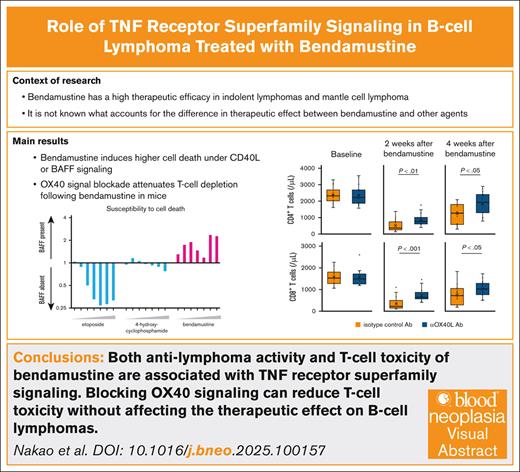
Key Points
Both antilymphoma activity and T-cell toxicity induced by bendamustine are closely associated with TNF receptor superfamily signaling.
T-cell toxicity of bendamustine can be reduced by blocking the OX40 signaling without affecting the therapeutic effect on B-cell lymphomas.
Visual Abstract
Bendamustine is one of the primary chemotherapeutic agents for the treatment of B-cell lymphomas, whereas its toxicity to T cells is associated with a high rate of infectious complications and a potential negative impact on subsequent immunotherapies. Bendamustine has previously been reported to be a potent in vitro inhibitor of HOIP, the catalytic subunit of the linear ubiquitin chain assembly complex (LUBAC). Because LUBAC functions as a checkpoint for tumor necrosis factor receptor superfamily (TNFRSF)–induced cell death, we hypothesized that bendamustine may have specific activity on the CD40 or BAFF-R signaling in B-cell lymphomas. In a cell culture assay, we found that bendamustine not only suppressed CD40L- and BAFF-driven NF-κB signaling in B-cell lymphomas but also induced higher cell death in the presence of these signals, a phenomenon not typical of other chemotherapeutic agents. Bendamustine resistance was shown to be driven by knockout of TRAF3, a mediator of CD40 and BAFF-R signaling. Importantly, the toxic effect of bendamustine on T cells was also found to be associated with another TNFRSF molecule, OX40. Pretreatment with an antibody blocking OX40-OX40L signaling attenuated bendamustine-induced T-cell depletion in mice, whereas preserving the cytotoxic effect of bendamustine on transplanted B-cell lymphoma. In conclusion, both the antilymphoma effect and the T-lymphopenia induced by bendamustine are associated with TNFRSF signaling, and the T-cell toxicity can be separately controlled by blocking OX40 signaling before bendamustine administration.
Introduction
B-cell lymphomas depend on the immune microenvironment to varying degrees according to histological subtype.1 Lymphoma cells receive survival and proliferation signals through direct cell-to-cell contact with or indirectly by receiving secreted molecules from surrounding cells, such as T cells, follicular dendritic cells, stromal cells, and macrophages. Members of the tumor necrosis factor receptor superfamily (TNFRSF), such as CD40 and B-cell activating factor belonging to the tumor necrosis factor family receptor (BAFF-R), are critically involved in B-cell physiology, and mediate essential microenvironmental signals to B-cell lymphoma cells. These signals that lymphoma cells receive from the microenvironment are generally recognized as protecting lymphoma cells from chemotherapy-induced cell death.2-6
Bendamustine is one of the key drugs in the treatment of B-cell lymphomas and is characterized by a high therapeutic efficacy particularly in indolent lymphomas and mantle cell lymphoma (MCL). We speculated that bendamustine may have a unique pharmacological activity that can effectively target lymphoma cells that are robustly supported by the immune microenvironment.
In a previous matrix-assisted laser desorption/ionization–time-of-flight (MALDI-TOF) mass spectrometry–based drug screening study, bendamustine was listed at the top of the 1430 screened drugs with the highest inhibitory activity on an E3 ubiquitin ligase HOIP, the catalytic subunit of the linear ubiquitin chain assembly complex (LUBAC).7 By specifically generating M1-linked ubiquitin chains, LUBAC plays important roles in the regulation of various immune signaling and cell-fate decisions.8 Some of the TNFRSFs are known to have dual functions in the cell-fate decisions, and the ubiquitin network is critically involved in the regulation of cell death checkpoints downstream of these receptors. Inhibition of LUBAC is one of the conditions that triggers the activation of these death signals.9-13 We, therefore, hypothesized that the unique treatment effect of bendamustine may be achieved through the induction of the death signals downstream of the TNFRSF signaling pathway by inhibiting the enzymatic activity of LUBAC.
We compared the biological effects of bendamustine on B-cell lymphoma cells in the presence or absence of 2 representative TNFRSF signals, CD40 and BAFF-R. Although lymphoma cell death induced by other chemotherapeutic agents is attenuated in the presence of these signals, bendamustine has been shown to conversely induce higher levels of cell death. In addition, T-cell depletion by bendamustine has been shown to be associated with OX40 signaling, another member of the TNFRSF expressed on T cells. Inhibition of the OX40 signaling pathway prior to bendamustine administration attenuated T-cell depletion in mice while maintaining the therapeutic efficacy against B-cell lymphoma cells. Our discovery of the unique mechanisms of action of bendamustine is expected to contribute to the development of safer and more effective treatments for B-cell lymphomas.
Materials and methods
Cell lines and chemotherapeutic agents
Human transformed follicular lymphoma (FL) lines FL18, FL218, and FL318,14 MCL cell lines MINO and Granta 519,15 and activated B-cell–like (ABC) diffuse large B-cell lymphoma (DLBCL) lines HBL1,16 OYB and DLBCL214 were cultured at 37°C in humidified air containing 5% CO2 in RPMI 1640 medium (Nacalai, Kyoto, Japan) containing 10% fetal bovine serum (FBS) with 1% penicillin-streptomycin-glutamine (PSG) mixed solution (Nacalai). All these cell lines express surface CD40 and BAFF-R (supplemental Figure 1).
A murine fibroblast line L cells and human CD40 ligand (CD40L)–expressing L cells (CD40L+ L cells)17 were cultured in DMEM (Nacalai) containing 10% FBS and 1% PSG. For the preparation of human BAFF–expressing L cells, the BAFF coding sequence was amplified by polymerase chain reaction and ligated into pLVSIN-EF1α-IRES-ZsGreen1 vector (Takara, Otsu, Japan). The BAFF-introduced or mock vectors were transfected into Lenti-X 293T cells (Takara) with pCAG-HIVgp, pCMV-VSV-G-RSV-Rev using TransIT-Lenti Transfection Reagent (Mirus, Madison, WI). L cells were infected with lentivirus contained in the ultracentrifuged supernatants with 5 μg/mL Polybrene (Nacalai), and ZsGreen1-positive L cells were sorted using SH800s Cell Sorter (Sony, San Jose, CA). HM876 is a murine lymphoma cell line that we previously established from B-cell lymphoma developed in HOIP and MYD88 L252P transgenic mice.18 HM876 was grown in DMEM containing 10% FBS and 1% PSG.
Etoposide (MedChemExpress, Monmouth Junction, NJ), 4-hydroxycyclophosphamide (4-OHCP; a cytotoxic metabolite of cyclophosphamide) (Toronto Research Chemicals, Toronto, Canada), bendamustine (Selleck, Houston, TX), and ibrutinib (Selleck) were used in the experiments at the indicated concentrations.
NF-κB luciferase reporter assay
B-cell lymphoma lines were introduced with the pNL3.2.NF-κB-RE [NlucP/NF-κB-RE/Hygro] vector (Promega) by electroporation using the 4D-Nucleofector system and a nucleofector kit (Lonza, Tokyo, Japan). The vector-transfected cells were selected with hygromycin. L cells were irradiated at 55 Gy and seeded the next day in 24-well plates at 0.5 × 105 cells per well. After incubation for 24 hours, lymphoma cells transfected with NF-κB reporter vector were seeded at 2.5 × 105 per well. After 4 hours of coculture, lymphoma cells were treated with etoposide, 4-OHCP, bendamustine, or vehicle (0.1% dimethyl sulfoxide) at various concentrations as indicated, and harvested at 0 (untreated), 4, and 8 hours after treatment. The NF-κB activity in each cell condition was evaluated using Nano-Glo Luciferase Assay Reagent (Promega) by measuring relative luminescence using a PerkinElmer ARVO ×3 (PerkinElmer).
Immunoblotting
Cells were lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% TritonX-100 (MP Biomedicals, Irvine, CA), 2 mM phenylmethylsulfonyl fluoride (Nacalai), protease inhibitor cocktail (Roche, Basel, Switzerland). The lysates were centrifuged, and the supernatants were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. After blocking with Blocking One (Nacalai), the membranes were immunoblotted with antibodies. The membranes were visualized with SuperSignal West Pico Plus chemiluminescent substrate (ThermoFisher Scientific, Waltham, MA) and analyzed on ImageQuant 800 (Cytiva, Tokyo, Japan). The following antibodies were used in the immunoblotting assay: antilinear polyubiquitin (clone LUB9; LifeSensors, Malvern, PA), anti-cFLIP (clone D5J1E; Cell Signaling, Danvers, MA), anti-TRAF2 (clone F2Y7K; Cell Signaling), anti-TRAF3 (clone D1N5B; Cell Signaling), anti-NEMO (clone W16070A; BioLegend, San Diego, CA), anti-RIPK1 (clone D94C12; Cell Signaling), anti-mouse immunoglobulin G (IgG; NA934; Cytiva, Tokyo, Japan), anti-rabbit IgG (NA931; Cytiva), anti-rat IgG (NA935V; GE Healthcare, Chicago, IL), anti–β-actin (clone AC15; Sigma-Aldrich, St Louis, MO).
Tandem ubiquitin binding entity assay
L cells and CD40L+ L cells were irradiated with 55 Gy and seeded the next day in 6-well plates at a concentration of 2 × 105 cells per ml in 2 mL each and cultured for 24 hours. FL18 and FL318 were then seeded at 1 × 106 cells per mL in 2 mL per well, and after 1 hour, bendamustine was added at the indicated concentration and the cells were treated for a further 1 hour. Cells were lysed with lysis buffer as described above with the addition of 10 mM N-ethylmaleimide (Nacalai) and 15 mM L-cysteine (Nacalai), and 200 μg of cell lysates were incubated with 10 μL slurry of M1 Linear Ubiquitin Magnetic Tubes (LifeSensors) at 4°C for 2 hours. Precipitates were washed with tris-buffered saline with Tween-20 (TBST) containing 150 mM NaCl, 20 mM Tris pH 8.0 (Nacalai), and 0.1% Tween-20 (MP biomedicals, Irvine, CA), boiled in Tris–sodium dodecyl sulfate sample buffer with 2-mercaptoethanol (Nacalai) and subjected to immunoblotting.
Analysis of apoptotic cell death
L cells were irradiated at 55 Gy and seeded the next day in 48-well plates at 300 μL per well at a concentration of 1 × 105 cells per mL. After 24 hours of incubation, B-cell lines were seeded at 5 × 105 cells per mL at 5 × 105 cells per mL. After 4 hours of coculture, the cells were treated with etoposide, 4-OHCP, bendamustine, or vehicle at various concentrations for 24 hours as indicated. The lymphoma cells were then labeled with Val-Ala-Asp(OMe)-fluoromethylketone (VAD-FMK) (Promega) at a final concentration of 5 μM and incubated for 20 minutes. The B-cell lines were then collected and washed with Dulbecco’s phosphate-buffered saline (D-PBS) containing 0.1% FBS, and dead cells were stained with DAPI (4',6-diamidino-2-phenylindole) or propidium iodide solution, and the cells were evaluated by flow cytometric analysis.
Analysis of peripheral T-cell subsets in mice
The mouse experiment in this study was conducted after approval by the institutional review board. Eight to 10-week-old female C57BL/6 mice were treated twice (day 1 and day 5) with an intraperitoneal injection of 100 μg anti-OX40L antibody (clone RM134L; BioLegend) or isotype control antibody (clone RTK4530; BioLegend), followed 3 hours later by an intravenous injection of 40 mg/kg bendamustine. Peripheral blood was collected from each mouse before treatment and 2 and 4 weeks after the first injection. Leukocyte counts were evaluated by an automated blood cell counter (Tokushima Iryouki, Fukuoka, Japan), and CD4+ and CD8+ T cells were analyzed by flow cytometry after erythrocyte lysis. CD4+ T cells were further classified into naive (CD44lowCD62Lhigh), central memory (CD44highCD62Lhigh), and effector (CD44highCD62Llow) T-cell subsets. The CD40L blocking experiment was performed using anti-mouse CD40L antibody (clone MR1; BioLegend) and isotype control antibody (clone HTK888; BioLegend) in the same manner as the OX40L blocking experiment. In the experiment to evaluate the effect of OX40L blockade in cyclophosphamide treatment, single doses of anti-OX40L antibody and 200 mg/kg cyclophosphamide were intravenously administered.
Mouse lymphoma transplantation model experiment
HM876 cells were injected subcutaneously at 5 × 106 cells each into the bilateral flanks of 4 Gy-irradiated 8- to 10-week-old female C57BL/6 mice (Clea Japan, Inc, Tokyo, Japan), and tumors with ≥6 mm in diameter developed were observed at the injection sites after 2 weeks. The mice were then treated twice (day 1 and day 5) with anti-mouse OX40L or isotype control antibody and bendamustine as in the analysis of peripheral T-cell subsets. The tumor volume of each mouse was evaluated as the sum of the bilateral tumor volumes calculated by the approximate formula V (mm3) = (W2 × L) ÷ 2 (where V indicates volume, W short diameter, and L long diameter). The CD40L blocking experiment was performed using anti-mouse CD40L antibody and isotype control antibody in the same manner as the OX40L blocking experiment.
Flow cytometry analysis and antibodies
Cells were stained with the following antibodies: anti–CD3-APC-Cy7 (clone 17A2; BD Biosciences, Franklin Lakes, NJ), anti–CD4-PerCP-Cy5.5 (clone RM4.5; BD Biosciences), anti–CD8a-FITC (clone 53-6.7; ThermoFisher Scientific, Waltham, MA), anti–CD44-PE (clone IM7; BD Biosciences), anti–CD62L-APC (clone MEL-14; BD Biosciences), anti–CD40L-PE (clone 24-31; BioLegend), anti–CD40-FITC (clone 5C3; BioLegend), anti–BAFF-PE (clone T7-241; BioLegend), and anti–BAFF-R-FITC (clone 11C1; BioLegend). Flow cytometry data were acquired on a FACSLyric (BD Biosciences), and the results were analyzed using the FlowJo software (Tree Star, Inc, Ashland, OR).
Results
Bendamustine inhibits CD40-induced NF-κB activation
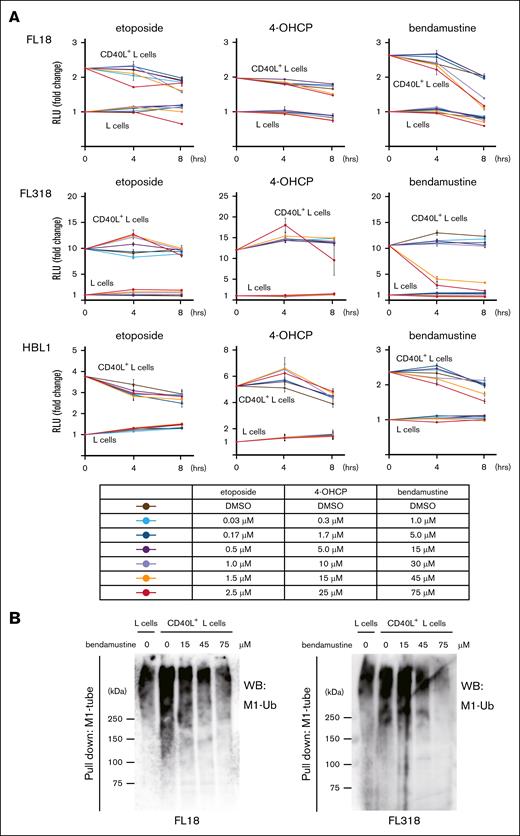
CD40 is a member of the TNFRSFs that is frequently expressed on B-cell lymphomas and is known to mediate survival and proliferative signals from the microenvironment. We first speculated that bendamustine might suppress NF-κB signaling downstream of CD40, a known target of LUBAC.19 We evaluated changes in NF-κB activity in lymphoma cells cocultured with L cells or CD40L+ L cells by luciferase reporter assay along with the time course after exposure to etoposide, 4-OHCP, or bendamustine. The cells cocultured with CD40L+ L cells showed higher baseline NF-κB activity compared to those cocultured with L cells, suggesting that CD40 signaling is activated by CD40L. As expected, bendamustine suppressed the NF-κB activity at higher concentrations in FL18 and FL318 cells cocultured with CD40L+ L cells, whereas no clear trend was observed in the etoposide and 4-OHCP groups (Figure 1A). The suppression of NF-κB activity is less significant in HBL1, an ABC-DLBCL line, and it is thought to be due to the high basal activation level of NF-κB.
Bendamustine suppresses NF-κB activity by inhibiting M1 ubiquitination. (A) Changes in NF-κB activity after treatment with etoposide, 4-OHCP, and bendamustine at parallel different concentrations in lymphoma cell lines. NF-κB activity was assessed by luciferase reporter assay. The basal NF-κB activity of each cell cocultured with L cells was set to 1, and relative changes in luciferase activity are shown in the line graphs. Error bars represent the standard error of the mean (SEM) of duplicate samples. (B) Immunoblotting for total M1 ubiquitination in the M1-TUBE enriched fraction in FL18 and FL318 cells cocultured with L cells or CD40L+ L cells and treated with different concentrations of bendamustine. DMSO, dimethyl sulfoxide; RLU, relative luciferase unit; Ub, ubiquitin; WB, western blotting.
Bendamustine suppresses NF-κB activity by inhibiting M1 ubiquitination. (A) Changes in NF-κB activity after treatment with etoposide, 4-OHCP, and bendamustine at parallel different concentrations in lymphoma cell lines. NF-κB activity was assessed by luciferase reporter assay. The basal NF-κB activity of each cell cocultured with L cells was set to 1, and relative changes in luciferase activity are shown in the line graphs. Error bars represent the standard error of the mean (SEM) of duplicate samples. (B) Immunoblotting for total M1 ubiquitination in the M1-TUBE enriched fraction in FL18 and FL318 cells cocultured with L cells or CD40L+ L cells and treated with different concentrations of bendamustine. DMSO, dimethyl sulfoxide; RLU, relative luciferase unit; Ub, ubiquitin; WB, western blotting.
We next investigated whether NF-κB inhibition by bendamustine is mediated by suppression of the catalytic activity of the E3 ligase LUBAC, as suggested by the MALDI-TOF mass spectrometry–based drug screening assay.7 We examined the changes in linear (M1)-ubiquitin chain formation after the addition of bendamustine to FL cells cocultured with CD40L+ L cells by immunoprecipitation and western blotting. Total M1-ubiquitin chain formation was increased in FL cells cocultured with CD40L+ L cells compared to those cocultured with L cells at baseline, which was inhibited by bendamustine in a dose dependent manner (Figure 1B). Known targets of M1-ubiquitin chains, NEMO and RIPK1, were deubiquitinated by increasing doses of bendamustine (supplemental Figure 2).
Bendamustine induces more apoptosis in lymphoma cells with active CD40 and BAFF-R signaling
The fate decision of the TNFRSF signaling is tightly regulated by a series of checkpoints that depend on several protein modification systems including ubiquitination. The typical TNFRSF molecule that is closely associated with live and death signals is TNF receptor 1. The downstream signals of TNF receptor 1 that induce cell death by apoptosis and necroptosis originate from a secondary cytoplasmic complex called complex II, and activation of caspase 8 triggers the apoptosis by activating the effector caspases. Other TNFRSF molecules without death domains, such as CD40, are also capable of mediating cell death signals depending on the cellular context and modulate the balance between life and death.20,21
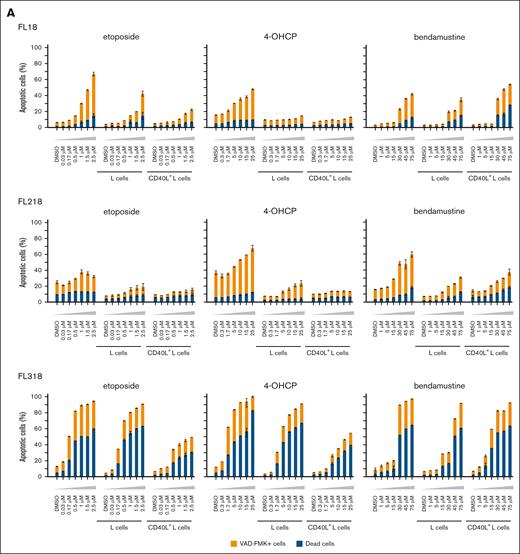
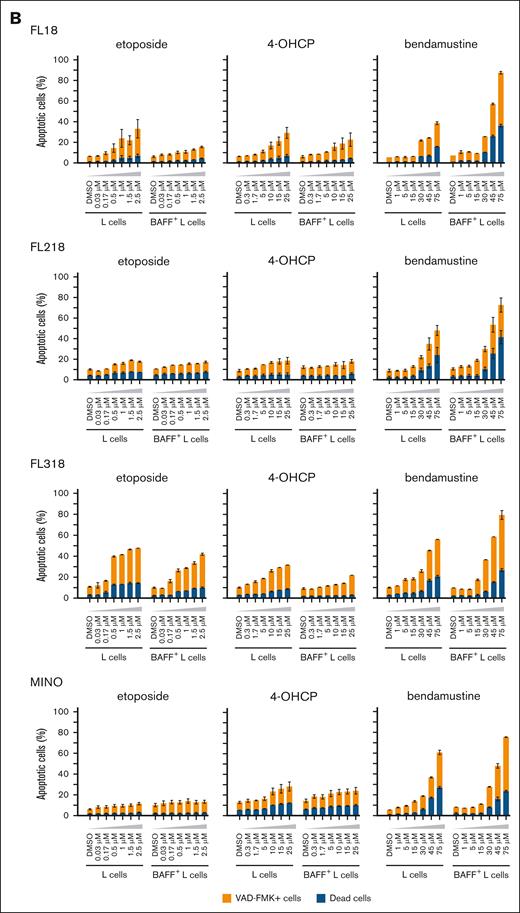
To evaluate the role of LUBAC inhibitory function of bendamustine on cell apoptosis, we compared the apoptotic and proapoptotic cell rates of B-cell lymphoma lines cocultured with L cells with or without CD40L or BAFF expression after treatment with chemotherapeutic agents including bendamustine. Etoposide and 4-OHCP invariably induced less apoptosis in the presence of L cells, especially those expressing CD40L or BAFF. On the contrary, bendamustine induced more apoptosis in these cell lines cocultured with CD40L+ or BAFF+ L cells than in those cocultured with L cells (Figure 2A-B). We also examined the effects of CD40L and BAFF on chemotherapy-induced cell death in 2 MCL cell lines, MINO and Granta 519. Although coculturing with CD40L+ L cells did not increase cell death by chemotherapeutic agents in these cells, coculturing with BAFF+ L cells increased cell death by bendamustine in MINO cells (Figure 2B).
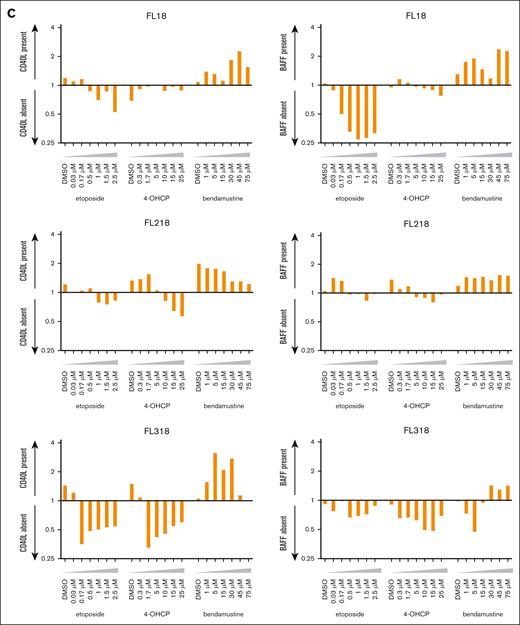
Apoptotic cell death rates of FL cell lines induced by chemotherapeutic agents in the different cell conditions. (A) Cell death rates of FL cell lines induced by etoposide, 4-OHCP, and bendamustine in the absence of feeder cells in the presence of L cells or CD40L+ L cells. Caspase-positive and dead cells were assessed by flow cytometry. Error bars represent the SEM of duplicate samples. (B) Cell death rates of FL and MCL cell lines induced by etoposide, 4-OHCP, and bendamustine treatment in the presence of L cells or BAFF+ L cells. Caspase-positive and dead cells were assessed by flow cytometry. Error bars represent the SEM of duplicate samples. (C) Ratio of cell death in FL cells cocultured with CD40L+ L cells to L cells (left panels), or those cocultured with BAFF+ L cells to L cells (right panels), at the indicated concentrations of the chemotherapeutic agents.
Apoptotic cell death rates of FL cell lines induced by chemotherapeutic agents in the different cell conditions. (A) Cell death rates of FL cell lines induced by etoposide, 4-OHCP, and bendamustine in the absence of feeder cells in the presence of L cells or CD40L+ L cells. Caspase-positive and dead cells were assessed by flow cytometry. Error bars represent the SEM of duplicate samples. (B) Cell death rates of FL and MCL cell lines induced by etoposide, 4-OHCP, and bendamustine treatment in the presence of L cells or BAFF+ L cells. Caspase-positive and dead cells were assessed by flow cytometry. Error bars represent the SEM of duplicate samples. (C) Ratio of cell death in FL cells cocultured with CD40L+ L cells to L cells (left panels), or those cocultured with BAFF+ L cells to L cells (right panels), at the indicated concentrations of the chemotherapeutic agents.
The ratio of apoptosis rates in FL cells with and without CD40 and BAFF signaling showed a clear tendency for specifically higher apoptosis rates with bendamustine under these signals (Figure 2C). These results suggest that, although CD40 and BAFF-R signaling generally attenuate chemotherapy-induced cell death of lymphoma cells, they promote cell death induced by bendamustine.
TRAF3-KO renders lymphoma cells resistant to bendamustine
In contrast to FL cells, neither CD40L nor BAFF stimulation enhanced bendamustine-induced cell death in HBL1 (Figure 3A and data not shown), although CD40 and BAFF-R were expressed at the same levels as those in FL cells (supplemental Figure 1). TNF receptor–associated factor (TRAF) 2 and TRAF3 are the major signaling mediators of CD40 and BAFF-R and are degraded upon activation of these signals.22 To evaluate whether the downstream signaling of CD40 and BAFF-R is active in HBL1, we compared the expression of TRAF2 and TRAF3 in FL cell lines and HBL1. Although both proteins were detected in the FL cell lines and were partially degraded by CD40L stimulation, only a high molecular weight form of the TRAF3 protein23,24 was detected and almost completely disappeared upon CD40L stimulation in HBL1 (Figure 3B). Although TRAF3 has been reported to be recurrently mutated in B-cell lymphomas,25-28 no genetic abnormalities in the TRAF3 gene were found in HBL1. Because HBL1 carries CD79B and MYD88 L265P mutations, we speculated that TRAF3 is constitutively degraded by the aberrant signals induced by these mutations.24,29,30
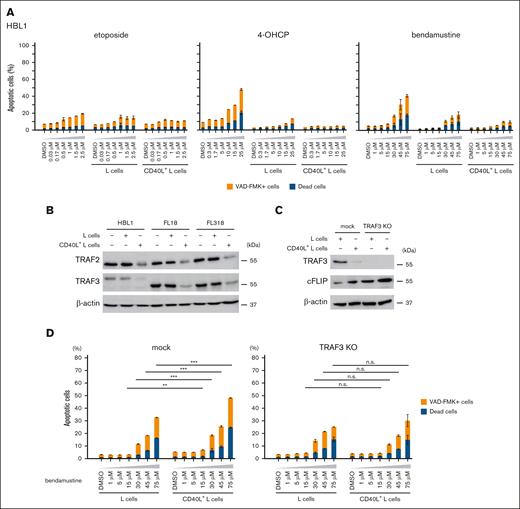
Loss of TRAF3 confers resistance of lymphoma cells to bendamustine. (A) Cell death rates of HBL1 induced by etoposide, 4-OHCP, and bendamustine treatment in the absence of feeder cells, in the presence of L cells or CD40L+ L cells. Caspase-positive and dead cells were assessed by flow cytometry. Error bars indicate the SEM of duplicate samples. (B) Western blot analysis for TRAF2, TRAF3, and β-actin (loading control) of HBL1, FL18, and FL318 cells cultured in the absence of feeder cells or in the presence of L cells or CD40L+ L cells. (C) Western blot analysis for TRAF3, cFLIP, and β-actin (loading control) of mock or TRAF3-KO FL18 cells cocultured with L cells or CD40L+ L cells. (D) Cell death rates of mock or TRAF3-KO FL18 cells induced by bendamustine at the indicated concentrations in the presence of L cells or CD40L+ L cells. Caspase-positive and dead cells were assessed by flow cytometry. Error bars indicate the SEM of duplicate samples. Statistical significance was evaluated by 2-way analysis of variance (ANOVA), followed by Tukey-Kramer test for multiple comparisons. The results are indicated as: n.s., P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. n.s., not significant.
Loss of TRAF3 confers resistance of lymphoma cells to bendamustine. (A) Cell death rates of HBL1 induced by etoposide, 4-OHCP, and bendamustine treatment in the absence of feeder cells, in the presence of L cells or CD40L+ L cells. Caspase-positive and dead cells were assessed by flow cytometry. Error bars indicate the SEM of duplicate samples. (B) Western blot analysis for TRAF2, TRAF3, and β-actin (loading control) of HBL1, FL18, and FL318 cells cultured in the absence of feeder cells or in the presence of L cells or CD40L+ L cells. (C) Western blot analysis for TRAF3, cFLIP, and β-actin (loading control) of mock or TRAF3-KO FL18 cells cocultured with L cells or CD40L+ L cells. (D) Cell death rates of mock or TRAF3-KO FL18 cells induced by bendamustine at the indicated concentrations in the presence of L cells or CD40L+ L cells. Caspase-positive and dead cells were assessed by flow cytometry. Error bars indicate the SEM of duplicate samples. Statistical significance was evaluated by 2-way analysis of variance (ANOVA), followed by Tukey-Kramer test for multiple comparisons. The results are indicated as: n.s., P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. n.s., not significant.
We hypothesized that the loss of TRAF3 is directly responsible for the resistance to CD40 and BAFF-R signaling dependent cell death induced by bendamustine. We generated TRAF3-knockout (KO) FL18 cells using the CRISPR-Cas9 system (Figure 3C) and found that bendamustine no longer enhanced cell death of TRAF3-KO FL18 cells cocultured with CD40L+ L cells (Figure 3D).
Ibrutinib enhances bendamustine-induced cell death by reducing cFLIP expression
Western blotting revealed the higher cellular FADD-like IL-1β–converting enzyme (FLICE)-inhibitory protein (cFLIP) expression in TRAF3-KO FL18 cells, especially in the presence of CD40L stimulation (Figure 3C). Because the cFLIP gene (CFLAR) is a known target of NF-κB, we hypothesized that HBL1 might be tolerant to bendamustine due to the high expression of cFLIP caused by constitutive NF-κB activation. We compared the expression levels of cFLIP in HBL1 and FL cell lines cocultured with or without L cells by western blotting and found that cFLIP expression was higher in HBL1 than that in FL cell lines in all conditions (Figure 4A).
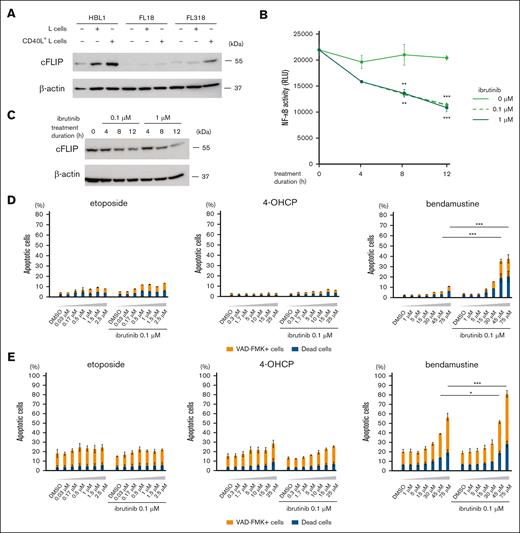
NF-κB activation leads to bendamustine resistance by increasing cFLIP expression, and ibrutinib can restore sensitivity of lymphoma cells to bendamustine. (A) Western blot analysis for cFLIP and β-actin (loading control) of HBL1, FL18, and FL318 in the absence of feeder cells or in the presence of L cells or CD40L+ L cells. (B) Changes in NF-κB activity of HBL1 after treatment with different concentrations of ibrutinib evaluated by luciferase reporter assay. RLU representing NF-κB activity are shown in the line graphs. Error bars represent the SEM of duplicate samples. Statistical significance between samples treated with 0 and 0.1 μM ibrutinib (upper stars) or those treated with 0 and 1 μM ibrutinib (lower stars) for 8 and 12 hours was evaluated by 2-way ANOVA, followed by Tukey-Kramer test for multiple comparisons. Results are shown as: ∗∗P < .01 or ∗∗∗P < .001. (C) Western blot analysis for cFLIP and β-actin (loading control) of HBL1 at different ibrutinib concentrations and durations of treatment. (D) Cell death rates of HBL1 cocultured with CD40L+ L cells treated with etoposide, 4-OHCP, and bendamustine at the indicated concentrations in combination with or without 0.1 μM ibrutinib. Error bars represent the SEM of duplicate samples. The results are indicated as: ∗∗∗P < .001. (E) Cell death rates of MINO cocultured with BAFF+ L cells treated with etoposide, 4-OHCP, and bendamustine at the indicated concentrations in combination with or without 0.1 μM ibrutinib. Error bars represent the SEM of duplicate samples. The results are indicated as: ∗P < .05 or ∗∗∗P < .001.
NF-κB activation leads to bendamustine resistance by increasing cFLIP expression, and ibrutinib can restore sensitivity of lymphoma cells to bendamustine. (A) Western blot analysis for cFLIP and β-actin (loading control) of HBL1, FL18, and FL318 in the absence of feeder cells or in the presence of L cells or CD40L+ L cells. (B) Changes in NF-κB activity of HBL1 after treatment with different concentrations of ibrutinib evaluated by luciferase reporter assay. RLU representing NF-κB activity are shown in the line graphs. Error bars represent the SEM of duplicate samples. Statistical significance between samples treated with 0 and 0.1 μM ibrutinib (upper stars) or those treated with 0 and 1 μM ibrutinib (lower stars) for 8 and 12 hours was evaluated by 2-way ANOVA, followed by Tukey-Kramer test for multiple comparisons. Results are shown as: ∗∗P < .01 or ∗∗∗P < .001. (C) Western blot analysis for cFLIP and β-actin (loading control) of HBL1 at different ibrutinib concentrations and durations of treatment. (D) Cell death rates of HBL1 cocultured with CD40L+ L cells treated with etoposide, 4-OHCP, and bendamustine at the indicated concentrations in combination with or without 0.1 μM ibrutinib. Error bars represent the SEM of duplicate samples. The results are indicated as: ∗∗∗P < .001. (E) Cell death rates of MINO cocultured with BAFF+ L cells treated with etoposide, 4-OHCP, and bendamustine at the indicated concentrations in combination with or without 0.1 μM ibrutinib. Error bars represent the SEM of duplicate samples. The results are indicated as: ∗P < .05 or ∗∗∗P < .001.
We hypothesized that the inhibition of NF-κB signaling by a Bruton tyrosine kinase (BTK) inhibitor, ibrutinib, would be able to reduce cFLIP expression and increase sensitivity to bendamustine in HBL1. In the luciferase NF-κB reporter assay, 0.1 μM and 1 μM ibrutinib had similar inhibitory effects on NF-κB activity in HBL1 (Figure 4B). Accordingly, NF-κB inhibition by ibrutinib resulted in a decrease in cFLIP protein expression in HBL1 over time (Figure 4C). Notably, the combination of 0.1 μM ibrutinib with bendamustine, but not with etoposide or 4-OHCP, effectively induced cell death of HBL1 cocultured with CD40L+ L cells (Figure 4D). We found that ibrutinib decreased cFLIP expression in 2 additional ABC-DLBCL cell lines OYB and DLBCL2, and increased bendamustine-induced cell death of these cells cocultured with CD40L+ L cells (supplemental Figure 3). Additionally, we found that ibrutinib increased cell death of bendamustine but not by etoposide and 4-OHCP in MINO cells cocultured with BAFF+ L cells (Figure 4E). These results suggest that ibrutinib makes B-cell lymphoma cells more sensitive to bendamustine under TNFRSF signaling.
OX40L-OX40 signaling blockade attenuates bendamustine-induced T-cell depletion
Bendamustine treatment is frequently associated with T-cell depletion, especially of CD4+ T cells. T-cell depletion occurs shortly after the initiation of bendamustine and persists for several months after the end of treatment, leading to an increased risk of opportunistic infections.31-33 We hypothesized that T-cell depletion by bendamustine may also be caused by increased cell death through activation of the TNFRSF pathway in T cells. Because OX40 signaling is closely involved in the functional activity of CD4+ T cells, we hypothesized that transient blockade of OX40L-OX40 signaling might be able to preserve CD4+ T cells during bendamustine treatment.
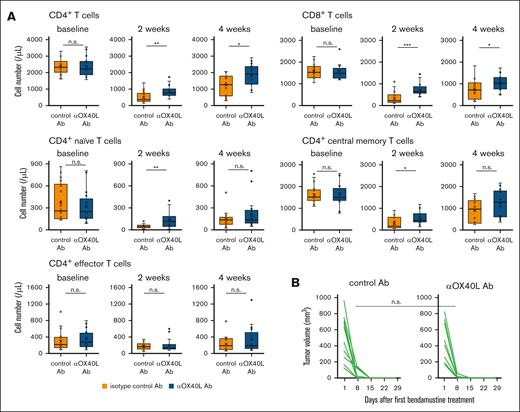
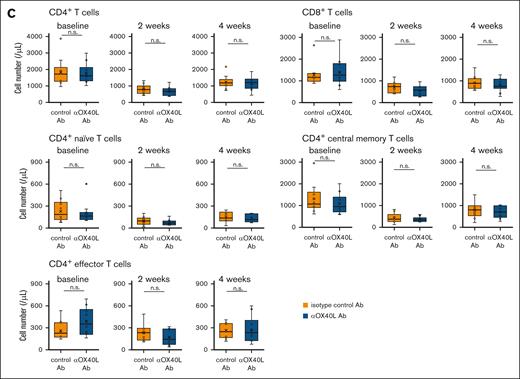
To test this hypothesis, we treated C57BL/6 mice with bendamustine twice after intraperitoneal injection of anti-OX40L blocking antibody or isotype control antibody, and evaluated peripheral T-cell counts. Notably, pretreatment with anti-OX40L blocking antibody significantly attenuated bendamustine-induced T-cell depletion in both the CD4+ and CD8+ T-cell populations at 2 and 4 weeks after treatment (Figure 5A). Although there were fewer differences in cell numbers between the anti-OX40 antibody and control groups in each CD4+ T-cell subset, an increasing trend was observed in the anti-OX40 antibody. In contrast, pretreatment with anti-OX40L blocking antibody had no effect on T-cell numbers after cyclophosphamide treatment (Figure 5C).
Blockade of OX40 signaling attenuates bendamustine-induced T-cell depletion. (A) T-cell counts at baseline and 2 and 4 weeks after bendamustine administration in C57BL/6 mice pretreated with anti-OX40L Ab (n = 17) or isotype control Ab (n = 18). Statistical significance was assessed by Welch t test based on T-cell counts at 2 and 4 weeks. The results are indicated as: n.s., P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. (B) Changes in the volume of transplanted B-cell lymphoma (HM876) in C57BL/6 mice after bendamustine administration with anti-OX40L Ab (n = 10) or isotype control Ab (n = 10) pretreatment. The tumor reduction rates at day 8 were compared between the 2 groups using the Brunner-Munzel test, which does not require assumptions of normality and homoscedasticity. (C) T-cell counts at baseline and 2 and 4 weeks after cyclophosphamide administration in C57BL/6 mice pretreated with anti-OX40L Ab (n = 10) or isotype control Ab (n = 10). Statistical significance was assessed by Welch t test based on T-cell counts at 2 and 4 weeks. The results are indicated as: n.s., P > .05. Ab, antibody.
Blockade of OX40 signaling attenuates bendamustine-induced T-cell depletion. (A) T-cell counts at baseline and 2 and 4 weeks after bendamustine administration in C57BL/6 mice pretreated with anti-OX40L Ab (n = 17) or isotype control Ab (n = 18). Statistical significance was assessed by Welch t test based on T-cell counts at 2 and 4 weeks. The results are indicated as: n.s., P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. (B) Changes in the volume of transplanted B-cell lymphoma (HM876) in C57BL/6 mice after bendamustine administration with anti-OX40L Ab (n = 10) or isotype control Ab (n = 10) pretreatment. The tumor reduction rates at day 8 were compared between the 2 groups using the Brunner-Munzel test, which does not require assumptions of normality and homoscedasticity. (C) T-cell counts at baseline and 2 and 4 weeks after cyclophosphamide administration in C57BL/6 mice pretreated with anti-OX40L Ab (n = 10) or isotype control Ab (n = 10). Statistical significance was assessed by Welch t test based on T-cell counts at 2 and 4 weeks. The results are indicated as: n.s., P > .05. Ab, antibody.
We next sought to determine whether blockade of OX40-OX40L signaling affects the antilymphoma effect of bendamustine. We subcutaneously injected C57BL/6 mice with the B-cell lymphoma line HM876,18 and after engraftment, bendamustine was administered twice after injection of anti-OX40L blocking antibody or isotype control antibody and changes in tumor volume were evaluated over time. The results showed that tumors regressed promptly and similarly in mice treated with anti-OX40L blocking antibody and isotype control antibody (statistical significance of tumor regression rates at day 8 between the groups, P = .44; Figure 5B), indicating that OX40L blockade did not affect the therapeutic efficacy of bendamustine against lymphoma cells.
We further evaluated the effect of injecting anti-CD40L blocking antibody prior to bendamustine administration. Pretreatment with the antibody had almost no effect on T-cell counts after bendamustine treatment, whereas differences in T-cell counts were observed between the anti-CD40L and control antibody groups under a few conditions (supplemental Figure 4A). We suspect this is because CD40 is expressed not only on B cells but also on a small population of T cells,34,35 and CD40 signaling may play a limited role in T-cell depletion caused by bendamustine.
Notably, anti-CD40L blocking antibody prolonged the time to regression of transplanted lymphoma after bendamustine treatment in some individuals (statistical significance of tumor regression rates at day 8 between the groups, P < .05) (supplemental Figure 4B), suggesting that CD40L-CD40 signaling plays a role in obtaining the therapeutic effect of bendamustine. These results suggest that T-cell depletion during bendamustine treatment is associated with OX40 signaling and that blockade of OX40L-OX40 signaling can attenuate T-cell depletion without apparently affecting the therapeutic effects of bendamustine.
Discussion
TNFRSFs are the cell-surface molecules that are essentially involved in the regulation of physiological functions of lymphocytes and in the survival and maintenance of lymphoma cells. Some members of the TNFRSFs are known to mediate signals for both cell survival and cell death, and LUBAC plays an important role as one of the switches for the cell-fate decision of TNFRSF signaling through the linear ubiquitination of downstream molecules. In this study, we have shown that bendamustine induces cell death in both B-cell lymphoma cells and T cells by switching TNFRSF signals received from the microenvironment from survival to death signals, most likely by inhibiting linear ubiquitination.
In FL, the importance of the immune microenvironment on outcome has long been recognized,36-38 but the inconsistency of high-risk gene signatures between patients receiving CHOP/CVP- and bendamustine-based regimens was demonstrated in the analysis of the FL patients enrolled in the GALLIUM study.39,40 In this analysis, none of the high-risk prognostic indicators identified in the CHOP/CVP-based regimens had a worse prognostic impact on the patients treated with bendamustine-based regimens, and some of the genes were shown to have an inverse prognostic impact on the patients treated with CHOP/CVP- and bendamustine-based regimens.39 By focusing on the TNFRSF signals that lymphoma cells receive from the microenvironment, our findings provide a clue to understanding the molecular background of the different therapeutic effects led by bendamustine and CHOP/CVP. Our findings may be useful in the prediction of treatment efficacy and in the selection of the optimal treatment for lymphoma patients.
Our data suggest that CD40 and BAFF-R signaling are important for the bendamustine-induced cell death. These findings would explain why bendamustine is more effective in indolent lymphomas and MCL, where lymphoma cells are more dependent on the signals from surrounding cells than in aggressive lymphomas. This phenomenon was more clearly observed in FL cells than in MCL cells in our cell line–based experiment. We hypothesize that MCL cells may have become less dependent on the tumor microenvironment than FL cells do during the process of transformation into cell lines.
TRAF3 is a mediator of CD40 and BAFF-R signaling, and loss of TRAF3 function has been reported to induce upregulation of NF-κB–inducing kinase and activation of noncanonical NF-κB signaling.41 We have shown that genetic ablation of TRAF3 leads to the abrogation of enhanced cell death induced by CD40 signaling upon bendamustine treatment, suggesting that defective genetic abnormality of TRAF3 is one of the mechanisms of bendamustine resistance.
Our results have shown that ibrutinib reduces cFLIP expression by inhibiting NF-κB activity and contributes to lowering the threshold for bendamustine-induced cell death. Combination therapy of bendamustine with BTK inhibitors has been clinically developed in MCL.42,43 BTK inhibitors have the ability to release lymphoma cells from their protective microenvironment in vivo,44 and bendamustine would target lymphoma cells that remain highly dependent on the microenvironment. A deeper understanding of the molecular mechanism of action of bendamustine is expected to facilitate the development of better combination therapies of bendamustine.
CD4+ T-cell depletion after bendamustine treatment leads to prolonged immunodeficiency and increased risk of opportunistic infections. In recent years, severe and sometimes life-threatening coronavirus infections after bendamustine treatment have become an emerging concern. In addition, the use of bendamustine shortly before chimeric antigen receptor T-cell apheresis has been shown to be associated with an increased risk of the manufacturing failure and poor outcomes,45,46 suggesting the possibility that bendamustine may reduce the efficacy of immunotherapy by affecting T cells.
Although there is not as much information on the death signals induced by OX4047 as there is on those induced by CD40 signaling, our results suggest that OX40 signaling is involved in the bendamustine-induced CD4+ T-cell depletion and that this can be partially rescued by blocking OX40L-OX40 signaling prior to bendamustine administration Fig. 6. Moreover, inhibition of OX40L-OX40 signaling did not affect the therapeutic effect of bendamustine against transplanted B-cell lymphoma in mice. OX40 signaling is generally essential for CD4+ T-cell survival and activation, and prolonged blockade of OX40 signaling may impair CD4+ T-cell fitness. Because bendamustine has a plasma half-life of ∼40 minutes48 and is rapidly hydrolyzed in the blood, short-term blockade of OX40 signaling may be sufficient to preserve peripheral T-cell counts. Incomplete T-cell recovery with blockade of OX40 signaling may be explained by the concomitant involvement of other TNFRSF members expressed on T cells, and it is possible that simultaneous blockade of T-cell–specific TNFRSF signaling in addition to OX40 may restore T-cell counts more completely.
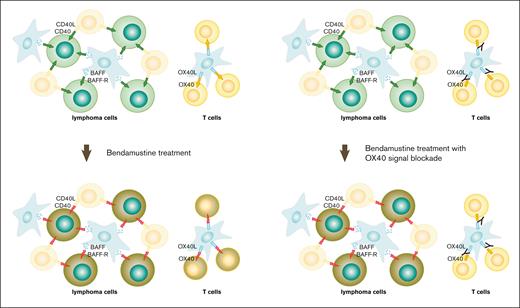
Schematic illustration of the mechanism of cell death induced by bendamustine. TNFRSF signaling generally provides survival signals (green and yellow arrows) to both lymphoma cells and T cells, but it is converted to death signals (red lightning bolts) after bendamustine treatment (left panels). Blockade of OX40L-OX40 signaling protects T cells under bendamustine treatment by preventing OX40-mediated death signals (right panels).
Schematic illustration of the mechanism of cell death induced by bendamustine. TNFRSF signaling generally provides survival signals (green and yellow arrows) to both lymphoma cells and T cells, but it is converted to death signals (red lightning bolts) after bendamustine treatment (left panels). Blockade of OX40L-OX40 signaling protects T cells under bendamustine treatment by preventing OX40-mediated death signals (right panels).
In this study, we have shown that both the antilymphoma effect and the T-cell depletion induced by bendamustine are associated with TNFRSF signaling and can be independently regulated by blocking the signaling specifically involved in lymphoma cells and T cells. Although further investigation and clinical validation in patients are required, our study sheds light on the basic molecular mechanism of action of bendamustine and is expected to provide important information for the more efficient and safer clinical use of bendamustine.
Acknowledgments
This work was supported by research grants from SymBio Pharmaceuticals and the Japan Society for the Promotion of Science (grant numbers 21K08392 and 24K11558 [M.N.]).
Authorship
Contribution: K.N. and Y.A. performed experiments; K.N., H.A., Y.A., Y.G., T.T. and I.M. contributed vital new reagents; K.N., H.A., T.J., T.K., A.T.-K., and M.N. analyzed and interpreted data; K.N. and M.N. designed the research and wrote the manuscript; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: A.T.-K. has received research funding from Shionogi and honoraria from Janssen Pharmaceuticals. M.N. has received research funding from SymBio Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Momoko Nishikori, Department of Hematology and Human Health Sciences, Graduate School of Medicine, Kyoto University, 53 Shogoin Kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan; email: nishikor@kuhp.kyoto-u.ac.jp.
References
Author notes
Data are available upon reasonable request to the corresponding author, Momoko Nishikori (nishikor@kuhp.kyoto-u.ac.jp).
The full-text version of this article contains a data supplement.