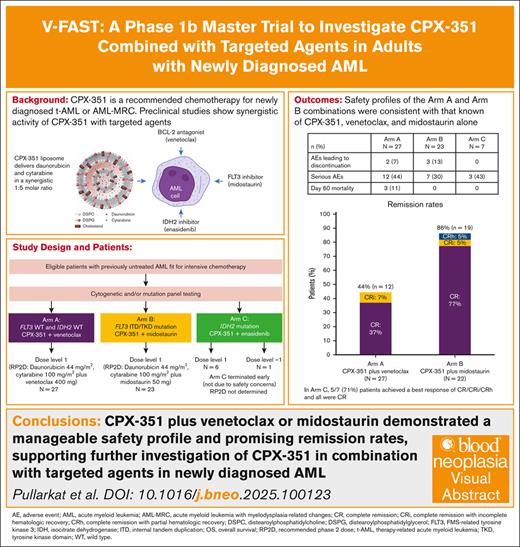
Key Points
CPX-351 combined with venetoclax or midostaurin demonstrated a manageable safety profile and promising remission rates.
These results support further investigation of CPX-351 in combination with targeted agents in patients with newly diagnosed AML.
Visual Abstract
Preclinical data suggest CPX-351, approved for patients with newly diagnosed therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes, may exhibit synergy with targeted agents, suggesting a rationale for combining targeted agents with CPX-351 as a chemotherapy backbone. The V-FAST (Vyxeos – First Phase ASsessment With Targeted Agents) phase 1b trial evaluated CPX-351 with the targeted agents venetoclax (arm A), midostaurin (arm B), and enasidenib (arm C) in adults with newly diagnosed AML fit for intensive chemotherapy. The dose exploration phase used a 3+3 design to determine the recommended phase 2 dose (RP2D) for each combination. The expansion phase enrolled additional patients to confirm the RP2D. Primary end points were the RP2D and safety. Secondary end points included initial efficacy assessments. Overall, 57 patients were enrolled (arm A, n = 27; arm B, n = 23; arm C, n = 7). In arms A and B, the RP2D was established: CPX-351 (daunorubicin 44 mg/m2 and cytarabine 100 mg/m2) plus venetoclax 400 mg or midostaurin 50 mg, respectively. Arm C was stopped early by the sponsor (not due to safety concerns), and the RP2D was not determined. The safety profiles of the combinations were consistent with those known for CPX-351, venetoclax, and midostaurin alone; the most common adverse events were hematologic and gastrointestinal. The best response (complete remission [CR]/CR with incomplete/partial hematologic recovery) was 44% (12/27 patients) and 86% (19/22 patients) in arms A and B, respectively. Although few patients received CPX-351 with enasidenib, these results suggest that CPX-351 may be safely combined with venetoclax or midostaurin. This trial was registered at www.clinicaltrials.gov as #NCT04075747.
Introduction
Patients with newly diagnosed acute myeloid leukemia (AML) who are fit for intensive chemotherapy (IC) are often treated with combination therapy, depending on the molecular subtype of the disease. The conventional 7+3 regimen has been the backbone of IC for newly diagnosed AML for decades.1-3 However, the AML treatment landscape continues to evolve, with several new therapies receiving approval.4 One of these therapies, CPX-351 (VYXEOS in the United States and Canada; Vyxeos liposomal in Europe and the United Kingdom), a dual-drug liposomal encapsulation of daunorubicin and cytarabine in a synergistic 1:5 molar ratio, is approved for newly diagnosed therapy-related AML or AML with myelodysplasia-related changes in adults and children aged ≥1 year in the United States and in adults in Europe, Canada, and the United Kingdom who are eligible for IC.5-8 Approval of CPX-351 was based on the pivotal phase 3 trial in adults aged 60 to 75 years with newly diagnosed, high-risk or secondary AML,9 in which CPX-351 significantly improved median overall survival (OS; 9.56 vs 5.95 months; 1-sided P = .003) and remission rate (complete response [CR] + CR with incomplete hematologic recovery [CRi]; 47.7% vs 33.3%; 2-sided P = .016) compared to 7+3, with a comparable safety profile. After 5 years of follow-up, the improved median OS and remission rate with CPX-351 vs 7+3 were maintained, with a 5-year Kaplan-Meier (KM) survival rate of 18% vs 8%.10
Recent advancements in the understanding of genetic involvement in AML facilitated the development of several molecularly targeted AML therapies, including the B-cell lymphoma 2 inhibitor venetoclax, the FMS-related tyrosine kinase 3 (FLT3) inhibitor midostaurin, and the isocitrate dehydrogenase 2 (IDH2) inhibitor enasidenib.2-4 Preclinical data suggest that CPX-351 plus venetoclax11 or midostaurin12 may have synergistic antitumor effects in AML. Although combining these targeted therapies with conventional chemotherapy demonstrated efficacy in adults with newly diagnosed AML,13-15 the feasibility and safety of combining CPX-351 with such targeted agents remains unclear. The V-FAST (Vyxeos – First Phase ASsessment With Targeted Agents) trial was designed to determine the recommended phase 2 dose (RP2D), safety, and preliminary efficacy of CPX-351 plus venetoclax, midostaurin, or enasidenib in adults with newly diagnosed AML who were fit for IC.
Methods
Study design
V-FAST was an open-label, multicenter, multiarm, nonrandomized phase 1b trial (clinicaltrials.gov identifier: NCT04075747) that enrolled patients from 9 US centers between 21 November 2019 and 29 September 2023. V-FAST investigated CPX-351 combined with targeted agents in patients with newly diagnosed AML who were fit for IC (supplemental Methods).
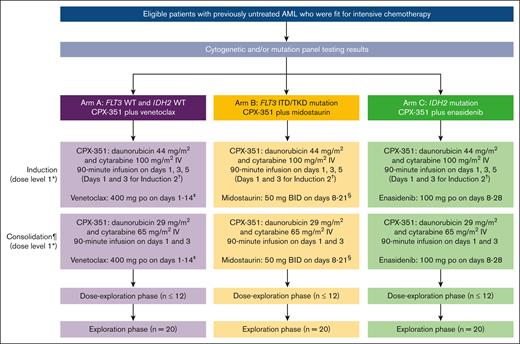
Three treatment arms (Figure 1) were included in V-FAST, designed to independently investigate CPX-351 with a different targeted agent, with no comparison planned between arms: CPX-351 plus venetoclax (arm A); CPX-351 plus midostaurin (arm B); and CPX-351 plus enasidenib (arm C). Enrolled patients were assigned to each arm according to cytogenetic and/or mutation panel testing (see “Patient eligibility”).
Study design and treatment. ∗For de-escalation to dose level –1 in arm C, patients received CPX-351 induction and consolidation at dose level 1 combined with enasidenib 100 mg on days 8 to 21. †Patients could have received up to 2 induction courses in total at the discretion of the treating investigator. A second induction was highly recommended for any patient with no response or documented reduction in leukemia burden. If safe to administer, a second induction was mandatory for patients who achieved >50% reduction in the percentage of blasts count in their BM assessment during the first induction on day 14 for arm A and on day 21 for arms B and C. ‡During the first induction, to mitigate the risk of potential tumor lysis syndrome, a dose ramp-up of venetoclax was administered from 100 mg/d on day 1 to 200 mg/d on day 2, and then 400 mg/d (target dose) on days 3 to 14. For subsequent inductions or consolidations, venetoclax was administered at the full target dose (400 mg on days 1 to 14). Based on previous PK studies, concomitant treatment with a strong CYP3A inhibitor was permitted after the venetoclax dose ramp-up with a dose reduction of venetoclax by 75%, whereas concomitant treatment with a moderate CYP3A inhibitor was permitted after the venetoclax dose ramp-up with a dose reduction of venetoclax by 50%. §Coadministration of midostaurin with strong CYP3A inhibitors may increase midostaurin concentrations, with increase more pronounced in the first week of midostaurin administration, and this may increase the risk of midostaurin-associated toxicity. Alternative therapies that do not strongly inhibit CYP3A activity were recommended. Alternatively, with coadministration of midostaurin and strong CYP3A inhibitors, monitoring patients for increased risk of adverse reactions, especially during the first week of midostaurin administration in each course of therapy was recommended. ¶Patients who achieved remission (CR, CRi, or CRh), neutrophils ≥0.5 × 109/L, and platelets ≥50 × 109/L could have received up to 2 consolidation courses at the discretion of the treating investigator. BID, twice daily; CYP, cytochrome P450 enzymes; ITD, internal tandem duplication; TKD, tyrosine kinase domain; WT, wild type.
Study design and treatment. ∗For de-escalation to dose level –1 in arm C, patients received CPX-351 induction and consolidation at dose level 1 combined with enasidenib 100 mg on days 8 to 21. †Patients could have received up to 2 induction courses in total at the discretion of the treating investigator. A second induction was highly recommended for any patient with no response or documented reduction in leukemia burden. If safe to administer, a second induction was mandatory for patients who achieved >50% reduction in the percentage of blasts count in their BM assessment during the first induction on day 14 for arm A and on day 21 for arms B and C. ‡During the first induction, to mitigate the risk of potential tumor lysis syndrome, a dose ramp-up of venetoclax was administered from 100 mg/d on day 1 to 200 mg/d on day 2, and then 400 mg/d (target dose) on days 3 to 14. For subsequent inductions or consolidations, venetoclax was administered at the full target dose (400 mg on days 1 to 14). Based on previous PK studies, concomitant treatment with a strong CYP3A inhibitor was permitted after the venetoclax dose ramp-up with a dose reduction of venetoclax by 75%, whereas concomitant treatment with a moderate CYP3A inhibitor was permitted after the venetoclax dose ramp-up with a dose reduction of venetoclax by 50%. §Coadministration of midostaurin with strong CYP3A inhibitors may increase midostaurin concentrations, with increase more pronounced in the first week of midostaurin administration, and this may increase the risk of midostaurin-associated toxicity. Alternative therapies that do not strongly inhibit CYP3A activity were recommended. Alternatively, with coadministration of midostaurin and strong CYP3A inhibitors, monitoring patients for increased risk of adverse reactions, especially during the first week of midostaurin administration in each course of therapy was recommended. ¶Patients who achieved remission (CR, CRi, or CRh), neutrophils ≥0.5 × 109/L, and platelets ≥50 × 109/L could have received up to 2 consolidation courses at the discretion of the treating investigator. BID, twice daily; CYP, cytochrome P450 enzymes; ITD, internal tandem duplication; TKD, tyrosine kinase domain; WT, wild type.
Each arm comprised 2 phases: a dose exploration phase and an expansion phase. The dose exploration phase used a 3+3 design to evaluate safety, determine dose-limiting toxicities (DLTs), and establish the RP2D for each combination. An independent safety assessment committee reviewed the safety data during the dose exploration phase. The DLT observation period was days 1 to 49 after starting treatment, with a minimum observation period of 28 days. Dose de-escalation to dose level –1 (Figure 1) was permitted if ≥2 of the first 3 evaluable patients or ≥2 of the 6 total evaluable patients at dose level 1 experienced a DLT. In the expansion phase, additional patients received study treatment to confirm the RP2D and to further evaluate safety and assess initial efficacy. Patients were followed for safety (1 month [±1 week] after end of treatment/early termination of treatment) and survival (≤2 years after the first administration of study treatment). After study treatment discontinuation, patients could receive subsequent therapies at the treating investigator’s discretion.
This trial was conducted in accordance with international guidelines, including the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Tripartite Guideline for Good Clinical Practice, and other applicable laws and regulations.
Patient eligibility
Eligible patients were aged 18 to 75 years with newly diagnosed AML per the World Health Organization 2017 pathologic criteria16 and deemed fit to receive IC (supplemental Methods). Eligible patients were those without FLT3 mutations and/or IDH2 mutation in arm A, with FLT3 mutations in arm B, or with an IDH2 mutation in arm C; thus, patients were not limited to the approved indication of CPX-351. Exclusion criteria included favorable risk cytogenetics: (t8;21), inv(16), t(16;16), or t(15;17) karyotype abnormalities.
Study treatment
At dose level 1 across all treatment arms, all patients received first induction with CPX-351 as a 90-minute IV infusion of daunorubicin 44 mg/m2 and cytarabine 100 mg/m2 on days 1, 3, and 5, plus a targeted agent depending on the treatment arm (Figure 1). In arm A, patients received venetoclax. During the first induction, to mitigate the risk of tumor lysis syndrome, a venetoclax dose ramp-up was administered (100 mg/d on day 1; 200 mg/d on day 2; and 400 mg/d on days 3-14); for subsequent inductions/consolidations, venetoclax was administered at the target dose (400 mg orally [po] on days 1-14). After the venetoclax dose ramp-up, concomitant treatment with a strong or moderate CYP3A inhibitor was permitted, with a dose reduction of venetoclax by 75% or 50%, respectively. In arm B, patients received midostaurin 50 mg po twice daily on days 8 to 21. In arm C, patients received enasidenib 100 mg po on days 8 to 28. Patients could have received ≤4 treatment courses (2 inductions and 2 consolidations; Figure 1).
Only patients who achieved CR or CRi/CR with partial hematologic recovery (CRh) with absolute neutrophil count (ANC) of ≥0.5 × 109/L and platelet count of ≥50 × 109/L after induction were eligible to receive consolidation. Consolidation with hematopoietic cell transplantation (HCT) or other therapies was permitted in place of or after study consolidation therapy. Patients who did not achieve CR or CRi/CRh after 2 inductions and those who did not recover ANC or platelet counts by day 75 discontinued study treatment and proceeded to end of treatment/early termination of treatment visit and follow-up.
Outcomes and assessments
Primary end points were the RP2D and safety/tolerability of CPX-351 with the targeted agents, determined by the dose de-escalation/dose escalation algorithm and incidence of adverse events (AEs) and DLTs. AEs or any worsening of preexisting conditions that occurred on or after the first dose of study treatment (CPX-351 and/or targeted agent) were recorded ≤30 days after the end of treatment/early termination of treatment visit. AEs were coded using the Medical Dictionary for Regulatory Activities version 21.1 and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. DLTs were evaluated for each combination during the first induction of the dose exploration phase. DLTs were defined as grade ≥3 hematologic toxicities (lasting ≥49 days after the first dose of therapy with a hypocellular bone marrow [BM; <20%] and <5% marrow blasts or a hypocellular BM with <5% blasts, ANC ≥0.5 × 109/L, and platelets ≥50 × 109/L by day 49) and grade ≥3 treatment-related nonhematologic toxicities. For treatment-related events to be considered DLTs, patients must have received ≥1 dose of CPX-351 and the targeted agent at the time of onset. Common events in patients with leukemia (often occurring in >50% of patients; eg, grade 3 neutropenic fever without infection), were not used to define DLTs.
Secondary end points included the proportion of patients who achieved CR, CRi, CRh, CR/CRi, CR/CRh, and morphologic leukemia-free state (MLFS) after ≤2 inductions; patients who achieved CR/CRi or CR/CRh with minimal/measurable residual disease (MRD)–negative status after ≤2 inductions; and pharmacokinetics (PK) of CPX-351 for each combination. Response was investigator assessed in patients with evaluable BM using modified criteria based on the 2017 European LeukemiaNet criteria1 and DiNardo et al,17 including MRD-negative status (CR/CRi/CRh and <0.1% leukemic cells) from local laboratory multicolor flow cytometry results (supplemental Table 1).
Exploratory end points were duration of remission (DOR); OS and event-free survival (EFS) 2 years after the first administration of the study treatment; and the proportion of patients who received HCT.
Statistical analyses
As a phase 1b trial with no hypothesis testing, formal sample size calculations were not undertaken. Each treatment arm planned to enroll ≤12 patients in the dose exploration phase and ≤20 patients at the RP2D in the expansion phase, for a total of ≤32 patients per arm.
All safety analyses were conducted for patients who received ≥1 dose of CPX-351/targeted agent. All efficacy analyses (response, DOR, OS, and EFS) were conducted for patients who received ≥1 dose of CPX-351/targeted agent and had ≥1 response assessment. DOR, OS, and EFS were estimated using the KM method (defined in the supplemental Methods). Descriptive PK summaries included plasma concentrations (per nominal time points by treatment arm and dose level) from all patients who received ≥1 dose of CPX-351/targeted agent and had ≥1 evaluable PK concentration for either study treatment.
Results
Patient disposition and characteristics
Overall, 57 patients were enrolled into V-FAST and received study treatment: 27 patients in arm A received dose level 1; 23 patients in arm B received dose level 1; and 7 patients in arm C, including 6 who received dose level 1 and 1 who received dose level –1 (Figure 1; supplemental Figure 1). Patient numbers by number of induction and consolidation cycles are provided in supplemental Figure 1. Further enrollment into arm C was stopped early based on sponsor decision, not due to safety or efficacy concerns. Patient demographics and baseline characteristics were consistent with a population of patients with newly diagnosed AML (Table 1). In arm A (n = 27), the median age was 57 years; 14 patients (52%) had poor-risk disease, 5 (19%) had mutated TP53, and 7 (26%) had antecedent hematologic disorder, including 4 (15%) with antecedent myelofibrosis. In arm B (n = 23), the median age was 66 years, and 7 patients (30%) had poor-risk disease. In arm C (n = 7), the median age was 50 years, and 2 patients (29%) had poor-risk disease.
Patient baseline demographics and clinical characteristics by treatment arm
| Characteristic . | Arm A CPX-351 plus venetoclax (N = 27) . | Arm B CPX-351 plus midostaurin (N = 23) . | Arm C CPX-351 plus enasidenib (N = 7) . |
|---|---|---|---|
| Age, median (range), y | 57 (35-71) | 66 (40-74) | 50 (39-68) |
| 18-59, n (%) | 16 (59) | 5 (22) | 5 (71) |
| ≥60, n (%) | 11 (41) | 18 (78) | 2 (29) |
| Sex at birth, n (%) | |||
| Male | 15 (56) | 11 (48) | 3 (43) |
| Female | 12 (44) | 12 (52) | 4 (57) |
| Race, n (%) | |||
| White | 20 (74) | 18 (78) | 6 (86) |
| Black or African American | 3 (11) | 2 (9) | 0 |
| Asian | 2 (7) | 2 (9) | 1 (14) |
| Declined to state | 2 (7) | 1 (4) | 0 |
| Disease risk classification, n (%) | |||
| Favorable | 5 (19) | 1 (4) | 1 (14) |
| Intermediate | 8 (30) | 15 (65) | 4 (57) |
| Poor | 14 (52) | 7 (30) | 2 (29) |
| Positive for mutations, n (%) | |||
| FLT3-ITD | 1 (4)∗ | 18 (78) | 0 |
| FLT3-TKD | 0 | 5 (22) | 0 |
| NPM1 | 4 (15) | 16 (70) | 2 (29) |
| CBPA | 4 (15) | 1 (4) | 1 (14) |
| IDH1 | 1 (4) | 3 (13) | 0 |
| IDH2 | 0 | 2 (9) | 7 (100) |
| RUNX1 | 1 (4) | 2 (9) | 1 (14) |
| ASXL1 | 3 (11) | 2 (9) | 2 (29) |
| TP53 | 5 (19) | 0 | 0 |
| Other | 19 (70) | 9 (39) | 5 (71) |
| AML subtype,†n (%) | |||
| Newly diagnosed AML per WHO criteria | 27 (100) | 22 (96)‡ | 7 (100) |
| De novo AML | 19 (70) | 17 (74) | 5 (71) |
| Antecedent hematologic disorder§ | 7 (26) | 4 (17) | 2 (29) |
| Therapy-related AML | 4 (15) | 3 (13) | 0 |
| ECOG PS, n (%) | |||
| 0 | 11 (41) | 6 (26) | 5 (71) |
| 1 | 14 (52) | 15 (65) | 1 (14) |
| 2 | 2 (7) | 2 (9) | 1 (14) |
| Characteristic . | Arm A CPX-351 plus venetoclax (N = 27) . | Arm B CPX-351 plus midostaurin (N = 23) . | Arm C CPX-351 plus enasidenib (N = 7) . |
|---|---|---|---|
| Age, median (range), y | 57 (35-71) | 66 (40-74) | 50 (39-68) |
| 18-59, n (%) | 16 (59) | 5 (22) | 5 (71) |
| ≥60, n (%) | 11 (41) | 18 (78) | 2 (29) |
| Sex at birth, n (%) | |||
| Male | 15 (56) | 11 (48) | 3 (43) |
| Female | 12 (44) | 12 (52) | 4 (57) |
| Race, n (%) | |||
| White | 20 (74) | 18 (78) | 6 (86) |
| Black or African American | 3 (11) | 2 (9) | 0 |
| Asian | 2 (7) | 2 (9) | 1 (14) |
| Declined to state | 2 (7) | 1 (4) | 0 |
| Disease risk classification, n (%) | |||
| Favorable | 5 (19) | 1 (4) | 1 (14) |
| Intermediate | 8 (30) | 15 (65) | 4 (57) |
| Poor | 14 (52) | 7 (30) | 2 (29) |
| Positive for mutations, n (%) | |||
| FLT3-ITD | 1 (4)∗ | 18 (78) | 0 |
| FLT3-TKD | 0 | 5 (22) | 0 |
| NPM1 | 4 (15) | 16 (70) | 2 (29) |
| CBPA | 4 (15) | 1 (4) | 1 (14) |
| IDH1 | 1 (4) | 3 (13) | 0 |
| IDH2 | 0 | 2 (9) | 7 (100) |
| RUNX1 | 1 (4) | 2 (9) | 1 (14) |
| ASXL1 | 3 (11) | 2 (9) | 2 (29) |
| TP53 | 5 (19) | 0 | 0 |
| Other | 19 (70) | 9 (39) | 5 (71) |
| AML subtype,†n (%) | |||
| Newly diagnosed AML per WHO criteria | 27 (100) | 22 (96)‡ | 7 (100) |
| De novo AML | 19 (70) | 17 (74) | 5 (71) |
| Antecedent hematologic disorder§ | 7 (26) | 4 (17) | 2 (29) |
| Therapy-related AML | 4 (15) | 3 (13) | 0 |
| ECOG PS, n (%) | |||
| 0 | 11 (41) | 6 (26) | 5 (71) |
| 1 | 14 (52) | 15 (65) | 1 (14) |
| 2 | 2 (7) | 2 (9) | 1 (14) |
ECOG PS, Eastern Cooperative Oncology Group performance status; ITD, internal tandem duplications; TKD, tyrosine kinase domain; WHO, World Health Organization.
A protocol deviation resulted in 1 patient with low FLT3-ITD allelic ratio being enrolled into arm A.
Patients may have had multiple AML subtypes.
One patient in arm B had mixed-lineage leukemia and did not have newly diagnosed AML per WHO criteria.
Including 4 patients who had antecedent myelofibrosis.
There were 8, 12, and 6 patients in arms A, B, and C, respectively, who completed the trial (ie, had survival follow-up for ≤2 years after the first administration of study treatment), whereas 19, 11, and 1 patient, respectively, discontinued from the trial due to death (supplemental Figure 1).
Determination of the RP2D
In arm A, 1 patient reported 2 DLT events while receiving dose level 1 (grade 4 nonserious events of thrombocytopenia and neutropenia, considered related to study treatment by the investigator). Three additional patients were added at dose level 1 with no further DLTs observed; therefore, dose level 1 was determined to be the RP2D for arm A.
In arm B, no DLTs were identified during dose exploration; therefore, dose level 1 was determined to be the RP2D for arm B.
In arm C, 1 patient experienced increased transaminases while receiving dose level 1, and 1 experienced significant hematologic toxicity (neutropenia). Although these 2 events were not considered a DLT by the investigator, review by the independent safety assessment committee determined these events met DLT criteria and recommended dose de-escalation to dose level –1. Enrollment was stopped early for the 3-patient cohort at dose level –1 based on a decision by the sponsor, not due to safety or efficacy concerns; therefore, the RP2D was not determined for arm C.
Safety
Across the 3 arms, all patients experienced ≥1 AE of any grade (Table 2; supplemental Table 2), and the most common AEs were hematologic and gastrointestinal events.
Most frequently reported AEs (in 20% of patients or more) by preferred term occurring in arm A or arm B
| AE . | Arm A CPX-351 plus venetoclax (N = 27) . | Arm B CPX-351 plus midostaurin (N = 23) . |
|---|---|---|
| Patients with any AE (all grades), n (%) | 27 (100) | 23 (100) |
| Patients with any hematologic AE (all grades), n (%) | ||
| Febrile neutropenia | 18 (67) | 18 (78) |
| Thrombocytopenia | 12 (44) | 16 (70) |
| Leukopenia | 11 (41) | 14 (61) |
| Neutropenia | 10 (37) | 9 (39) |
| Anemia | 7 (26) | 8 (35) |
| Patients with any nonhematologic AE (all grades), n (%) | ||
| Constipation | 14 (52) | 4 (17) |
| Nausea | 10 (37) | 15 (65) |
| Fatigue | 10 (37) | 6 (26) |
| Hypokalemia | 9 (33) | 8 (35) |
| Peripheral edema | 9 (33) | 7 (30) |
| Diarrhea | 8 (30) | 8 (35) |
| Maculopapular rash | 8 (30) | 5 (22) |
| Headache | 7 (26) | 10 (43) |
| Pyrexia | 7 (26) | 5 (22) |
| Hypocalcemia | 6 (22) | 4 (17) |
| Blood alkaline phosphatase increased | 5 (19) | 7 (30) |
| AST increased | 4 (15) | 8 (35) |
| Hyperphosphatemia | 4 (15) | 5 (22) |
| Vomiting | 4 (15) | 5 (22) |
| ALT increased | 3 (11) | 12 (52) |
| Cough | 3 (11) | 5 (22) |
| Hypoalbuminemia | 3 (11) | 8 (35) |
| Hyponatremia | 3 (11) | 10 (43) |
| Blood bilirubin increased | 2 (7) | 5 (22) |
| Hyperglycemia | 2 (7) | 6 (26) |
| AE . | Arm A CPX-351 plus venetoclax (N = 27) . | Arm B CPX-351 plus midostaurin (N = 23) . |
|---|---|---|
| Patients with any AE (all grades), n (%) | 27 (100) | 23 (100) |
| Patients with any hematologic AE (all grades), n (%) | ||
| Febrile neutropenia | 18 (67) | 18 (78) |
| Thrombocytopenia | 12 (44) | 16 (70) |
| Leukopenia | 11 (41) | 14 (61) |
| Neutropenia | 10 (37) | 9 (39) |
| Anemia | 7 (26) | 8 (35) |
| Patients with any nonhematologic AE (all grades), n (%) | ||
| Constipation | 14 (52) | 4 (17) |
| Nausea | 10 (37) | 15 (65) |
| Fatigue | 10 (37) | 6 (26) |
| Hypokalemia | 9 (33) | 8 (35) |
| Peripheral edema | 9 (33) | 7 (30) |
| Diarrhea | 8 (30) | 8 (35) |
| Maculopapular rash | 8 (30) | 5 (22) |
| Headache | 7 (26) | 10 (43) |
| Pyrexia | 7 (26) | 5 (22) |
| Hypocalcemia | 6 (22) | 4 (17) |
| Blood alkaline phosphatase increased | 5 (19) | 7 (30) |
| AST increased | 4 (15) | 8 (35) |
| Hyperphosphatemia | 4 (15) | 5 (22) |
| Vomiting | 4 (15) | 5 (22) |
| ALT increased | 3 (11) | 12 (52) |
| Cough | 3 (11) | 5 (22) |
| Hypoalbuminemia | 3 (11) | 8 (35) |
| Hyponatremia | 3 (11) | 10 (43) |
| Blood bilirubin increased | 2 (7) | 5 (22) |
| Hyperglycemia | 2 (7) | 6 (26) |
All AEs were coded using Medical Dictionary for Regulatory Activities, version 21.1. Multiple entries for an individual patient under each preferred term were only counted once.
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
In arm A, the most common AEs were febrile neutropenia (18/27 patients [67%]), constipation (14/27 patients [52%]), and thrombocytopenia (12/27 patients [44%]; Table 2). Most patients (25/27 [93%]) experienced grade ≥3 AEs; the most common were febrile neutropenia (17/27 patients [63%]), thrombocytopenia (12/27 patients [44%]), and leukopenia (11/27 patients [41%]; Table 3). Two patients (7%) experienced AEs leading to discontinuation of both CPX-351 and venetoclax: cardiac failure congestive (n = 1), considered by the investigator as related to CPX-351; and worsening of AML (n = 1), which was not considered by the investigator as related to CPX-351 or venetoclax. No AEs led to dose reduction of study treatment (CPX-351, venetoclax, or both) or dose interruption of CPX-351. In total, 12 of 27 patients (44%) experienced serious AEs, and the most common serious AE was febrile neutropenia (5/27 patients [19%]; Table 4). Three patients (11%) experienced AEs leading to death: sepsis (n = 2), both of which were considered by the investigator as related to CPX-351 and venetoclax; and ischemic stroke (n = 1), which was considered by the investigator as not related to CPX-351 or venetoclax. Early mortality within 60 days of the first dose of study treatment occurred in 3 patients (11%) due to disease progression (n = 2) and sepsis (n = 1; patient died within 30 days of the first dose of study treatment). In patients who achieved CR/CRi (n = 12), the median time to ANC (≥0.5 × 109/L) and platelet recovery (≥50 × 109/L) was 35.5 (interquartile range [IQR], 32.5-36.5) and 35.5 days (IQR, 27-38.5), respectively.
Most frequently reported grade 3 or higher AEs (in more than 10% of patients) by preferred term occurring in arm A or arm B
| AE . | Arm A CPX-351 plus venetoclax (N = 27) . | Arm B CPX-351 plus midostaurin (N = 23) . |
|---|---|---|
| Patients with any grade ≥3 AE, n (%) | 25 (93) | 23 (100) |
| Patients with grade ≥3 hematologic AE, n (%) | ||
| Febrile neutropenia | 17 (63) | 16 (70) |
| Thrombocytopenia | 12 (44) | 15 (65) |
| Leukopenia | 11 (41) | 14 (61) |
| Neutropenia | 10 (37) | 9 (39) |
| Anemia | 5 (19) | 8 (35) |
| Lymphopenia | 3 (11) | 0 |
| Patients with grade ≥3 nonhematologic AE, n (%) | ||
| Hypokalemia | 3 (11) | 1 (4) |
| Hypoxia | 3 (11) | 1 (4) |
| Sepsis | 3 (11) | 1 (4) |
| Syncope | 3 (11) | 0 |
| Pneumonia | 2 (7) | 4 (17) |
| AE . | Arm A CPX-351 plus venetoclax (N = 27) . | Arm B CPX-351 plus midostaurin (N = 23) . |
|---|---|---|
| Patients with any grade ≥3 AE, n (%) | 25 (93) | 23 (100) |
| Patients with grade ≥3 hematologic AE, n (%) | ||
| Febrile neutropenia | 17 (63) | 16 (70) |
| Thrombocytopenia | 12 (44) | 15 (65) |
| Leukopenia | 11 (41) | 14 (61) |
| Neutropenia | 10 (37) | 9 (39) |
| Anemia | 5 (19) | 8 (35) |
| Lymphopenia | 3 (11) | 0 |
| Patients with grade ≥3 nonhematologic AE, n (%) | ||
| Hypokalemia | 3 (11) | 1 (4) |
| Hypoxia | 3 (11) | 1 (4) |
| Sepsis | 3 (11) | 1 (4) |
| Syncope | 3 (11) | 0 |
| Pneumonia | 2 (7) | 4 (17) |
All AEs were coded using Medical Dictionary for Regulatory Activities, version 21.1. Severity grade was coded using Common Terminology Criteria for Adverse Events, version 5.0. Multiple entries for an individual patient under each preferred term were only counted once.
All serious AEs by preferred term in arm A or arm B
| AE . | Arm A CPX-351 plus venetoclax (N = 27) . | Arm B CPX-351 plus midostaurin (N = 23) . |
|---|---|---|
| Patients with any serious AE, n (%) | 12 (44) | 7 (30) |
| Patients with serious hematologic AE, n (%) | ||
| Febrile neutropenia | 5 (19) | 1 (4) |
| AML | 1 (4) | 0 |
| Patients with serious nonhematologic AE, n (%) | ||
| Sepsis | 3 (11) | 0 |
| Congestive cardiac failure | 1 (4) | 0 |
| Ischemic stroke | 1 (4) | 0 |
| Skin infection | 1 (4) | 0 |
| Acute respiratory failure | 0 | 1 (4) |
| Anemia | 0 | 1 (4) |
| Blood bilirubin increased | 0 | 1 (4) |
| Coronavirus infection | 0 | 1 (4) |
| Delirium | 0 | 1 (4) |
| Ejection fraction decreased | 0 | 1 (4) |
| Myocarditis | 0 | 1 (4) |
| Pericarditis | 0 | 1 (4) |
| Small intestinal obstruction | 0 | 1 (4) |
| Thrombosis | 0 | 1 (4) |
| AE . | Arm A CPX-351 plus venetoclax (N = 27) . | Arm B CPX-351 plus midostaurin (N = 23) . |
|---|---|---|
| Patients with any serious AE, n (%) | 12 (44) | 7 (30) |
| Patients with serious hematologic AE, n (%) | ||
| Febrile neutropenia | 5 (19) | 1 (4) |
| AML | 1 (4) | 0 |
| Patients with serious nonhematologic AE, n (%) | ||
| Sepsis | 3 (11) | 0 |
| Congestive cardiac failure | 1 (4) | 0 |
| Ischemic stroke | 1 (4) | 0 |
| Skin infection | 1 (4) | 0 |
| Acute respiratory failure | 0 | 1 (4) |
| Anemia | 0 | 1 (4) |
| Blood bilirubin increased | 0 | 1 (4) |
| Coronavirus infection | 0 | 1 (4) |
| Delirium | 0 | 1 (4) |
| Ejection fraction decreased | 0 | 1 (4) |
| Myocarditis | 0 | 1 (4) |
| Pericarditis | 0 | 1 (4) |
| Small intestinal obstruction | 0 | 1 (4) |
| Thrombosis | 0 | 1 (4) |
All AEs were coded using Medical Dictionary for Regulatory Activities, version 21.1. Multiple entries for an individual patient under each preferred term were only counted once.
In arm B, the most common AEs were febrile neutropenia (18/23 patients [78%]), thrombocytopenia (16/23 patients [70%]), and nausea (15/23 patients [65%]; Table 2). All 23 patients (100%) experienced grade ≥3 AEs, and the most common were febrile neutropenia (16/23 patients [70%]), thrombocytopenia (15/23 patients [65%]), and leukopenia (14/23 patients [61%]; Table 3). Three patients (13%) experienced AEs leading to study treatment discontinuation: ejection fraction decreased (n = 1) and acute respiratory failure (n = 1), both considered by the investigator as related to CPX-351 and midostaurin and led to discontinuation of both treatments in both patients; and colitis (n = 1), which was considered by the investigator as related to CPX-351 but not midostaurin; however, midostaurin was discontinued. One patient (4%) experienced an AE leading to dose reduction of study treatment (electrocardiogram QT prolonged, considered by the investigator as related to midostaurin). Two patients (9%) experienced AEs leading to dose interruption of study treatment: lung infection (COVID-19) and hypoxia (n = 1), not considered related to study treatment; and blood bilirubin increased (n = 1), considered by the investigator as related to CPX-351. Seven patients (30%) experienced serious AEs, but no serious AE was reported in more than 1 patient (Table 4). No patients experienced AEs leading to death. No patients in arm B died within 30 or 60 days of the first dose of study treatment. In patients who achieved CR/CRi (n = 18), the median time to ANC and platelet recovery was 34.5 days (IQR, 30-36) and 32.5 days (IQR, 28-41), respectively.
In arm C, the most common any-grade AEs are summarized in supplemental Table 2. All 7 patients (100%) experienced grade ≥3 AEs, and the most common were febrile neutropenia (7/7 patients [100%]), thrombocytopenia (6/7 patients [86%]), and neutropenia (5/7 patients [71%]; Table 5). No patients experienced AEs leading to discontinuation or dose reduction of study treatment (CPX-351, enasidenib, or both) or dose interruption of CPX-351. Three patients (43%) experienced serious AEs (Table 5): febrile neutropenia (n = 2 [29%]) and pyrexia (n = 1 [14%]). No patients experienced AEs leading to death. No patients experienced differentiation syndrome. No patients in arm C died within 30 or 60 days of the first dose of study treatment. In patients who achieved CR/CRi (n = 5), the median time to ANC and platelet recovery was 37 days (IQR, 31-38) and 28 days (IQR, 21-28), respectively.
All grade 3 or higher AEs and serious AEs in arm C
| AE . | Arm C CPX-351 plus enasidenib (N = 7) . |
|---|---|
| Patients with any grade ≥3 AE by preferred term, n (%) | 7 (100) |
| Grade ≥3 hematologic AE, n (%) | |
| Febrile neutropenia | 7 (100) |
| Thrombocytopenia | 6 (86) |
| Neutropenia | 5 (71) |
| Anemia | 4 (57) |
| Leukopenia | 1 (14) |
| Grade ≥3 nonhematologic AE, n (%) | |
| Allergic transfusion reaction | 1 (14) |
| ALT increased | 1 (14) |
| Clostridium difficile infection | 1 (14) |
| Diarrhea | 1 (14) |
| Drug eruption | 1 (14) |
| Maculopapular rash | 1 (14) |
| Streptococcal bacteremia | 1 (14) |
| Patients with any serious AE by preferred term, n (%) | 3 (43) |
| Febrile neutropenia | 2 (29) |
| Pyrexia | 1 (14) |
| AE . | Arm C CPX-351 plus enasidenib (N = 7) . |
|---|---|
| Patients with any grade ≥3 AE by preferred term, n (%) | 7 (100) |
| Grade ≥3 hematologic AE, n (%) | |
| Febrile neutropenia | 7 (100) |
| Thrombocytopenia | 6 (86) |
| Neutropenia | 5 (71) |
| Anemia | 4 (57) |
| Leukopenia | 1 (14) |
| Grade ≥3 nonhematologic AE, n (%) | |
| Allergic transfusion reaction | 1 (14) |
| ALT increased | 1 (14) |
| Clostridium difficile infection | 1 (14) |
| Diarrhea | 1 (14) |
| Drug eruption | 1 (14) |
| Maculopapular rash | 1 (14) |
| Streptococcal bacteremia | 1 (14) |
| Patients with any serious AE by preferred term, n (%) | 3 (43) |
| Febrile neutropenia | 2 (29) |
| Pyrexia | 1 (14) |
All AEs were coded using Medical Dictionary for Regulatory Activities, version 21.1. Multiple entries for an individual patient under each preferred term were only counted once.
ALT, alanine aminotransferase.
Efficacy
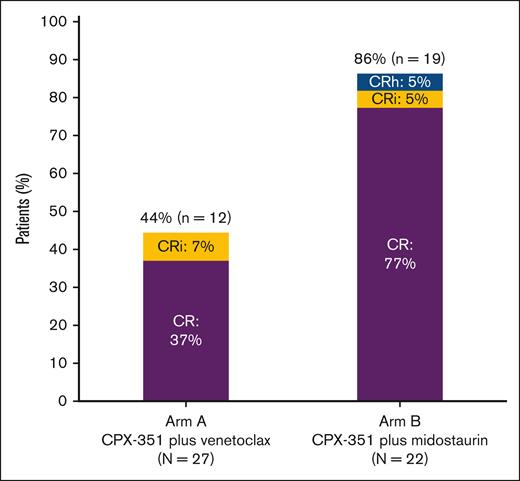
In arm A, 12 of 27 patients (44%) achieved a best response of CR/CRi, of whom 10 (37%) achieved a best response of CR (Figure 2). Of the patients with a CR/CRi who were tested for MRD status (n = 6), all were MRD negative. For the 5 patients with mutated TP53 in arm A, 4 had no response, and 1 achieved CR with MRD negativity. One patient in arm A had a CRi at the end of first induction, with ∼30% blast-like monocytic cells. The investigator confirmed that these immature cells appeared to be regenerating myeloid cells. The percentage of aberrant blasts (CD7+) by flow and CD34+ cells by immunohistochemistry were <5%; therefore, the response was classified as CRi. A best response of MLFS was achieved by 3 of 27 patients (11%). In patients who achieved remission (CR/CRi/CRh), the KM estimate of DOR rate was 67% at 12 months and 58% at 24 months; the median time in remission was not estimable (NE; 95% confidence interval [CI], 7.0 months to NE]). The KM estimate of OS rate was 44% at 12 months and 30% at 24 months, and the median OS time was 10.1 months (95% CI, 3.8-21.0; supplemental Figure 2A). The KM estimate of EFS rate was 30% at 12 months and 26% at 24 months, with a median EFS time of 3.3 months (95% CI, 0.03-10.8). Within 2 years after the first dose of study treatment, 8 of 27 patients (30%) underwent HCT. In these patients, the median OS from treatment initiation was NE (95% CI, 6.2 months to NE), and the KM-estimated 2-year OS was 50%.
Remission rates. Remission rates shown for patients in the efficacy analysis set who were treated with CPX-351 plus venetoclax (arm A) or CPX-351 plus midostaurin (arm B); n represents the number of patients who achieved a best response of CR, CRi, or CRh; N, the number of patients who received ≥1 dose of study drug and had an evaluable BM result.
Remission rates. Remission rates shown for patients in the efficacy analysis set who were treated with CPX-351 plus venetoclax (arm A) or CPX-351 plus midostaurin (arm B); n represents the number of patients who achieved a best response of CR, CRi, or CRh; N, the number of patients who received ≥1 dose of study drug and had an evaluable BM result.
In arm B, 19 of 22 patients (86.4%) achieved a best response of CR/CRi/CRh, with 17 of 22 (77%) achieving a best response of CR (Figure 2). Of those patients with a CR/CRi who were tested for MRD status (n = 12), 5 (42%) were MRD negative. No patients achieved a best response of MLFS. In patients who achieved remission, the KM estimate of DOR rate was 63% at 12 months and was not available at 24 months; the median time in remission was 20.4 months (95% CI, 4.8 to NE). The KM estimate of OS rate was 68% at 12 months and 55% at 24 months, and the median OS time was NE (95% CI, 8.2 months to NE; supplemental Figure 2B). The KM estimate of EFS rate was 55% at 12 months and 41% at 24 months, with a median EFS time of 15.3 months (95% CI, 5.3 to NE). Within 2 years after the first dose of study treatment, 15 of 22 patients (68%) underwent HCT. In these patients, the median OS from treatment initiation was NE (95% CI, 14.6 months to NE), and the KM-estimated 2-year OS was 73%.
In arm C, 5 of 7 patients (71%) achieved a best response of CR/CRi/CRh, and all were CRs. Of those patients with CR/CRi who were tested for MRD status (n = 3), all were MRD negative. One patient (14%) achieved a best response of MLFS. The KM estimate of DOR rate was 100% at both 12 and 24 months; the median time in remission was NE (95% CI, NE to NE). The KM estimate of OS rate was 100% at 12 months and 86% at 24 months; the median OS time was NE (95% CI, 19.5 months to NE; supplemental Figure 2C). The KM estimate of EFS rate was 86% at 12 months and 71% at 24 months; the median EFS time was NE (95% CI, 0.03 months to NE). Within 2 years after the first dose of study treatment, 4 of 7 patients (57%) underwent HCT. In these patients, the median OS from treatment initiation was NE (95% CI, 19.5 months to NE), and the KM-estimated 2-year OS was 75%.
PK
The PK of CPX-351 with venetoclax and midostaurin in this trial (supplemental Table 3) was consistent with the known PK of CPX-351 as a monotherapy.18
Discussion
We investigated the safety and preliminary efficacy of CPX-351 plus venetoclax, midostaurin, or enasidenib in adults with newly diagnosed AML who were fit to receive IC. We determined the RP2D of CPX-351 with venetoclax or midostaurin, and overall, CPX-351 plus either targeted agent was generally well tolerated, with the safety profile of each combination consistent with the known safety profiles of the drugs as single-agent treatments.9,19,20 There was no worsening of ANC (≥0.5 × 109/L) and platelet count (≥50 × 109/L) recovery with CPX-351 plus the targeted agents in this trial (CPX-351 + venetoclax, 35.5 and 35.5 days; CPX-351 + midostaurin, 34.5 and 32.5 days; CPX-351 + enasidenib, 37 and 28 days, respectively) vs CPX-351 alone in the pivotal phase 3 trial9 (35 and 36.5 days, respectively), confirming previous findings that suggest ANC and platelet recovery with CPX-351 takes longer than with 7+3.9 The prolonged neutropenia with CPX-351 relative to 7+3 in the pivotal phase 3 trial was not associated with excessive infectious complications. Longer count recovery observed with CPX-351 may be attributed to the prolonged drug exposure achieved with CPX-351 liposomes, which maintain the synergistic 1:5 drug ratio, which, in turn, may increase cytotoxicity vs its free-drug counterpart.9,21
Best response rate (CR/CRi) with CPX-351 plus venetoclax was 44%, which is promising considering the high-risk population in this trial, including 52% of patients with poor-risk disease, 41% with secondary AML, and 15% with antecedent myelofibrosis, all of which are known clinically challenging features.22,23 Patients with TP53-mutated AML treated with CPX-351 plus venetoclax had a poor remission rate (1/5 achieved CR with MRD negativity), highlighting the difficulty in treating this subset.24 The VIALE-A study reported a response rate of 65% with azacitidine plus venetoclax in patients ineligible for IC, most of whom had de novo AML (75%) and intermediate-risk disease (64%), whereas patients with antecedent myelofibrosis were excluded.25 Furthermore, 41% of patients in VIALE-A who achieved remission and tested for MRD status were MRD negative, and overall, 1% of patients underwent HCT.25,26 In this trial, 100% of patients who achieved remission after CPX-351 plus venetoclax and had MRD testing achieved MRD negativity; additionally, 30% of patients underwent HCT. Relative to azacitidine plus venetoclax, the CPX-351 plus venetoclax combination is a more intensive regimen, which may yield better MRD negativity early on and/or allow for patients to transition to HCT.27 In a phase 1b trial (N = 35) of adults with AML unfit for IC, lower-intensity CPX-351 (∼30% of the standard dose of CPX-351) plus venetoclax was shown to be a promising alternative to hypomethylating agents plus venetoclax for induction therapy.28 Of the 17 patients who achieved a CR/CRi, nearly all achieved a CR (16/17 [94%]). Most patients (82%) who achieved CR/CRi also achieved MRD negativity, which was often reached after only 1 treatment cycle. Nevertheless, given the small sample size in both V-FAST and the lower-intensity CPX-351 study, larger studies are needed to further investigate these findings.
For the small subset of patients with FLT3 mutations in the pivotal trial, efficacy data suggested a trend toward improved survival with CPX-351 vs 7+3.9 In this trial, treatment with CPX-351 plus midostaurin demonstrated a best response rate (CR/CRi/CRh) of 86% and HCT rate of 68% in patients with FLT3 mutations, both higher than previously observed with 7+3 plus midostaurin (59% and 57%, respectively), which is the current standard therapy for this patient population.14 Furthermore, there was no worsening of hematologic grade ≥3 AEs in patients treated with the CPX-351 plus midostaurin combination compared to those previously reported for 7+3 plus midostaurin14 (febrile neutropenia, 70% vs 82%; thrombocytopenia, 65% vs 97%; neutropenia, 39% vs 95%; anemia, 35% vs 93%; and lymphopenia, 0% vs 19%), except for leukopenia (61% vs 19%). Additionally, there were no cases of grade ≥3 diarrhea with CPX-351 plus midostaurin, compared to 16% with 7+3 plus midostaurin. These results suggest CPX-351 plus midostaurin may offer an effective and possibly less toxic alternative to 7+3 plus midostaurin. The promising results of CPX-351 in combination with midostaurin in patients with FLT3 mutations may prompt investigations of CPX-351 in combination with other FLT3 inhibitors. A phase 1/2 trial of CPX-351 plus quizartinib in patients with newly diagnosed or relapsed/refractory AML and high-risk myelodysplastic syndrome (clinicaltrials.gov identifier: NCT04128748) and a phase 1 trial of CPX-351 plus gilteritinib in patients with relapsed/refractory FLT3-mutated AML (clinicaltrials.gov identifier: NCT05024552) are ongoing. Notably, preliminary results of the CPX-351 plus gilteritinib trial suggest the combination may achieve higher response rates than gilteritinib alone.29
This trial had several limitations. The small number of patients enrolled in each arm makes it difficult to interpret the data. In particular, due to early termination of arm C (CPX-351 plus enasidenib) and with only 7 patients enrolled in this arm, there were insufficient data to determine the RP2D for this combination. Additionally, there are limited data on safety during consolidation treatment because consolidation was optional.
In conclusion, data from V-FAST suggest that CPX-351 has the potential to be combined with venetoclax or midostaurin, having demonstrated a manageable safety profile and promising remission and HCT rates. These encouraging results support further investigation of CPX-351 in combination with targeted agents in larger populations of patients with newly diagnosed AML, including those with poor-risk disease or FLT3 mutations.
Acknowledgments
The authors thank the trial participants. Medical writing support, under the direction of the authors, was provided by Trina Soluta, and editorial support was provided by Victoria Evans of CMC Affinity, a division of IPG Health Medical Communications, with funding from Jazz Pharmaceuticals, in accordance with Good Publication Practice (GPP 2022) guidelines.
This trial was supported by Jazz Pharmaceuticals.
Authorship
Contribution: S.F. and R.S.C. contributed to study conceptualization; R.S.C., A.T.F., V.R.B., D.C., and Y.L. contributed to data curation; R.S.C., T.V., D.C., and Y.L. contributed to the formal analysis of data; V.A.P., M.L., J.M., G.N.M., S.A.S., A.T.F., T.L.L., V.R.B., R.S.C., and H.P.E. were the study investigators; R.S.C. and T.V. contributed to study methodology; V.A.P., A.T.F., T.L.L., V.R.B., and R.S.C. contributed to project administration; V.A.P., S.A.S., A.T.F., T.L.L., and V.R.B. contributed study resources; T.V. contributed software; V.A.P., S.A.S., A.T.F., T.L.L., V.R.B., D.C., Y.L., S.F., and R.S.C. supervised the study; V.A.P., A.T.F., T.L.L., V.R.B., and T.V. contributed to study validation; V.A.P., A.T.F., and T.V. contributed to study visualization; V.A.P., A.T.F., D.C., Y.L., and R.S.C. contributed to writing and original draft preparation of the manuscript; and all authors contributed to writing, reviewing, and editing the manuscript and approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: V.A.P. has served on advisory committees/speakers’ bureaus for and received honoraria from AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, Rigel, and Servier. M.L. has received institutional research funding from Astellas Pharma and Fujifilm; and honoraria from Amgen, Astellas Pharma, Daiichi Sankyo, Fujifilm, and Menarini. J.M. has served on speakers’ bureaus for AbbVie, Amgen, Bristol Myers Squibb, Incyte, Jazz Pharmaceuticals, Stemline, and Takeda; and has served in a consulting or advisory role for AbbVie, CTI BioPharma, and Novartis. G.N.M. has received institutional research support from Forty Seven, Inc/Gilead, GlycoMimetics, and Jazz Pharmaceuticals; and has served in a consulting or advisory role for AbbVie, Agios, Astellas Pharma, Bristol Myers Squibb, Genentech, Pfizer, and Stemline. S.A.S. has served on speakers’ bureaus for GlaxoSmithKline; and has served in a consulting or advisory role for AbbVie, BerGenBio, Ellipses, Genentech, Kura Oncology, Nkarta, Novartis, Senti Bio, Sobi, Syros, and Terns. A.T.F. has served in a consulting or advisory role for AbbVie, Agios, Amgen, Astellas Pharma, AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, Daiichi Sankyo, Foghorn Therapeutics, Forty Seven, Inc, Genentech, Gilead, Ipsen, Kite Pharma, Kura Oncology, MorphoSys, Novartis, Pfizer, Schrodinger, Seagen, Syndax, Takeda, Trillium Therapeutics, and Trovagene; and received institutional research funding from AbbVie, Agios, Bristol Myers Squibb, Servier, and Takeda. T.L.L. has received institutional research funding from AbbVie, Aptevo, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, CicloMed, Cleave Biosciences, Roche Genentech, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Mateon Therapeutics, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seagen, Tolero Pharmaceuticals, and Cardiff Oncology; and served in an advisory role for Servier. V.R.B. reports participating in the safety monitoring committee for Protagonist; serving as an associate editor for the journal Current Problems in Cancer and as a contributor for BMJ Best Practice; consulting fees from Imugene, Sanofi, and Taiho; institutional research funding from AbbVie, Actinium Pharmaceutical, Incyte, Jazz Pharmaceuticals, MEI Pharma, National Marrow Donor Program, Pfizer, and Sanofi US Services; and institutional drug support from Chimerix for a trial. T.V. is a contingent worker for Jazz Pharmaceuticals. D.C., Y.L., S.F., and R.S.C. are employees of and hold stock ownership/options in Jazz Pharmaceuticals. H.P.E. has received institutional research support from AbbVie, Daiichi Sankyo, Forma, ImmunoGen, Jazz Pharmaceuticals, MacroGenics, Novartis, and PTC Therapeutics; served as a speaker for AbbVie, Agios, Celgene, Incyte, Jazz Pharmaceuticals, and Novartis; served in a consulting or advisory role for AbbVie, Agios, Amgen, Astellas Pharma, Celgene, Daiichi Sankyo, GlycoMimetics, ImmunoGen, Incyte, Jazz Pharmaceuticals, MacroGenics, Novartis, and Pfizer; and serves as a member of the scientific steering committee for GlycoMimetics, the chair of the scientific steering committee for Celgene, and the chair of the independent review committee for Covance (AbbVie).
The current affiliation for S.A.S. is Canon Research Institute, TriStar Centennial, Nashville, TN.
Correspondence: Vinod A. Pullarkat, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope Medical Center, 1500 East Duarte Rd, Duarte, CA 91010; email: vpullarkat@coh.org.
References
Author notes
All relevant data are provided within the article and supporting files. Jazz Pharmaceuticals has established a process to review requests from qualified external researchers for data from Jazz-sponsored clinical trials in a responsible manner, which includes protecting patient privacy, assuring data security and integrity, and furthering scientific and medical innovation. Additional details on Jazz Pharmaceuticals data sharing criteria and process for requesting access can be found at https://www.jazzpharma.com/science/clinical-trial-data-sharing/.
The full-text version of this article contains a data supplement.