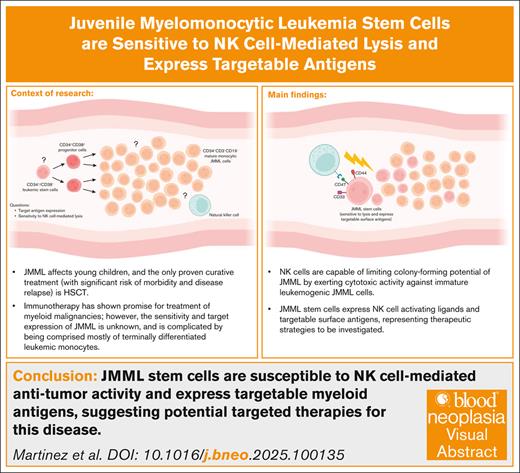
Key Points
JMML comprises distinct subpopulations, and the regenerating leukemic stem cell population represents an important therapeutic target.
NK cells have the ability to eliminate leukemic stem cells, and leukemia stem cells express antigens that may be targeted via cell therapy.
Visual Abstract
Juvenile myelomonocytic leukemia (JMML) is a myeloproliferative neoplasm for which hematopoietic stem cell transplantation is the only curative treatment. Innovative therapies are needed to address high rates of morbidity, treatment-related mortality, and relapse. Monoclonal antibodies and adoptive cell transfer of natural killer (NK) cells or T cells are approved or being investigated for other myeloid leukemias; however, their activity against JMML warrants further investigation. In this study, we hypothesized that NK cells may effectively target JMML tumor cells. Mass cytometry was used to evaluate the expression of NK cell–activating and –inhibitory ligands and candidate target antigens on the monocytic and stem cell subsets of JMML. Monocytes from healthy donors and monocytic subsets of JMML were similar in NK cell ligand expression, and JMML monocytes were resistant to NK cell cytotoxicity in vitro. However, NK cells effectively controlled proliferation of JMML colony-forming cells. CD34+CD38– JMML stem cells express a broad repertoire of NK cell ligands similar to that of acute myeloid leukemia stem cells; and CD33, CD44, and CD47 were expressed by both CD34+CD38– stem cells and CD34+CD38+ progenitors in JMML. This suggests that JMML may be responsive to NK cell–mediated activity, and that targeting CD33, CD44, or CD47 may facilitate eradication of JMML.
Introduction
Juvenile myelomonocytic leukemia (JMML) is a clonal mixed myelodysplastic/myeloproliferative disorder of young children that arises from pluripotent hematopoietic stem cells (HSCs).1-5 Tumorigenesis is driven by mutations in RAS or RAS regulatory components such as NF1, PTPN11, or CBL that interfere with downstream regulation of the RAS signaling pathway.5-9 Allogeneic HSC transplant (HSCT) is the only proven curative therapy for JMML; however, 5-year overall survival after transplant is only 52% to 64%, with high rates of leukemia relapse and graft failure.6,10
Allogeneic HSCT is an important therapeutic modality for most myeloid malignancies, wherein donor-derived immune cells elicit a potent antitumor effect.11 Natural killer (NK) cells are innate lymphoid cells that serve as a critical component of first-line immunity, providing host defense against pathogens and mediating immune surveillance and antitumor activity.12,13 Importantly, NK cells contribute to the graft-versus-leukemia (GVL) effect without directly inducing graft-versus-host disease.14 Moreover, donors with killer immunoglobulin-like receptors (KIRs) licensed to HLA ligands that are absent in the recipient (referred to as KIR mismatch) are associated with more effective GVL activity, higher engraftment rates, and reduced graft-versus-host disease.15
Recently, the transplant literature has supported an immunologic role in the treatment of JMML.16-19 Because NK cells have a natural ability to differentially target tumor cells and spare healthy cells, they are attractive candidates for cancer immunotherapy in this clinical setting,20,21 and the adoptive transfer of ex vivo–expanded haploidentical NK cells is safe and can induce remission in patients with acute myeloid leukemia (AML).19,22-24 Although we demonstrated a lack of influence by KIR-related factors in HSCT for JMML,25 the efficacy of NK cell activity against JMML has not been reported.
Leukemia stem cells (LSCs) represent a self-renewing pool of cells that can become quiescent and escape chemotherapy and therefore serve as the source of leukemia recurrence. Particularly in myeloproliferative diseases that maintain a spectrum of differentiated cell subsets, the LSC subset must be targeted in order for treatment to be effective. Identifying LSC-specific antigens may lead to new approaches for targeted therapy.26,27 Previous studies have shown the cytolytic potential of NK cells against LSCs in AML28; the LSCs in JMML have only recently been characterized.29
NK cell activity is regulated by a series of signals relayed from activating and inhibitory receptors engaged through target cell ligand binding.30 The primary inhibitory NK cell receptors include KIRs and natural killer cell protein group 2A (NKG2A) that recognize self in the form of the major histocompatibility complex, including HLA-E. Many tumor cells downregulate major histocompatibility complex class I expression to escape recognition by cytotoxic T cells, although conversely, this reduces inhibitory signaling and renders tumor cells susceptible to NK cell recognition.31-34 Natural cytotoxicity receptors (NKp30, NKp44, NKp46, and NKp80), NKG2D, and DNAX accessory molecule 1 (DNAM-1) are the primary activating receptors expressed by human NK cells.35,36 Furthermore, NK cell killing may be activated through antibody (Ab)-dependent cell-mediated cytotoxicity, which is propagated through the low-affinity immunoglobulin γ Fc region receptor III-A (FcγRIIIA [CD16a]) that may be engaged by the Fc portion of immunoglobulin G (IgG) Abs bound to specific antigens on target cells.37,38 NK cell function is essential for effective cancer treatment with Abs targeting tumor-associated antigens.39,40
The goal of this study was to determine whether NK cells might have a direct role in JMML antitumor activity, and whether tumor-associated antigens identified for targeted AML therapies may be used in the treatment of JMML. We investigated these aspects of JMML LSCs using high-dimension phenotyping, cytotoxicity assays, and colony-forming assays to determine ligand expression, NK cell cytotoxic effects, and targetable antigens.
Methods
Cells and cell lines
Spleens were obtained from children with JMML undergoing splenectomy as part of their routine clinical care after informed consent under an institutional review board (IRB)–approved protocol at the University of Arkansas for Medical Sciences (n = 11 separate clinical samples). The patient-derived AML xenograft cell line used in experimentation for this study was isolated from an 8-year-old patient at diagnosis; AML M1, cytogenetics 47, XX, t(1;19)(p22;q13), del(2)(q33), del(3)(q21), +I(5)(p10), add(6)(p23); immunophenotype CD4 (58%), HLA-DR, CD13, CD33, CD11b (31%). Mononuclear cells (MNCs) were isolated by density gradient centrifugation. The parental K562 cell line was obtained from the American Type Culture Collection. Clone9.mbIL21 was derived as previously described.41,42 Cells were cultured in RPMI 1640 media (CellGro/Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (Life Technologies, Carlsbad, CA), 1× GlutaMAX (Gibco/Life Technologies, Carlsbad, CA) and 1× penicillin/streptomycin (CellGro/Mediatech), at 37°C in a 5% CO2 incubator.
Isolation and expansion of primary human NK cells
Buffy coats from healthy donors were obtained from Gulf Coast Regional Blood Center (Houston, TX). Exemption and waiver of consent for the research use of buffy coat fractions obtained from anonymized donors at Gulf Coast Regional Blood Center was granted by the IRB of The University of Texas MD Anderson Cancer Center under protocol PA13-0978. Anonymized cord blood units were obtained from the MD Anderson Cord Blood Bank after being deemed unacceptable for clinical use and released for research. NK cells were isolated using the RosetteSep Human NK cell enrichment cocktail (STEMCELL Technologies; resulting in consistently >95% pure CD56+ NK cell products) and expanded, as described previously, using K562 Clone9.mbIL21 as feeder cells for 21 days.42 Expanded NK cells were cryopreserved, and subsequently thawed and recovered for 1 to 2 days before their use. During recovery, NK cells were cultured in NK cell media consisting of RPMI 1640 (Corning) supplemented with 50 IU/mL recombinant human interleukin-2 (Proleukin, Novartis Vaccines and Diagnostics Inc), 20% fetal bovine serum (Thermo Fisher), L-glutamine (Gibco), and penicillin/streptomycin (Corning).
Flow cytometry
MNCs of healthy donors and patients with JMML were stained with CD14-Fluorescein isothiocyanate (FITC), CD33-Allophycocyanin (APC) and phycoerythrin (PE)-labeled anti-major histocompatibility complex class I polypeptide-related sequence A/B (MIC A/B), CD112, CD155, HLA-ABC, or HLA-E Abs (all mouse anti-human [m-a-h] IgG1), or isotype control Abs for 30 minutes at 4°C, then washed and resuspended in staining buffer. Data were acquired using a fluorescence-activated cell sorting cytometer (FACSCalibur, BD Biosciences) and analyzed using FlowJo software (Tree Star Inc, Ashland, OR) to gate live cells based on side and forward scatter and 7-aminoactinomycin D exclusion. The expanded NK cell subpopulations were determined by CD3– and CD16+ or CD56+ gating strategy.
Cytotoxicity assay
The fluorescence-based calcein release assay was used to assess cytotoxicity, as previously described.41,42 Target cells were labeled with 5 μg/mL of calcein-acetoxymethyl (Sigma-Aldrich) for 1 hour at 37°C with occasional shaking. NK cells were cocultured with target cells at different effector-to-target (E:T) ratios for 4 hours at 37°C. Supernatant fluorescence was determined at 485 nm/530 nm (excitation per emission) using the SpectraMax Plus384 spectrophotometer. The percent lysis was calculated according to the formula [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
CFU assay
Expanded NK cells from healthy donors were preincubated with JMML samples and their effect on growth of JMML colony-forming units (CFUs) was tested. JMML MNCs were incubated with NK cells at an E:T ratio of 1:2 for 4 hours and then seeded into MethoCult with growth factors. CFUs were counted on day 12 with an inverted microscope. Given the variability in CFU capacity of individual samples, data were normalized to CFU numbers obtained in untreated control plates.
NK cell–JMML splenocyte coculture
Cells were cocultured in serum-free expansion medium II (STEMCELL Technologies, Cambridge, MA) supplemented with StemSpan CD34+ expansion supplement (STEMCELL Technologies). A deidentified primary AML sample was obtained from The Ohio State University Leukemia Tissue Bank consistent with the Declaration of Helsinki, and healthy donor peripheral blood mononuclear cells (PBMCs) were isolated from a Red Cross buffy coat through Ficoll gradient centrifugation. Splenocyte samples from patients with JMML were obtained from the University of California, San Francisco under IRB protocol CC number 06151 (for the 2 patient samples used in this experiment, 1 harbored a CBL C384R mutation, whereas the other harbored a KRAS G13D mutation). Expanded NK cells were cocultured with either JMML splenocytes, AML cells, or PBMCs at a target-to-effector ratio of 1:2 for 4 hours. After culture, cells were collected and stained for cytometry by time-of-flight (CyTOF) analysis with heavy-metal–conjugated Abs to evaluate various immune cell subtypes, including NK cells, monocytes and myeloid progenitors, T cells, and B cells (supplemental Table 1).
Mass cytometry
Abs for mass cytometry were labeled with heavy metals using Maxpar-X8 labeling reagent kits (DVS Sciences) according to the manufacturer’s instructions and titrated for determination of optimal concentration. Because NK cell receptors may have multiple ligands, or unknown ligands, chimeric receptor–IgG-Fc fusion proteins were tagged with heavy metals and used for identification of ligands on JMML cells. Splenic cells (1.5 × 106) were stained for viability with 2.5 μM Cell-ID Cisplatin (Fluidigm, 201064) in serum-free RPMI 1640 for 1 minute and washed twice with complete media. Surface staining was performed as previously described.43 Cells were fixed with paraformaldehyde, stained with an iridium metallointercalator, and then measured on the CyTOF mass cytometer (DVS Sciences and Fluidigm/Standard BioTools). Cell events were acquired at an event rate of ∼200 to 400 events per second with internally calibrated dual count detection. Noise reduction was used, and cell extraction parameters were set as follows: cell length, 8 to 150; convolution threshold, 600; and dual count start, 1. During event collection, samples were suspended in water containing 1:20 Fluidigm EQ beads. These bead events were removed according to an algorithm developed by Finck et al, enabling correction of short- and long-term fluctuations in signal intensity.44
SPADE clustering analysis
Pooled data of 3 separately acquired patient samples were analyzed in spanning-tree progression analysis of density-normalized events (SPADE) simultaneously. For the monocyte population, a minimum spanning tree was constructed based on expression of CD33 and CD14. CD14+CD33+ nodes were identified as monocytes. Each SPADE tree node represents a separate cell subpopulation. Expression of each marker was obtained for each node of each sample within the gated population and divided by expression of the control of the same node to normalize the data. Each dot on the graph represents the normalized expression of the corresponding node. For the stem cell population, a minimum spanning tree was constructed based on expression of CD3, CD4, CD8a, CD11b, CD14, CD15, CD19, CD25, CD34, CD38, CD45, CD56, CD117, CD133, and CD164. Putative cell populations were annotated manually based on known coexpression of lineage markers. Nodes representing JMML hematopoietic stem/progenitor cells were identified by CD34, CD38, and the absence of lineage markers (Lin−), and expression of LSC-associated markers were analyzed. Each dot on the graph represents the average expression of the analyzed marker in the corresponding nodes. The dotted line is the threshold of expression.
Statistical analysis
Results are expressed as the mean ± standard deviation or mean ± standard error of the mean, as indicated. Student t tests were performed for pair-wise comparisons. Cytotoxicity across E:T ratios were compared between groups using 2-way repeated measures analysis of variance. Statistical analysis was performed using Prism 6 for Mac (GraphPad Software). P values are reported as <.05 (∗), <.01 (∗∗), <.001 (∗∗∗), or <.0001 (∗∗∗∗).
Results
Mature monocytic JMML cells express similar NK cell ligands compared with healthy donor monocytes and are relatively resistant to NK cell–mediated lysis
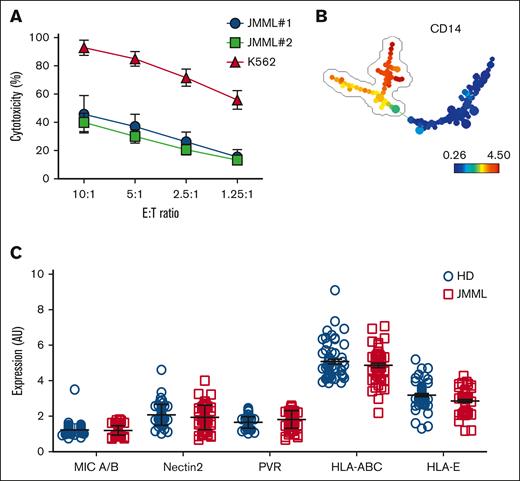
JMML cells and healthy donor MNCs were evaluated by mass cytometry for the expression of ligands for activating and inhibitory NK cell receptors. Cytotoxicity assays were performed to determine whether JMML monocytes are susceptible to NK cell–mediated lysis. Mature JMML monocytes were relatively resistant to NK cell–mediated cytotoxicity when compared to the NK cell–sensitive K562 cell line (Figure 1A). To evaluate common receptors mediating cytotoxicity, a minimum spanning tree was constructed based on forward and side scatter and expression of CD33 and CD14 (Figure 1B), and the CD14+ population was selected for further analysis. Major Histocompatibility Complex (MHC) class I chain-related protein A and B (MIC A/B) (ligands for the activating receptor NKG2D); Nectin2 and poliovirus receptor (PVR) (engaging both the activating receptor DNAM-1 and also the inhibitory receptor TIGIT); HLA-A, -B, -C, and -E expression levels on CD14+CD33+ monocytes from healthy donors and patients with JMML were not significantly different (Figure 1C).
The major population of mature monocytic JMML cells are similar to HD peripheral blood monocytes in NK cell ligand expression and are relatively resistant to NK cell lysis. (A) NK cells were cocultured with target cells at different E:T ratios (10:1, 5:1, 2.5:1, and 1.25:1). JMML cells (n = 2 patient samples) were used as targets in a standard cytotoxicity assay. K562 cells were used as a positive control for NK cell cytotoxicity. (B) PBMCs of 5 HDs and 5 patients with JMML were stained with CD14-FITC, CD33-APC, and PE-labeled anti-MIC A/B, CD112, CD155, HLA-ABC, or HLA-E Abs (all m-a-h IgG1), or isotype control, washed and analyzed immediately by flow cytometry. Data were acquired using a fluorescence-activated cell sorting (FACSCalibur) cytometer. Data were preanalyzed using FlowJo to gate live cells based on side and forward scatter and 7-aminoactinomycin D exclusion. Gated events were exported into a new FCS3 file, totaling 60 files. FCS3 files were uploaded into SPADE and analyzed collectively. A minimum spanning tree was constructed based on forward and side scatter and expression of CD33 and CD14. CD14+CD33+ nodes were identified as monocytes. (C) MIC A/B, Nectin2, PVR, HLA-ABC, and HLA-E expression levels on CD14+CD33+ monocytes were analyzed from HDs and patients with JMML. AU, arbitrary units; HD, healthy donor; MIC, Major histocompatibility complex class I-related chain A and B; PVR, poliovirus receptor.
The major population of mature monocytic JMML cells are similar to HD peripheral blood monocytes in NK cell ligand expression and are relatively resistant to NK cell lysis. (A) NK cells were cocultured with target cells at different E:T ratios (10:1, 5:1, 2.5:1, and 1.25:1). JMML cells (n = 2 patient samples) were used as targets in a standard cytotoxicity assay. K562 cells were used as a positive control for NK cell cytotoxicity. (B) PBMCs of 5 HDs and 5 patients with JMML were stained with CD14-FITC, CD33-APC, and PE-labeled anti-MIC A/B, CD112, CD155, HLA-ABC, or HLA-E Abs (all m-a-h IgG1), or isotype control, washed and analyzed immediately by flow cytometry. Data were acquired using a fluorescence-activated cell sorting (FACSCalibur) cytometer. Data were preanalyzed using FlowJo to gate live cells based on side and forward scatter and 7-aminoactinomycin D exclusion. Gated events were exported into a new FCS3 file, totaling 60 files. FCS3 files were uploaded into SPADE and analyzed collectively. A minimum spanning tree was constructed based on forward and side scatter and expression of CD33 and CD14. CD14+CD33+ nodes were identified as monocytes. (C) MIC A/B, Nectin2, PVR, HLA-ABC, and HLA-E expression levels on CD14+CD33+ monocytes were analyzed from HDs and patients with JMML. AU, arbitrary units; HD, healthy donor; MIC, Major histocompatibility complex class I-related chain A and B; PVR, poliovirus receptor.
Immature JMML cells are susceptible to lysis by NK cells
Because monocytic JMML cells represent a terminally differentiated bulk population and not the leukemogenic JMML population, we evaluated the effect of NK cells on the colony-forming properties of LSCs, comparing spleen MNCs from patients with JMML to cord blood MNCs. Incubation of JMML MNCs with NK cells at E:T ratios as low as 0.5:1 significantly reduced JMML CFUs by 74.4% ± 7.85% but did not have an effect on the CFU potential of allogeneic cord blood cells (Figure 2A). To evaluate NK cell activity against JMML subpopulations, healthy donor NK cells were cocultured for 48 hours with splenocytes from patients with JMML (or control AML cells or control healthy donor PBMCs), then collected and analyzed via CyTOF. Clustering analysis with SPADE was performed, and bubbles were drawn to group immune cell subsets based on surface marker expression (for monocytes and myeloid cells, NK cells, etc). NK cells, PBMC, AML, and JMML cells were examined alone and after coculture, and changes in the CD34+CD38− stem, CD34+CD38+ progenitor, and CD34− bulk populations were evaluated. The percentage of total cells significantly decreased after coculture for both stem and progenitor populations, although the bulk population of JMML cells did not significantly decrease after coculture with NK cells (Figure 2B-C). These findings suggest that NK cells exert preferential cytotoxic activity against immature leukemogenic JMML cells.
Immature JMML cells are susceptible to lysis by NK cells. (A) Expanded NK cells from 3 HDs were preincubated with spleen MNCs from patients with JMML or cord blood MNCs. NK cell effect on growth of CFUs was tested. Either 250 000 JMML or cord blood MNCs were incubated with 125 000 NK cells for 4 hours and then seeded into MethoCult with growth factors. CFUs were counted on day 12. Data were normalized to CFU numbers obtained in untreated control plates. The experiment was performed twice, with both data sets shown. (B) Expanded NK cells from 3 healthy donors were cocultured with JMML splenocytes (n = 2 patient samples), AML cells, or allogeneic healthy donor PBMCs. After 48 hours, cells were examined by mass cytometry. Cells were classified in clusters based on immune cell phenotype, and subpopulations then were evaluated to compare effects of NK cell–mediated lysis on different subpopulations. (C) Percent of total cells for the bulk, progenitor, and stem populations comparing JMML splenocytes cultured alone vs with NK cells are plotted, with asterisks denoting significant decreases in population size after coculture (P < .05).
Immature JMML cells are susceptible to lysis by NK cells. (A) Expanded NK cells from 3 HDs were preincubated with spleen MNCs from patients with JMML or cord blood MNCs. NK cell effect on growth of CFUs was tested. Either 250 000 JMML or cord blood MNCs were incubated with 125 000 NK cells for 4 hours and then seeded into MethoCult with growth factors. CFUs were counted on day 12. Data were normalized to CFU numbers obtained in untreated control plates. The experiment was performed twice, with both data sets shown. (B) Expanded NK cells from 3 healthy donors were cocultured with JMML splenocytes (n = 2 patient samples), AML cells, or allogeneic healthy donor PBMCs. After 48 hours, cells were examined by mass cytometry. Cells were classified in clusters based on immune cell phenotype, and subpopulations then were evaluated to compare effects of NK cell–mediated lysis on different subpopulations. (C) Percent of total cells for the bulk, progenitor, and stem populations comparing JMML splenocytes cultured alone vs with NK cells are plotted, with asterisks denoting significant decreases in population size after coculture (P < .05).
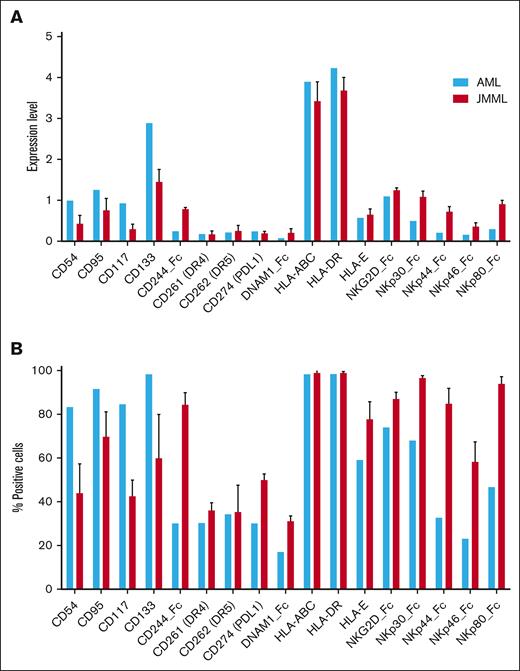
JMML stem cells express a broad repertoire of NK cell ligands similar to that of AML stem cells
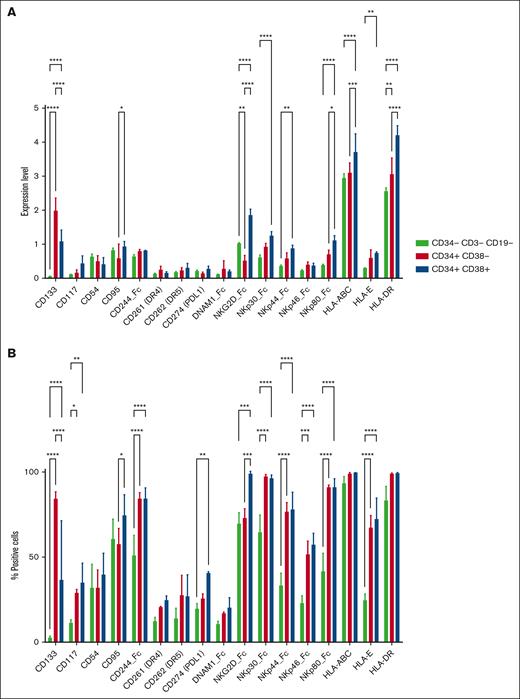
The finding of significant NK cell antitumor activity against JMML colony-forming cells suggested that JMML LSCs express ligands for NK cell–activating receptors, so we aimed to characterize the phenotype of JMML stem cells and compare this with the phenotype of AML stem cells. Immature JMML and AML CD34+ populations were examined for surface expression of ligands for various NK cell–activating and –inhibitory receptors. Multiple NK cell–activating and –inhibitory ligands expressed by AML cells also were detected on the surface of JMML cells (Figure 3A-B). We then evaluated NK cell ligand expression differences among JMML subpopulations, comparing the CD34−CD3−CD19− bulk, CD34+CD38− stem, and CD34+CD38+ progenitor cell populations. As expected, expression of the cancer stem cell marker CD133 was significantly higher on the stem and progenitor populations than the bulk population. Expression of NK cell ligands for the activating receptor NKG2D were significantly lower on the CD34+CD38− LSC population than the bulk or progenitor populations, but activating ligands for several natural cytotoxicity receptors (NKp30, NKp44, and NKp80) were higher on stem and progenitor populations than the bulk mature population (Figure 4A-B). These differences in ligand expression are mechanistically congruent with the differences observed in NK cell–mediated cytotoxicity against the mature monocytic population vs the immature compartment of JMML.
JMML stem cells express a broad repertoire of NK cell ligands similar to that of AML cells. Immature JMML and AML CD34+ populations (n = 3 JMML samples and n = 1 AML sample) were examined for surface expression of ligands for various NK cell–activating and –inhibitory receptors by mass cytometry. Results are plotted as normalized expression of median metal intensity (MMI) (A) or percent positive cells (B). PDL1, Programmed Death-Ligand 1.
JMML stem cells express a broad repertoire of NK cell ligands similar to that of AML cells. Immature JMML and AML CD34+ populations (n = 3 JMML samples and n = 1 AML sample) were examined for surface expression of ligands for various NK cell–activating and –inhibitory receptors by mass cytometry. Results are plotted as normalized expression of median metal intensity (MMI) (A) or percent positive cells (B). PDL1, Programmed Death-Ligand 1.
JMML subpopulations differentially express NK cell ligands. Subpopulations (CD34–CD3–CD19– bulk, CD34+CD38– stem, and CD34+CD38+ progenitor cell populations) from 3 samples from patients with JMML were analyzed for surface expression of ligands for various NK cell–activating and –inhibitory receptors by mass cytometry. Results are plotted as average normalized expression of MMI (A) or percent positive cells (B) with error bars representing standard deviation and statistically significant differences represented by asterisks for ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. PDL1, Programmed Death-Ligand 1.
JMML subpopulations differentially express NK cell ligands. Subpopulations (CD34–CD3–CD19– bulk, CD34+CD38– stem, and CD34+CD38+ progenitor cell populations) from 3 samples from patients with JMML were analyzed for surface expression of ligands for various NK cell–activating and –inhibitory receptors by mass cytometry. Results are plotted as average normalized expression of MMI (A) or percent positive cells (B) with error bars representing standard deviation and statistically significant differences represented by asterisks for ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. PDL1, Programmed Death-Ligand 1.
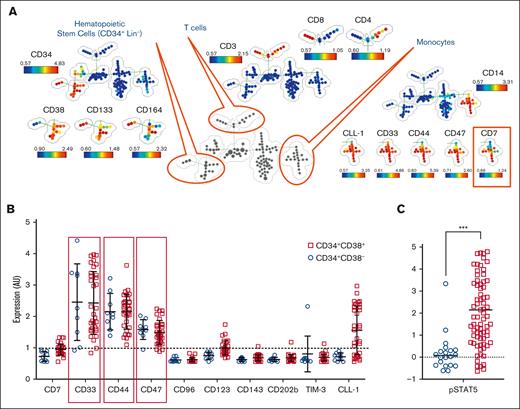
Immature JMML populations express targetable surface markers
In order to evaluate potential immunotherapeutic targets in JMML, a panel of 25 Abs for mass cytometry was used to phenotypically profile putative JMML stem cell populations.
The panel included 15 Abs for subset inclusion/exclusion including CD3, CD4, CD8a, CD11b, CD14, CD15, CD19, CD25, CD34, CD38, CD45, CD56, CD117, CD133, and CD164, as well as 10 Abs for antigens previously described as important markers of leukemia/cancer stem cells including CD7, CD33, CD44, CD47, CD96, CD123, CD143, CD202b, T cell immunoglobulin and mucin domain-3 (TIM-3), and C-type lectin-like molecule-1 (CLL-1) (Figure 5A). SPADE analyses were used to differentiate subpopulations in an unsupervised manner, and stem cell populations from other subpopulations, among which we identified monocytes with aberrant expression of CD7 as is common in myelodysplastic syndromes. CD33, CD44, and CD47 were noted to be expressed well above baseline in JMML CD34+CD38− stem cells as well as CD34+CD38+ progenitor cells, whereas substantial expression of CLL-1 and CD123 was noted on the progenitor but not the stem cell population (Figure 5B). Phosphorylated STAT5 measurement was included to confirm an activation signal consistent with JMML to support the gating strategy of the JMML population. Although the progenitor population more highly expressed phosphorylated STAT5, signal intensity was increased in both the stem and progenitor populations of JMML cells (Figure 5C).
Immature JMML populations express targetable surface markers. Splenic MNCs from 3 patients were stained with metal-conjugated Ab, fixed with paraformaldehyde, stained with an iridium metallointercalator, and then measured on the cytometry by time-of-flight mass cytometer. (A) Pooled data of 3 separately acquired samples were analyzed in SPADE simultaneously. A minimum spanning tree was constructed based on differentiating surface markers. Putative cell populations were annotated manually based on known coexpression of lineage markers. (B) Nodes representing JMML hematopoietic stem/progenitor cells were identified by CD34, CD38, and the absence of lineage markers (Lin–), and expression of LSC-associated markers (CD7, CD33, CD44, CD47, CD96, CD123, CD143, CD202b, TIM-3, and CLL-1) were analyzed. (C) Nodes representing JMML hematopoietic stem/progenitor cells were evaluated for pSTAT5 expression. Each dot on the graphs in panels B-C represents the average expression of the analyzed marker in the corresponding nodes. Dotted line represents threshold of expression. ∗∗∗P < .001. AU, arbitrary units; pSTAT5, phosphorylated STAT5.
Immature JMML populations express targetable surface markers. Splenic MNCs from 3 patients were stained with metal-conjugated Ab, fixed with paraformaldehyde, stained with an iridium metallointercalator, and then measured on the cytometry by time-of-flight mass cytometer. (A) Pooled data of 3 separately acquired samples were analyzed in SPADE simultaneously. A minimum spanning tree was constructed based on differentiating surface markers. Putative cell populations were annotated manually based on known coexpression of lineage markers. (B) Nodes representing JMML hematopoietic stem/progenitor cells were identified by CD34, CD38, and the absence of lineage markers (Lin–), and expression of LSC-associated markers (CD7, CD33, CD44, CD47, CD96, CD123, CD143, CD202b, TIM-3, and CLL-1) were analyzed. (C) Nodes representing JMML hematopoietic stem/progenitor cells were evaluated for pSTAT5 expression. Each dot on the graphs in panels B-C represents the average expression of the analyzed marker in the corresponding nodes. Dotted line represents threshold of expression. ∗∗∗P < .001. AU, arbitrary units; pSTAT5, phosphorylated STAT5.
Discussion
The only proven curative therapy for JMML is HSCT; however, side effects are significant, and only half of patients achieve a 5-year event-free survival.6 Through efforts to identify innovative and effective treatment options for patients, NK cell adoptive immunotherapy has emerged as an attractive approach to treating patients with myeloid malignancies. The impact of NK cells on transplant survival has been associated with increased numbers of NK cells in the stem cell graft, rapid recovery of NK cells in the early posttransplant period, and alloreactivity of NK cells in the GVL direction predicted by KIR-ligand mismatch.15,45,46 However, the impact of NK cell activity in the setting of JMML has not yet been characterized, although we have previously reported the absence of KIR/HLA-based predictors.25 In this study, we sought to determine whether JMML is an NK cell–responsive malignancy. We found that the mature monocytic population of JMML expresses a similar profile of NK cell ligands as healthy donor monocytes and is resistant to NK cell–mediated cytotoxicity. However, the impact of NK cells on the colony-forming properties of LSCs was impressive, with JMML LSCs displaying a significant loss in clonogenicity when cocultured in the presence of NK cells, while CFU capacity of cord blood was unaffected.
LSCs typically are quiescent and resistant to chemotherapy and are considered to have a central role in relapse and the refractory nature of AML; hence, developing therapeutic strategies specifically to target the LSC population is critical to achieve cure in patients with AML.47 NK cell responses may be triggered by binding to tumor ligands capable of engaging NK cell receptors.48,49 Various tumor cell types express ligands for the activating NKG2D receptor, and these ligands trigger NK cell–mediated cytotoxicity.50,51 Paczulla et al previously demonstrated lack of expression of NKG2D ligands on LSCs by examining xenografts of human AML and syngeneic mouse models of leukemia. These findings suggest that AML LSCs lacking NKG2D ligand expression may evade NK cell immune surveillance.52 In this study, to evaluate the antileukemic effects of NK cells against JMML stem cells, we investigated the surface expression of NK cell ligands for various activating and inhibitory receptors by high dimensional phenotyping. JMML stem cells, defined as Lin−CD34+CD38−, exhibited similar expression of ligands for NKG2D, DNAM-1, NKp30, NKp44, and NKp46 compared with that of AML stem cells. JMML stem cells express similar levels of HLA class 1 and HLA-E, suggesting that inhibitory mechanisms may be relevant for JMML, similar to AML. In addition, when subpopulations of JMML cells were examined, the expression of NK cell ligands for several activating receptors was significantly lower in the CD34−CD3−CD19− subgroup than in JMML stem and progenitor cells. Lower expression of activating NK cell ligands on monocytes provides a potential mechanism to support the resistance of this cell population to NK cell–mediated lysis.
Investigation of the LSC surface antigen profile to identify suitable markers for targeted therapy has attracted much attention in the field of oncology research. Although AML LSCs share a similar CD34+CD38− surface phenotype as HSCs, a number of cell surface markers such as CD123, CD47, CD96, CD44, TIM-3, CLL-1, CD90, CD32, CD25, and CD33 have been identified to differentiate AML LSCs from HSCs.53,54 Monoclonal Abs targeting several of these antigens have been investigated in preclinical models of AML.55 Moreover, immunotherapies targeting CD33 and CD123 have been evaluated in clinical trials and have been shown to mediate NK cell Ab-dependent cell-mediated cytotoxicity.56,57 In this study, it was demonstrated that CD33, CD44, and CD47 are differentially expressed by JMML stem and progenitor cells, whereas CLL-1 is expressed only by the progenitor cell population. Of note, given the relatively limited availability of samples from pediatric patients with JMML with sufficient cell counts to facilitate laboratory investigation in this study, future investigation encompassing a wider variety of patient samples will add to our knowledge base and support the development of more effective therapies for pediatric patients with JMML.
Here, we demonstrate that the JMML stem cell population is uniquely susceptible to NK cell cytotoxic activity. To our knowledge, this is the first investigation to identify the potential impact of targeted NK cell activity against JMML, and one of the first studies to focus on LSC vulnerabilities in JMML. Using commercially available Abs or engineered cell therapies targeting CD33, CD44, or CD47 may have promising therapeutic potential for enhancing NK cell–based cancer immunotherapy in the setting of JMML. Furthermore, these results support that NK cells may serve as an effective adjunct to HSCT for the treatment of JMML and could lead to better outcomes for pediatric patients with cancer.
Acknowledgments
The authors thank Justin Lyberger at The Ohio State University Mass Cytometry Shared Resource for his assistance with cytometry by time-of-flight experiments. The visual abstract was created in BioRender. Campbell, A. (2025) https://BioRender.com/4t5jc12. There is a second image in the 2-part visual abstract for which I also have a Biorender Confirmation of Publication and Licensing Rights. The document was created earlier, and Biorender did not provide the exact link in the citation as what is provided for the image in the more recent License (for the second image in the visual abstract). The other image is available via https://app.biorender.com/illustrations/65e628642a590d8b89f3460d?slideId=683d7c9f-1612-4636-a638-d09e4c832bd7.
This work has been supported by the Alex's Lemonade Stand Foundation Pediatric Oncology Student Training program award and the American Society of Hematology’s Minority Medical Student Award Program. A.A.-L. received funding support from the American Legion Auxiliary Fellowship in Cancer Research, the George M. Stancel, PhD Fellowship in Biomedical Sciences, and the University of Puerto Rico/The University of Texas MD Anderson Cancer Center (UPR/MDACC) Partnership for Excellence in Cancer Research (CA096297-300). A.R.C. received funding support from the Alex’s Lemonade Stand Young Investigator award and the St. Baldrick’s Foundation Fellow Award.
Authorship
Contribution: S.M., V.V.S., and A.A.-L. contributed to conceptualization, experimentation, data analysis, and writing, reviewing, and editing of the manuscript; S.H., G.B., and A.R.C. contributed via data analysis, and writing, reviewing, and editing of the manuscript; P.D.E., Y.L.L., and E.S. contributed through conceptualization, providing clinical samples, and reviewing and editing of the manuscript; D.A.L. contributed via conceptualization, funding acquisition, supervision, data analysis, and reviewing and editing of the manuscript.
Conflict-of-interest disclosure: D.A.L. serves on the scientific advisory board of, and consults for, Avidicure; receives consultancy, licensing, and royalty fees from Kiadis Pharma, a Sanofi company; and has intellectual property interests related to natural killer cell therapy. The remaining authors declare no competing financial interests.
Correspondence: Dean A. Lee, Hematology, Oncology, and Blood and Marrow Transplant, Abigail Wexner Research Institute at Nationwide Children’s Hospital, 700 Children’s Dr, Columbus, OH 43205; email: dean.lee@nationwidechildrens.org.
References
Author notes
S.M. and V.V.S. contributed equally to this study.
Cytometry by time-of-flight data has been uploaded to provide public access through Cytobank.
Original data are available on request from the corresponding author, Dean A. Lee (dean.lee@nationwidechildrens.org).
The full-text version of this article contains a data supplement.