Visual Abstract
TO THE EDITOR:
Both subcutaneous panniculitis-like T-cell lymphoma (SPTCL) and cutaneous gamma/delta T-cell lymphoma (CGDTCL) are rare diseases of aggressive nature and incurable with conventional therapies.1-7 Here, we report a retrospective review of 9 patients (5 with SPTCL and 4 with CGDTCL) who received allogeneic hematopoietic cell transplantation (HCT) at Stanford University between 2013 and 2022. Our review focused on the course before and after transplantation to highlight the clinically relevant information. All patients provided informed consent for outcome analysis approved by institutional review board in accordance with the Declaration of Helsinki.
Between 2013 and 2022, 8 of 16 patients with SPTCL and 6 of 11 patients with CGDTCL evaluated at the Stanford Multidisciplinary Cutaneous Lymphoma Clinic were referred for allogeneic HCT. The reasons for not referring for HCT consideration were age of >80 years (n = 2), indolent disease (n = 1), sustained remission after first therapy (n = 5), consultation only (n = 2), loss of follow-up after initial consultation (n = 2), and uncontrolled disease (n = 1). Of 14 patients referred for HCT, 9 patients received allogeneic HCT between 2013 and 2022, and were included in this report. The remaining 5 patients included 1 patient who received allogeneic HCT in 2024, 1 patient without adequate social support for HCT, 2 patients with uncontrolled diseases, and 1 patient remaining in first complete remission (CR) after 8 years. Most of the 9 (78%) patients in this report had a history of hemophagocytic lymphohistiocytosis (HLH), and 5 patients had extracutaneous involvement. At the time of conditioning for allogeneic HCT, 4 patients were in CR, 4 were in partial remission (PR), and 1 patient had stable disease (SD) (Table 1).
Seven patients had nonmyeloablative conditioning with total lymphoid irradiation and antithymocyte globulin (n = 5)8,9 or fludarabine with total body irradiation (TBI) (n = 2),10 with either HLA-matched sibling (n = 3) or unrelated donor (n = 2), or HLA-mismatched unrelated donor (n = 2). The 2 patients with haploidentical donor graft had reduced intensity fludarabine-cyclophosphamide–TBI conditioning.11 One patient with SPTCL and 3 patients with CGDTCL had radiation boost (24-34 Gy) to multiple large residual skin lesions, immediately before conditioning.12,13
All patients received granulocyte colony–stimulating factor mobilized peripheral blood hematopoietic cells with median donor CD34+ cell dose of 7.7 × 106 cells per kg (range, 4.9 × 106 to 14.3 × 106 cells per kg) and median donor CD3+ cell dose of 240 × 106 cells per kg (range, 130 × 106 to 513 × 106 cells per kg). At day +90, 3 patients (33%) achieved full donor chimerism, and 5 patients had mixed chimerism.14 One patient had primary graft failure on day +24. Four of 5 patients with mixed chimerism at day +90 subsequently achieved full donor chimerism, and 1 patient had secondary graft failure on day +297. The median time to achieving full donor chimerism was 157 days (range, 32-283). The 2 patients with graft failure recovered autologous hematopoiesis.
Seven patients received their hematopoietic cell infusions as outpatients, with the 2 haploidentical graft recipients managed as inpatients. Overall, patients tolerated the HCT well with limited toxicities including infectious complications (supplemental Table 1). The 2 patients with haploidentical donors developed sinusoidal occlusive syndrome, 1 of whom had sinusoidal occlusive syndrome associated renal failure, and prolonged hospital stay of 14 and 47 days, respectively; both patients recovered fully.
Two patients developed grade 1 acute graft-versus-host disease (GVHD; both skin) at day +27 and day +62, respectively. Another patient developed grade 3 acute GVHD (stage 2 skin, stage 1 liver, and stage 3 gastrointestinal) at day +168 after early immunosuppression withdrawal due to disease relapse. This patient’s acute GVHD resolved with treatment, but the patient subsequently developed chronic GVHD of the skin and joints. Two additional patients developed de novo chronic GVHD at day +311 and day +341, respectively, 1 of whom is now off all immunosuppression after GVHD resolution. At last follow-up, 2 patients were still on immunosuppression for persistent chronic GVHD.
At day +90, all patients achieved/maintained a clinical CR. Two patients with CGDTCL experienced disease relapse limited to the skin at day +138 and day +146, respectively. The first patient was managed by quick withdrawal of immunosuppression, followed by a short course of lenalidomide (10 mg daily, ×10 days), resulting in a CR within 2 months while developing chronic GVHD. The second patient was also managed by quick withdrawal of immunosuppression, which resulted in grade 3 acute GVHD as described earlier and a CR with subsequent chronic GVHD.
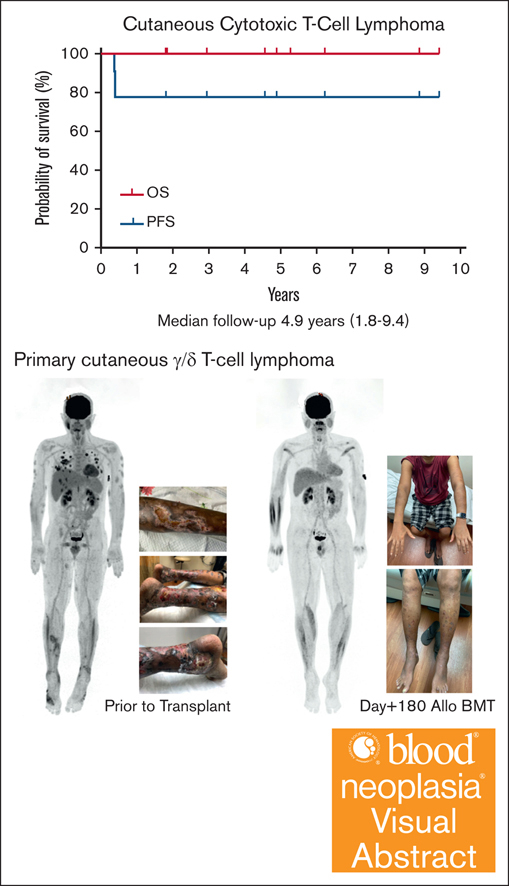
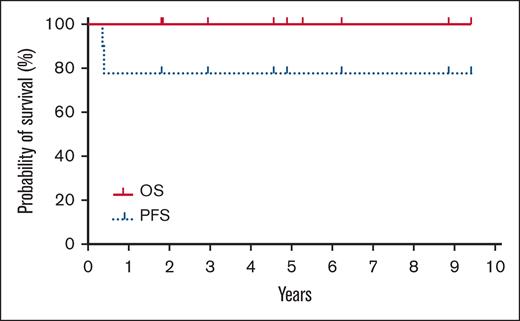
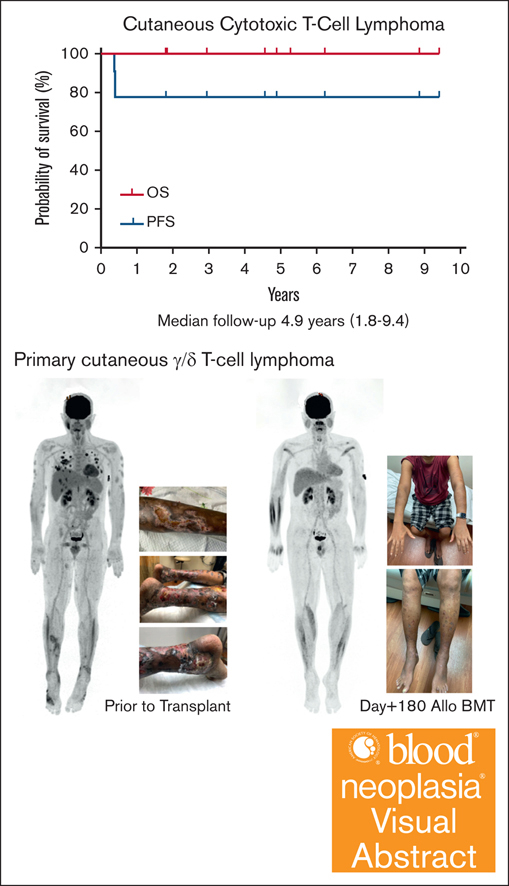
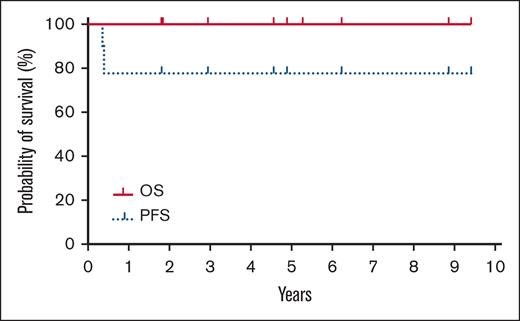
The median follow-up after HCT is 4.9 years (range, 1.8-9.4). The nonrelapsed mortality (NRM) was 0%. Neither the median progression-free survival (PFS) nor overall survival (OS) were reached with all patients alive without clinical evidence of disease at last follow-up (Figure 1; supplemental Table 2). The incidence/severity of acute GVHD was low in this cohort. Three patients, all with CGDTCL, developed chronic GVHD (1 moderate and 2 with severe with extensive sclerodermoid presentations). To clear residual cutaneous/subcutaneous disease, radiation was used before HCT. As opposed to the electron-beam radiation used in mycosis fungoides (MF) or Sézary syndrome (SS),15 patients in this cohort were treated with photons because of the depth of their cutaneous lesions.12,13 One patient who experienced severe lower extremity sclerodermoid GVHD had required substantive radiation of his lower extremities to successfully bridge to allogeneic HCT because of highly refractory disease.12 Although this more intensive radiation therapy may contribute to severe sclerosis, radiotherapy remains an effective modality for establishing pre-HCT disease control in selected patients.
Kaplan-Meier estimates of OS (thick solid line) and progression-free survival (dotted line).
Kaplan-Meier estimates of OS (thick solid line) and progression-free survival (dotted line).
Patients with relapsed or refractory cytotoxic CTCL, in particular with HLH, have poor prognosis with median OS of ∼1 year and 5-year OS of only 10% to 20%.1-5 Responses to systemic therapies are often short-lived, and most patients die of their disease within a few years of diagnosis if unable to bridge to allogeneic HCT.7 Our institutional practice is to consider allogeneic HCT in patients with aggressive diseases such as extracutaneous involvement or HLH, or in patients who have limited or short-lived responses to several lines of systemic therapies. The timing of HCT is usually individualized and primarily based on the clinical course, responsiveness to therapies, and availability of suitable donors.
In previous reports, the benefit of allogeneic HCT in patients with SPTCL is mixed, with some patients maintaining long-term remission.5,16,17 However, most of the reports showed high risk of complication including GVHD and high NRM.18 The largest series of allogeneic stem cell transplant (SCT) in CGDTCL reported 7 patients19; 3 patients died from transplant-related toxicity (2 from acute GVHD), with 100-day NRM of 29%. In comparison, our series has 0% NRM and manageable GVHD, partially because of our approach using nonmyeloablative or reduced intensity conditioning. The choice of nonmyeloablative conditioning is determined by multiple factors. We prefer total lymphoid irradiation and antithymocyte globulin conditioning with HLA-mismatched donors because of its lower incidence of GVHD with preserved graft-versus-leukemia effect,9 whereas fludarabine-TBI is preferred in patients with highly aggressive or residual active disease because of its quicker engraftment.10
Eight patients had available samples for HTS to determine the unique malignant T-cell receptor CDR3 sequences, with dominant CDR3 sequence found in 5 cases (2 with SPTCL and 3 with CGDTCL) for measurable residual disease (MRD) monitoring.20 All 5 patients achieved sustained MRD negativity (<106) in both the skin and blood after day +34, +87, +191, +248, and +283, respectively, including the 2 patients who needed quick immunosuppression withdrawal for disease relapse. Based on our experience in other CTCL populations,15 it is reasonable to consider sustained MRD negativity to be a predictor for long-term clinical remission with very low rate of future relapse.
Our patients with refractory, high-risk cytotoxic CTCL who received allogeneic SCT had excellent outcome despite adverse prognostic factors including extracutaneous involvement and/or HLH. All patients received a low-intensity conditioning regimen, and radiation therapy was often used to successfully bridge to transplant. As in MF/SS type of CTCL, monitoring MRD remains useful in clinical management. Allogeneic SCT should be considered an important and effective option for this subset of aggressive cytotoxic CTCLs.
Contribution: W.-K.W. and Y.H.L. designed the research, performed research, collected data, analyzed and interpreted data, and wrote the manuscript; G.M.W. collected and analyzed data, and wrote the manuscript; C.I. analyzed and interpreted data, and wrote the manuscript; R.T.H. performed research, and collected and analyzed data; and S.H. and M.S.K. designed the research, and analyzed and interpreted data.
Conflict-of-interest disclosure: Y.H.K. served on the advisory board for Seattle Genetics (now Seagen), Galderma, Eisai, Actelion, Kyowa Hakko Kirin, Celgene, and Millennium; and received research funding from Seattle Genetics, Millennium, Eisai, Merck, Kyowa Hakko Kirin, and TetraLogic Pharmaceuticals. The remaining authors declare no competing financial interests.
The current affiliation for G.M.W. is Homestead High School, Cupertino, CA.
Correspondence: Wen-Kai Weng, Division of Blood and Marrow Transplantation & Cellular Therapy, Department of Medicine, Stanford University School of Medicine, 780 Welch Rd, CJ 250H, Palo Alto, CA 94304; email: wkweng@stanford.edu.
References
Author notes
Presented, in part, at the Transplantation & Cellular Therapy Meetings, February 2021.
Data that support the findings of this study are available upon reasonable request from the corresponding author, Wen-Kai Weng (wkweng@stanford.edu).
The full-text version of this article contains a data supplement.