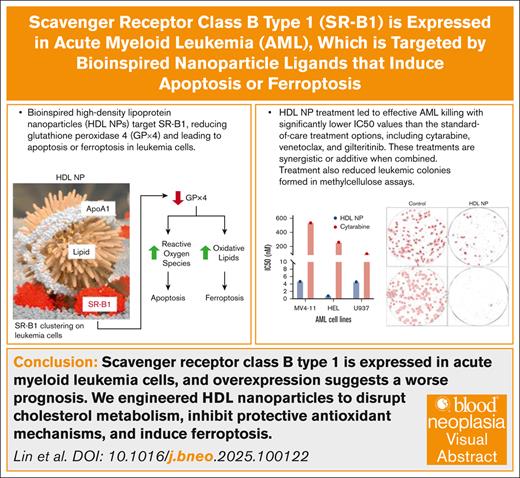
Key Points
SR-B1 is expressed in AML, and its overexpression may suggest a worse prognosis.
We engineered HDL NPs to disrupt cholesterol metabolism, inhibit protective antioxidant mechanisms, and induce ferroptosis.
Visual Abstract
Despite progress in research and treatment strategies for acute myeloid leukemia (AML), the prognosis for patients with AML, particularly for individuals aged >60 years and those with adverse risk factors, remains poor. Cellular receptors that affect cholesterol homeostasis may present a new target for treating AML. Scavenger receptor class B type 1 (SR-B1), which plays an important role in cellular cholesterol uptake and redox balance, is expressed by AML cells and correlates with poor patient outcomes. Previously, we targeted SR-B1 in various hematologic and solid malignancies with a synthetic bioinspired high-density lipoprotein nanoparticle (HDL NP) ligand that disrupted cholesterol metabolism, inhibited protective antioxidant mechanisms, and induced ferroptosis. This study demonstrates that HDL NPs are effective at low nanomolar drug concentrations in AML, surpassing the effectiveness of cytarabine, a standard-of-care chemotherapy agent. The HDL NP reduced glutathione peroxidase 4, leading to reactive oxygen species accumulation, which causes some AML cells to undergo ferroptosis while others undergo apoptosis and pyroptosis. HDL NP treatment was synergistic with standard AML therapies, including cytarabine, venetoclax, and gilteritinib for fms-like tyrosine kinase 3–mutated leukemia cells. Notably, HDL NP treatment induced the differentiation of AML cells into mature granulocytes. Overall, this study provides a foundation for further investigations into the underlying mechanisms and clinical applications of SR-B1 targeting in AML treatment.
Introduction
Despite significant advances in the understanding of acute myeloid leukemia (AML) pathogenesis and treatment modalities, the overall prognosis remains unsatisfactory, particularly for patients >60 years of age and those with adverse risk factors. AML accounts for a significant proportion of leukemias, representing ∼80% of acute leukemias in adults and 15% to 20% of childhood leukemias.1 Patients with AML have a 5-year overall survival (OS) rate of only 31.7%, which is lower in relapsed/refractory setting.2 The 5-year OS rate ranges from 20% to 40% in younger patients, but it drops to <10% for patients aged >60 years.3 In addition, a substantial number of patients experience relapse after achieving initial remission, even after allogeneic hematopoietic stem cell transplantation, contributing to high mortality rates.4 AML is characterized by its heterogeneity, with multiple subtypes classified according to morphological, cytogenetic, and molecular features. The biological basis of AML remains complex, involving genetic predisposition, exposure to environmental toxins, including previous chemotherapy or radiation therapy, and age-related mutational changes. These challenges underscore the need for innovative therapeutic approaches to enhance treatment efficacy and minimize toxicities.
Growing evidence suggests that cholesterol metabolism may play a role in AML biology, and the cell surface receptor scavenger receptor class B type 1 (SR-B1) may be a therapeutic target.5 Cholesterol is essential for cell membranes and is a building block for steroid hormones. Cholesterol can either be produced within the body or acquired from the diet. As a lipid, cholesterol requires transport within the bloodstream to different cells and tissues by lipoproteins, such as high-density lipoprotein (HDL), that bind to specific cell surface receptors, such as SR-B1. Cellular cholesterol is tightly regulated by a complex network of enzymes and transporters. When cholesterol metabolism is disrupted in AML, it can lead to increased cell proliferation, maintenance of leukemia stem cells, and resistance to cell death.5 In addition, leukemic blasts have been shown to have abnormally high levels of cholesterol, suggesting that AML outsized uptake of cholesterol is critical for growth, survival, and resistance to conventional treatment.5 Preclinical models have shown that targeting cholesterol metabolism can selectively decrease the viability of leukemic cells and reduce disease progression.6 However, clinical trials combining statins, drugs that inhibit de novo cellular cholesterol biosynthesis, with standard therapy were inconclusive and mostly negative.7,8
Contrary to the mechanism of cholesterol reduction by statins, we found that certain cancer cells are highly dependent on exogenous cholesterol uptake and express SR-B1, high-affinity receptor for cholesterol-rich HDL.9-15 Cell membrane-integrated SR-B1 receptors play a crucial role in cellular cholesterol and redox balance.16-18 Increased expression of SR-B1 has been observed in leukemic cells, particularly in AML subtypes associated with adverse prognostic factors.5,19 Increased expression of SR-B1 may promote aberrant acquisition of cholesterol from extracellular environment and fuel the growth and survival of leukemic cells. Based on this hypothesis, we previously engineered a bioinspired HDL nanoparticle (NP) using a 5 nm gold NP core as a scaffold to enable synthesis of a spherical NP with similar size, surface charge, and surface composition compared with native HDLs. HDL NPs specifically target and bind to SR-B1, preventing uptake of cholesterol and other lipophilic cargo via the receptor (Figure 1A).9,12,13,15 These HDL NPs mimic the physical and surface chemical features of native HDLs, which enables active targeting of SR-B1. Characteristics of HDL NPs are noted in supplemental Figure 1. Accordingly, HDL NP excludes native cholesterol-rich ligand binding to SR-B1 and modulates cholesterol uptake and metabolism. In addition, we recently demonstrated that HDL NP treatment of lymphoma cells modulates redox pathways and is accompanied by a reduction in the expression of glutathione peroxidase 4 (GPx4).9 GPx4 is the sole enzyme responsible for detoxifying cell membrane lipid peroxides and preventing cell death through ferroptosis, an iron-dependent redox form of cell death.20 We found that HDL NPs target SR-B1 and potently induce ferroptosis in lymphoma cells, as well as in other ferroptosis-sensitive malignancies, including anaplastic large T-cell lymphomas, Ewing sarcoma, medulloblastoma, and ovarian cancer.10,11,21 Given the critical role of SR-B1 in cholesterol metabolism, redox biology, and AML pathogenesis, the development of therapeutic agents targeting SR-B1 or its downstream effectors holds immense promise. In this article, we evaluated the effects of HDL NP treatment on AML cell lines and samples from patients with AML.
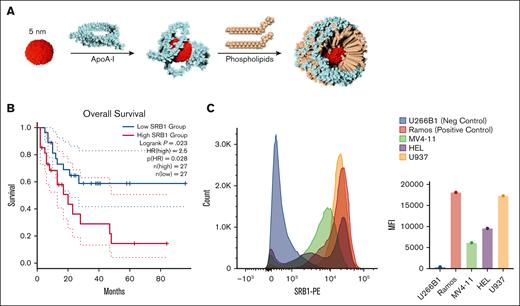
SR-B1 expression is elevated in AML cells, targeted by bioinspired gold-core HDL NPs. (A) HDL NPs comprise a 5 nm gold NP core surface functionalized with ApoA-I and phospholipids. After the stepwise addition of the components and self-assembly, HDL NPs are purified by tangential flow filtration. (B) In the LAML database in Gene Expression Profiling Interactive Analysis, there was a significant reduction in OS with patients expressing higher SR-B1 levels than those with AML with lower SR-B1 expression. (C) SR-B1 expression by flow cytometry in AML cell lines MV4-11, HEL, U937 compared with SR-B1– U266B1 and SR-B1+ Ramos cells presented as a histogram (left) and MFI (right). ApoA-I, apolipoprotein A1; HR, hazard ratio.
SR-B1 expression is elevated in AML cells, targeted by bioinspired gold-core HDL NPs. (A) HDL NPs comprise a 5 nm gold NP core surface functionalized with ApoA-I and phospholipids. After the stepwise addition of the components and self-assembly, HDL NPs are purified by tangential flow filtration. (B) In the LAML database in Gene Expression Profiling Interactive Analysis, there was a significant reduction in OS with patients expressing higher SR-B1 levels than those with AML with lower SR-B1 expression. (C) SR-B1 expression by flow cytometry in AML cell lines MV4-11, HEL, U937 compared with SR-B1– U266B1 and SR-B1+ Ramos cells presented as a histogram (left) and MFI (right). ApoA-I, apolipoprotein A1; HR, hazard ratio.
Methods
HDL NP synthesis
HDL NPs were synthesized and characterized as previously described.11,13,18,22 Briefly, 5 nm citrate-stabilized gold NPs (nanoComposix) were surface functionalized with apolipoprotein A-I and the PDP PE (phospholipids 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[3-(2-pyridyldithio)propionate]; Avanti Polar Lipids) and DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine; Avanti Polar Lipids). HDL NPs were purified using the KrosFlo tangential flow filtration system, using a 50 kDa cutoff polyethersulfone module (Repligen). HDL NP concentration was calculated using UV-Visable spectroscopy and the Beer law.
Flow cytometric analysis of SR-B1 expression
AML cell line (MV4-11 [RRID:CVCL_0064], HEL [RRID:CVCL_0001], and U937 [RRID:CVCL_0007]), Ramos (SR-B1 and Burkitt lymphoma; RRID:CVCL_0597), and U266B1 (SR-B1 negative myeloma; RRID:CVCL_0566) cells were analyzed for SR-B1 expression using an allophycocyanin (APC)-labeled anti–SR-B1 antibody (BioLegend). All cells were purchased and thus authenticated through the American Type Culture Collection (lot: MV4-11 no. 70039150; HEL no. 70048921; U937 no. 70029716). Cells (5 × 105) were washed with ice-cold fluorescence-activated cell sorting (FACS) buffer, then resuspended in FACS buffer with Fc Block (BioLegend) and incubated on ice for 10 minutes. After incubation, SR-B1 antibody diluted in FACS buffer was added to each tube and incubated in the dark on ice for 30 minutes. The cells were washed twice with FACS buffer and resuspended in fresh buffer. The SR-B1 fluorescence was measured using the BD LSRII Fortessa flow cytometer. Data were analyzed using FCS Express software.
Cell death assays
The MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assays (CellTiter, Promega) were performed as described previously.9,11,13 MV4-11, HEL, or U937 cells were incubated for 5 days under treatment conditions. Then, MTS reagent was added to each well, and absorbance was recorded at 0-minute and 90-minute time points using a 96-well plate reader at 490 nm. Viability results were normalized to the untreated (phosphate-buffered saline [PBS]) controls (100%). Ferrostatin-1 (Fer-1) and deferoxamine (DFO) were obtained from Sigma Aldrich (RRID:SCR_008988).
Apoptosis assays
MV4-11 apoptosis assays were performed according to manufacturer’s guidelines. Dead cell apoptosis kits: treated cells were washed with PBS and resuspended in annexin V buffer and stained for 15 minutes. Pyroptosis 660 caspase-1 kit: 5 μL FLICA (fluorochrome inhibitor of caspases) was added to 300 μL cells and incubated for 30 minutes. Cells were washed with 2 mL wash buffer. CellEvent caspase-3/7 green flow cytometry assay kit: caspase-3/7 (500 μM) was added to 106 cells and aminoactinomycin D (AAD) (1 mM) was added after. CellROX deep red reagent: CellRox was added to cells at a final concentration of 5 μM for 30 minutes and stained with Aqua Live/Dead. All samples were analyzed on a FACSymphony A5.2 spectral analyzer and FlowJo software (RRID:SCR_008520).
CFU assays
MethoCult were plated with Ficoll-separated peripheral blood mononuclear cells (PBMCs) at 20 000 or 200 000 cells per well. Cell lines were plated at 10 000 cells per well. Wells were imaged in gray scale using Nikon Ti2 widefield microscope on days 14 and 21. Images were imported into Fiji (RRID:SCR_002285), cropped, and the background subtracted. A Fiji Labkit plugin was used to train the classifier defining leukemic progenitor cells or colony-forming units (CFUs) compared with the background.23 Generated probability map was thresholded to 80% confidence, and particles of >2000 pixels2 were analyzed for total area or CFU count and graphed using Prism.
Our protocols to collect patient samples used in this article were approved by the institutional review board at Northwestern University.
Additional methods are listed in the supplemental Data.
Results
SR-B1 is expressed in AML
Using the Cancer Genome Atlas Program-Acute Myeloid Leukemia database in Gene Expression Profiling Interactive Analysis, we found that AML samples from 173 patients had a lower SR-B1 expression (median, 36.76 transcripts per million [TPM]) compared with normal control cells (n = 70; median, 125.3 TPM; P < .01). However, there was a significant OS detriment in patients demonstrating high SR-B1 expression (≥75th percentile) compared with low SR-B1 expression (≤25th percentile; P = .023; hazard ratio, 2.5; Figure 1B). Using cBioPortal24 and selecting AML-related trials, elevated SR-B1 messenger RNA (mRNA) expression suggested an association of worse OS (supplemental Figure 2A-B). The lowest quartile of SR-B1 mRNA expression appears to have the highest OS. However, when altering the variable to “mRNA expression (TPM),” the OS survival difference no longer persists (supplemental Figure 2C). Using University of California Santa Cruz (UCSC) Xena,25 the Cancer Genome Atlas Program-Acute Myeloid Leukemia, and Target AML database did not indicate any OS differences between SR-B1 expression levels (supplemental Figure 2D-E). This highlights the need for a prospective multivariate analysis adjusted for other covariates.
To further evaluate SR-B1 as a target, we measured SR-B1 expression in 3 commonly studied AML cell lines: MV4-11 (a biphenotypic myelomonocytic leukemia cell line that carries an fms-like tyrosine kinase 3 [FLT3] with internal tandem duplication mutation), HEL (an erythroblastic AML), and U937 (a promonocytic AML). SR-B1 expression, as measured by flow cytometry, had a median fluorescent intensity (MFI) of 6146 in MV4-11, MFI of 9555 in HEL, and MFI of 17286 in U937 (U266B1, MFI 396; Ramos, and MFI 18115; Figure 1C). MV4-11 and HEL had elevated SR-B1 expression detected by western blot (supplemental Figure 3). Using BloodSpot,26 SR-B1 expression was much more varied in samples from patients with AML compared with normal hematopoietic cells (supplemental Figure 4).
HDL NPs induce AML cell death at nanomolar concentrations
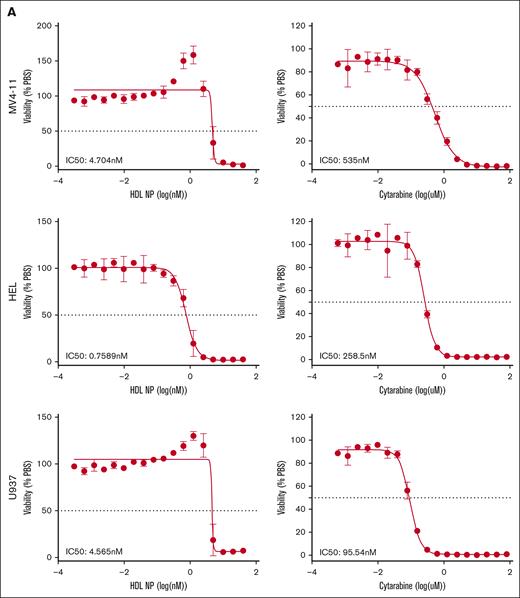
We treated AML cell lines with HDL NPs, effectively target SR-B1, for 5 days and compared the efficacy with that of cytarabine, using a colorimetric MTS assay (Figure 2A). The half-maximal inhibitory concentration (IC50) of HDL NPs toward MV4-11, HEL, and U937 were in the low nanomolar range, 4.7 nM, 0.8 nM, and 4.6 nM, respectively. By comparison, IC50 for cytarabine is 535 nM for MV4-11, 259 nM for HEL, and 95.5 nM for U937. We observed an increase in metabolism in MV4-11 and U937 cells just below the IC50 dose. MV4-11 cells had an MTS viability readout at 135%, whereas the cell viability, quantified using trypan blue staining, was only 47%, suggesting that increased MTS signal may be due to increased metabolic activity of the cells rather than increased proliferation relative to PBS controls. Binding of HDL NPs to SR-B1 disrupts the cholesterol and cholesteryl ester uptake mechanisms.22,23 Of note, HDL NP treatment did not alter the viability of SR-B1– cell lines, U266B1 (supplemental Figure 5A) and Jurkat.23,24
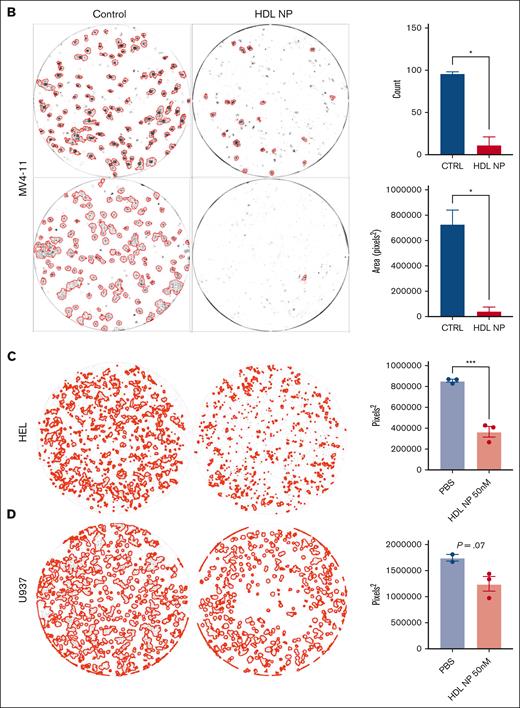
AML cell lines are exquisitely sensitive to HDL NP treatment. (A) MTS viability assay of MV4-11 (top), HEL (middle), and U937 (bottom) treated with HDL NPs (left) or cytarabine (right) at various nanomolar or micromolar concentrations. (B) Colony-forming assays with MV4-11 cells grown in methylcellulose treated with or without HDL NPs at 50 nM. CFUs are highlighted by red outlines on well images (left). The total CFU count (top right) and area (bottom right) were significantly reduced when treated with HDL NPs. (C-D) Representative images for HEL and U937 with or without HDL NP and total CFU areas. ∗P < .05; ∗∗∗P < .001. CTRL, control.
AML cell lines are exquisitely sensitive to HDL NP treatment. (A) MTS viability assay of MV4-11 (top), HEL (middle), and U937 (bottom) treated with HDL NPs (left) or cytarabine (right) at various nanomolar or micromolar concentrations. (B) Colony-forming assays with MV4-11 cells grown in methylcellulose treated with or without HDL NPs at 50 nM. CFUs are highlighted by red outlines on well images (left). The total CFU count (top right) and area (bottom right) were significantly reduced when treated with HDL NPs. (C-D) Representative images for HEL and U937 with or without HDL NP and total CFU areas. ∗P < .05; ∗∗∗P < .001. CTRL, control.
Statins have been studied in AML due to abnormal cholesterol homeostasis in leukemia cells, leading to drug resistance and disturbance of differentiation.5,27,28 Treatment with atorvastatin, lovastatin, or rosuvastatin at doses up to 10 μM did not affect viability in MV411 and HEL (supplemental Figure 5B). Finally, we used colony-forming assays to investigate the ability of HDL NPs to induce AML cell death in vitro using a more physiologically relevant model (Figure 2B). MV4-11 cells cultured with HDL NPs in methylcellulose had dramatically reduced CFUs measured as counts (11 vs 95.5 for control; P = .015) and total area (39 596 pixels2 vs 72 861 pixels2 for control; P = .029) after 2 weeks of incubation. Longer incubation times of MV4-11, up to 4 weeks, continue to show growth inhibition (supplemental Figure 6). Similarly, treatment reduced CFU total areas for HEL and U937 (Figure 2C-D).
Previously, we showed that primary human hepatocytes and macrophages had no observable toxicity when treated with HDL NPs and effectively preserved cholesterol homeostasis.24,25 Here, we acquired CD34+ mobilized human peripheral blood cells and observed a low expression level of SRB1 when compared with a sample from a patient with AML processed concurrently. Colony-forming assays indicated that treatment with HDL NPs did not decrease CFUs, implying that HDL NPs do not affect the viability of normal stem cells (supplemental Figure 7).
HDL NPs reduce GPx4 and induce ferroptosis and apoptosis in AML
Previously, we found that cholesterol auxotrophic lymphomas meet their cholesterol requirements exclusively through cholesterol uptake from circulating lipoproteins, such as HDLs. Typically, mature cholesterol-rich HDLs bind to SR-B1 and deliver their cargo of cholesterol and cholesterol esters to the lymphoma cells. However, in the setting of HDL NP treatment, cholesterol uptake is blocked, and thus, lymphoma cells increase the expression of de novo cholesterol biosynthesis genes, which is accompanied by a decrease in the expression of GPx4, a critical enzyme that converts toxic lipid peroxides to their corresponding nontoxic lipid alcohols.29,30 Reduced GPx4 expression leads to accumulation of oxidized phospholipids, ultimately resulting in cell death by ferroptosis. We previously found that HDL NP reduced GPx4 in U937 and triggered ferroptosis,26,27 which were also observed in this study (supplemental Figure 8). Here, we also evaluated whether the other 2 AML cell lines undergo ferroptosis when treated with HDL NPs.
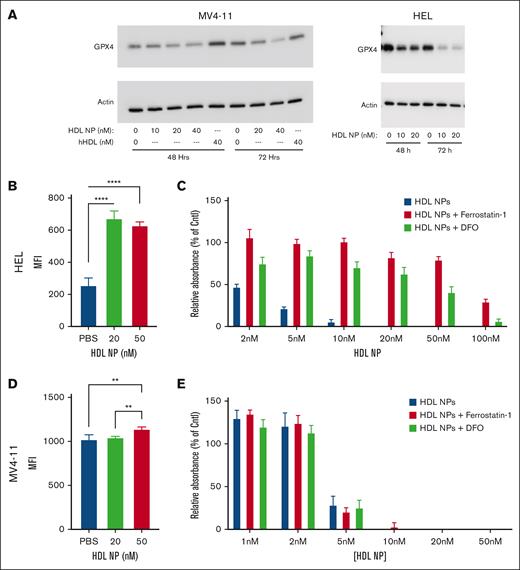
When treated with HDL NPs, MV4-11 showed a reduction of GPx4 expression at 48 hours, which was more pronounced at 72 hours (Figure 3A). Conversely, treatment with human HDL did not reduce GPx4. Similarly, HEL cells treated with HDL NP demonstrated reduced GPx4 expression, with only a faint band at 72 hours. To verify ferroptosis as a mechanism of cell death, we used the lipophilic C11-BODIPY fluorescent dye, whose emission wavelength shifts from red to green in the presence of lipid peroxides. Like our previously reported results in U937 cells, HEL cells treated with HDL NPs showed a significant increase in lipid peroxide accumulation by flow cytometry (supplemental Figure 9; MFI, 672 ± 27 and 628 ± 13, respectively) compared with PBS (MFI, 256 ± 27; P < .0001; Figure 3B). MV4-11 had only a minor increase in oxidative lipid accumulation at 50 nM (MFI, 1139 ± 12) compared with 20 nM (MFI, 1043 ± 7) or PBS (MFI, 1022 ± 26; P < .01; Figure 3D). Furthermore, HDL NP–induced cell death was rescued by adding lipophilic antioxidant Fer-1 and iron chelator, DFO, in HEL but not MV4-11 cell line (Figure 3C,E). HDL NPs at 5 nM reduced HEL viability to 21.4% ± 4.7%, but in the presence of Fer-1 (1 μM) or DFO (1 μM), viability improved to 99.1% ± 11.9% and 84.3% ± 14.6%, respectively. Conversely, HDL NPs at 5 nM reduced MV4-11 viability to 28.5% ± 25.4%, and addition of Fer-1 and DFO did not change the viability (20.41% ± 11.9% and 25.24% ± 21.7%, respectively). Together, these data show that HDL NPs induce ferroptosis in HEL but not in MV4-11 despite reducing GPx4 in both cell lines.
HDL NPs reduced GPx4 expression in leukemia cell lines leading to ferroptosis in HEL cells but not MV4-11 cells. (A) Western blot of GPx4 protein expression in MV4-11 (left) or HEL (right) leukemia cells treated with either PBS; HDL NPs at 10, 20, or 40 nM; or human native HDL for 48 and 72 hours. (B) C11 BODIBY oxidative lipid assay in HEL cells after treatment with HDL NPs at 20 or 50 nM. (C) Viability (MTS) assay of HEL cells treated at 2 to 100 nM HDL NPs without (red bars) or with the iron chelator Fer-1 (blue bars) and DFO (green bars). (D) C11 BODIBY oxidative lipid assay in MV4-11 cells after treatment with HDL NPs at 20 or 50 nM. (E) Viability (MTS) assay of MV4-11 cells treated at 2 to 100 nM HDL NPs without (red bars) or with iron chelator Fer-1 (blue bars) and DFO (green bars). Cntl, control; hHDL, human HDL.
HDL NPs reduced GPx4 expression in leukemia cell lines leading to ferroptosis in HEL cells but not MV4-11 cells. (A) Western blot of GPx4 protein expression in MV4-11 (left) or HEL (right) leukemia cells treated with either PBS; HDL NPs at 10, 20, or 40 nM; or human native HDL for 48 and 72 hours. (B) C11 BODIBY oxidative lipid assay in HEL cells after treatment with HDL NPs at 20 or 50 nM. (C) Viability (MTS) assay of HEL cells treated at 2 to 100 nM HDL NPs without (red bars) or with the iron chelator Fer-1 (blue bars) and DFO (green bars). (D) C11 BODIBY oxidative lipid assay in MV4-11 cells after treatment with HDL NPs at 20 or 50 nM. (E) Viability (MTS) assay of MV4-11 cells treated at 2 to 100 nM HDL NPs without (red bars) or with iron chelator Fer-1 (blue bars) and DFO (green bars). Cntl, control; hHDL, human HDL.
Targeting SR-B1 with HDL NPs causes apoptosis in MV4-11
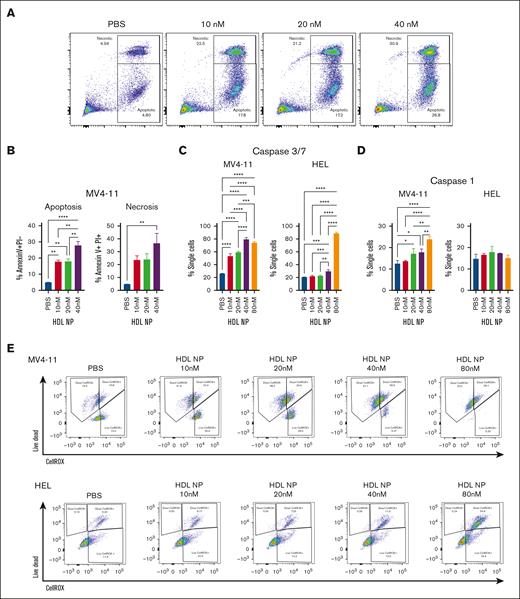
Our previous lymphoma studies suggest that HDL NPs can induce both ferroptosis and apoptosis.27,28 HDL NP exposure causes an increase in annexin V expression on MV4-11 cells, suggesting these leukemia cells undergo apoptosis (annexin V positive propidium iodide [PI] negative) as well as necrosis (annexin V positive PI positive; Figure 4A-B). We also found that HDL NP treatment raised levels of caspase-3/7, which supports the hypothesis that MV4-11 undergoes apoptosis (Figure 4C). Caspases 3 and 7 are essential for breaking down cellular structures during apoptosis. In contrast, HEL exhibited less dose dependency and demonstrated increased staining at only higher HDL NP doses. Additionally, MV4-11 cells undergo pyroptosis through increased levels of caspase-1 whereas HEL exhibited no increase in pyroptosis when treated with HDL NP (Figure 4D). Pyroptosis holds significance in cancer treatment because it can trigger antitumor immune responses and enhance the effectiveness of immunotherapies.
Mechanisms of cell death of MV4-11 and HEL treated with HDL NPs. (A) Annexin V–PI staining after MV4-11 cells were treated with HDL NPs from 10 to 40 nM. Apoptosis is represented by the annexin V positive PI negative population, and necrosis by the annexin V positive PI positive population. (B) Bar graph analysis of the apoptosis and necrosis results. (C) Caspase-3 and 7 levels in MV4-11 (left) and HEL (right) were detected by flow cytometry after treatment of HDL NPs from 10 to 80 nM. (D) Caspase-1 levels of MV4-11 (left) and HEL (right) after treatment of HDL NPs from 10 to 40 nM as detected by flow cytometry. (E) Representative flow cytometry results of MV4-11 (top) and (bottom) HEL cells were treated with PBS and HDL NPs (10-80 nM) stained with Live/Dead and CellROX.
Mechanisms of cell death of MV4-11 and HEL treated with HDL NPs. (A) Annexin V–PI staining after MV4-11 cells were treated with HDL NPs from 10 to 40 nM. Apoptosis is represented by the annexin V positive PI negative population, and necrosis by the annexin V positive PI positive population. (B) Bar graph analysis of the apoptosis and necrosis results. (C) Caspase-3 and 7 levels in MV4-11 (left) and HEL (right) were detected by flow cytometry after treatment of HDL NPs from 10 to 80 nM. (D) Caspase-1 levels of MV4-11 (left) and HEL (right) after treatment of HDL NPs from 10 to 40 nM as detected by flow cytometry. (E) Representative flow cytometry results of MV4-11 (top) and (bottom) HEL cells were treated with PBS and HDL NPs (10-80 nM) stained with Live/Dead and CellROX.
Although there was a minor increase in oxidative lipid accumulation, HDL NPs have previously been shown to increase the total reactive oxygen species (ROS) burden in various lymphomas as measured with the CellROX fluorescent assay. When the Live/Dead stain was included, MV4-11 cells showed a slight increase in ROS levels with HDL NP treatment, alongside a higher number of dying cells with negative CellROX staining. In contrast, HEL exhibited significantly more ROS in the dying cells after treatment with HDL NPs (Figure 4E; supplemental Figure 10). MV4-11 resistance to ferroptosis is not specific to HDL NPs. GPx4 inhibitors (ML210 and RSL3), which traditionally induce ferroptosis, were not as efficacious in MV4-11 compared with HEL and U937 cells (supplemental Figure 11).
Targeting SR-B1 causes differentiation in MV4-11
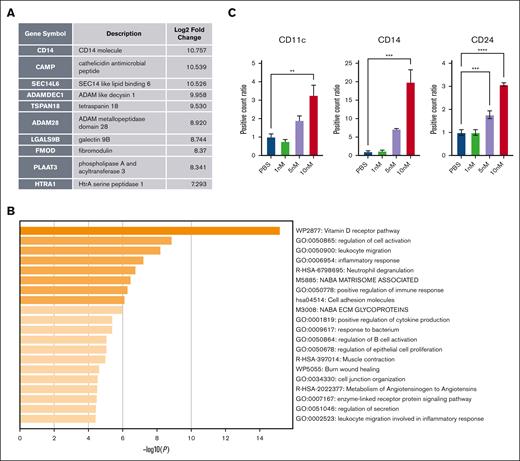
Cholesterol modulation has previously been shown to cause leukemia cell differentiation.31 Several targeted leukemia therapies, such as all-trans retinoic acid and isocitrate dehydrogenase inhibitors, can also cause differentiation.32 Using bulk RNA sequencing, we found that HDL NP treatment resulted in 3 differentially expressed genes (DEGs) with log2 changes of >10: CD14 molecule, which is a mature neutrophil marker; cathelicidin antimicrobial peptide, which relates to neutrophil function; and SEC14-like lipid binding 6, which is involved in lipid metabolism. The top 10 increased DEGs are shown in Figure 5A. The top 5 enhanced pathways are the vitamin D pathway, regulation of cell activation, leukocyte migration, inflammatory response, and neutrophil degranulation (Figure 5B). The DEGs and pathway analysis suggest that the leukemia cells may differentiate into more mature granulocytes in the presence of HDL NPs. Conversely, only 2 genes had a negative log2 fold change of <2, collagen type VI α2 chain (−3.06) and immediate early response 3 (−5.47), a negative prognostic marker.33 Furthermore, surface marker staining for mature granulocyte markers showed that HDL NP treatment increased CD11c, CD14, and CD24 expression on these MV4-11 cells, suggesting leukemia cell differentiation. In addition, we noticed subtle morphologic changes in blast cells when treated with HDL NPs (supplemental Figure 12).
Pathway changes of MV4-11 after treatment with HDL NPs. (A) Top 10 DEGs from bulk RNA sequencing analysis. (B) Top pathways that are enhanced from pathway analysis from DEGs. (C) Mature granulocyte surface marker staining of CD11c, CD14, and CD24 of MV4-11 after HDL NP treatment from 1 to 10 nM. ∗∗P < .01; ∗∗∗P < .01; ∗∗∗∗P < .0001.
Pathway changes of MV4-11 after treatment with HDL NPs. (A) Top 10 DEGs from bulk RNA sequencing analysis. (B) Top pathways that are enhanced from pathway analysis from DEGs. (C) Mature granulocyte surface marker staining of CD11c, CD14, and CD24 of MV4-11 after HDL NP treatment from 1 to 10 nM. ∗∗P < .01; ∗∗∗P < .01; ∗∗∗∗P < .0001.
HDL NP can be synergistic with SOC agents in AML
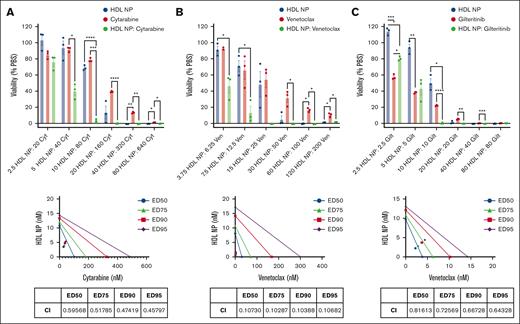
We evaluated the effects of combining HDL NPs with standard-of-care (SOC) agents for the treatment of AML, including cytarabine, venetoclax (a B-cell lymphoma 2 [BCL2] inhibitor), and gilteritinib (a FLT3 inhibitor). FLT3 mutation affects 20% to 30% of patients with AML.33,34 Analyzing the data with SynergyFinder,34,35 combinations of HDL NPs with cytarabine, venetoclax, and gilteritinib had overall additive effects (supplemental Figure 13). However, at concentration ratios with the highest synergy scores, there was synergy (Figure 6). Viability of MV4-11 cells was 68.9% ± 1.8% when treated with HDL NP at 10 nM and 78.6% ± 1.4% with cytarabine at 80 nM. The viability of combination therapy was 2.92% ± 1.5%. Calculated combination index by CompuSyn was as low as 0.46. Similarly, the viability after treatment with HDL NPs at 15 nM and venetoclax at 25 nM was 48.3% ± 15.8% and 54.4% ± 10.2%, respectively, compared with 0% when treated with the combination of HDL NP and venetoclax. The viability of HDL NPs at 10 nM and gilteritinib at 10 nM was 50.1% ± 5.4% and 22.1% ± 0.5%, respectively, compared with 0.2% ± 0.4% when treated with the combination. HDL NP combined with venetoclax or gilteritinb had combination index values well below 1. By comparison, when HEL cells were treated with a dose matrix of HDL NP in combination with cytarabine or venetoclax, the score was additive by SynergyFinder, and the graphs did not have a specific point of high synergy scores (supplemental Figure 14). HDL NPs were not synergistic in MV4-11 or HEL cells when combined with statins such as atorvastatin and rosuvastatin (supplemental Figure 15).
Synergy experiments to treat MV4-11 with HDL NPs combined with currently used agents to treat AML. MV4-11 cells were treated with HDL NPs and Cyt (A), Ven (B), and Gilt (C). Viability of the combinations at various concentrations (nanomolar) with a fixed ratio (top). Isobologram analysis of the combinations and the table represent the CI calculated by CompuSyn (bottom). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CI, combination index; Cyt, cytarabine; ED, effective dose; Gilt, gilteritinib; Ven, venetoclax.
Synergy experiments to treat MV4-11 with HDL NPs combined with currently used agents to treat AML. MV4-11 cells were treated with HDL NPs and Cyt (A), Ven (B), and Gilt (C). Viability of the combinations at various concentrations (nanomolar) with a fixed ratio (top). Isobologram analysis of the combinations and the table represent the CI calculated by CompuSyn (bottom). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CI, combination index; Cyt, cytarabine; ED, effective dose; Gilt, gilteritinib; Ven, venetoclax.
HDL NPs induced cell death in samples from patients with AML
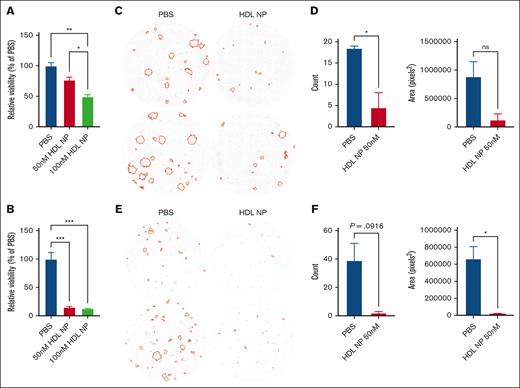
To demonstrate HDL NP efficacy beyond cell lines, we collected blood from patients with AML and isolated PBMCs, including leukemia blasts. Patients 1 and 2 had additional CD34 enrichment, whereas unselected PBMCs were used for patients 3 and 4 (supplemental Table 1). Isolated leukemic blasts from patients 1 and 2 expressed SR-B1, as detected by flow cytometry (supplemental Figure 16A-B). After treating leukemia cells with HDL NPs for 3 days, the viability was significantly reduced (defined as annexin V−PI− population) for patient 1 (49.45% ± 3.1%) and 2 (12.94% ± 0.4%; Figure 7A-B). To reduce effects of natural cell death during culture, we performed colony-forming assays with whole PBMCs from patients 3 and 4 instead of isolated populations. The imaged wells with and without treatment with HDL NPs are shown in Figure 7C-D. We used CD33 and HLA-DR to identify the leukemic blasts in the PBMCs and found that 46% of cells from patient 3 and 54.4% of cells from patient 4 expressed SR-B1 (supplemental Figure 16C-D). PBMCs cultured with HDL NPs in methylcellulose dramatically reduced CFUs (P < .05) and total area (P = not significant) for patient 3, and counts (P = .09) and total area (P = .04) for patient 4. In addition, we evaluated 2 patient bone marrow samples in the colony-forming assays (supplemental Figure 17). Both patient samples were CD34+ and isolated with a CD34 selection kit. One patient sample did not respond to HDL NP therapy. The other patient might have responded, but the images were hard to quantify because there were many individual cells, with only a few that formed colonies. However, the cells were visibly darker because of the HDL NP binding.
HDL NP inhibits leukemia cell growth with samples from patients with AML. Relative viability (Annexin V– PI–) of leukemia cells isolated from patient 1 (A) and patient 2 (B) after treatment with HDL NPs at 50 or 100 nM. Colony-forming assays of patient 3 (C) or patient 4 (D) treated with PBS or HDL NP at 50 nM. Images highlight the leukemic colonies in red as well as the CFU count or area measured by the images (E-F). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, not significant.
HDL NP inhibits leukemia cell growth with samples from patients with AML. Relative viability (Annexin V– PI–) of leukemia cells isolated from patient 1 (A) and patient 2 (B) after treatment with HDL NPs at 50 or 100 nM. Colony-forming assays of patient 3 (C) or patient 4 (D) treated with PBS or HDL NP at 50 nM. Images highlight the leukemic colonies in red as well as the CFU count or area measured by the images (E-F). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ns, not significant.
Discussion
Despite US Food and Drug Administration approval of new drugs over the last 5 years, AML management continues to be challenging, as evidenced by the OS rate of <40% at 5 years. Nanotechnology has already entered the AML treatment landscape.36,37 Statins may have promise in AML and have been linked to reduced progression from myelodysplastic syndrome to AML.28,38 However, the clinical benefits of statins combined with induction chemotherapy have not improved outcomes,35,36 and we saw no benefit in our studies. We demonstrate that the expression of SR-B1 and the dependence on cholesterol and enhanced antioxidant mechanisms for survival and treatment resistance are novel targets and treatment strategies for AML, respectively. We previously found that targeting SR-B1 with HDL NPs in cholesterol uptake-dependent malignancies potently induced cell death.36,37 Unlike CPX-351 and other nanotechnology platforms in cancer therapy, HDL NPs are not carriers but are bioinspired synthetic ligands designed to actively target SR-B1. The HDL NP therapy antagonizes native cholesterol-rich HDL binding to SR-B1 and inhibits enhanced cancer cell proliferation and survival maintained by HDL support of cholesterol uptake and redox balance. The HDL NP can disrupt these processes as a standalone therapeutic by targeting SR-B1, and our data suggest this may be a successful new paradigm for drug design.
SR-B1 could be considered both a target for AML cells and an important biomarker. It is important to note that normal cells expressing SR-B1 are not dependent on cholesterol uptake or increase reduction of lipid oxidation; thus, HDL NPs do not induce cell death.37,38 AML with high expression of SR-B1 may be more aggressive because it suggests more rapid uptake and use of cholesterol, which is a poor prognostic marker in lymphoma.39,40 The mechanism of cell death is predominantly ferroptosis and correlates with a reduction in GPx4 expression at both the mRNA and protein levels.39 HDL NPs target SR-B1 on AML cells and also cause GPx4 reduction. The mechanism of cell death was ferroptosis for HEL and U937 cells, as in our previous reports with lymphoma and ovarian cancer cells, along with GPx4 reduction. In AML, high GPx4 expression is associated with worse OS.41 Thus, targeting SR-B1 and reduction of GPx4 is a logical, novel, and attractive strategy. However, we found that MV4-11 cells underwent apoptosis and pyroptosis rather than ferroptosis despite the reduction of GPx4. The reason that MV4-11 cell death in our model is not related to ferroptosis is unclear, but there was nevertheless an increase in ROS accumulation. Theoretically, increasing ROS in the leukemia cells would improve the efficacy of chemotherapy.42 We found that cytarabine, venetoclax, and gilteritinib were synergistic with HDL NPs for MV4-11 cells. Finally, other difficult-to-treat and highly resistant cancers have been found to demonstrate stemness. They are highly resistant to oxidative stress, which renders them particularly sensitive to ferroptosis.43,44
From the RNA sequencing data, when evaluating the pathways in MV4-11 leukemia cells altered by HDL NPs, we found that increased pathways were related to neutrophil and monocyte activity, suggesting that the leukemia cells might be differentiating. The vitamin D receptor pathway exhibited the highest increased expression. The vitamin D pathway was associated with differentiation when leukemia cells were treated with isocitrate dehydrogenase inhibitors,45 similar to HDL NPs. Vitamin D receptor expression is reciprocal with AML stemness and leukemic propagation,46 so increasing vitamin D receptors would suggest less stemness and growth. Moreover, the increased expression of markers for mature granulocytes supports the differentiation of leukemia into neutrophils and monocytes. By contrast, HEL cells, which are erythroblasts, showed a different pattern. HEL cells demonstrated increases in the following cholesterol-related membrane pathways after treatment: nonintegrin membrane extracellular matrix interactions pathway, regulation of plasma membrane–bound cell projection organism pathway, and response to hormone pathway. It is unclear why HDL NPs cause some leukemias to differentiate whereas altering the membrane profile of others. This is an active and interesting area of pursuit.
Overall, we have developed a synthetic bioinspired platform, an HDL-like NP ligand, to target SR-B1 as a novel treatment strategy for AML by simultaneously regulating cell cholesterol and redox balance. The treatment potently induced ferroptosis and apoptosis in AML cell lines and samples from patients with AML. We found that HDL NPs are synergistic when combined with SOC agents, such as cytarabine, venetoclax, and gilteritinib. These studies open a new paradigm and strategy for developing targeted synthetic biologics, such as the HDL NP for SR-B1, and cancer therapy in AML. Currently, we are planning to proceed to phase 1 clinical trials with the HDL NPs, which will include an AML cohort. More work is needed to understand the mechanisms by which HDL NP ligands bind to SR-B1, reduce GPx4 expression, and cause cell death or differentiation in AML.
Acknowledgments
The authors thank the Robert H. Lurie Comprehensive Cancer Center (RHLCC) of Northwestern University in Chicago, Illinois, for the use of the Flow Cytometry Core Facility.
This work was supported by an RHLCCC through the National Cancer Institute Cancer Center Support Grant (P30 CA060553). A.Y.L. and E.Y. were supported by the Cancer Research Foundation Young Investigator Award. S.S. is supported by the American Society of Hematology Research Training Award for Fellows. A.Y.L., J.A., Y.A., L.C.P., C.S.T., and L.I.G. are supported by the RHLCCC Support Grant (P30 CA060553). L.C.P. is supported by National Institutes of Health grants NCI R01-CA121192 and VA I01CX000916. C.S.T. is grateful for a grant support from the V Foundation. L.I.G. is supported by the Shannahan, Brookstone, and Mander Foundations. This work was supported by the Northwestern University NUSeq Core Facility.
Authorship
Contribution: A.Y.L. was the project's lead scientist, performed and managed the experiments and data interpretation, and wrote the manuscript; J.S.R. manufactured the nanoparticles and performed experiments; E.Y. performed the experiments and analyzed the data; J.J.G. assisted in database analysis and creating figures; J.A. and Y.A. assisted in patient sample collection; S.S. and L.C.P. contributed new assays and analytical tools; T.J.Z. assisted in pathological evaluations of leukemia cells; C.S.T. and L.I.G. assisted in experimental design and the writing of this manuscript.
Conflict-of-interest disclosure: C.S.T. and L.I.G. are cofounders of a biotechnology company that licensed the technology from Northwestern University. A.Y.L. reports participation in speakers bureaus with AbbVie and Genmab. J.A. reports research support from Astellas and Lumanity; and participation in advisory boards with AbbVie, Astellas, BioSight, bluebird bio, Curio, Gilead, Kura Oncology, Kymera, Stemline Therapeutics, Syros, Dark Blue Therapeutics, Treadwell Therapeutics, Daiichi Sankyo, Rigel, Aptitude Health, MD Education, and VJHemOnc. Y.A. serves on scientific advisory boards or consults for Astellas, Bristol Myers Squibb (BMS), Geron, Kite, Pfizer, Rigel, and Servier; and receives institutional trial support from AbbVie, ALX Oncology, Biomea, BioSight, Curis, and Novartis. L.I.G. has participated in advisory boards for Kite/Gilead, Juno/Celgene/BMS, and Bayer; and reports research funding from Juno/Celgene/BMS. The remaining authors declare no competing financial interests.
Correspondence: Adam Y. Lin, Division of Hematology/Oncology, Department of Medicine, Feinberg School of Medicine, Northwestern University, 676 N Saint Clair, Arkes Suite 850, Chicago, IL 60611; email: adam.lin@northwestern.edu; and C. Shad Thaxton, Department of Urology, Feinberg School of Medicine, Northwestern University, 676 N Saint Clair, Arkes Suite 2300, Chicago, IL 60611; email: cthaxton003@northwestern.edu.
References
Author notes
Data are available on request from the corresponding authors, Adam Y. Lin (adam.lin@northwestern.edu) and C. Shad Thaxton (cthaxton003@northwestern.edu).
The full-text version of this article contains a data supplement.