Key Points
The orally available decitabine prodrug OR-2100 inhibits proliferation of DHL through the perturbation of mitosis.
The combination of OR-2100 and CHOP might be an effective and feasible treatment for DHL.
Visual Abstract
Double-hit lymphoma (DHL), an aggressive B-cell lymphoma with a poor prognosis, harbors rearrangements of MYC and BCL2. The standard chemoimmunotherapy comprising R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) yields an unsatisfactory treatment response. Hypomethylating agents increase the susceptibility to malignant lymphoma via the restoration of tumor suppressor genes. Decitabine not only inhibits DNA methylation but also causes mitotic disruption, which leads to antileukemia effects through the covalent binding of DNA methyltransferase 1 to DNA. Previously, we developed an orally bioavailable prodrug of decitabine (OR-2100). Here, we investigated the efficacy and underlying mechanism of OR-2100 as a treatment for DHL. OR-2100 alone or in combination with key antilymphoma drugs, including doxorubicin and vincristine, suppressed the in vitro proliferation of DHL cell lines, particularly those with wild-type TP53. OR-2100 induced downregulation of CDCA8 and BIRC5 (baculoviral IAP [inhibitor of apoptosis] repeat–containing 5), the knockdown of which suppressed the proliferation of DHL cell lines. Both OR-2100 treatment and CDCA8 knockdown led to mitotic perturbation, suggesting that the disruption of mitosis may underlie the antitumor mechanism of OR-2100 given that the efficacy of OR-2100 was dependent on the TP53 status and that CDCA8 and BIRC5 are downstream targets of the E2F1 pathway. These findings suggest that the antitumor activity of OR-2100 may be mediated through inhibition of the E2F1 pathway. The combination of CHOP and OR-2100 reduced the tumor weight significantly in a xenograft mouse model without increased toxicities. The induction of mitotic perturbation might be a key antilymphoma mechanism for OR-2100, and the combination of OR-2100 and CHOP might be a promising treatment strategy for DHL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for approximately one-third of non-Hodgkin lymphoma cases.1 R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) is the established first-line chemoimmunotherapy, achieving long-term remission in nearly 60% of patients. Double-hit lymphoma (DHL), which is characterized by the rearrangement of MYC and BCL2, is a more aggressive type of BCL that has a poor prognosis; as such, it is designated as a distinct clinical entity called DLBCL/high-grade BCL with MYC and BCL2 rearrangements.2 The 2-year progression-free survival of patients with DHL is only 40%, and the median overall survival is 20 months if treated with conventional R-CHOP; therefore, intensive chemotherapy, such as DA-EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), is recommended for a better clinical outcome.3-5 However, the prognosis of DHL remains dismal, making the development of improved therapeutic strategies a priority. Given that DHL falls within the EZB subtype of the LymphGen classification,1 which is marked by deregulated epigenetic pathways, we explored the potential clinical efficacy of OR-2100.
Hypomethylating agents (HMAs), such as decitabine (DAC) and azacitidine (AZA), are used routinely to treat acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS). The main mechanism of action is not only the direct cytotoxic effect but also a demethylating effect through the inhibition of DNA methyltransferase 1 when used at noncytotoxic doses.6,7 Aberrant hypermethylation of cytosine-guanine (CpG)–rich sites (CpG islands) within tumor suppressor genes is frequently observed in cancer,8 and HMAs exert demethylating effects on the lesions, thereby exhibiting anticancer activity. Recently, other anticancer mechanism of HMAs have been reported; among these, mitotic disruption (referred to mitotic perturbation), is a newly discovered and unique mechanism through which DAC ameliorates AML/MDS.9
HMAs are being evaluated for their clinical efficacy as a treatment for DLBCL when administered together with R-CHOP (ClinicalTrials.gov identifiers: NCT02343536, NCT02951728, and NCT04025593).10,11 The combination of HMAs and R-CHOP demonstrates clinical efficacy in patients with DLBCL; however, there are limited data on the effectiveness of the combination of HMAs and R-CHOP in patients with DHL who have a poor prognosis primarily because of the small number of cases. Previous studies have shown that transcriptional activation of SMAD1, a mediator of the transforming growth factor β signaling pathway, or endogenous retroviruses that stimulate the stimulator of interferon genes pathway, underlies the activity of HMAs against DLBCL.12,13
Previously, we developed OR-2100, an orally bioavailable, single-compound DAC prodrug,14 and reported its efficacy as a monotherapy or combination therapy that targets hematologic malignancies. In addition, OR-2100 was less toxic than DAC.15-21 Based on these preclinical results, a phase 1 clinical trial of OR-2100 is currently underway for high-risk MDS (jRCT2041220035). In this study, we investigated the efficacy and safety of OR-2100 as a monotherapy and as a combination treatment with CHOP for DHL, as well as its mechanism of action.
Materials and methods
Cell lines and cultures
Six DHL cell lines were used (RC, DOHH2, OCI-Ly18, SU-DHL-10, WSU-DLCL2, and SU-DHL-6).
Reagents
OR-2100 was synthesized by Ohara Pharmaceutical Co (Shiga, Japan). AZA, DAC, and HLM006474 (an E2F inhibitor) were purchased from Sigma-Aldrich (St. Louis, MO). All reagents were dissolved in dimethyl sulfoxide and stored at −20°C.
Bisulfite pyrosequencing
The methylation status of the CpG sites of LINE-1 and SMAD1 was determined using pyrosequencing-based analysis, described in detail in the supplemental Methods.
Western blot analysis
Cells were treated with 500 nM AZA, DAC, and OR-2100 for 3 consecutive days or with 500 nM HLM006474 for 2 consecutive days. The protein was extracted 4 or 3 days after exposure to the drugs, respectively. Whole-cell lysates were extracted using Radioimmunoprecipitation Assay (RIPA) buffer (Santa Cruz Biotechnology), and the total protein content was quantified in a protein assay (Bio-Rad). Equal amounts of each whole-cell lysate were resolved in 4% to 12% n-polyacrylamide gels (Invitrogen), and the proteins were transferred to nitrocellulose membranes. Each protein was detected using an Odyssey imaging system (LI-COR Biosciences). The antibodies are listed in the supplemental Methods.
Quantitative real-time PCR
RNA isolation was performed using a phenol-chloroform extraction method. The sample RNA was extracted using a spin column, and genomic DNA was removed using DNase I (Direct-zol RNA MiniPrep; Zymo Research, Orange, CA). Complementary DNA was prepared using ReverTra Ace (Toyobo), and quantitative real-time polymerase chain reaction (PCR) was performed using TaqMan Gene Expression Master Mix (Applied Biosystems) with primer/probe sets (cdca8, 4331182; birc5, 4351372). The expression of β-actin (401846) was measured as an internal control.
Mice
Animal studies were conducted in accordance with the Saga University–approved animal protocols (A2024-002-0) and in accordance with the German Animal Welfare Act. All mice received humane care, and the humane end points decided on for the mice were a continuous decrease in body weight of more than 25% of its maximum body weight or marked debilitation. Immunodeficient BALB/c Rag-2/Jak3 double Knockout (KO) (BRJ) female mice (6 weeks of age) were provided by Seiji Okada (Kumamoto University, Kumamoto, Japan).
IHC staining
Immunohistochemistry (IHC) analysis was performed on cell blocks of cell lines. The formalin-fixed paraffin-embedded specimens were stained with monoclonal mouse antihuman p53 protein (clone DO-7; Ready-to-Use; Dako, Glostrup, Denmark).
Confocal microscopy
Cultured cells were blocked with 0.2% bovine serum albumin for 1 hour at room temperature. The cells were then labeled with an anti-α-tubulin (B-5-1-2) Alexa Fluor 488 Mouse Monoclonal Antibody (Thermo Fisher Scientific; 2 μg/mL in 0.1% bovine serum albumin) and incubated for 3 hours at room temperature. Nuclei were stained with ProLong-Gold Antifade Mountant with DNA Stain DAPI (4′,6-diamidino-2-phenylindole; Thermo Fisher Scientific) and observed under a confocal microscope (LSM 880+Airyscan Fast; Carl Zeiss, Oberkochen, Germany).
DLBCL xenograft mouse model (OR-2100 monotherapy)
OCI-Ly18 cells were infected with empty vector (short hairpin [sh] control [shControl]) or shCDCA8 to yield CDCA8 OCI-Ly18 cells. Next, 5 × 106 sh-control cells or CDCA8 OCI-Ly18 cells were inoculated subcutaneously in the back of BRJ mice at the same time (n = 6). Tumor volume, calculated as the (short axis)2 × (long axis)/2, was measured twice per week. All mice were euthanized by cervical dislocation 28 days after inoculation of sh-control cells or CDCA8 OCI-Ly18 cells and the tumors were resected and weighed.
RC cells (5 × 106) were inoculated subcutaneously into 6-week-old female BRJ male mice. Two weeks later, when the average xenograft tumor volume reached 120 mm3, the mice were randomized into 2 groups, namely control (n = 10) and OR-2100 (n = 9). A total of 3.39 mg/kg (9.9 μmol/kg) of OR-2100 or 10% HP-β-CD (2-hydroxypropyl-β-cyclodextrin) as a vehicle control, was injected intraperitoneally (IP) for 5 consecutive days. OR-2100 was developed to allow oral administration, but the human formulation has large granules and does not pass through the sonde in mice and thus it was administered IP. All mice were euthanized by cervical dislocation at 14 days after the first treatment. At the time of euthanasia, subcutaneous tumor samples and peripheral blood samples were collected.
DLBCL xenograft mouse model (combination therapy)
RC cells (5 × 106) were inoculated subcutaneously into 6-week-old female BRJ mice to evaluate the efficacy of combination therapy. Two weeks later, when the average xenograft tumor volume reached 150 mm3, the mice were randomized into 4 groups, namely control (n = 5), OR-2100 (n = 6), CHOP (n = 5), and OR-2100 plus CHOP (n = 5). A total of 3.39 mg/kg (9.9 μmol/kg) of OR-2100 or 10% HP-β-CD as a vehicle control was injected IP for 5 consecutive days from 1 week before administration of dose-attenuated CHOP. It has been confirmed that the pharmacokinetics are almost equivalent between IP administration and orally administered formulations.14,17,19 The dose of OR-2100 was calculated based on an area under the curve–guided comparison to ensure equivalence with DAC (1.25 mg/kg). Dose-attenuated CHOP comprised 100 mg/kg of cyclophosphamide (day 1), 6.75 mg/kg of doxorubicin (day 1), 3.75 μg of vincristine (day 1), and 13.5 mg/kg of prednisolone (days 1-5); these 4 drugs were halved from the previously described method.22 Tumor volume was measured twice per week. All mice were euthanized by cervical dislocation at 22 days after the first treatment. At the time of euthanasia, subcutaneous tumor samples and peripheral blood samples were collected.
RNA sequencing
Total RNA was isolated using the Direct-zol RNA MiniPrep kit (Zymo Research). The NEBNext Ultra II RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA) was used for library preparation. RNA sequencing using a NovaSeq 6000 system (Illumina, San Diego, CA) was performed by Veritas Genetics (Danvers, MA). Analysis of the differentially expressed genes (DEGs) was conducted by cBioinformatics Inc (Tokyo, Japan). DEGs were detected by edgeR (version 3.40.2), and genes with low expression were filtered using the filterByExpr function. Trimmed Mean of M-values (TMM) correction was applied thereafter. The samples were assigned to 1 of 3 groups, namely control, AZA monotherapy, and OR-2100 monotherapy. The cell lines were treated with 500 nM OR-2100 or AZA for 3 consecutive days, and RNA was extracted on day 5. Upregulated genes were defined as those showing a fold change >2. Downregulated genes were defined as those showing a fold change <0.5. Gene Ontology (GO) analysis was performed for both upregulated and downregulated DEGs using gprofiler2 (version 0.2.1) and clusterProfiler (version 4.6.2). The clusters were sorted in descending order of the gene ratio (count data divided by number of genes showing variable expression). In gprofiler2, Fisher exact probability tests were performed on the terms in the database, and a statistical analysis was performed to determine which terms were biased toward genes that showed variable expression. Corrected P values were obtained using the Benjamini-Hochberg method. A balloon plot was created using terms that were sorted in descending order of the gene ratio.
RRBS
Total DNA was isolated using the QIAprep Spin Miniprep kit (Qiagen, Hilden, Germany). The library DNA was constructed using the Zymo Research Zymo-Seq Reduced Representation of Bisulfite Sequencing (RRBS) Library Kit, and sequencing was performed using a Illumina NovaSeq X Plus. The reads were then converted to FASTQ-formatted files and applied to the nf-core methylseq pipelines, version 3.0.0.23 The obtained BAM-formatted mapping data were then applied to methylKit, version 1.32.1, in R, version 4.4.3, for Mac to analyze the differentially methylated CpG regions.24
Statistical analyses
Data are expressed as the mean ± standard deviation. Differences between groups were evaluated using Student t tests (2-sided) or 1-way analysis of variance. Calculations were performed using GraphPad Prism, version 10.2.2 (GraphPad Software, MA). The results were considered significant at P value <.05.
Cell lines and cultures, fluorescence in situ hybridization, cell growth assay, cell apoptosis, cell cycle analysis, chromosomal analysis, lentivirus preparation and Infection, flow cytometry, and cell sorting
A detailed description of these experiments is provided in the supplemental Methods.
Results
OR-2100 with/without doxorubicin/vincristine inhibits growth of DHL tumor cells in vitro
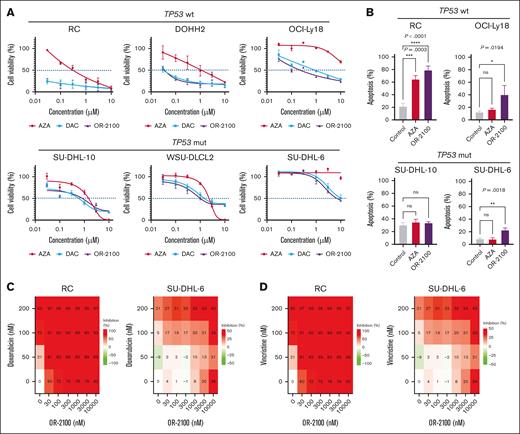
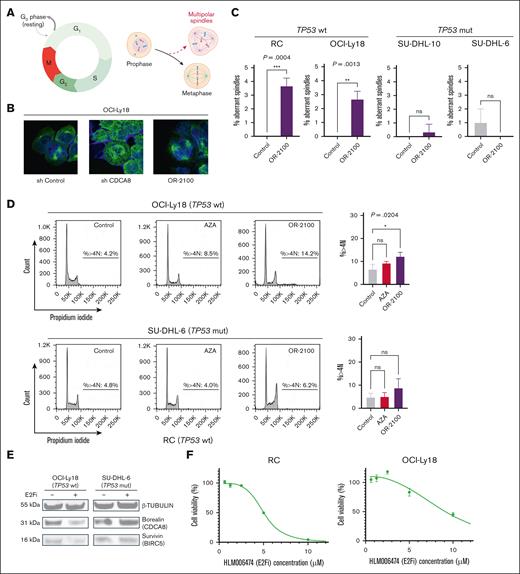
Fluorescence in situ hybridization was performed to confirm the status of MYC and BCL2 rearrangements in the 5 DHL cell lines. RC, DOHH2, OCI-Ly18, SU-DHL-10, WSU-DLCL-2, and SU-DHL-6 cells all harbored MYC and BCL2 rearrangements, fulfilling the definition of DHL. Next, we performed cell growth assays with the 5 DHL cell lines (Figure 1A) and found that the 3 DHL cell lines with wild-type TP53 (RC, DOHH2, and OCI-Ly18) were much more sensitive to DAC or OR-2100 than AZA at a drug concentration of <1 μM. In contrast, SU-DHL-6 and SU-DHL-10, which carry the TP53 mutation, were resistant to AZA, DAC, and OR-2100. The expression levels of p53 protein, which reflect the TP53 mutational status in each cell line, were assessed by IHC25 (supplemental Figure 1A). The data suggest that the effects of OR-2100 monotherapy might be dependent on the TP53 mutational status (supplemental Table 1) and showed that OR-2100 induced cell apoptosis to a greater extent than AZA, particularly in TP53 wild-type cells (Figure 1B).
Sensitivity of DHL cell lines to OR-2100 when used as a single agent or as combination therapy. (A) RC cells, DOHH2 cells, OCI-Ly18 cells, SU-DHL-10 cells, WSU-DLCL2 cells, and SU-DHL-6 cells were treated with HMAs (AZA, DAC, or OR-2100) for 72 hours, followed by cell viability assays. (B) The proportion of apoptotic cells in SU-DHL-6, SU-DHL-10, RC, and OCI-Ly18 cells after 72 hours of treatment with vehicle, 500 nM AZA, or OR-2100. The combined effect of OR-2100 and doxorubicin (C) or vincristine (D) in RC cells and SU-DHL-6 (calculated by SynergyFinder Plus). The combination of OR-2100 and doxorubicin has an additive effect against both RC cells and SU-DHL-6 cells. The combination of OR-2100 and vincristine has an additive effect against RC cells and SU-DHL-6 cells. mut, mutation; ns, not significant; wt, wild type.
Sensitivity of DHL cell lines to OR-2100 when used as a single agent or as combination therapy. (A) RC cells, DOHH2 cells, OCI-Ly18 cells, SU-DHL-10 cells, WSU-DLCL2 cells, and SU-DHL-6 cells were treated with HMAs (AZA, DAC, or OR-2100) for 72 hours, followed by cell viability assays. (B) The proportion of apoptotic cells in SU-DHL-6, SU-DHL-10, RC, and OCI-Ly18 cells after 72 hours of treatment with vehicle, 500 nM AZA, or OR-2100. The combined effect of OR-2100 and doxorubicin (C) or vincristine (D) in RC cells and SU-DHL-6 (calculated by SynergyFinder Plus). The combination of OR-2100 and doxorubicin has an additive effect against both RC cells and SU-DHL-6 cells. The combination of OR-2100 and vincristine has an additive effect against RC cells and SU-DHL-6 cells. mut, mutation; ns, not significant; wt, wild type.
Next, we investigated the combined effects of OR-2100 and doxorubicin or vincristine in vitro. SynergyFinder Plus was used to calculate the potential combined effects. The Zero Interaction Potency (ZIP) synergy scores for RC and SU-DHL-6 cells treated with OR-2100 and doxorubicin were 3.74 and −0.55, respectively (Figure 1C), and for cells treated with OR-2100 and vincristine, the scores were 0.17 and −0.3, respectively (Figure 1D).26,27 Although OR-2100 monotherapy did not inhibit tumor growth in TP53-mutated DHL cells at lower drug concentrations (<1 μM), it did show additive effects against TP53-mutated cells when combined with doxorubicin or vincristine.
OR-2100 downregulates the mitosis-related genes CDCA8 and BIRC5 in vitro
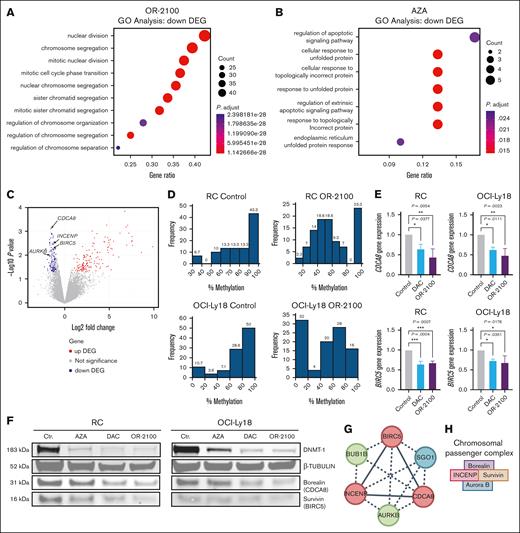
To explore the mechanism through which OR-2100 exerts effects against DHL, we investigated the gene expression profiles by sequencing RNA from 3 DHL cell lines (SU-DHL-6, SU-DHL-10, and RC). GO analysis and DEG analysis revealed that the upregulated GO terms were similar between the AZA-treated group and the OR-2100-treated group (supplemental Figure 2A-B). Meanwhile, mitosis-related GO terms were significantly more downregulated in the OR-2100–treated group than in the AZA-treated group (Figure 2A-B). CDCA8 and BIRC5 (baculoviral IAP [inhibitor of apoptosis] repeat–containing 5), both components of the chromosomal passenger complex (CPC), were significantly downregulated mitosis-related genes (P = .0007 and P = .0059, respectively; Figure 2C). Other components of the CPC, namely INCENP (inner centromere protein) and AURKB (Aurora kinase B), were also significantly downregulated (P = .0035 and P = .0101, respectively). Among these, CDCA8 exhibited the most significant downregulation, prompting us to focus on this gene in subsequent analyses. Given that BIRC5, which encodes survivin, has been implicated in aggressive DLBCL, we also included BIRC5 in our investigation.28
CDCA8 and BIRC5 are downregulated in DHL cells after treatment with OR-2100. GO analysis of the genes that were downregulated by OR2100 monotherapy (A) or AZA monotherapy (B). (C) Volcano plot showing the DEGs between the Ctr group and OR-2100–treated samples. |log2 fold change| > 2; adjusted P <.05. Red indicates the upregulated genes, and blue indicates the downregulated genes. CDCA8, BIRC5, INCENP, and AURKB were identified as significantly downregulated genes. (D) Histograms of the percentage CpG methylation of RC cells and OCI-Ly18 cells after treatment with vehicle (Ctr) or OR2100. (E) The relative expression of CDCA8 and BIRC5 was determined by real-time PCR after treatment with the vehicle (Ctr), DAC, or OR-2100. The relative expression is shown as the fold change relative to the untreated cells after normalization to the expression of β-actin. (F) The expression of DNMT-1, β-tubulin, borealin (CDCA8), and survivin (BIRC5) proteins in RC cells and OCI-Ly18 cells that were treated with vehicle (Ctr) or the HMAs AZA, DAC, or OR-2100 was determined by western blotting. The representative images show that DAC and OR-2100 reduced the expression of the DNMT-1, borealin, and survivin proteins when compared with AZA. (G) STRING network analysis of the functional association between CDCA8 and BIRC5. INCENP and AURKB, parts of the CPC together with CDCA8 and BIRC5, show mutual protein association. Red, positive regulation of mitotic cytokinesis; green, mitotic spindle assembly checkpoint signaling; blue, SGO1. (H) A schematic image of the CPC. Ctr, control; DNMT-1 DNA methyltransferase inhibitor 1. Created with BioRender.com.
CDCA8 and BIRC5 are downregulated in DHL cells after treatment with OR-2100. GO analysis of the genes that were downregulated by OR2100 monotherapy (A) or AZA monotherapy (B). (C) Volcano plot showing the DEGs between the Ctr group and OR-2100–treated samples. |log2 fold change| > 2; adjusted P <.05. Red indicates the upregulated genes, and blue indicates the downregulated genes. CDCA8, BIRC5, INCENP, and AURKB were identified as significantly downregulated genes. (D) Histograms of the percentage CpG methylation of RC cells and OCI-Ly18 cells after treatment with vehicle (Ctr) or OR2100. (E) The relative expression of CDCA8 and BIRC5 was determined by real-time PCR after treatment with the vehicle (Ctr), DAC, or OR-2100. The relative expression is shown as the fold change relative to the untreated cells after normalization to the expression of β-actin. (F) The expression of DNMT-1, β-tubulin, borealin (CDCA8), and survivin (BIRC5) proteins in RC cells and OCI-Ly18 cells that were treated with vehicle (Ctr) or the HMAs AZA, DAC, or OR-2100 was determined by western blotting. The representative images show that DAC and OR-2100 reduced the expression of the DNMT-1, borealin, and survivin proteins when compared with AZA. (G) STRING network analysis of the functional association between CDCA8 and BIRC5. INCENP and AURKB, parts of the CPC together with CDCA8 and BIRC5, show mutual protein association. Red, positive regulation of mitotic cytokinesis; green, mitotic spindle assembly checkpoint signaling; blue, SGO1. (H) A schematic image of the CPC. Ctr, control; DNMT-1 DNA methyltransferase inhibitor 1. Created with BioRender.com.
We explored RRBS and revealed that whole genomic demethylation was induced in both RC and OCI-Ly18 cells by OR-2100 (Figure 2D). Common significantly demethylated CpG regions were at intron regions of FMN1 and an exon region of COX1(MT-CO1), both of which are not relevant to mitosis or cell cycle regulation (supplemental Table 2). The upregulation of SMAD1 via demethylation of promoter lesions forms part of the mechanism through which HMAs exert anti-DLBCL activity.12,13 An evaluation of the LINE-1 methylation levels revealed a picture representative of global genomic DNA methylation. AZA, DAC, and OR-2100 significantly reduced methylation of LINE-1 in RC cells (AZA, 60.5% [P < .0001]; DAC, 67.2% [P < .0001]; OR-2100, 63.9% [P < .0001]; supplemental Figure 3A). Despite the methylation levels, the gene expression levels of SMAD1, which is involved in the antilymphoma effects through DNA demethylation, did not change following treatment with AZA, DAC, or OR-2100 (supplemental Figure 3B-C). The observed changes in the DNA methylation status and gene expression related to the antilymphoma effect did not demonstrate a consistent pattern, suggesting that the antilymphoma mechanism of OR-2100 might not be primarily attributed to its demethylation effect. The transcription of CDCA8 and BIRC5 in RC cells, as measured by quantitative real-time PCR, decreased significantly after DAC or OR-2100 treatment (DAC, P = .0377 and P = .0004; OR-2100, P = .0054 and P = .0007, respectively), as well as in OCI-Ly18 cells (DAC, P = .0111 and P = .0351; OR-2100:, P = .0023 and P = .0176, respectively), whereas the transcription of CDCA8 and BIRC5 in SU-DHL-6 cells was not decreased (Figure 2E; supplemental Figure 4A-B). The protein expression levels of borealin and survivin also decreased following OR2100 treatment, indicating that OR-2100 might downregulate mitosis-related genes in DHL (Figure 2F). Furthermore, the analysis of the publicly available data set for DLBCL (GSE31312) revealed that patients with higher expression levels of CDCA8 messenger RNA had significantly worse event-free survival than those with lower expression (P = .024; supplemental Figure 5A). Higher expression levels of BIRC5 messenger RNA also translated to significantly worse event-free survival than lower expression levels (P = .0027; supplemental Figure 5B). This data set included patients who were MYC high or BCL2 high, both of which are associated with poor progression-free survival (supplemental Figure 5C-D), suggesting that it likely includes patients with DHL. Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) network analysis revealed a functional association between CDCA8 and BIRC5, along with INCENP and AURKB (Figure 2G). These 4 proteins together constitute the CPC, which plays a critical role in ensuring accurate chromosomal segregation (Figure 2H).
Targeting of the mitosis-related genes CDCA8 and BIRC5 might be a crucial antitumor role of OR-2100 in DHL
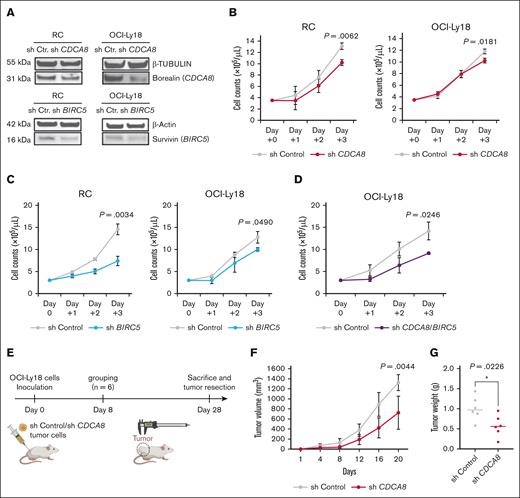
Next, we investigated the biological impact of CDCA8 and BIRC5 on DHL. Knockdown of CDCA8 reduced the proliferation of both RC and OCI-Ly18 cells in vitro (RC, 10.2 × 105/mL vs 13.1 × 105/mL [P = .0062]; OCI-Ly18, 10.2 × 105/mL vs 11.8 × 105/mL [P = .0181], respectively; Figure 3A-B). Similarly, knockdown of BIRC5 inhibited proliferation of both RC and OCI-Ly18 cells in vitro (RC, 7.4 × 105/mL vs 14.6 × 105/mL [P = .0034]; OCI-Ly18, 10.0 × 105/mL vs 12.8 × 105/mL [P = .0490]; Figure 3C). Simultaneous knockdown of CDCA8 and BIRC5 led to a complete loss of cell proliferation in RC cells and lethality, indicating that these 2 molecules are essential for cell survival. Double knockdown of CDCA8 and BIRC5 additively inhibited proliferation of both RC and OCI-Ly18 cells (Figure 3D), indicating a potential interrelationship among these genes. Next, we used a xenograft model based on CDCA8-knockdown OCI-Ly18 cells (Figure 3E). Knockdown of CDCA8 reduced proliferation of OCI-Ly18 cells, which led to smaller tumor volumes and weights relative to the control tumors (724.4 mm3 vs 1323.2 mm3 [P = .0044]; and 0.573 g vs 1.008 g [P = .0226]; Figure 3F-G). These results suggest that CDCA8 and BIRC5 play a critical role in proliferation in DHL. Notably, the publicly available data set of AML/MDS that we reported previously (GSE141677) showed that the mitosis-related genes CDCA8 and BIRC5 are downregulated after OR-2100 treatment, similar to the findings in DHL cells (supplemental Figure 6A-B). The induction of mitotic perturbation by altering the expression of mitosis-related genes via HMAs is associated with the antitumor mechanism in AML/MDS.9 Taken together, these findings suggested that mitotic perturbations might be a crucial antitumor mechanism of HMAs.
Functional analysis of CDCA8 or BIRC5 in DHL cell lines in vitro and in vivo. (A) RC cells and OCI-Ly18 cells were transduced with shRNA targeting CDCA8 and BIRC5, respectively. A reduction in the expression of borealin (CDCA8) and survivin (BIRC5) was confirmed by immunoblotting. (B) Cell counts for the shCtr cells and shCDCA8 cells. Red lines indicate the cells infected with lentiviral vectors that lacked CDCA8. Gray lines indicate the cells infected with empty vectors. (C) Cell counts for the shCtr cells and shBIRC5 cells. Blue lines indicate the cells infected with lentiviral vectors lacking BIRC5. Gray lines indicate the cells infected with empty vectors. (D) A comparison of the cell counts after knockdown of both CDCA8 and BIRC5 with Ctr (gray, Ctr; purple, shCDCA8 and BIRC5). (E) Experimental schema for the OCI-Ly18 cell xenograft mouse model used to evaluate CDCA8 knockdown. Volume (F) or weight (G) of xenografts formed by OCI-Ly18 cells infected with empty vector (shCtr; gray line) or shCDCA8 (red line). cont, control.
Functional analysis of CDCA8 or BIRC5 in DHL cell lines in vitro and in vivo. (A) RC cells and OCI-Ly18 cells were transduced with shRNA targeting CDCA8 and BIRC5, respectively. A reduction in the expression of borealin (CDCA8) and survivin (BIRC5) was confirmed by immunoblotting. (B) Cell counts for the shCtr cells and shCDCA8 cells. Red lines indicate the cells infected with lentiviral vectors that lacked CDCA8. Gray lines indicate the cells infected with empty vectors. (C) Cell counts for the shCtr cells and shBIRC5 cells. Blue lines indicate the cells infected with lentiviral vectors lacking BIRC5. Gray lines indicate the cells infected with empty vectors. (D) A comparison of the cell counts after knockdown of both CDCA8 and BIRC5 with Ctr (gray, Ctr; purple, shCDCA8 and BIRC5). (E) Experimental schema for the OCI-Ly18 cell xenograft mouse model used to evaluate CDCA8 knockdown. Volume (F) or weight (G) of xenografts formed by OCI-Ly18 cells infected with empty vector (shCtr; gray line) or shCDCA8 (red line). cont, control.
OR-2100 induces mitotic perturbation via the E2F1 signaling pathway, and this is dependent on the TP53 mutational status
Next, we investigated whether mitotic perturbation may be involved in the antitumor mechanism of OR-2100 in the context of DHL.9 Mitotic perturbation induces irregularly separated chromosomes, such as multipolar spindles (Figure 4A). Observations under a fluorescence microscope revealed that knockdown of CDCA8 induced mitotic abnormalities, as did treatment with OR-2100 (Figure 4B). These multipolar spindles were significantly increased by OR-2100 in RC and OCI-Ly18 cells (0% vs 3.7% [P = .004]; 0% vs 2.7% [P = .0013], respectively), whereas there was no increase in SU-DHL-10 and SU-DHL-6 cells that harbored a TP53 mutation (Figure 4C). Flow cytometry analysis revealed that the percentage of OCI-Ly18 cells with a >4N karyotype was significantly higher after OR-2100 treatment than after AZA treatment (14.2% vs 8.5%, respectively), whereas the proportion of SU-DHL-6 cells with a >4N karyotype increased modestly (Figure 4D). In addition, the percentage of TP53 wild-type RC cells with a 4N karyotype increased, whereas that of the TP53 mutated SU-DHL-10 cells did not (supplemental Figure 7A-B), suggesting that mitotic perturbation is dependent on the TP53 mutational status. The percentage of cells with 4N karyotypes increased in a dose-dependent manner and was proportional to the increase in apoptotic cells, indicating that apoptosis is linked to mitotic abnormalities (supplemental Figure 8A-B).
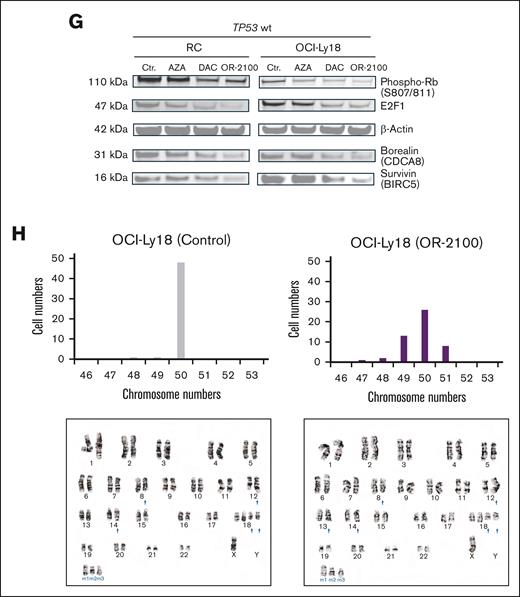
OR-2100 downregulates mitosis-related molecules via an E2F-mediated pathway, leading to mitotic perturbation and chromosomal aberration. (A) Schematic image of the cell cycle and multipolar spindles. (B) Representative images of OCI-Ly18 cells (left, shCtr; middle, shCDCA8; right, OR-2100-treated). Immunofluorescence images were captured at the original magnification ×100; α-tubulin (green) and DAPI (blue). (C) The quantification of aberrant spindles was conducted using IHC; the percentage of aberrant spindles were significantly increased among TP53 wt cells. (D) Flow cytometry analysis was performed to detect the percentage of cells with a >4N karyotype. The percentage of abnormal OCI-Ly18 cells that harbored a >4N karyotype was significantly increased by OR-2100 treatment when compared with AZA treatment, whereas that of SU-DHL-6 cells was increased modestly (gray, Ctr; red, AZA; purple, OR-2100). (E) The expression of β-tubulin, borealin (CDCA8), and survivin (BIRC5) in OCI-Ly18 cells and SU-DHL-6 cells treated with HLM006474 (an E2F inhibitor), as measured by western blotting. The expression of borealin and survivin protein decreased in OCI-Ly18 cells but not in SU-DHL-6 cells. (F) The cell viability assays conducted for RC cells and OCI-Ly18 cells treated with HLM006474. HLM006474 inhibited the growth of RC cells and OCI-Ly18 cells. (G) The phosphorylation of Rb (S807/811), E2F1, β-actin, borealin (CDCA8), and survivin (BIRC5) in RC cells and OCI-Ly18 cells treated with HMAs (AZA, DAC, and OR-2100) was measured by western blotting. Representative images show that the expression of phosphorylated Rb (S807/811), E2F1, borealin, and survivin decreased in the presence of DAC and OR-2100 to a greater extent than in the presence of AZA. (H) Chromosomal analysis of the OCI-Ly18 cells. (gray, Ctr; purple, OR-2100 treated). Cell and chromosome numbers are shown with the images of G-banding analysis (left, Ctr; right, OR-2100 treated). E2Fi, E2F1 inhibitor; Mut, mutation; ns, not significant; Phospho-Rb, phosphorylated retinablastoma protein; wt, wild type. Panel A created with BioRender.com.
OR-2100 downregulates mitosis-related molecules via an E2F-mediated pathway, leading to mitotic perturbation and chromosomal aberration. (A) Schematic image of the cell cycle and multipolar spindles. (B) Representative images of OCI-Ly18 cells (left, shCtr; middle, shCDCA8; right, OR-2100-treated). Immunofluorescence images were captured at the original magnification ×100; α-tubulin (green) and DAPI (blue). (C) The quantification of aberrant spindles was conducted using IHC; the percentage of aberrant spindles were significantly increased among TP53 wt cells. (D) Flow cytometry analysis was performed to detect the percentage of cells with a >4N karyotype. The percentage of abnormal OCI-Ly18 cells that harbored a >4N karyotype was significantly increased by OR-2100 treatment when compared with AZA treatment, whereas that of SU-DHL-6 cells was increased modestly (gray, Ctr; red, AZA; purple, OR-2100). (E) The expression of β-tubulin, borealin (CDCA8), and survivin (BIRC5) in OCI-Ly18 cells and SU-DHL-6 cells treated with HLM006474 (an E2F inhibitor), as measured by western blotting. The expression of borealin and survivin protein decreased in OCI-Ly18 cells but not in SU-DHL-6 cells. (F) The cell viability assays conducted for RC cells and OCI-Ly18 cells treated with HLM006474. HLM006474 inhibited the growth of RC cells and OCI-Ly18 cells. (G) The phosphorylation of Rb (S807/811), E2F1, β-actin, borealin (CDCA8), and survivin (BIRC5) in RC cells and OCI-Ly18 cells treated with HMAs (AZA, DAC, and OR-2100) was measured by western blotting. Representative images show that the expression of phosphorylated Rb (S807/811), E2F1, borealin, and survivin decreased in the presence of DAC and OR-2100 to a greater extent than in the presence of AZA. (H) Chromosomal analysis of the OCI-Ly18 cells. (gray, Ctr; purple, OR-2100 treated). Cell and chromosome numbers are shown with the images of G-banding analysis (left, Ctr; right, OR-2100 treated). E2Fi, E2F1 inhibitor; Mut, mutation; ns, not significant; Phospho-Rb, phosphorylated retinablastoma protein; wt, wild type. Panel A created with BioRender.com.
These results suggest that OR-2100 induces nuclear aneuploidy in DHL cells by downregulating CDCA8 to a greater extent than AZA. Mitotic perturbation was induced to a greater extent in TP53 wild-type cells that were more sensitive to OR-2100. CDCA8 and BIRC5, which are clustered genes, function downstream of the p53-p21-DREAM-E2F/CHR axis (ie, the p53-DREAM pathway) in which their expression levels are tightly regulated to ensure proper cell division and to prevent uncontrolled proliferation.29,30 HLM006474 decreased the protein expression levels of borealin and survivin proteins in OCI-Ly18 cells (TP53 wild-type) but not in SU-DHL-6 (TP53-mutated) cells (Figure 4E). HLM006474 showed antiproliferative effects in TP53 wild-type cells (RC and OCI-Ly18 cells) that were highly sensitive to OR-2100 (Figure 4F). The phosphorylation of Retinoblastoma protein (Rb) (S807/811) and E2F1 was downregulated by DAC or OR-2100, corresponding with the downregulation of borealin and survivin (Figure 4G). These results suggest that the mitotic perturbation that underlies the anti-DHL effects of OR-2100 might be dependent on the p53-E2F1–mediated signaling pathway.
Chromosomal analysis using the G-banding technique revealed that knockdown of CDCA8 or treatment with OR-2100 induced chromosomal aberrations. The number of abnormal chromosomes was higher in OR-2100–treated cells than in control cells (Figure 4H), indicating that shCDCA8 and OR-2100 may induce mitotic aberrations. Notably, an abnormality, add(13)(q14), which involves the RB1-encoding region, was observed in OR-2100–treated cells but was absent in control cells.
The efficacy of OR-2100 and the associated suppression of mitosis-related gene expression are governed by the TP53 mutational status
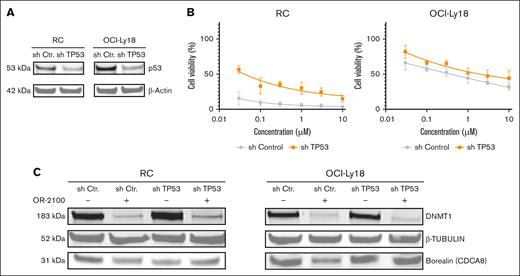
To investigate whether the anti-DHL efficacy of OR-2100 was associated with the TP53 mutational status, we conducted TP53 knockdown experiments in vitro (Figure 5A). OR-2100 effectively inhibited cell growth in shControl RC and OCI-Ly18 cells; however, this inhibitory effect was attenuated in TP53-knockdown RC and OCI-Ly18 cells (Figure 5B). Consistently, immunoblotting showed that the protein levels of borealin was reduced by OR-2100 in shControl RC and OCI-Ly18 cells but not in shTP53 cells (Figure 5C). These findings suggest that both the sensitivity to OR-2100 and the downregulation of mitosis-related genes are dependent on functional TP53.
Evaluation of the TP53-dependent mechanism of OR-2100. (A) RC and OCI-Ly18 cells were transduced with shRNA that targeted TP53, and the reduction in TP53 expression was confirmed by immunoblotting. (B) The cells were treated with OR-2100 for 72 hours, followed by assessment of the cell viability. (C) The protein expression levels of β-tubulin and borealin (CDCA8) in RC and OCI-Ly18 cells treated with OR-2100 was assessed by western blotting. The representative images demonstrate that the protein levels of borealin was more profoundly downregulated in shCtr cells than in shTP53 cells.
Evaluation of the TP53-dependent mechanism of OR-2100. (A) RC and OCI-Ly18 cells were transduced with shRNA that targeted TP53, and the reduction in TP53 expression was confirmed by immunoblotting. (B) The cells were treated with OR-2100 for 72 hours, followed by assessment of the cell viability. (C) The protein expression levels of β-tubulin and borealin (CDCA8) in RC and OCI-Ly18 cells treated with OR-2100 was assessed by western blotting. The representative images demonstrate that the protein levels of borealin was more profoundly downregulated in shCtr cells than in shTP53 cells.
OR-2100 with/without CHOP inhibits the growth of DHL tumors in a xenograft model
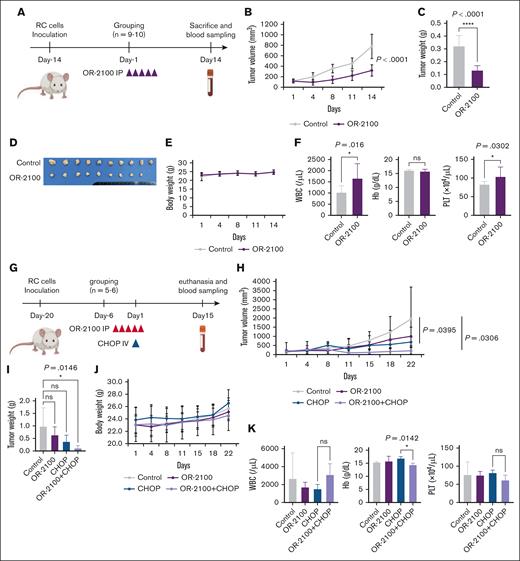
Finally, to confirm the anti-DHL effect in vivo, we performed OR-2100 monotherapy using immunodeficient mice. RC cells were inoculated subcutaneously into BRJ double-deficient mice (Figure 6A). OR-2100 monotherapy significantly decreased the tumor size (320.0 vs 778.1 mm3; P < .0001) without body weight loss (Figure 6B-E). Apparent myelosuppression was not observed (Figure 6F), indicating that OR2100 monotherapy was effective and well tolerated.
Xenograft experiment to test the OR-2100 plus CHOP regimen. (A) Experimental schema for the RC cell xenograft mouse model. BRJ double-deficient mice were inoculated subcutaneously with 5 × 106 RC cells and then treated with vehicle (n = 10), OR-2100 (3.39 mg/kg; n = 9), as indicated by the red arrowheads. (B) The mean tumor volume was measured twice per week, starting on day 1. Ctr, gray; OR-2100, purple. (C) The mean tumor weight in each treatment group. Differences between the vehicle and each treatment group were evaluated using Dunnett test. (D) Image of the resected tumors in each treatment group. (E) Mean body weight of mice. No body weight loss was noted in any of the groups Ctr, gray; OR-2100, purple. (F) The concentration of Hb and complete blood counts, including WBC and PLT counts, in mice euthanized at 14 days after the first treatment. (G) Experimental schema for the RC cell xenograft mouse model. BRJ double-deficient mice were inoculated subcutaneously with 5 × 106 RC cells and then treated with vehicle (n = 5), OR-2100 (3.39 mg/kg; n = 6), CHOP (n = 5), or OR-2100 + CHOP (n = 6), as indicated by the red and blue arrowheads. (H) The mean tumor volume was measured twice per week, starting on day 1. Ctr, gray; OR-2100: purple; CHOP: blue; OR-2100 + CHOP, lavender. (I) The mean tumor weight in each treatment group. Differences between the vehicle and each treatment group were tested using Dunnett’s test. ns, not significant. (J) The mean body weight of mice. No body weight loss was noted in any of the 4 groups. Ctr, gray; OR-2100, purple; CHOP, blue; OR-2100 + CHOP, lavender. (K) The concentration of Hb and complete blood counts, including WBC and PLT counts, in mice sacrificed at 22 days after the first treatment. The differences between the CHOP and OR-2100 + CHOP group were tested using Dunnett test. Hb, hemoglobin; ns, not significant; PLT, platelet; WBC, white blood cell.
Xenograft experiment to test the OR-2100 plus CHOP regimen. (A) Experimental schema for the RC cell xenograft mouse model. BRJ double-deficient mice were inoculated subcutaneously with 5 × 106 RC cells and then treated with vehicle (n = 10), OR-2100 (3.39 mg/kg; n = 9), as indicated by the red arrowheads. (B) The mean tumor volume was measured twice per week, starting on day 1. Ctr, gray; OR-2100, purple. (C) The mean tumor weight in each treatment group. Differences between the vehicle and each treatment group were evaluated using Dunnett test. (D) Image of the resected tumors in each treatment group. (E) Mean body weight of mice. No body weight loss was noted in any of the groups Ctr, gray; OR-2100, purple. (F) The concentration of Hb and complete blood counts, including WBC and PLT counts, in mice euthanized at 14 days after the first treatment. (G) Experimental schema for the RC cell xenograft mouse model. BRJ double-deficient mice were inoculated subcutaneously with 5 × 106 RC cells and then treated with vehicle (n = 5), OR-2100 (3.39 mg/kg; n = 6), CHOP (n = 5), or OR-2100 + CHOP (n = 6), as indicated by the red and blue arrowheads. (H) The mean tumor volume was measured twice per week, starting on day 1. Ctr, gray; OR-2100: purple; CHOP: blue; OR-2100 + CHOP, lavender. (I) The mean tumor weight in each treatment group. Differences between the vehicle and each treatment group were tested using Dunnett’s test. ns, not significant. (J) The mean body weight of mice. No body weight loss was noted in any of the 4 groups. Ctr, gray; OR-2100, purple; CHOP, blue; OR-2100 + CHOP, lavender. (K) The concentration of Hb and complete blood counts, including WBC and PLT counts, in mice sacrificed at 22 days after the first treatment. The differences between the CHOP and OR-2100 + CHOP group were tested using Dunnett test. Hb, hemoglobin; ns, not significant; PLT, platelet; WBC, white blood cell.
Next, we evaluated the combined effects of CHOP and OR-2100 in a xenograft model based on RC cells. (Figure 6G). On day 14 after inoculation, the tumor volume had increased to approximately 150 mm3, after which the mice were injected with OR-2100 or vehicle. OR-2100 monotherapy inhibited the growth of tumor cells to a greater extent than that of vehicle treatment, but CHOP or OR-2100 plus CHOP inhibited the tumor growth more markedly (Figure 6H). Notably, tumor weight was significantly lower in the OR-2100 plus CHOP group than in the control group (P = .0146; Figure 6I). Regarding the toxicity of the combination therapy, the OR-2100 plus CHOP–treated group exhibited lower hemoglobin levels than the CHOP-treated group (14.3 vs 16.8 g/dL, respectively; P = .0142). Meanwhile, no body weight loss was noted in any of the 4 groups (Figure 6J), and there were no significant changes in the white blood cell or platelet counts (Figure 6K), suggesting that the combination of OR-2100 and CHOP is an effective and feasible treatment for DHL.
Discussion
In this study, we demonstrated the antitumor (ie, DHL) activity of OR-2100 and its mechanism of action. OR-2100 inhibited the growth of DHL tumor cells when used as monotherapy in vitro, particularly that of cell lines without TP53 mutations; however, when used in combination with doxorubicin or vincristine, it inhibited the growth of both TP53-mutated and nonmutated cells. Notably, OR-2100 demonstrated more potent efficacy than AZA against DHL cells when used at relatively low dose (<1 μM). Furthermore, OR-2100 plus CHOP inhibited tumor cell growth significantly and without severe toxicity in vivo. These data suggest that OR-2100 exerts antitumor effects against DHL, both in vitro and in vivo, and at a concentration deemed clinically achievable in real patients.
We found that OR-2100 significantly downregulated mitosis-related genes, such as CDCA8 and BIRC5. CDCA8 encodes borealin, which functions during cytokinesis, whereas BIRC5 encodes survivin, which is involved in centromere targeting. Because both CDCA8 and BIRC5 play crucial roles in the CPC to ensure proper chromosome segregation during mitosis in collaboration with INCENP and AURKB, the impairment of these mitosis-related molecules might lead to chromosomal instability and aneuploidy, which leads to cell death. The knockdown of CDCA8 or BIRC5 inhibited tumor cell growth, and knockdown of CDCA8 or treatment of OR-2100 triggered nuclear aneuploidy in DHL cells, suggesting that the antitumor mechanism of OR-2100 might involve mitotic perturbation via the downregulation of mitosis-related genes.
Conventionally, DNA HMAs exert antitumor effects through DNA demethylation and the reactivation of gene expression of silenced critical genes. However, as demonstrated by Goyama et al, decitabine may additionally induce antitumor effects through mitotic disruption in MDS/AML.9 Notably, the regions that underwent substantial DNA demethylation by OR-2100 were not directly implicated in mitotic perturbation.
The expression of mitosis-related genes in DHL is elevated in patients with lymphoma,29,31 and upregulation of CDCA8 and BIRC5 signifies a poor prognosis in patients with DLBCL, suggesting that they could be a therapeutic target for DHL. Although genomic instability may promote tumor progression among indolent tumors,32 our findings suggest that mitotic perturbation and subsequent mitotic catastrophe actually suppress the tumor growth in aggressive lymphomas, including DHL.33
The p53-Rb-E2F1 pathway is a tumor suppressor mechanism that regulates cell cycle arrest and maintains genomic stability. It functions by repressing transcription of genes involved in cell cycle progression and mitosis, thereby helping to prevent uncontrolled cell division and tumor development. CDCA8 and BIRC5 are downstream targets of the p53-DREAM pathway.29,30 In this study, we found that OR-2100 suppressed the upstream Rb-E2F1 pathway (decreasing phosphorylation of Rb and stabilizing E2F1), which, in turn, downregulated the downstream activity of CDCA8 and BIRC5. Because the Rb-E2F1 pathway is p53-dependent, TP53 wild-type DHL might be highly sensitive to OR-2100. Furthermore, DNA methyltransferase 1 is essential for cell survival and proliferation, mediated by the transcription factor c-MYC,34 which facilitates the binding of E2F1 protein to E2F gene promoters.35 These results suggest that the antitumor mechanism of OR-2100 against DHL might be mediated via the transcriptional c-MYC and p53-Rb-E2F1 pathway and that OR-2100 might be a promising treatment for DHL, especially in TP53 wild-type cases. OR-2100 that induces mitotic perturbation via the downregulation of highly expressed c-MYC in DHL could represent a novel therapeutic strategy for patients with this challenging disease.
A TP53 mutation occurs in 10% to 20% of DLBCL cases and confers resistance to immunochemotherapy. The combination of OR-2100 with doxorubicin or vincristine also showed efficacy against TP53 mutated DHLs, suggesting that OR-2100 may have antitumor efficacy against TP53-mutated DHL, possibly associated with transcriptional immune activation.36 We also confirmed that OR-2100 upregulated IRF7, an inducer of interferon responses, in TP53-mutated DHL cells to a greater extent than in than TP53 wild-type cells, particularly when compared with AZA (supplemental Figure 9A-B).
The prognostic impact of DHL is poor37,38; thus, an intensive chemoimmunotherapy regimen, such as dose-adjusted EPOCH-R, is the recommended treatment.5 However, even DA-EPOCH-R can be insufficient, and some patients are unable to tolerate intensive chemotherapy.38 Recently, a molecularly high-risk group, namely those with the double-hit signature, was also found to have a poor prognosis, similar to DHL.37,39
This study has several limitations. First, all experiments were based on cell lines, and the results were not validated using primary samples from patients. Although OR-2100 demonstrated striking efficacy in vitro, its effect was more modest in vivo, particularly in combination with CHOP. This discrepancy may be attributed to several unknown in vivo cofactors. Moreover, given that CHOP is a highly effective and potentially curative regimen for DLBCL, the additive benefit of OR-2100 may have been obscured. Second, the antitumor mechanism of OR-2100 against TP53-mutated cells when combined with doxorubicin or vincristine was not elucidated. Third, the safety and efficacy of OR-2100 plus CHOP remains uncertain in humans.
To summarize, we demonstrated that OR-2100 exerts potent antitumor activity against DHL via the p53-DREAM pathway. Mitotic perturbation might be a key antitumor mechanism through which OR-2100 inhibits DHL. OR-2100 might be of clinical benefit as a treatment for DHL, particularly in combination with CHOP.
Acknowledgments
Flow cytometry analyses, cell sorting, and pyrosequencing were conducted at the Analytical Research Center for Experimental Sciences, Saga University. The authors thank Tatsuro Watanabe (Meiji Seika Pharma Co, Ltd, Tokyo, Japan) for his assistance throughout the study.
This work was partially supported by Ohara Pharmaceutical Co, Ltd and partially by research grants from the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (21K16245 [H.U.]) and the Takeda Science Foundation (H.U.).
Authorship
Contribution: K.K. was responsible for conceptualization, data curation, the use of software, formal analysis, validation, investigation, methodology, writing the original draft, project administration, and review and editing of the manuscript; H.U. was responsible for conceptualization, data curation, the use of software, formal analysis, funding acquisition, validation, investigation, methodology, writing the original draft, project administration, and review and editing; Y.Y. and R.Y. were responsible for data curation and investigation; Y.F.-K. was responsible for data curation and supervision; Y.K. was responsible for data curation, supervision, and review and editing of the manuscript; Y.M., S.A., K.N., and H.M. were responsible for methodology, data curation, and investigation; K.O. and A.K. were responsible for methodology, data curation, investigation, and supervision; and S.K. was responsible for supervision, funding acquisition, writing the original draft, and review and editing of the manuscript.
Conflict-of-interest disclosure: H.U. reports receiving honoraria from Novartis. Y.K. and Y.F.-K. reports being full-time employees of Ohara Pharmaceutical Co, Ltd. K.O. reports receiving honoraria and research funding from Chugai Pharmaceutical Co, Ltd and Takeda Pharmaceutical Co, Ltd. S.K. reports receiving honoraria from Pfizer, Otsuka Pharmaceuticals, Novartis, and Bristol Myers-Squibb; and receiving research funding from Pfizer, Bristol Myers-Squibb, and Ohara Pharmaceutical Co, Ltd. The remaining authors declare no competing financial interests.
Correspondence: Hiroshi Ureshino, Department of Drug Discovery and Biomedical Sciences, Faculty of Medicine, Saga University, 5-1-1, Nabeshima, Saga city, Saga 849-8501, Japan; email: sr0795@cc.saga-u.ac.jp; and Shinya Kimura, Division of Hematology, Respiratory Medicine and Oncology, Department of Internal Medicine, Faculty of Medicine, Saga University, 5-1-1, Nabeshima, Saga city, Saga 849-8501, Japan; email: shkimu@cc.saga-u.ac.jp.
References
Author notes
RNA-sequencing data and comprehensive methylation data are available from the DNA Data Bank of Japan (accession numbers PRJDB 19646 and PRJDB 20401, respectively).
All data can be accessed by contacting the corresponding author, Hiroshi Ureshino (sr0795@cc.saga-u.ac.jp).
The full-text version of this article contains a data supplement.