Key Points
Rituximab treatment in SOT recipients with EBV DNAemia may be associated with a reduction in the rate of PTLD over time.
Increasing EBV viral load, lung or multivisceral transplant, and high-risk serostatus were associated with higher rates of PTLD over time.
Visual Abstract
The association between Epstein-Barr virus (EBV) DNAemia after solid organ transplantation (SOT) and posttransplant lymphoproliferative disorder (PTLD) is well described. Published data support preemptive rituximab for EBV DNAemia after bone marrow transplant. However, there are inadequate data to support any specific preemptive strategy for DNAemia after SOT. The goal of this single-center retrospective cohort study was to explore the association between posttransplant rituximab and development of PTLD in SOT recipients with EBV DNAemia. This study included 1386 patients with EBV DNAemia after SOT at Columbia University Irving Medical Center from 2008 to 2023. There were 129 patients who received rituximab for various indications (eg, organ rejection, EBV DNAemia). Across all patients, 82 of 1386 (6%) developed PTLD and the 5-year PTLD-free survival rate was 95%. In multivariable analysis, posttransplant rituximab exposure for any indication was independently associated with a reduced rate of PTLD over time as a time-independent (hazard ratio [HR], 0.16; P = .011) but not time-dependent (HR, 0.25; P = .056) variable. When limiting the rituximab-exposed cohort to the 60 patients who received rituximab after documented EBV DNAemia, time-independent (HR, 0.25; P = .06) and time-dependent (HR, 0.43; P = .2) rituximab exposure were not associated with PTLD-free survival. Higher EBV peak viral load, high-risk organ transplant type, and high-risk EBV serostatus were independently associated with increased rates of PTLD. This retrospective study suggests that posttransplant rituximab may reduce the rate of PTLD in SOT recipients. Prospective, randomized studies are needed to more rigorously determine the benefit of preemptive rituximab for the prevention of PTLD in patients with EBV DNAemia.
Introduction
Epstein-Barr virus (EBV) reactivation occurs in up to 45% of solid organ transplant (SOT) recipients.1-3 Immunosuppression and EBV DNAemia can lead to uncontrolled proliferation of EBV-infected lymphocytes (typically B cells) given the iatrogenic reduction in immune surveillance in transplant recipients. The incidence of posttransplant lymphoproliferative disorder (PTLD) after SOT is 1% to 20%, varying by organ transplant type, immunosuppression regimen, and EBV serostatus.1,4-8 PTLD is EBV-associated in up to 60% of cases and contributes significantly to morbidity and mortality after SOT, with an overall survival of 59% at 2 years in high-risk patients, and 30% in very high-risk patients.1,9,10 Furthermore, there is insufficient evidence to support consensus recommendations for any preventive strategy for PTLD in the setting of EBV DNAemia.11
Reduction in immunosuppression (RIS) is generally the first line of treatment for EBV DNAemia and has been associated with a reduced rate of PTLD after SOT.11 Lee et al reported a reduction in incidence of PTLD from 16% to 2% in pediatric liver transplant recipients.12,13 However, RIS alone is not always effective in clearing EBV or preventing PTLD and can be associated with a significant risk of graft rejection. Nucleoside analogues (eg, valganciclovir) have limited efficacy in treatment of EBV DNAemia.14 These agents have modest activity in lytic phase replication of EBV by inhibiting viral DNA polymerase but do not eradicate latent-phase EBV infection, in which EBV replicates within B cells using the host cell DNA polymerase.15-17
Epigenetic modulation has been shown to induce EBV lytic reactivation, potentially improving clearance. A clinical trial combining nanatinostat with valganciclovir in patients with relapsed/refractory EBV-related lymphomas showed an overall disease response rate of 40%.18 EBV-specific cytotoxic T lymphocytes have been studied in EBV DNAemia after bone marrow transplant (BMT), and tabelecleucel is approved by the European Medicines Agency for relapsed/refractory EBV+ PTLD. However, EBV cytotoxic T lymphocytes have been less studied in EBV DNAemia in the absence of lymphoma in SOT recipients.19-27
Rituximab is a monoclonal antibody targeting CD20 on B lymphocytes, depleting the EBV-infected CD20+ B cells.28 In the peritransplant and posttransplant setting, rituximab is used for a variety of indications including donor antibody desensitization therapy, induction therapy, treatment of rejection, treatment of PTLD, and EBV DNAemia.29,30 Published data support the use of rituximab for EBV DNAemia after BMT.31-37 However, there is a paucity of data regarding the benefits of rituximab for EBV DNAemia after SOT.
A retrospective study of 4765 SOT recipients reported no cases of PTLD among patients who received rituximab for induction therapy or organ rejection.38 A study of pediatric heart transplant recipients (n = 6) found that rituximab in addition to RIS led to a complete response (EBV viral load of <1000 IU/mL) in 83.3%.39 A prospective study of rituximab for EBV DNAemia after heart transplantation demonstrated reduction in viral load to of <105 copies per mL in 87.5% (n = 8) after 1 dose. A decreased incidence of PTLD was observed as compared with a historical cohort though was underpowered to draw definitive conclusions.40 A study of rituximab for EBV DNAemia in renal transplant recipients (n = 6) with high-risk donor-recipient EBV seromismatch (donor positive [D+]/recipient negative [R−]) reported that none developed PTLD, however the study was underpowered to detect a significant reduction in risk of PTLD.41 In this analysis, the objective was to determine whether rituximab use in SOT recipients with EBV DNAemia is associated with a reduced rate of PTLD over time.
Methods
The study was approved by the institutional review board at Columbia University Irving Medical Center (CUIMC). Data were obtained using structured query language in an open-source database query tool. Patients who underwent SOT and had EBV DNAemia from 1 January 2008 to July 2023 were included. International Classification of Diseases codes were used to identify SOT recipients, transplant type, and PTLD diagnoses (supplemental Table 1). Patients who received a transplant at an outside institution and transferred care to CUIMC were included. Pretransplant EBV serology (EBV anti-viral capsid antigen [VCA] immunoglobulin G [IgG]/IgM) and polymerase chain reaction (PCR) viral load positivity were recorded. Patient demographics included age, sex, and self-reported race. Pathology report data were queried to identify biopsy-proven PTLD diagnoses. Pathology reports were manually reviewed, and patients who did not meet criteria for PTLD were analyzed in the non-PTLD cohort. PTLD disease characteristics collected included EBV positivity by in situ hybridization for EBV-encoded small RNAs (EBERs), and immunophenotypic features including CD20, CD30, and CD138 expression. PTLD subtype was classified according to the fifth edition of the World Health Organization Classification of Hematolymphoid Tumors.42 Biopsy and imaging data within 2 months before rituximab were manually recorded. Biopsies done any time before rituximab to rule out PTLD were also recorded.
EBV DNAemia was defined as any positive PCR assay in the peripheral blood (whole blood or plasma). Peak EBV viral load was defined as maximum plasma EBV viral load in international unit per milliliter. Patients with a detectable viral load in the whole blood but who either did not have a plasma assay sent or had plasma assays with undetectable viral load were included in the analysis; these patients were categorized as having EBV DNAemia with an unknown or peak EBV viral load of 0 IU/mL, respectively. Patients without a viral load value in international unit per milliliter were not included in analyses of peak EBV viral load. Duration of EBV DNAemia was calculated by recording date of first and last positive EBV viral load (all assays). Date of EBV reactivation was the first detectable EBV PCR. Date of last EBV PCR was the last recorded EBV PCR within the follow-up period for patients who did not develop PTLD, or as last detectable EBV PCR within 4 weeks of PTLD diagnosis. If the first EBV PCR was sent after diagnosis of PTLD but within 4 weeks, patients were included because they likely had EBV DNAemia before PTLD diagnosis. Patients who received rituximab as empiric treatment for PTLD in the setting of EBV DNAemia before their diagnostic biopsy, and who were then pathologically confirmed to have PTLD, were categorized as having not received pre-PTLD rituximab. For patients who received rituximab, average EBV viral load in the 3 months before and after the first dose was recorded if available. Rituximab dosing data were queried from the medication administration record. Indications for rituximab were obtained via chart review.
Patient characteristics were summarized with descriptive statistics. Time to the development of PTLD (PTLD-free survival) was defined as time from SOT to diagnosis of PTLD and estimated using the Kaplan-Meier method; patients were censored at date of last follow-up or date of death without PTLD. High-risk serostatus was defined as EBV D+/R−, intermediate-risk serostatus was defined as EBV D+/R+ or D−/R+, and low-risk serostatus was defined as EBV D−/R−. Median levels of cyclosporine, tacrolimus, and sirolimus were recorded within 3 months before and after peak EBV viral load. Immunosuppression agents dosed before and after rituximab were recorded for patients who had a documented plasma EBV viral load within 3 months before and after rituximab. Antiviral use (ganciclovir, valganciclovir, acyclovir, or valacyclovir) was recorded within 3 months before and after the peak plasma EBV viral load and was capped at the time of PTLD diagnosis.
Cox proportional hazard regression models were used to investigate the relationship between risk factor variables and time to diagnosis of PTLD. Univariable and multivariable time-independent Cox regression models were used to identify significant risk factors associated with PTLD. Rituximab administration was analyzed as a time-independent as well as a time-dependent covariate accounting for prerituximab and postrituximab exposure periods. All other variables were analyzed as time-independent variables. These analyses were conducted using the survival R package (version 3.6.4; Therneau et al).43 To determine the optimal cutoff point for peak EBV viral load, maximizing sensitivity and specificity in predicting the development of PTLD, receiver operating characteristic (ROC) curve analysis with 1000 bootstrap iterations using the “cutpointr” R package (version 1.1.2; Thiele et al)44 was performed. Peak EBV viral load was divided into bins using 2 approaches: (1) ROC curve analysis to determine the lower cutoff point by maximizing the sum of sensitivity and specificity and (2) identification of the upper cutoff point by selecting the value that produced an hazard ratio (HR) closest to 5.0, based on evaluations at 1-unit increments. All analyses were performed using R statistical software (version 4.4.1; R Core Team 2024).
Results
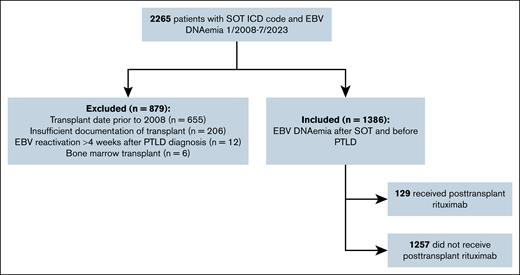
Of 2265 SOT recipients with EBV DNAemia, 879 patients were excluded for the following reasons: transplant before 2008 (n = 655), transplant at an outside institution with insufficient records (n = 206), EBV reactivation >4 weeks after PTLD diagnosis (n = 12), history of BMT (n = 6). The remaining 1386 patients were included (Figure 1).
The average age at time of transplant was 40 years, and 388 (28%) patients were aged <18 years. There were 803 (58%) male transplant recipients. The cohort included 452 (33%) liver, 441 (32%) kidney, 309 (22%) heart, 110 (8%) lung, 20 (1%) intestinal and/or multivisceral, and 3 (0.2%) pancreas transplant recipients. The remaining 51 (4%) patients received multiple transplants, including simultaneous and sequential organ transplants. Self-reported race included 620 (45%) White, 242 (17%) Black, 77 (6%) Asian, and 447 (32%) patients of other/unreported races (Table 1). Ethnicity data were not recorded due to inconsistencies in patient-reported ethnicity.
Patient characteristics
| Characteristic . | n = 1386 n (%) . |
|---|---|
| Transplant type | |
| Liver | 452 (33) |
| Kidney | 441 (32) |
| Heart | 309 (22) |
| Lung | 110 (8) |
| Intestinal and/or multivisceral | 20 (1) |
| Pancreas | 3 (0.2) |
| Multiple∗ | 51 (4) |
| Sex | |
| Male | 803 (58) |
| Female | 583 (42) |
| Race | |
| White | 620 (45) |
| Black | 242 (17) |
| Asian | 6 (77) |
| Other/unknown | 447 (32) |
| Age at organ transplant, y | |
| Average age (range) | 40 years (21 days to 80 years) |
| <10 | 257 (19) |
| 10-18 | 131 (9) |
| 18-60 | 609 (44) |
| ≥60 | 389 (28) |
| EBV serostatus | |
| High risk (D+/R−) | 229 (17) |
| Intermediate risk (D+/R+, D−/R+) | 1074 (77) |
| Low risk (D−/R−) | 42 (3) |
| Unknown | 41 (3) |
| Peak plasma EBV viral load (IU/mL) | |
| Detectable but not quantifiable | 280 (20) |
| <1000 | 801 (58) |
| 1000-4000 | 139 (10) |
| >4000 | 127 (9) |
| Unknown† | 39 (3) |
| Characteristic . | n = 1386 n (%) . |
|---|---|
| Transplant type | |
| Liver | 452 (33) |
| Kidney | 441 (32) |
| Heart | 309 (22) |
| Lung | 110 (8) |
| Intestinal and/or multivisceral | 20 (1) |
| Pancreas | 3 (0.2) |
| Multiple∗ | 51 (4) |
| Sex | |
| Male | 803 (58) |
| Female | 583 (42) |
| Race | |
| White | 620 (45) |
| Black | 242 (17) |
| Asian | 6 (77) |
| Other/unknown | 447 (32) |
| Age at organ transplant, y | |
| Average age (range) | 40 years (21 days to 80 years) |
| <10 | 257 (19) |
| 10-18 | 131 (9) |
| 18-60 | 609 (44) |
| ≥60 | 389 (28) |
| EBV serostatus | |
| High risk (D+/R−) | 229 (17) |
| Intermediate risk (D+/R+, D−/R+) | 1074 (77) |
| Low risk (D−/R−) | 42 (3) |
| Unknown | 41 (3) |
| Peak plasma EBV viral load (IU/mL) | |
| Detectable but not quantifiable | 280 (20) |
| <1000 | 801 (58) |
| 1000-4000 | 139 (10) |
| >4000 | 127 (9) |
| Unknown† | 39 (3) |
Multiple transplant group includes both sequential and simultaneous organ transplants in the following combinations: liver/lung, kidney/pancreas, kidney/liver, heart/liver, and heart/kidney.
Patients who had EBV DNAemia detected in whole blood (copies per milliliter) but who did not have a plasma assay to confirm EBV viral load in international units per milliliter.
When evaluating EBV serostatus of donors and recipients, 229 (17%) were high risk (D+/R−), 1074 (77%) were intermediate risk (D+/R+ and D−/R+), 42 (3%) were low risk (D−/R−), and 41 (3%) had unknown risk for EBV reactivation/infection.45 There were 278 (20%) patients who were R− and therefore considered to have had a primary EBV infection. Peak EBV viral load using plasma PCR assays included 280 (20%) with detectable but unquantifiable EBV DNAemia, 801 (58%) with peak EBV of <1000 IU/mL, 139 (10%) with peak EBV of 1000 to 4000 IU/mL, and 127 (9%) with peak EBV of >4000 IU/mL. There were 39 (3%) transplant recipients with an unknown peak EBV viral load; these patients had EBV DNAemia detected in the whole blood (copies per milliliter) but did not have a plasma assay to confirm EBV viral load in international units per milliliter (Table 1). Median peak EBV viral load was 130 (<50 to 2 900 000 IU/mL). There were a total of 196 689 EBV viral load assays (any unit of measurement) sent during the study period. Among patients with measurable EBV DNAemia in the plasma compartment (international units per milliliter), there were a median of 1.24 tests performed per patient per year (range, 0-104).
Immunosuppression was managed according to organ-specific standard protocols (supplemental Table 2) and was further individualized to the specific recipient. Median levels (nanogram per milliliter) of tacrolimus, sirolimus, and cyclosporine were similar 3 months before and after peak EBV viral load (n = 1067; Table 2). In addition, the use of antiviral therapy did not correlate with a reduction in risk of PTLD over time, and was not associated with the receipt of rituximab (supplemental Table 3).
Immunosuppression: drug levels
| Immunosuppressant . | Cyclosporine . | Tacrolimus . | Sirolimus . |
|---|---|---|---|
| Median EBV level before peak (ng/ml)∗ | 137 | 7.6 | 5.5 |
| Median EBV level after peak EBV (ng/mL)∗ | 144 | 7.1 | 5.1 |
| Immunosuppressant . | Cyclosporine . | Tacrolimus . | Sirolimus . |
|---|---|---|---|
| Median EBV level before peak (ng/ml)∗ | 137 | 7.6 | 5.5 |
| Median EBV level after peak EBV (ng/mL)∗ | 144 | 7.1 | 5.1 |
Median level was captured within 3 months before and 3 months after documented peak EBV viral load in the plasma compartment (international units per milliliter).
A total of 129 of 1386 (9%) patients received posttransplant rituximab, and of these, 2 (2%) subsequently developed PTLD (Figure 2A). Available prerituximab biopsy and imaging data are described in supplemental Table 4. Of 129 patients treated with rituximab, 60 had recorded EBV DNAemia before at least 1 of their rituximab doses, and 69 patients received all of their doses of rituximab before EBV DNAemia was recorded. Rituximab was given for treatment of rejection in 56 (43%; median of 2 doses over 9 days), antibody desensitization or induction therapy in 46 (36%; median of 1 dose over 1 day), EBV DNAemia in 16 (12%,;median of 1 dose over 1 day), other non–transplant-related indications (eg, lupus nephritis) in 9 (7%; median of 2 doses over 14 days), and for multiple indications in 2 patients (2%; receiving 2 doses and 6 doses, respectively, over 2 years; Figure 2B-C). Rituximab was administered within 1 month of transplant in 34 patients (26%), between 1 month and 1 year after transplant in 32 (25%), between 1 year and 5 years in 35 (27%), and after 5 years in 28 (22%) patients (Figure 2D). Median time from transplant to rituximab was 285 days (range, 0-4328).
Descriptive data for PTLD rate and rituximab dosing. (A) PTLD rates between the posttransplant rituximab group and nonrituximab groups. (B) Indications for posttransplant rituximab. (C) Dosing details by indication. (D) Timing of rituximab dosing.
Descriptive data for PTLD rate and rituximab dosing. (A) PTLD rates between the posttransplant rituximab group and nonrituximab groups. (B) Indications for posttransplant rituximab. (C) Dosing details by indication. (D) Timing of rituximab dosing.
Among 32 patients with measured EBV viral loads within 3 months before and after the first rituximab dose, 21 patients (66%) had a >90% reduction in mean EBV viral load. In this group of 32 patients, the mean EBV viral load was 3003 IU/mL in the 3 months before the first dose (median, 226 IU/mL) and 970 IU/mL (median, 7 IU/mL) in the 3 months after the first dose (Figure 3). The immunosuppression regimens for this cohort included a median of 4 drugs within 3 months before rituximab, and a median of 3 drugs after rituximab. The specific immunosuppressive agents dosed did not change appreciably from before to after rituximab when evaluating all treated patients (Table 3).
Change in EBV viral load after rituximab dosing. EBV viral load (international unknit per milliliter) collected from 3 months before rituximab to 3 months after rituximab. (A) Histogram, with each bar representing 1 patient. (B) Spider plot, with each line representing 1 patient.
Change in EBV viral load after rituximab dosing. EBV viral load (international unknit per milliliter) collected from 3 months before rituximab to 3 months after rituximab. (A) Histogram, with each bar representing 1 patient. (B) Spider plot, with each line representing 1 patient.
Immunosuppression: specific agents dosed before and after rituximab
| Drug . | Before rituximab . | After rituximab . |
|---|---|---|
| Prednisone | 29 | 26 |
| Tacrolimus | 28 | 27 |
| Mycophenolate | 23 | 20 |
| Sirolimus | 6 | 8 |
| Cyclosporine | 6 | 6 |
| Azathioprine | 4 | 3 |
| Basiliximab | 2 | 2 |
| IV immunoglobulin | 2 | 2 |
| Bortezomib | 1 | 2 |
| Antithymocyte globulin∗ | 14 | 2 |
| Drug . | Before rituximab . | After rituximab . |
|---|---|---|
| Prednisone | 29 | 26 |
| Tacrolimus | 28 | 27 |
| Mycophenolate | 23 | 20 |
| Sirolimus | 6 | 8 |
| Cyclosporine | 6 | 6 |
| Azathioprine | 4 | 3 |
| Basiliximab | 2 | 2 |
| IV immunoglobulin | 2 | 2 |
| Bortezomib | 1 | 2 |
| Antithymocyte globulin∗ | 14 | 2 |
Includes IS agents dosed 3 months before and after rituximab for the 32 patients who had a documented EBV viral load in the plasma compartment (international units per milliliter) within 3 months before and after rituximab.
IS, immunosuppression.
Antithymocyte globulin was given either for induction immunosuppression or for acute rejection in all cases.
There were 1257 of 1386 patients (91%) who did not receive posttransplant rituximab, and of these, 80 (6%) developed PTLD. There was a significant difference (P < .001) in median peak EBV viral load between patients treated with posttransplant rituximab (median, 287 IU/mL) and patients who did not receive rituximab (median, 124 IU/mL). Median time from EBV reactivation to PTLD diagnosis was 88 days (range, 0 days to 13 years). Median time from peak EBV viral load to diagnosis of PTLD was 8 days (range, 0 days to 7.7 years). In 2 patients who were diagnosed with PTLD after receiving posttransplant rituximab, diagnosis occurred at 6 and 12 years after transplant.
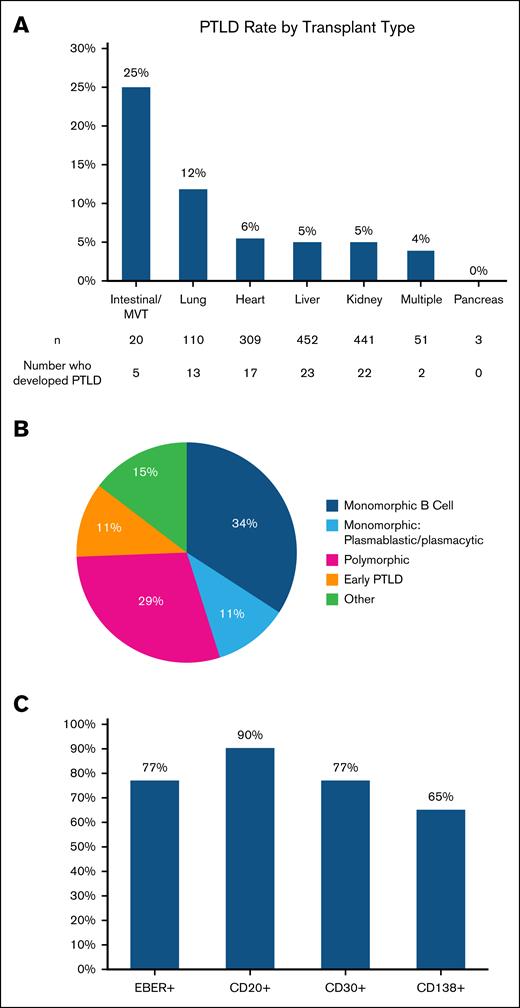
Morphologic subtypes of the 82 cases of PTLD included 37 (45%) monomorphic B cell (of which 9 cases [11% overall] were plasmablastic/plasmacytic), 24 (29%) polymorphic, 12 (15%) with other morphologies (eg, Hodgkin, Burkitt), and 9 (11%) with nondestructive PTLD (Figure 4B). Immunohistochemistry staining demonstrated that 70 of 78 (90%) patients tested were at least partially CD20+, 36 of 47 (77%) were CD30+, and 13 of 20 (65%) were CD138+. There were 60 of 68 (77%) cases of EBER+ PTLD (Figure 4C).
Characteristics of patients who developed PTLD. (A) PTLD rates by organ type. (B) PTLD morphology subtypes. MVT, multivisceral transplant.
Characteristics of patients who developed PTLD. (A) PTLD rates by organ type. (B) PTLD morphology subtypes. MVT, multivisceral transplant.
The rate of PTLD during the follow-up period was highest in recipients of intestinal and multivisceral transplants at 25%, followed by 12% in lung, 6% in heart, 5% in kidney, and 5% in liver transplant recipient, 4% in recipients of multiple organ transplants, and 0% in pancreas transplant recipient (Figure 4A). Using liver transplant recipients as a reference point, lung and multivisceral/intestinal transplant recipients had a significantly higher rate of PTLD over time (HR, 2.59 [95% confidence interval [CI], 1.31-5.11; P = .006] and HR, 7.24 [95% CI, 2.75-19.09; P < .0001], respectively). Therefore, lung and multivisceral/intestinal transplant recipients were considered high-risk organ transplant types.
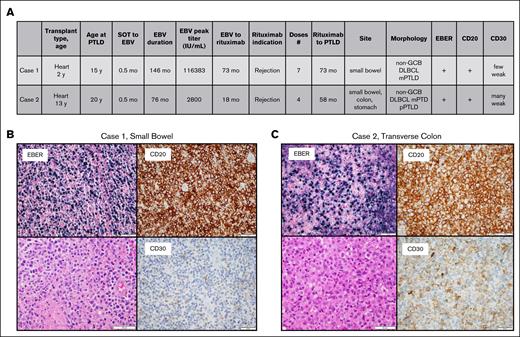
There were 2 patients who received posttransplant rituximab and developed PTLD. Both were heart transplant recipients, aged 2 and 13 years at the time of transplantation, and 15 and 20 years at PTLD diagnosis, respectively. The first patient received 7 doses of rituximab for treatment of rejection and developed PTLD 73 months later. The second patient received 4 doses of rituximab, also for treatment of rejection, and developed PTLD 58 months later. Both patients had EBER+, CD20+, weak CD30+, non–germinal center diffuse large B-cell lymphoma/PTLD of the gastrointestinal tract with clonal IgH rearrangements. The second patient had a series of biopsies from different sites in the gastrointestinal tract that also demonstrated polymorphic morphology (Figure 5).
Characteristics and histology for the 2 patients who developed PTLD after rituximab. (A) Patient characteristics. (B) Histologic characteristics of case 1. (C) Histologic characteristics of case 2. DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B cell; mPTLD, monomorphic PTLD; pPTLD, polymorphic PTLD.
Characteristics and histology for the 2 patients who developed PTLD after rituximab. (A) Patient characteristics. (B) Histologic characteristics of case 1. (C) Histologic characteristics of case 2. DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B cell; mPTLD, monomorphic PTLD; pPTLD, polymorphic PTLD.
In univariable analysis (Table 4), posttransplant rituximab was associated with a lower rate of PTLD over time (HR, 0.23; 95% CI, 0.06-0.94; P = .040) when analyzed as a time-independent variable. When analyzing rituximab treatment as a time-dependent variable, the association with rate of PTLD was no longer statistically significant (HR, 0.41; 95% CI, 0.10-1.66; P = .2). Log-transformed peak EBV viral load was associated with a higher rate of PTLD (HR, 1.31; 95% CI, 1.20-1.42; P < .001). When EBV peak viral loads were divided into bins, EBV viral load of >4000 IU/mL was associated with a 4.90-fold higher rate of PTLD than a viral load that was detectable but not quantifiable (95% CI, 2.56-9.40; P < .001). EBV viral load of 1000 to 4000 IU/mL and <1000 IU/mL were not statistically associated with increased rate of PTLD (P = .092 and P = .2, respectively). Longer duration of EBV DNAemia was associated with a nonmeaningful increased rate of PTLD (HR, 1.01; 95% CI, 1.00-1.01; P = .003). Longer time from SOT to EBV reactivation was associated with a slightly decreased rate of PTLD (HR, 0.99; 95% CI, 0.98-0.99; P < .001). Patients with high-risk serostatus had a greater than fivefold increased rate of PTLD (HR, 5.13; 95% CI, 3.29-8.00; P < .001) as compared with low- and intermediate-risk groups combined. When compared with the intermediate-risk group, high-risk serostatus was associated with an increased rate of PTLD (HR, 5.34; 95% CI, 3.39-8.40; P < .001), whereas low-risk serostatus was not significantly different (P = .2). High-risk organ transplant type (lung, multivisceral/intestinal) was associated with an increased rate of PTLD (HR, 3.27; 95% CI, 1.93-5.52; P < .001) compared with non–high-risk organ transplant type. Age of <18 years at transplantation was associated with a higher rate of PTLD (HR, 2.31; 95% CI, 1.50-3.57; P < .001).
Cox regression analyses
| Variable . | Time-independent analyses . | Time-dependent analyses . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Univariable Cox regression analyses | ||||||
| Preemptive rituximab | 0.23 | 0.06-0.94 | .040 | 0.41 | 0.10-1.66 | .2 |
| Log-transformed peak EBV viral load (IU/mL) | 1.31 | 1.20-1.42 | <.001 | - | - | - |
| Peak plasma EBV viral load categories (IU/mL) | - | - | - | - | - | - |
| Detectable but not quantifiable | - | - | - | - | - | - |
| <1000 | 0.62 | 0.32-1.20 | .2 | - | - | - |
| 1000-4000 | 1.94 | 0.90-4.20 | .092 | - | - | - |
| >4000 | 4.90 | 2.56-9.40 | <.001 | - | - | - |
| Duration of EBV DNAemia (mo) | 1.01 | 1.00-1.01 | .003 | - | - | - |
| Time from SOT to EBV reactivation (mo) | 0.99 | 0.98-0.99 | <.001 | - | - | - |
| High-risk D/R serostatus | 5.13 | 3.29-8.00 | <.001 | - | - | - |
| D/R serostatus∗ | - | - | - | - | - | - |
| Intermediate risk | - | - | - | |||
| High risk | 5.34 | 3.39-8.40 | <.001 | - | - | - |
| Low risk | 2.02 | 0.62-6.56 | .2 | - | - | - |
| High-risk organ type† | 3.27 | 1.93-5.52 | <.01 | |||
| Age of <18 years at transplant | 2.31 | 1.50-3.57 | <.001 | - | - | - |
| Multivariable Cox regression analysis | ||||||
| Preemptive rituximab | 0.16 | 0.04-0.65 | .011 | 0.25 | 0.06-1.04 | .056 |
| Log-transformed peak EBV viral load (IU/mL) | 1.26 | 1.15-1.38 | <.001 | 1.26 | 1.15-1.37 | <.001 |
| Duration of EBV DNAemia (mo) | 0.99 | 0.99-1.00 | .076 | 0.99 | 0.99-1.00 | .043 |
| Time from SOT to EBV reactivation (mo) | 0.99 | 0.98-1.00 | <.001 | 0.99 | 0.98-0.99 | <.001 |
| D/R serostatus∗ | - | - | - | - | - | - |
| Intermediate risk | - | - | - | - | - | - |
| High risk | 3.08 | 1.78-5.31 | <.001 | 3.11 | 1.82-5.32 | <.001 |
| Low risk | 1.62 | 0.47-5.57 | .4 | 1.71 | 0.50-5.85 | .4 |
| High-risk organ† | 3.71 | 2.03-6.78 | <.001 | 3.69 | 2.03-6.71 | <.001 |
| Age of <18 years at transplant | 1.14 | 0.65-2.01 | .7 | 1.10 | 0.63-1.94 | .7 |
| Variable . | Time-independent analyses . | Time-dependent analyses . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Univariable Cox regression analyses | ||||||
| Preemptive rituximab | 0.23 | 0.06-0.94 | .040 | 0.41 | 0.10-1.66 | .2 |
| Log-transformed peak EBV viral load (IU/mL) | 1.31 | 1.20-1.42 | <.001 | - | - | - |
| Peak plasma EBV viral load categories (IU/mL) | - | - | - | - | - | - |
| Detectable but not quantifiable | - | - | - | - | - | - |
| <1000 | 0.62 | 0.32-1.20 | .2 | - | - | - |
| 1000-4000 | 1.94 | 0.90-4.20 | .092 | - | - | - |
| >4000 | 4.90 | 2.56-9.40 | <.001 | - | - | - |
| Duration of EBV DNAemia (mo) | 1.01 | 1.00-1.01 | .003 | - | - | - |
| Time from SOT to EBV reactivation (mo) | 0.99 | 0.98-0.99 | <.001 | - | - | - |
| High-risk D/R serostatus | 5.13 | 3.29-8.00 | <.001 | - | - | - |
| D/R serostatus∗ | - | - | - | - | - | - |
| Intermediate risk | - | - | - | |||
| High risk | 5.34 | 3.39-8.40 | <.001 | - | - | - |
| Low risk | 2.02 | 0.62-6.56 | .2 | - | - | - |
| High-risk organ type† | 3.27 | 1.93-5.52 | <.01 | |||
| Age of <18 years at transplant | 2.31 | 1.50-3.57 | <.001 | - | - | - |
| Multivariable Cox regression analysis | ||||||
| Preemptive rituximab | 0.16 | 0.04-0.65 | .011 | 0.25 | 0.06-1.04 | .056 |
| Log-transformed peak EBV viral load (IU/mL) | 1.26 | 1.15-1.38 | <.001 | 1.26 | 1.15-1.37 | <.001 |
| Duration of EBV DNAemia (mo) | 0.99 | 0.99-1.00 | .076 | 0.99 | 0.99-1.00 | .043 |
| Time from SOT to EBV reactivation (mo) | 0.99 | 0.98-1.00 | <.001 | 0.99 | 0.98-0.99 | <.001 |
| D/R serostatus∗ | - | - | - | - | - | - |
| Intermediate risk | - | - | - | - | - | - |
| High risk | 3.08 | 1.78-5.31 | <.001 | 3.11 | 1.82-5.32 | <.001 |
| Low risk | 1.62 | 0.47-5.57 | .4 | 1.71 | 0.50-5.85 | .4 |
| High-risk organ† | 3.71 | 2.03-6.78 | <.001 | 3.69 | 2.03-6.71 | <.001 |
| Age of <18 years at transplant | 1.14 | 0.65-2.01 | .7 | 1.10 | 0.63-1.94 | .7 |
As compared with intermediate-risk serostatus. High-risk serostatus, D+/R−; intermediate-risk serostatus, D−/R+, D+/R+; low-risk serostatus, D−/R−.
High-risk organ transplant types: lung and multivisceral/intestinal transplants.
In multivariable analysis (Table 4) adjusting for time from SOT to EBV reactivation, duration of EBV DNAemia, peak EBV viral load, age at transplantation, EBV serostatus, and high-risk organ transplant type, posttransplant rituximab was associated with a lower rate of PTLD (HR, 0.16; 95% CI, 0.04-0.65; P = .011) when analyzed as a time-independent variable. When analyzing rituximab treatment as a time-dependent variable, there was a trend toward association with reduced rate of PTLD that did not reach statistical significance (HR, 0.25; 95% CI, 0.06-1.04; P = .056). Higher log-transformed peak EBV viral load remained associated with higher rate of PTLD (HR, 1.26; P < .001 when adjusting for either time-dependent or time-independent rituximab exposure). Longer interval from transplant to EBV reactivation was associated with a slightly reduced rate of PTLD (HR, 0.99; P < .001 when adjusting for either time-dependent or time-independent rituximab exposure). Longer duration of EBV DNAemia was slightly associated with a lower rate of PTLD when adjusting for time-dependent rituximab exposure (HR, 0.99; P = .043) but not time-independent rituximab exposure (P = .076). Compared with the intermediate-risk group, high-risk serostatus was associated with an increased rate of PTLD when adjusting for time-independent or time-dependent rituximab exposure (HR, 3.08 and HR, 3.11, respectively; P < .001). Low-risk serostatus did not have a significant association with rate of PTLD (P = .4). High-risk organ transplant type was associated with an increased rate of PTLD when adjusting for time-independent or time-dependent rituximab exposure (HR, 3.71 and HR, 3.69, respectively; P < .001). Age at transplantation was not associated with rate of PTLD when analyzing rituximab as a time-independent and time-dependent variable (P = .7). Because 69 patients received all of their rituximab doses before documented EBV reactivation (eg, for desensitization of donor-specific antibodies peritransplant), a sensitivity analysis was performed including the subset of 60 patients who received at least 1 dose of rituximab after initial documentation of EBV DNAemia in the posttransplant rituximab cohort, which did not demonstrate a statistically significant relationship between time-independent and time-dependent posttransplant rituximab and the development of PTLD (HR, 0.25 [95% CI, 0.06-1.06; P = .060]; and HR, 0.43 [95% CI, 0.10-1.80; P = .2]). The other variables maintained their associations with the rate of PTLD over time as described earlier and in Table 5.
Cox regression sensitivity analysis of patients receiving rituximab after documented EBV reactivation
| Multivariable Cox regression analysis . | ||||||
|---|---|---|---|---|---|---|
| Variable . | Time-independent analyses . | Time-dependent analyses . | ||||
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Preemptive rituximab | 0.25 | 0.06-1.06 | .060 | 0.43 | 0.10-1.80 | .2 |
| Log-transformed peak EBV viral load (IU/mL) | 1.26 | 1.15-1.38 | <.001 | 1.25 | 1.15-1.37 | <.001 |
| Duration of EBV DNAemia (mo) | 0.99 | 0.99-1.00 | .078 | 0.99 | 0.99-1.00 | .053 |
| Time from SOT to EBV reactivation (mo) | 0.99 | 0.98-1.00 | <.001 | 0.99 | 0.98-0.99 | <.001 |
| D/R serostatus∗ | - | - | - | - | - | - |
| Intermediate risk | - | - | - | - | - | - |
| High risk | 3.08 | 1.79-5.32 | <.001 | 3.15 | 1.84, 5.39 | <.001 |
| Low risk | 1.64 | 0.48-5.61 | .4 | 1.71 | 0.50, 5.85 | .4 |
| High-risk organ† | 3.83 | 2.09-7.02 | <.001 | 3.95 | 2.16, 7.23 | <.001 |
| Age of <18 years at transplant | 1.14 | 0.65-2.01 | .7 | 1.11 | 0.63, 1.95 | .7 |
| Multivariable Cox regression analysis . | ||||||
|---|---|---|---|---|---|---|
| Variable . | Time-independent analyses . | Time-dependent analyses . | ||||
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Preemptive rituximab | 0.25 | 0.06-1.06 | .060 | 0.43 | 0.10-1.80 | .2 |
| Log-transformed peak EBV viral load (IU/mL) | 1.26 | 1.15-1.38 | <.001 | 1.25 | 1.15-1.37 | <.001 |
| Duration of EBV DNAemia (mo) | 0.99 | 0.99-1.00 | .078 | 0.99 | 0.99-1.00 | .053 |
| Time from SOT to EBV reactivation (mo) | 0.99 | 0.98-1.00 | <.001 | 0.99 | 0.98-0.99 | <.001 |
| D/R serostatus∗ | - | - | - | - | - | - |
| Intermediate risk | - | - | - | - | - | - |
| High risk | 3.08 | 1.79-5.32 | <.001 | 3.15 | 1.84, 5.39 | <.001 |
| Low risk | 1.64 | 0.48-5.61 | .4 | 1.71 | 0.50, 5.85 | .4 |
| High-risk organ† | 3.83 | 2.09-7.02 | <.001 | 3.95 | 2.16, 7.23 | <.001 |
| Age of <18 years at transplant | 1.14 | 0.65-2.01 | .7 | 1.11 | 0.63, 1.95 | .7 |
As compared with intermediate-risk serostatus. High-risk serostatus, D+/R−; intermediate-risk serostatus, D−/R+, D+/R+; low-risk serostatus, D−/R−.
High-risk organ transplant types: lung and multivisceral/intestinal transplants.
ROC curve analysis of the 1347 patients with a documented EBV viral load assay in the plasma compartment (international unit per milliliter) identified an EBV viral load of 1176 IU/mL as the cutoff point for maximizing sensitivity and specificity for predicting rate of PTLD over time (area under the curve of 0.7; supplemental Figure 1). Patients with EBV viral loads of >1176 IU/mL had a higher rate of PTLD (HR, 5.56; 95% CI, 3.35-9.24; P < .001; specificity 84%, sensitivity 50%). For those with peak viral load of >1176 IU/mL (n = 239), PTLD-free survival was 93% at 1 year (95% CI, 0.90-0.96) and 88% at 5 years (95% CI, 0.84-0.92), as compared with 99% at 1 year (95% CI, 0.98-0.99) and 97% at 5 years (95% CI, 0.96-0.98) for those with peak viral load ≤1176 IU/mL (n = 1108; Table 6; Figure 6D).
PTLD-free survival
| PTLD-free survival . | ||||
|---|---|---|---|---|
| Cohort . | 1 year . | 95% CI . | 5 years . | 95% CI . |
| All patients | 0.98 | 0.97-0.98 | 0.95 | 0.94-0.97 |
| Low-risk serostatus (D−/R−) | 0.95 | 0.89-1.00 | 0.93 | 0.85-1.00 |
| Intermediate-risk serostatus (D−/R+, D+/R+) | 0.99 | 0.99-1.00 | 0.98 | 0.97-0.99 |
| High-risk serostatus (D+/R−) | 0.90 | 0.87-0.94 | 0.86 | 0.82-0.91 |
| EBV detected but not quantifiable | 0.98 | 0.96-0.99 | 0.97 | 0.95-0.99 |
| Peak EBV viral load of <1000 IU/mL | 0.99 | 0.98-1.00 | 0.98 | 0.97-0.99 |
| Peak EBV viral load of 1000-4000 IU/mL | 0.99 | 0.97-1.00 | 0.93 | 0.88-0.97 |
| Peak EBV viral load of >4000 IU/mL | 0.88 | 0.83-0.94 | 0.84 | 0.78-0.91 |
| Peak EBV viral load of ≤1176 IU/mL | 0.99 | 0.98-0.99 | 0.97 | 0.96-0.98 |
| Peak EBV viral load of >1176 IU/mL | 0.93 | 0.90-0.96 | 0.88 | 0.84-0.92 |
| High-risk organ transplant types∗ | 0.90 | 0.85-0.95 | 0.87 | 0.81-0.93 |
| Non–high-risk organ transplant types | 0.98 | 0.98-0.99 | 0.96 | 0.95-0.97 |
| PTLD-free survival . | ||||
|---|---|---|---|---|
| Cohort . | 1 year . | 95% CI . | 5 years . | 95% CI . |
| All patients | 0.98 | 0.97-0.98 | 0.95 | 0.94-0.97 |
| Low-risk serostatus (D−/R−) | 0.95 | 0.89-1.00 | 0.93 | 0.85-1.00 |
| Intermediate-risk serostatus (D−/R+, D+/R+) | 0.99 | 0.99-1.00 | 0.98 | 0.97-0.99 |
| High-risk serostatus (D+/R−) | 0.90 | 0.87-0.94 | 0.86 | 0.82-0.91 |
| EBV detected but not quantifiable | 0.98 | 0.96-0.99 | 0.97 | 0.95-0.99 |
| Peak EBV viral load of <1000 IU/mL | 0.99 | 0.98-1.00 | 0.98 | 0.97-0.99 |
| Peak EBV viral load of 1000-4000 IU/mL | 0.99 | 0.97-1.00 | 0.93 | 0.88-0.97 |
| Peak EBV viral load of >4000 IU/mL | 0.88 | 0.83-0.94 | 0.84 | 0.78-0.91 |
| Peak EBV viral load of ≤1176 IU/mL | 0.99 | 0.98-0.99 | 0.97 | 0.96-0.98 |
| Peak EBV viral load of >1176 IU/mL | 0.93 | 0.90-0.96 | 0.88 | 0.84-0.92 |
| High-risk organ transplant types∗ | 0.90 | 0.85-0.95 | 0.87 | 0.81-0.93 |
| Non–high-risk organ transplant types | 0.98 | 0.98-0.99 | 0.96 | 0.95-0.97 |
High-risk organ transplant types: lung and multivisceral/intestinal transplants.
Survival curves. (A) PTLD-free survival (all patients). (B) PTLD-free survival stratified by EBV serostatus of patient and donor (high-risk, D+/R−; intermediate-risk, D−/R+, D+/R+; low-risk, D−/R−). (C) PTLD-free survival stratified by high-risk organ transplant type (lung, multivisceral/intestinal). (D) PTLD-free survival stratified by peak EBV viral load of >1176 vs ≤1176 IU/mL. (E) PTLD-free survival stratified by peak plasma EBV viral load (IU/mL): detected, <1000, 1000 to 4000, and >4000.
Survival curves. (A) PTLD-free survival (all patients). (B) PTLD-free survival stratified by EBV serostatus of patient and donor (high-risk, D+/R−; intermediate-risk, D−/R+, D+/R+; low-risk, D−/R−). (C) PTLD-free survival stratified by high-risk organ transplant type (lung, multivisceral/intestinal). (D) PTLD-free survival stratified by peak EBV viral load of >1176 vs ≤1176 IU/mL. (E) PTLD-free survival stratified by peak plasma EBV viral load (IU/mL): detected, <1000, 1000 to 4000, and >4000.
Median follow-up time was 6 years. Of 1386 patients in this cohort, 295 died, 33 of whom had PTLD. PTLD-free survival (Table 6; Figure 6A) for all patients was 98% at 1 year after transplant (95% CI, 0.97-0.98) and 95% at 5 years (95% CI, 0.94-0.97). PTLD-free survival data stratified by EBV serostatus and EBV viral load bins are described in Table 6 and Figure 6B,E. For most groups, PTLD-free survival at 1 and 5 years was >90%, with the exception of patients with high-risk serostatus (n = 229), who had PTLD-free survival of 90% at 1 year (95% CI, 0.87-0.94) and 86% at 5 years (95% CI, 0.82-0.91); patients with peak EBV viral load of >4000 IU/mL (n = 127), who had PTLD-free survival of 88% at 1 year (95% CI, 0.83-0.94) and 84% at 5 years (95% CI, 0.78-0.91); and patients with high-risk organ transplant types (n = 130), who had PTLD-free survival of 90% at 1 year (95% CI, 0.85-0.95) and 87% at 5 years (95% CI, 0.81-0.93; Table 6; Figure C).
Discussion
This retrospective study sought to explore the effect of posttransplant rituximab on the development of PTLD in SOT recipients with EBV DNAemia at CUIMC over 15.5 years. Increasing peak EBV viral load, high-risk serostatus, high-risk organ transplant type, and shorter time from SOT to EBV reactivation were independently associated with a higher rate of PTLD in multivariable analyses. Posttransplant rituximab was associated with a reduction in rate of PTLD in time-independent analyses. However, statistical significance was lost in time-dependent analyses. The loss of significance when analyzing rituximab as a time-dependent variable is likely because rituximab exposure was not uniform, and because patients were not exposed to rituximab for the same proportion of time because they were at risk for PTLD. A sensitivity analysis of 60 patients who received rituximab after first documented EBV PCR did not demonstrate a significant relationship between posttransplant rituximab and PTLD. This loss of significance could be influenced by decrease of power when reducing the cohort size from 129 to 60, and the low HR with borderline P value in the time-independent analysis suggests a trend toward a significant relationship with PTLD-free survival. Furthermore, given the lack of standardized EBV monitoring, it is possible that patients who received rituximab for indications other than EBV DNAemia and who were excluded in this sensitivity analysis did in fact have earlier onset EBV DNAemia that was not documented.
The observation that peak plasma EBV viral load was associated with increased rates of PTLD suggests that patients with high viral load would potentially benefit from preemptive rituximab. An EBV viral load of 1176 IU/mL was identified as the cutoff point on the ROC curve for maximizing specificity (84%) and sensitivity (50%) in distinguishing risk of PTLD. Low sensitivity was likely due to a low event rate. This analysis was not powered to distinguish the cutoff point between different organ transplant types. Furthermore, interinstitutional variability in EBV PCR assays could adversely affect the generalizability of EBV viral load threshold.
Although high-risk serostatus was associated with a greater than fivefold increased rate of PTLD when compared with low- and intermediate-risk serostatus, there was no significant difference in association with PTLD between low- and intermediate-risk serostatus groups. The low-risk group was small, and it is possible that differences in physician behavior (ie, increased surveillance for intermediate-risk groups) modified the risk of PTLD.
Duration of EBV DNAemia did not have a consistent association with rate of PTLD between univariable and multivariable analysis. This variable was subject to bias because EBV viral load was not checked at standardized time points. Patients with EBV DNAemia may have experienced increased surveillance and greater RIS leading to a lower rate of PTLD despite a longer duration of DNAemia. Furthermore, capping duration of EBV DNAemia at PTLD diagnosis could have led to shorter duration of EBV DNAemia for those who developed PTLD.
The fact that there was no appreciable change in immunosuppression regimens or drug levels before and after rituximab dosing suggests that the reduction in the rate of PTLD is more attributable to the effect of rituximab. It was not feasible to collect more detailed data on immunosuppression regimens because changes were made without adequate documentation of the timing and duration of reduction, and with significant heterogeneity across organs and recipients.
This analysis is not without limitations. Patients received rituximab for various indications other than EBV DNAemia with heterogeneous dosing. Given this heterogeneity, this analysis sought to capture whether exposure to rituximab for any indication was associated with rate of PTLD. Because EBV viral load was not evaluated at standardized time points, it is likely that some patients with EBV DNAemia were excluded, potentially leading to overestimation of the rate of PTLD in the setting of EBV DNAemia. However, the rate of PTLD in this study was consistent with previous publications. Because peak EBV viral load did not occur at a uniform time across patients, it was not possible to simultaneously adjust for overall exposure time for both rituximab and EBV in a time-dependent analysis. Therefore, only rituximab exposure was analyzed as a time-dependent variable. Additionally, there is heterogeneity between assays measuring EBV DNAemia. Studies have found that EBV DNA can be detected 5 to 25 times higher in whole blood than in plasma EBV DNA.46 In this analysis, data to analyze peak EBV level were limited to plasma-based EBV PCRs (international unit per milliliter). Plasma assays were used because although whole blood surveillance is overall more sensitive for early detection of EBV DNAemia, plasma testing may be more specific for disease detection.45,47 Standardization in EBV measurement compartments, timing, and potential lack of generalizability between centers must be considered when interpreting the results.
The findings of this retrospective analysis suggest there may be a role for preemptive rituximab in SOT recipients with EBV DNAemia to reduce the risk of PTLD. There is also a need for better understanding of biologic risk factors for developing PTLD, such as HLA status, T-cell repertoire, and T-cell function.48-51 A prospective randomized clinical trial of preemptive rituximab for EBV DNAemia is needed to determine whether rituximab use is an effective strategy for mitigating PTLD.
Acknowledgments
E.H.O. acknowledges Sarah Rutherford for her mentorship and advisement. The visual abstract was created with BioRender.com.Orlando, E. (2025) https://BioRender.com/m87c71a.
J.E.A. received funding from The Stewart Family Fund and The Esther and Oded Aboodi Lymphoma Research Fund.
Authorship
Contribution: E.H.O. and P.G. contributed to study design, collection, analysis, and interpretation of data, and wrote the manuscript; B.C. contributed to data analysis and critically reviewed the manuscript; Y.C. provided the biostatistical analyses and critically reviewed the manuscript; M.F. contributed to data analysis, wrote the institutional review board protocol, and critically reviewed the manuscript; A Sanjurjo, S.J., and D.T. contributed to data collection; B.M. designed the software data abstraction methodology; R.J.L.-N. and G.B. provided expert pathology review and critically reviewed the manuscript; G.K.D., H.M., S.A., F.L., M.M., G.Y.I., M.R.P., P.K.S., M.O.-G., A.H.L., A.S., R.R., and B.P. contributed to study design, and critically reviewed the manuscript; M.M.P. and E.M.D. critically reviewed the manuscript; and H.-J.J.C. and J.E.A. contributed to study and data compilation, biostatistical analyses, interpretation of data, and wrote the manuscript.
Conflict-of-interest disclosure: G.Y.I. discloses consultancy with Alimentiv, Altimmune, and Korro Bio. M.M.P. discloses consultancy with AstraZeneca. A.H.L. discloses consultancy with AstraZeneca, AbbVie, Synthekine, and BeiGene. A. Sawas discloses employment with Flatiron Health Inc; equity holdings with Roche; consultancy and speakers bureau role with Seagen and Acrotech Biopharma; speakers bureau role with Daiichi Sankyo; and research funding from Affimed. R.R. reports a consulting or advisory role with Allogene, Gilead Sciences, Incyte, TScan, Orca Bio, Quell Biotherapeutics, Sana Biotechnology, Bayer, and Autolus; and research funding from Atara Biotherapeutics, Incyte, Sanofi, Immatics, AbbVie, TCR2 Therapeutics, Takeda, Gilead Sciences, CareDx, TScan, Cabaletta, Synthekine, Bristol Myers Squibb, Johnson & Johnson, Allogene, Genentech, and Imugene. B.P. discloses honoraria with Seattle Genetics and Bio Secura. H.-J.J.C. discloses honoraria from ADC Therapeutics. J.E.A. discloses consultancy with AstraZeneca, Ipsen, and ADC Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Jennifer E. Amengual, Division of Hematology and Oncology, Columbia University Irving Medical Center, William Black Building Room 901, 650 W 168th St, New York, NY 10032; email: Jea2149@columbia.edu.
References
Author notes
E.H.O. and P.G. contributed equally to this study.
H.-J.J.C. and J.E.A. contributed equally to this study.
Data are available on request from the corresponding author, Jennifer E. Amengual (jea2149@columbia.edu).
The full-text version of this article contains a data supplement.