 | Nadir platelet counts increased | |||||||||
| Basser et al99 105 | Advanced cancer | PEG-rHuMGDF = 31 | Carboplatin, cyclophosphamide | 0.03-5.0 | SC | Daily up to 10 d | Dose-related increase in platelets† 0.03-0.1 μg/kg per day | Daily for 7 to 20 d | Increased recovery of platelets to baseline level | |
| Placebo = 10 | → no increase in platelets 0.3-1.0 μg/kg per day | Platelet nadir earlier but nadir platelet count unaffected | ||||||||
| → median 3-fold increase in platelets | ||||||||||
| Crawford et al100 | Non-small-cell lung cancer | PEG-rHuMGDF = 30 | Carboplatin, paclitaxel | 2.5-5.0 | SC | NA | NA | Daily up to 7 d | Dose-related increase in platelet nadir | |
| Placebo = 10 | Reduction in number of platelet transfusions required in first 2 cycles | |||||||||
| Dose-limiting thrombocytopenia in later cycles | ||||||||||
| Anti-TPO antibodies in 2 patients | ||||||||||
| Basser et al106 | Advanced cancer | PEG-rHuMGDF = 68 | Carboplatin, cisplatin | 1.0-10.0 | SC | NA | NA | Multiple daily doses up to 7 d | Increased platelet nadir (47.5 × 109/L versus 35.5×109/L; P =.003) and decreased duration of grade 3 or 4 thrombocytopenia (0 versus 3 days; P =.004) with PEG-rHuMGDF | |
| rhTPO | Vadhan-Raj et al101 102 | Sarcoma | rhTPO = 12 | Doxorubicin, ifosfamide | 0.3-2.4 | IV | Single | Dose-related 1.3-3.6-fold increase in platelets | 1, 2, 3, or 7 daily doses | Decreased (schedule-dependent) thrombocytopenia Increased platelet recovery |
| Vadhan-Raj et al103 104 | Gynecologic malignancy | rhTPO = 29 | Carboplatin | 0.6-3.6 | SC | Single | Dose-related 1.1-3.5-fold increase in platelets | 4 daily doses | Decrease in both degree and duration of severe thrombocytopenia and the need for platelet transfusions | |
| Form . | Investigator . | Disease . | No. of patients . | Chemotherapy . | Dose (μg/kg) . | Route . | Prechemotherapy phase . | Postchemotherapy phase . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Dosing . | Outcome . | Dosing . | Outcome . | |||||||
| PEG-rHuMGDF | Fanucchi et al98 | Non-small-cell lung cancer | PEG-rHuMGDF = 41 | Carboplatin, paclitaxel | 0.03-5.0 | SC | Daily up to 10 d | Increase in platelets in 2 of 3 patients treated* | Daily up to 16 d | Increased recovery of platelets to baseline level |
| Placebo = 12 | Nadir platelet counts increased | |||||||||
| Basser et al99 105 | Advanced cancer | PEG-rHuMGDF = 31 | Carboplatin, cyclophosphamide | 0.03-5.0 | SC | Daily up to 10 d | Dose-related increase in platelets† 0.03-0.1 μg/kg per day | Daily for 7 to 20 d | Increased recovery of platelets to baseline level | |
| Placebo = 10 | → no increase in platelets 0.3-1.0 μg/kg per day | Platelet nadir earlier but nadir platelet count unaffected | ||||||||
| → median 3-fold increase in platelets | ||||||||||
| Crawford et al100 | Non-small-cell lung cancer | PEG-rHuMGDF = 30 | Carboplatin, paclitaxel | 2.5-5.0 | SC | NA | NA | Daily up to 7 d | Dose-related increase in platelet nadir | |
| Placebo = 10 | Reduction in number of platelet transfusions required in first 2 cycles | |||||||||
| Dose-limiting thrombocytopenia in later cycles | ||||||||||
| Anti-TPO antibodies in 2 patients | ||||||||||
| Basser et al106 | Advanced cancer | PEG-rHuMGDF = 68 | Carboplatin, cisplatin | 1.0-10.0 | SC | NA | NA | Multiple daily doses up to 7 d | Increased platelet nadir (47.5 × 109/L versus 35.5×109/L; P =.003) and decreased duration of grade 3 or 4 thrombocytopenia (0 versus 3 days; P =.004) with PEG-rHuMGDF | |
| rhTPO | Vadhan-Raj et al101 102 | Sarcoma | rhTPO = 12 | Doxorubicin, ifosfamide | 0.3-2.4 | IV | Single | Dose-related 1.3-3.6-fold increase in platelets | 1, 2, 3, or 7 daily doses | Decreased (schedule-dependent) thrombocytopenia Increased platelet recovery |
| Vadhan-Raj et al103 104 | Gynecologic malignancy | rhTPO = 29 | Carboplatin | 0.6-3.6 | SC | Single | Dose-related 1.1-3.5-fold increase in platelets | 4 daily doses | Decrease in both degree and duration of severe thrombocytopenia and the need for platelet transfusions | |
NA indicates not applicable; PEG-rHuMGDF, pegylated recombinant human megakaryocyte growth and development factor; rhTPO, recombinant human thrombopoietin; SC subcutaneous; and IV, intravenous.
Six patients (3 placebo, 3 PEG-rHuMGDF) were evaluated in the prechemotherapy phase.
Seventeen patients (4placebo, 13 PEG-rHuMGDF) were evaluated in the prechemotherapy phase.
Subsequent trials evaluated the effects of PEG-rHuMGDF on hematologic recovery after chemotherapy. A randomized, placebo-controlled, dose-escalation study evaluated the effects of PEG-rHuMGDF after carboplatin-paclitaxel chemotherapy in 53 patients with lung cancer.98 Patients treated with PEG-rHuMGDF after chemotherapy had a higher median nadir platelet count (188 × 109/L) than did placebo-treated patients (111 × 109/L) and also showed more rapid recovery of platelet counts (14 days versus > 21 days). The need for platelet transfusions was unaffected because the chemotherapy regimen used did not frequently generate severe thrombocytopenia. In another randomized study of 41 patients with advanced cancer undergoing chemotherapy with carboplatin and cyclophosphamide, treatment with PEG-rHuMGDF enhanced platelet recovery in a dose-related manner.99 Although the platelet nadir occurred earlier in the PEG-rHuMGDF–treated group, its depth was unchanged. Similar results were observed in a dose-scheduling trial of PEG-rHuMGDF with G-CSF carried out in patients with non–small-cell lung cancer treated with carboplatin-paclitaxel.100 PEG-rHuMGDF–treated patients had a higher platelet nadir than did placebo-treated patients (89 × 109/L versus 27 × 109/L in cycle 1). Moreover, the need for transfusion was lower in the PEG-rHuMGDF group than in the placebo group (17% versus 64% in the first 2 cycles). However, in the later cycles, thrombocytopenia became dose limiting in all treatment groups.
A recent study examined the efficacy of different doses and schedules of PEG-rHuMGDF in 68 patients with advanced cancer.106Patients received 1 cycle of carboplatin and cyclophosphamide and were then randomly assigned to receive PEG-rHuMGDF or placebo after the second and subsequent cycles of carboplatin and cyclophosphamide chemotherapy. The platelet nadir was higher and the duration of grade 3 or 4 thrombocytopenia shorter when PEG-rHuMGDF was administered to patients who received the same dose of chemotherapy for at least 2 cycles. No evidence of an effect on platelet nadir was observed when PEG-rHuMGDF was given before chemotherapy. Unlike in animal chemotherapy models (in which multilineage responses are often seen), no effect of recombinant TPOs on red or white blood cell recovery has been seen in humans.
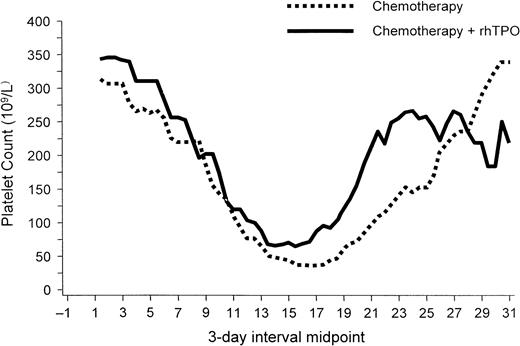
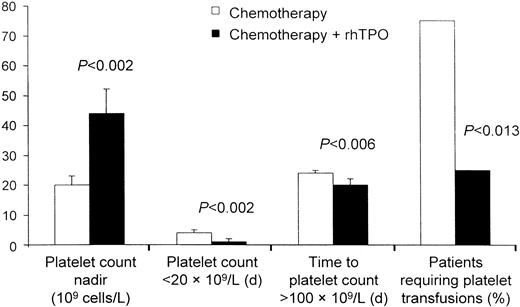
rhTPO has also produced a dose-dependent increase in platelet counts in patients with sarcomas and gynecologic malignancies.101-104,109 A phase 1 and 2 study examined the effect of rhTPO on megakaryocyte and platelet production before and after chemotherapy with doxorubicin and ifosfamide in patients with sarcomas who were at high risk of developing chemotherapy-induced thrombocytopenia. When given intravenously before chemotherapy, a single dose of rhTPO was associated with a dose-dependent increase in peripheral platelets that began on day 4 and peaked on day 12 in most patients.102This increase in platelet number was accompanied by a 4-fold increase in bone marrow megakaryocytes and a marked expansion and mobilization of erythroid, myeloid, and megakaryocyte progenitor cells. A single dose of rhTPO given intravenously after chemotherapy with doxorubicin and ifosfamide decreased the incidence of thrombocytopenia in some patients.101 A second trial investigated the clinical safety and activity of rhTPO administered subcutaneously to previously treated patients with gynecologic malignancies before and after chemotherapy with carboplatin.104 As observed in the previous study, administration of a single subcutaneous dose of rhTPO before chemotherapy produced a modest dose-dependent rise in circulating platelet counts. Administration of multiple doses of rhTPO after carboplatin chemotherapy produced an earlier platelet count nadir but effectively reduced the depth of the platelet nadir and the duration of severe thrombocytopenia (Figure3). The need for platelet transfusions decreased by 75% (Figure4).104
rhTPO increases nadir platelet count.
In patients undergoing intensive chemotherapy for gynecologic malignancy, rhTPO (given on days 2, 4, 6, and 8 after chemotherapy) increased the nadir platelet count. The platelet nadir also occurred earlier in patients treated with rhTPO than in untreated patients. Figure provided by Pharmacia (Peapack, NJ) from data in study by Vadhan-Raj et al.104
rhTPO increases nadir platelet count.
In patients undergoing intensive chemotherapy for gynecologic malignancy, rhTPO (given on days 2, 4, 6, and 8 after chemotherapy) increased the nadir platelet count. The platelet nadir also occurred earlier in patients treated with rhTPO than in untreated patients. Figure provided by Pharmacia (Peapack, NJ) from data in study by Vadhan-Raj et al.104
rhTPO decreases the need for platelet transfusions.
In patients undergoing intensive chemotherapy for gynecologic malignancy, rhTPO decreased thrombocytopenia and the need for platelet transfusions. Figure provided by Pharmacia (Peapack, NJ) from data in study by Vadhan-Raj et al.104
rhTPO decreases the need for platelet transfusions.
In patients undergoing intensive chemotherapy for gynecologic malignancy, rhTPO decreased thrombocytopenia and the need for platelet transfusions. Figure provided by Pharmacia (Peapack, NJ) from data in study by Vadhan-Raj et al.104
Myeloablative treatments
Prolonged and severe chemotherapy-induced thrombocytopenia is a major cause of morbidity in patients receiving intensive chemotherapy for acute leukemia and those undergoing blood stem cell transplantation.110,111 In recent years, several studies have evaluated the safety and efficacy of PEG-rHuMGDF and rhTPO in the management of thrombocytopenia associated with chemotherapy for acute leukemia and stem cell transplantation (Tables3 and4).112-125
Clinical studies of 2 forms of recombinant thrombopoietin as adjunct to induction/consolidation chemotherapy in patients with newly diagnosed acute myelogenous leukemia
| Form . | Investigator . | No. of patients . | Chemotherapy . | Dose (μg/kg) . | Route . | Induction . | Consolidation . | ||
|---|---|---|---|---|---|---|---|---|---|
| Dosing . | Outcome . | Dosing . | Outcome . | ||||||
| PEG-rHuMGDF | Archimbaud et al112 | PEG-rHuMGDF = 38 | Daunorubicin, cytarabine, etoposide | 2.5 or 5.0 | SC | Multiple or single | Dose-related increase in median platelet count | Multiple or single | Dose-related increase in remission median platelet count |
| Placebo = 12 | No difference between groups in time to platelet recovery or platelet transfusion requirements | No difference between groups in time to platelet recovery or platelet transfusion requirements | |||||||
| Schiffer et al114 | PEG-rHuMGDF = 24 | Daunorubicin, cytarabine +/− high-dose cytarabine | 2.5 or 5.0 | SC | Multiple | No difference between groups in time to platelet count ≥ 20 × 109/L or platelet transfusion requirements | Multiple | No difference between groups in time to platelet recovery to ≥ 20 × 109/L and ≥ 50 × 109/L or platelet transfusion requirements | |
| Placebo = 11 | |||||||||
| rhTPO | Cripe et al115 | rhTPO = 28 | Idarubicin, cytarabine | 2.5-5.0 | IV | Multiple | No difference between rhTPO and historical controls in time to platelet count ≥ 20 × 109/L or ≥ 50 × 109/L | NA | NA |
| Form . | Investigator . | No. of patients . | Chemotherapy . | Dose (μg/kg) . | Route . | Induction . | Consolidation . | ||
|---|---|---|---|---|---|---|---|---|---|
| Dosing . | Outcome . | Dosing . | Outcome . | ||||||
| PEG-rHuMGDF | Archimbaud et al112 | PEG-rHuMGDF = 38 | Daunorubicin, cytarabine, etoposide | 2.5 or 5.0 | SC | Multiple or single | Dose-related increase in median platelet count | Multiple or single | Dose-related increase in remission median platelet count |
| Placebo = 12 | No difference between groups in time to platelet recovery or platelet transfusion requirements | No difference between groups in time to platelet recovery or platelet transfusion requirements | |||||||
| Schiffer et al114 | PEG-rHuMGDF = 24 | Daunorubicin, cytarabine +/− high-dose cytarabine | 2.5 or 5.0 | SC | Multiple | No difference between groups in time to platelet count ≥ 20 × 109/L or platelet transfusion requirements | Multiple | No difference between groups in time to platelet recovery to ≥ 20 × 109/L and ≥ 50 × 109/L or platelet transfusion requirements | |
| Placebo = 11 | |||||||||
| rhTPO | Cripe et al115 | rhTPO = 28 | Idarubicin, cytarabine | 2.5-5.0 | IV | Multiple | No difference between rhTPO and historical controls in time to platelet count ≥ 20 × 109/L or ≥ 50 × 109/L | NA | NA |
NA indicates not applicable; PEG-rHuMGDF, pegylated recombinant human megakaryocyte growth and development factor; rhTPO, recombinant human thrombopoietin; SC, subcutaneous; and IV, intravenous.
Clinical studies of 2 forms of recombinant thrombopoietin in patients receiving myeloablative chemotherapy followed by stem cell transplantation
| Form . | Investigator . | No. of patients . | Chemotherapy . | Dose (μg/kg) . | Route . | Stem cell mobilization . | Stem cell engraftment . | ||
|---|---|---|---|---|---|---|---|---|---|
| Dosing . | Outcome . | Dosing . | Outcome . | ||||||
| PEG-rHuMGDF | Glaspy et al116 | PEG-rHuMGDF = 39 | STAMP (high-dose) | 1, 3, 10, or 30 | SC | NA | NA | Multiple | Dose-related increase in median platelet count with PEG-rHuMGDF |
| Placebo = 14 | No difference between groups in time to platelet recovery to ≥ 20 × 109/L or platelet transfusion requirements | ||||||||
| Bolwell et al117 | PEG-rHuMGDF = 36 | STAMP I | 1.0, 2.5, 5.0, 7.5, or 10.0 | SC | NA | NA | Multiple (until platelet recovery ≥ 50 × 109/L) | Dose-related increase in platelet counts with PEG-rHuMGDF as compared with placebo | |
| Placebo = 11 | No difference between groups in time to platelet recovery to ≥ 20 × 109/L or platelet transfusion requirements | ||||||||
| Beveridge et al118 | PEG-rHuMGDF = 40 Placebo = 10 | STAMP V | 5 or 10.0 | SC | NA | NA | Multiple (until platelet recovery ≥ 25 × 109/L) | No difference between groups in time to platelet recovery, severe thrombocytopenia, or platelet transfusion requirements | |
| rhTPO | Nash et al120 | rhTPO = 38 with delayed platelet recovery after stem cell transplantation | NA | 0.6-2.4 | IV | NA | NA | Single and multiple until platelet recovery ≥ 20 × 109/L | No effect on time to platelet recovery to ≥ 20 × 109/L or platelet transfusion requirements |
| Somlo et al121 | rhTPO plus G-CSF = 26 G-CSF alone = 20 | Cisplatin, etoposide, cyclophosphamide | 0.6-2.4 | IV | Single and multiple | Increased CD34+ cell yields with rhTPO plus G-CSF as compared with G-CSF alone | NA | Reduced time to platelet recovery and platelet transfusion requirements with rhTPO plus G-CSF-mobilized cells | |
| Linker et al123 | rhTPO plus G-CSF = 101 G-CSF alone = 28 | 1.5 | IV | Single or multiple | Increased CD34+ cell number per apheresis and number of patients reaching minimum graft (CD34+ > 2 × 106/kg) with rhTPO plus G-CSF as compared with G-CSF alone | Single or multiple | No effect on time to platelet recovery to ≥ 20 × 109/L or platelet transfusion requirements | ||
| Form . | Investigator . | No. of patients . | Chemotherapy . | Dose (μg/kg) . | Route . | Stem cell mobilization . | Stem cell engraftment . | ||
|---|---|---|---|---|---|---|---|---|---|
| Dosing . | Outcome . | Dosing . | Outcome . | ||||||
| PEG-rHuMGDF | Glaspy et al116 | PEG-rHuMGDF = 39 | STAMP (high-dose) | 1, 3, 10, or 30 | SC | NA | NA | Multiple | Dose-related increase in median platelet count with PEG-rHuMGDF |
| Placebo = 14 | No difference between groups in time to platelet recovery to ≥ 20 × 109/L or platelet transfusion requirements | ||||||||
| Bolwell et al117 | PEG-rHuMGDF = 36 | STAMP I | 1.0, 2.5, 5.0, 7.5, or 10.0 | SC | NA | NA | Multiple (until platelet recovery ≥ 50 × 109/L) | Dose-related increase in platelet counts with PEG-rHuMGDF as compared with placebo | |
| Placebo = 11 | No difference between groups in time to platelet recovery to ≥ 20 × 109/L or platelet transfusion requirements | ||||||||
| Beveridge et al118 | PEG-rHuMGDF = 40 Placebo = 10 | STAMP V | 5 or 10.0 | SC | NA | NA | Multiple (until platelet recovery ≥ 25 × 109/L) | No difference between groups in time to platelet recovery, severe thrombocytopenia, or platelet transfusion requirements | |
| rhTPO | Nash et al120 | rhTPO = 38 with delayed platelet recovery after stem cell transplantation | NA | 0.6-2.4 | IV | NA | NA | Single and multiple until platelet recovery ≥ 20 × 109/L | No effect on time to platelet recovery to ≥ 20 × 109/L or platelet transfusion requirements |
| Somlo et al121 | rhTPO plus G-CSF = 26 G-CSF alone = 20 | Cisplatin, etoposide, cyclophosphamide | 0.6-2.4 | IV | Single and multiple | Increased CD34+ cell yields with rhTPO plus G-CSF as compared with G-CSF alone | NA | Reduced time to platelet recovery and platelet transfusion requirements with rhTPO plus G-CSF-mobilized cells | |
| Linker et al123 | rhTPO plus G-CSF = 101 G-CSF alone = 28 | 1.5 | IV | Single or multiple | Increased CD34+ cell number per apheresis and number of patients reaching minimum graft (CD34+ > 2 × 106/kg) with rhTPO plus G-CSF as compared with G-CSF alone | Single or multiple | No effect on time to platelet recovery to ≥ 20 × 109/L or platelet transfusion requirements | ||
G-CSF indicates granulocyte colony-stimulating factor; NA, not applicable; PEG-rHuMGDF, pegylated recombinant human megakaryocyte growth and development factor; rhTPO, recombinant human thrombopoietin; STAMP, solid tumor autologous marrow program.
In contrast to their effect in the nonmyeloablative setting, PEG-rHuMGDF and rhTPO have not had a clinically significant effect on platelet production when administered to patients receiving dose-intensive therapy for acute leukemia and those undergoing stem cell transplantation after chemotherapy. Moderate increase in peak platelet counts and reduction in time to full platelet recovery were often achieved in patients treated with PEG-rHuMGDF and rhTPO. However, no improvement in time to recovery to a platelet count more than or equal to 20 × 109/L and no reduction in the need for platelet transfusions were observed in these studies.112-114,119 120
In preclinical studies, treatment with TPO before bone marrow harvesting accelerated platelet reconstitution in recipient mice after transplantation, suggesting that this approach may be effective in shortening the time to platelet independence after stem cell transplantation.126 With this approach, one study showed that administration of rhTPO to patients during mobilization of peripheral blood progenitor cells increased CD34+ yield before stem cell transplantation. Small, statistically significant improvements in neutrophil recovery and platelet and erythrocyte transfusion requirements were noted after transplantation.121
MDS
Hematopoietic growth factors have had some success in ameliorating the neutropenia and anemia associated with myelodysplastic syndrome (MDS). The recombinant TPOs may have a similar benefit: some in vitro studies have shown that bone marrow cells of patients with MDS can differentiate into the megakaryocytic lineage when exposed to recombinant TPO.127,128 Because of the underlying heterogeneity of MDS, some individuals might have responsive marrow whereas others might not. Endogenous TPO levels are normal to slightly elevated in MDS,129 so whether they can help predict responsiveness to exogenous TPO requires further investigation. In a preliminary report, various intravenous doses of PEG-rHuMGDF were given daily for 14 days to 21 Japanese patients with MDS (refractory anemia and refractory anemia with ringed sideroblasts) with platelet counts less than 30 × 109/L. The peak effect of PEG-rHuMGDF occurred 5 to 6 weeks later with an average doubling of platelet count; responses were seen in a third of the patients, and a multilineage effect was observed in a few patients.130
Human immunodeficiency virus
Several studies have examined thrombocytopenia in patients or primates infected with human immunodeficiency virus (HIV) with respect to peripheral platelet mass turnover, marrow megakaryocytopoiesis, and endogenous TPO levels.3,131,132 A 10-fold disparity between the reduced platelet production and expanded megakaryocyte mass was observed in the bone marrow of HIV patients with thrombocytopenia.132 This suggests that, despite the expanded megakaryocyte mass, HIV-infected megakaryocytes have a high rate of apoptosis and resultant ineffective thrombopoiesis and thrombocytopenia. Harker and colleagues131 showed that, in thrombocytopenic chimpanzees infected with HIV, administration of PEG-rHuMGDF rapidly eliminated thrombocytopenia. With normal or slightly elevated endogenous TPO levels in 6 HIV-infected patients, platelet counts in all patients increased 10-fold within 14 days of the start of PEG-rHuMGDF treatment.132 This increase was not associated with change in the megakaryocyte mass, platelet life span, or viral load. What appeared to occur was an increase in the rate of effective platelet production from the bone marrow megakaryocytes of these individuals. These data suggest that, in HIV-related immune thrombocytopenic purpura, TPO can be expected to produce clinically beneficial increases in platelet counts.
Liver disease
Recent understanding of TPO biology suggests that reduced hepatic production of TPO may play a major role in thrombocytopenia associated with liver disease. TPO is produced primarily in the liver, and thrombocytopenia in animals seems to be proportional to the extent of liver resection.135 In addition, after transplantation of healthy livers into TPO−/− mice, platelet counts returned toward normal, suggesting that the majority of TPO is produced in the liver.59 An association between low platelet counts (median, 84 × 109/L; range, 26 × 109/L-112 × 109/L) and low levels of TPO (median, < 20 pg/mL; range, < 20-182 pg/mL) has been reported in patients before orthotopic liver transplantation.60 61Within 4 days of orthotopic liver transplantation, TPO levels rose above normal and were accompanied by increased amounts of reticulated platelets. At 14 days after transplantation, platelet counts were normal in 14 of 18 patients (median, 254 × 109/L; range, 70 × 109/L-398 × 109/L) and TPO levels returned to normal in 14 of 18 patients (median, 59 pg/mL; range, < 20-639 pg/mL). No appreciable change in spleen size was observed. In multivariate analysis, the increase in TPO was the only variable that correlated with the increase in platelet count. Thus, TPO could potentially be used to reduce hemorrhage in patients with thrombocytopenia due to liver disease or to prepare such patients for liver transplantation.
Surgery
Approximately 40% of all platelet transfusions are used in surgical settings.136 Preoperative and postoperative thrombocytopenia complicates surgical procedures and mandates platelet transfusions. No clinical studies have targeted this important area. However, in dogs, the administration of PEG-rHuMGDF 4 days before surgery decreased thrombocytopenia after cardiopulmonary bypass.137 Despite its 5-day lag time before platelet rise, judicial administration of TPO before surgery may ameliorate preoperative and postoperative thrombocytopenia and reduce the need for platelet transfusions.
Transfusion medicine
The striking effect of TPO on the mobilization of CD34+ cells and expansion of multilineage stem cell progenitor pools in vivo led to an evaluation of its activity in 3 areas: mobilization of peripheral blood stem cells before stem cell transplantation, ex vivo expansion of pluripotent stem cells from umbilical cord blood or bone marrow, and increase in the yield of platelet apheresis from healthy donors.
Stem cell mobilization.
Recently, several pilot studies evaluated the activity of various doses and schedules of rhTPO or PEG-rHuMGDF in combination with G-CSF and chemotherapy as part of a mobilization regimen for stem cell transplantation (Table 4).99,121-123,138,139 In contrast to peak progenitor cell numbers on days 5 to 7 usually obtained with G-CSF alone, a peak on days 12 to 15 was produced by the combination of PEG-rHuMGDF and G-CSF. However, since a full pharmacodynamic response profile to PEG-rHuMGDF was not performed in this study, the exact day of peak stem cell mobilization was not determined. The addition of rhTPO to G-CSF for chemotherapy mobilization regimens substantially increased CD34+ yields (Figure5). The promising results observed in these early studies were recently confirmed in a large randomized phase 2 study of rhTPO in patients undergoing high-dose chemotherapy and transplantation of peripheral blood stem cells (Table4).121 Treatment with rhTPO in various doses and schedules reduced the number of aphereses needed to reach a target graft (ie, CD34+ > 5 × 106/kg) and, compared with placebo treatment, increased the percentage of patients reaching a target graft (from 46% in the placebo group to 79% in the rhTPO group), as well as the percentage of patients reaching the minimum target graft (ie, CD34+ > 2 × 106/kg) (from 75% in the placebo group to 94% in the rhTPO group). These studies demonstrate the ability of rhTPO to mobilize CD34+cells safely and effectively and increase the harvest of CD34+ cells used for stem cell transplantation.
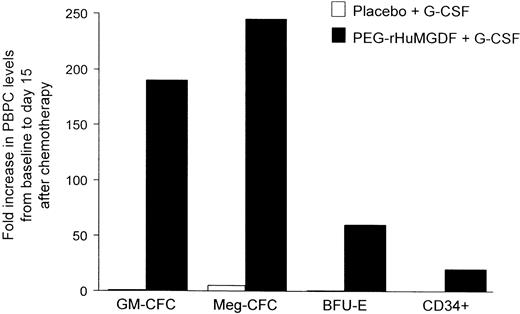
PEG-rHuMGDF increases peripheral blood progenitor cells (PBPCs).
Patients undergoing PBPC transplantation underwent stem cell mobilization with chemotherapy and G-CSF with or without PEG-rHuMGDF. Use of PEG-rHuMGDF increased granulocyte-macrophage colony-forming cells (GM-CFCs), megakaryocyte colony-forming cells (Meg-CFCs), erythroid burst-forming units (BFU-Es), and CD34+ cells. Adapted from Basser et al99 with permission.
PEG-rHuMGDF increases peripheral blood progenitor cells (PBPCs).
Patients undergoing PBPC transplantation underwent stem cell mobilization with chemotherapy and G-CSF with or without PEG-rHuMGDF. Use of PEG-rHuMGDF increased granulocyte-macrophage colony-forming cells (GM-CFCs), megakaryocyte colony-forming cells (Meg-CFCs), erythroid burst-forming units (BFU-Es), and CD34+ cells. Adapted from Basser et al99 with permission.
Ex vivo expansion of primitive stem cells.
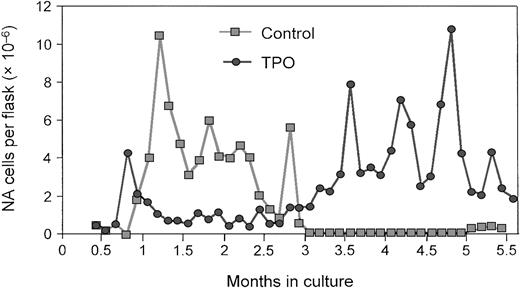
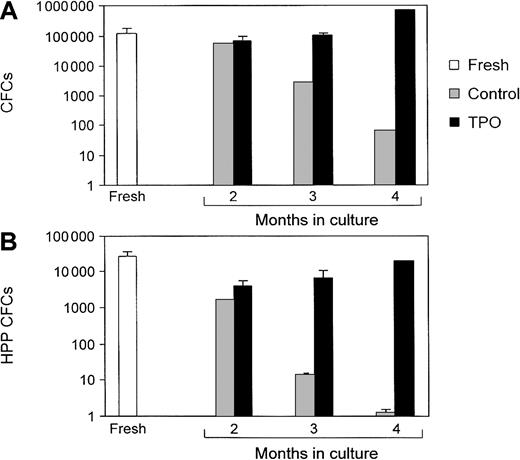
The role of TPO in the expansion and prolonged survival of primitive stem cells derived from bone marrow or umbilical cord blood has been the focus of several recent investigations. Yagi and colleagues140 demonstrated that administration of TPO alone can sustain ex vivo expansion of hematopoietic stem cells in long-term bone marrow cultures (LTBMCs) from mice (Figure6). The continuous presence of TPO resulted in the generation of long- and short-term colony-forming cells and maintained the relative amount of high-proliferative-potential colony-forming cells (Figure 7). Most important, competitive repopulation studies found that the TPO-treated LTBMC cells were as effective as fresh marrow. Subsequent data from this research group suggest that the expanded population of stem cells, when transplanted into recipient mice, is adequate for the long-term repopulation.
TPO promotes ex vivo stem cell expansion.
During a 5-month, long-term murine bone marrow culture, the total number of nonadherent (NA) cells increased in the presence of TPO. Reprinted from Yagi et al140 with permission.
TPO promotes ex vivo stem cell expansion.
During a 5-month, long-term murine bone marrow culture, the total number of nonadherent (NA) cells increased in the presence of TPO. Reprinted from Yagi et al140 with permission.
Colony-forming cells (CFCs) and high-proliferative-potential (HPP) CFCs are maintained during ex vivo stem cell expansion.
The total number of CFCs (A) and HPP CFCs (B) were measured in long-term bone marrow cultures. Totals were compared between cultures grown in the presence (TPO) or absence (control) of TPO and uncultured (fresh) bone marrow. Reprinted from Yagi et al140 with permission.
Colony-forming cells (CFCs) and high-proliferative-potential (HPP) CFCs are maintained during ex vivo stem cell expansion.
The total number of CFCs (A) and HPP CFCs (B) were measured in long-term bone marrow cultures. Totals were compared between cultures grown in the presence (TPO) or absence (control) of TPO and uncultured (fresh) bone marrow. Reprinted from Yagi et al140 with permission.
Piacibello and colleagues141 showed that the use of growth factors could expand human cord blood CD34+ cells ex vivo by many million-fold in total number; the CD34+ component and the lineage-specific progenitors increased proportionately. Although TPO alone and Flt 3 ligand alone were insufficient in stimulating sustained growth, a combination of the 2 growth factors accounted for this rapid increase in cell number during 24 weeks in culture. However, whether the expanded cell population can be used clinically for transplantation has not been demonstrated.
Platelet apheresis.
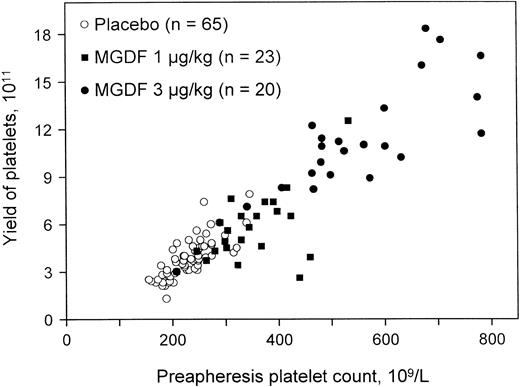
Extensive studies have shown that healthy apheresis donors maximally increase their platelet count 10 to 14 days after a single injection of PEG-rHuMGDF.136,142 143 The rise in platelet count is dose dependent and leads directly to an increase in the apheresis platelet yield (Figure 8). The platelets collected have normal aggregation responses and normal function on transfusion into thrombocytopenic recipients. Transfusions with the higher platelet doses extended the duration of transfusion independence and possibly reduced bleeding episodes when compared with standard doses. The corrected count increment was also improved when patients were transfused with platelets mobilized by PEG-rHuMGDF rather than with those harvested from control donors.
PEG-rHuMGDF increases the yield of platelet apheresis.
Administration of a single dose of PEG-rHuMGDF produced a dose-dependent increase in platelet count and apheresis yield 15 days later. Reprinted from Kuter et al142 with permission.
PEG-rHuMGDF increases the yield of platelet apheresis.
Administration of a single dose of PEG-rHuMGDF produced a dose-dependent increase in platelet count and apheresis yield 15 days later. Reprinted from Kuter et al142 with permission.
Radioprotection
Although there are no studies in humans, the potential for TPO to function as a radioprotectant is another area of clinical interest. In mice, administration of TPO 2 hours after exposure to sublethal total body irradiation (TBI) dramatically ameliorates the thrombocytopenia that is seen at day 10 in these mice.144 Mice treated with rhTPO before irradiation have a higher platelet count nadir (739 × 109/L) than do those that are untreated before irradiation (144 × 109/L); unirradiated control mice had a platelet count of 1123 × 109/L. This protective effect was enhanced when rhTPO was administered close to TBI. Stem cells appeared to be highly sensitive to the effects of rhTPO, possibly because it prevented apoptosis when given from 2 hours before until 2 hours after TBI. This very narrow window of protection underscores the importance of the timing of the administration of rhTPO vis-à-vis the irradiation. Furthermore, red and white blood cell counts also appeared to be protected somewhat by the administration of rhTPO close to the time of TBI (Figure9).144 These results suggest a major radioprotective effect of rhTPO on progenitor cells in the bone marrow. This finding is in line with previous data suggesting that pluripotential stem cells are sensitive to the presence of TPO and that TPO can support their survival.145,146 Similar results have been noted in subsequent studies that investigated the effect of rhTPO in mice exposed to lethal doses of TBI.147 Almost all the mice that received rhTPO close to the time of irradiation survived, whereas all the mice that received placebo died within 30 days of receiving TBI. Furthermore, recovery of blood counts in all lineages improved in those mice that received rhTPO within several hours of TBI.
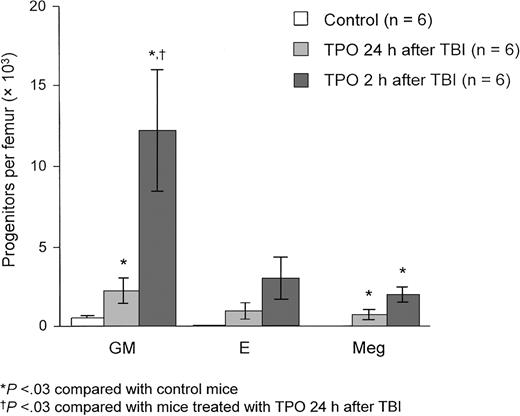
TPO improves regeneration of bone marrow progenitors.
Mice receiving TBI with 6 Gy were treated with TPO 2 hours and 24 hours after irradiation. After 7 days, GM-CFUs (GM), BFU-Es (E), and Meg-CFCs (Meg) were measured per femur. Regeneration of bone marrow progenitors improved with the 2-hour but not the 24-hour post-TBI administration of TPO. Reprinted from Neelis et al144 with permission.
TPO improves regeneration of bone marrow progenitors.
Mice receiving TBI with 6 Gy were treated with TPO 2 hours and 24 hours after irradiation. After 7 days, GM-CFUs (GM), BFU-Es (E), and Meg-CFCs (Meg) were measured per femur. Regeneration of bone marrow progenitors improved with the 2-hour but not the 24-hour post-TBI administration of TPO. Reprinted from Neelis et al144 with permission.
Whether these results can be expanded to the chemotherapy setting has not been fully explored. Conceivably the antiapoptotic effects of TPO might lessen chemotherapy-induced apoptosis of pluripotential stem cells and thereby ameliorate the pancytopenia of chemotherapy.
Safety of recombinant TPO
In most of the studies with TPO in myeloablative and nonmyeloablative chemotherapy, patients also received myeloid growth factors. Although interactions of TPO with myeloid growth factors had been seen in one animal model,148 none have been noted an any clinical study.
Administration of multiple doses of PEG-rHuMGDF to some cancer patients and healthy volunteers was associated with an abrogation of its pharmacologic effect as a result of the development of neutralizing antibodies.85,100,149,150 These antibodies neutralized both the recombinant and endogenous TPO, resulting in thrombocytopenia. Thrombocytopenia occurred in 4 of 665 cancer/stem cell transplantation/leukemia patients given multiple doses and in 2 (1.2%) of 210 healthy volunteers who received 2 doses and in 11 (8.9%) of 124 healthy volunteers given 3 doses of PEG-rHuMGDF.85,149 No subject developed neutralizing antibodies or thrombocytopenia after a single injection. Evaluation of these thrombocytopenic subjects showed that the thrombocytopenia was due to the formation of an IgG antibody to PEG-rHuMGDF that cross-reacted with endogenous TPO and neutralized its biologic activity.85,149,150 In 2 patients, thrombocytopenia was associated with anemia and neutropenia, suggesting an effect on a stem cell population.85,150 Because endogenous TPO is produced in a constitutive fashion by the liver, thrombocytopenia ensues. PEG-rHuMGDF was withdrawn from clinical trials in the United States by Amgen in September 1998 because of this side effect.151
A possible explanation for the immunogenicity of PEG-rHuMGDF administration may be that this molecule is truncated, nonglycosylated, and pegylated, in contrast to the full-length, glycosylated rhTPO molecule (Table 1). In addition, PEG-rHuMGDF has usually been administered subcutaneously, whereas full-length native rhTPO has been injected intravenously. Because TPO is a potent mobilizer of dendritic cells, injection of any form of TPO subcutaneously might enhance its immunogenicity. Support for this latter hypothesis comes from recent experiments in which PEG-ratMGDF was injected into rats once monthly for 3 months by either a subcutaneous or intravenous route. Most animals treated subcutaneously developed neutralizing antibodies and thrombocytopenia whereas those treated intravenously did not (personal communication, Dr A. Shimosaka, April 2002).
Summary
The development of recombinant TPO has led to a wide number of discoveries describing the underlying biology of platelet production in normal and pathologic settings. Recombinant TPO has demonstrated a unique pharmacology, unlike other hematopoietic growth factors. Both rhTPO and PEG-rHuMGDF have a prolonged half-life of about 40 hours, and thus continuous dosing with TPO does not seem to be required; one or more appropriately timed doses may even be superior to multiple doses. TPO does not increase the platelet count for 5 days and has its peak effect 10 to 12 days later. It has little effect on mature megakaryocytes and may actually inhibit their shedding of platelets.
TPO is the most specific and effective growth factor identified to date for the prevention and treatment of thrombocytopenia. Preliminary clinical evidence indicates that TPO administration may be a helpful adjunct to the conventional approach of platelet transfusion therapy for some cancer patients with chemotherapy-induced thrombocytopenia. However, the studies with TPO, as with IL-11, mostly involved nonconventional chemotherapy regimens that caused considerable thrombocytopenia. For most routine chemotherapy regimens, clinically significant thrombocytopenia is relatively uncommon. The overall impact of TPO on the need for platelet transfusions will probably not be great, especially with the recent reduction in the threshold “trigger” for platelet transfusions to 10 × 109/L.152-154
The failure to find any biologic effect in myeloablative regimens is still surprising given the success of the myeloid growth factors in these same settings. This is probably not simply due to inadequate dosing schemes; many have been tried. Rather it may reflect aspects of the clinical biology of TPO that are not yet recognized. The elevated endogenous TPO concentration in all of these settings may have already saturated the TPO receptor or, alternatively, may even prevent platelet shedding.155
The ultimate clinical indications for recombinant TPO or TPO mimetics will certainly depend on the results of continuing and future studies. Further studies to elucidate their complex and unique biology will help to determine their optimal application in the treatment of thrombocytopenia. While the potential of TPO to reduce the extent of chemotherapy-induced thrombocytopenia and reduce the need for platelet transfusions in the nonmyeloablative chemotherapy setting may be enhanced with innovative dosing schemes, TPO will probably have its greatest impact in nononcology settings such as the stimulation of directed platelet apheresis donors. The thrombocytopenia associated with HIV infection, ITP, MDS, liver disease, radiation, surgery, and intensive care patients accounts for over half of all platelet transfusions136 and remains in need of an effective thrombopoietic growth factor.
Supported in part by grants from the National Institutes of Health HL54838 and HL61272 (D.J.K.), the National Institutes of Health, Medical Research Council, Canberra, Australia; Amgen Inc, Thousand Oaks, CA (C.G.B.); and Pharmacia Inc, Peapack, NJ (D.J.K., C.G.B.).
D.J.K. has been a consultant for and received research support from Amgen Inc, Kirin Inc, and Pharmacia Inc. C.G.B. was a consultant to Amgen Inc during the course of the clinical studies with PEG-rHuMGDF.
References
Author notes
David J. Kuter, Hematology/Oncology Unit, Massachusetts General Hospital, Harvard Medical School, 100 Blossom St, Boston, MA 02114; e-mail:kuter.david@mgh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal