TO THE EDITOR:
The core spliceosome component SF3B1 is one of the most frequently mutated genes in myeloid neoplasms (MN), including myelodysplastic syndromes (MDS).1,2 Similarly to other MN mutations, SF3B1 mutations (SF3B1mt) also confer a fitness advantage to mutant hematopoietic stem cells (HSCs).3,4 The molecular mechanisms underlying this competitive advantage of SF3B1mt cells and how they relate to clinical disease development are not yet understood. However, recent large-scale studies of myeloproliferative neoplasms and acute myeloid leukemia have demonstrated that initiating fitness advantage-granting mutations frequently become clinically relevant only after expansion.3-5
Although several studies have investigated the impact of different SF3B1mt, these have been limited to comparing cells from separate patients with SF3B1mt and could therefore be confounded by differences in genetic background and clone-extrinsic factors.6,7 Albeit less frequent than expected by single mutation frequencies in myeloid malignancies (supplemental Table 1), the co-occurrence of separate SF3B1mt clones within the same patient provides a unique opportunity to investigate this question.
Herein, we pursue a long-term investigation of the clinical profile, fitness ability, and clonal/phylogenetic dynamics of independent SF3B1mt clones found in one individual with MDS with ring sideroblasts (MDS-RS) followed up in the clinic over 14 years (Patient 1, SF3B1N626D/K666N) and a second individual with dual SF3B1mt MDS-RS sampled only at diagnosis (Patient 2, SF3B1K700E/K666N). Through longitudinal analysis of Patient 1, we demonstrate that RNA mis-splicing is prevalent in the HSC, the cell of disease origin;8 and that SF3B1K666N and SF3B1N626D HSC display mutation-specific RNA mis-splicing profiles with distinct functional enrichment results.
Bone marrow (BM) samples from patients with MDS-RS were collected, subjected to myeloid panel sequencing at Karolinska University Hospital and processed according to established protocols.9 Control samples were collected from 4 healthy age- and sex-matched normal BM donors. An overview of patients with dual-SFmt (SF3B1, SRSF2, and U2AF1) in the clinical cohorts of Karolinska University Hospital and Kyoto University is provided in supplemental Table 1 and supplemental Figure 1. All material was provided with written informed consent for research use, given in accordance with the Declaration of Helsinki, and the study was approved by the ethics research committees at Karolinska Institutet (2010/427-31/3, 2017/1090-31/4) and Kyoto University (G608).
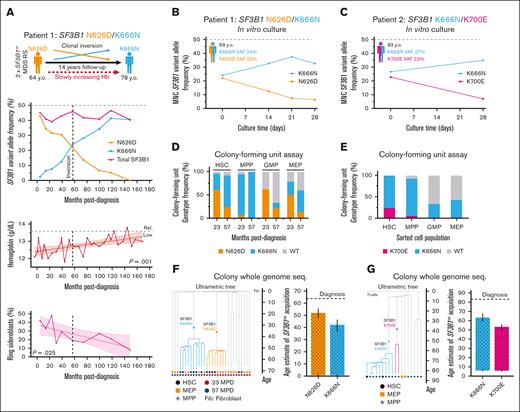
Patient 1 was investigated at 64 years of age because of mild anemia. Clinical investigation confirmed MDS-RS with hemoglobin at 12.1 g/dL, mean corpuscular volume of 103 fL, >40% RS, and 2 independent SF3B1 mutations (variant allele frequency [VAF] of 43% SF3B1N626D and 4.6% SF3B1K666N) without any other recurrent driver mutations. The patient remained untreated but was regularly followed up. Over 14 years, both clones inverted in frequency (Figure 1A; SF3B1N626D: 43%→0% VAF; and SF3B1K666N: 4.6%→41% VAF), with a slow, consistent improvement of erythroid parameters (supplemental Figure 2) coinciding with a mild increase in megakaryocyte-erythroid progenitor (MEP) frequencies (supplemental Figure 3), as has been described on aggregate for SF3B1K666N.10
Separate SF3B1-mutant clones arise independently decades before clinical disease and active clonal competition. (A) Kinetics of ddPCR-assessed VAF of separate SF3B1mt clones in BM mononuclear cells, clinical hemoglobin levels and BM ring sideroblast frequency of Patient 1, a patient with MDS-RS with separate SF3B1 N626D/K666N clones followed up over 14 years. (B-C) ddPCR–assessed SF3B1 VAFs from 3-dimensional in vitro culture time points of BM mononuclear cells seeded at (B) the inversion point of Patient 1’s long-term follow-up, and (C) the diagnostic visit of Patient 2, a patient with MDS-RS with separate SF3B1 K700E/K666N clones. (D-E) Genotype frequencies of colonies derived from sorted HSPCs and cultured in colony-forming unit assays from Patient 1 (D) and Patient 2 (E). (F-G) Clonal composition dendrograms and estimated age intervals for acquisition of each SF3B1 mutation by Patient 1 (F) and Patient 2 (G), relative to time of MDS-RS diagnosis. Error bars indicate 95% lower and upper bound errors for the age interval estimate. Comutations shared in all colonies of the same clone: P1-SF3B1N626D: GPATCH1N787S, EEIG2R172S, RLBP1K270Q; P1-SF3B1K666N: INTSV124F; P2-SF3B1K666N: CPNER517C, PCSK1E638K, SLC4A5P30S, FBXO34S320N; P2-SF3B1K700E: TTC12A389V, OR4N2T259M, KAT2AP691L. ddPCR, digital droplet polymerase chain reaction; MPD, months post diagnosis.
Separate SF3B1-mutant clones arise independently decades before clinical disease and active clonal competition. (A) Kinetics of ddPCR-assessed VAF of separate SF3B1mt clones in BM mononuclear cells, clinical hemoglobin levels and BM ring sideroblast frequency of Patient 1, a patient with MDS-RS with separate SF3B1 N626D/K666N clones followed up over 14 years. (B-C) ddPCR–assessed SF3B1 VAFs from 3-dimensional in vitro culture time points of BM mononuclear cells seeded at (B) the inversion point of Patient 1’s long-term follow-up, and (C) the diagnostic visit of Patient 2, a patient with MDS-RS with separate SF3B1 K700E/K666N clones. (D-E) Genotype frequencies of colonies derived from sorted HSPCs and cultured in colony-forming unit assays from Patient 1 (D) and Patient 2 (E). (F-G) Clonal composition dendrograms and estimated age intervals for acquisition of each SF3B1 mutation by Patient 1 (F) and Patient 2 (G), relative to time of MDS-RS diagnosis. Error bars indicate 95% lower and upper bound errors for the age interval estimate. Comutations shared in all colonies of the same clone: P1-SF3B1N626D: GPATCH1N787S, EEIG2R172S, RLBP1K270Q; P1-SF3B1K666N: INTSV124F; P2-SF3B1K666N: CPNER517C, PCSK1E638K, SLC4A5P30S, FBXO34S320N; P2-SF3B1K700E: TTC12A389V, OR4N2T259M, KAT2AP691L. ddPCR, digital droplet polymerase chain reaction; MPD, months post diagnosis.
To assess clone fitness in a context with minimal stromal, microenvironmental, and paracrine factors, we used an erythroid scaffold system8,9 for 28-day culture of BM mononuclear cells from Patient 1 at clonal inversion and from Patient 2 (VAF: SF3B1K700E, 23%; and SF3B1K666N, 27%). Despite a relatively short culture time, genotyping of both cultures demonstrated increased competition of SF3B1K666N clones to the detriment of the coexisting SF3B1mt clone (Figure 1B-C). Although clone-extrinsic elements may partially drive SF3B1mt clonal expansion, these in vitro clonal competition dynamics support the hypothesis that the fitness advantage of SF3B1K666N is mediated, at least in part, through a clone-intrinsic mechanism of hematopoietic origin.
Hematopoietic stem and progenitor cells (HSPCs) from both patients were then purified by fluorescence-activated cell sorting (supplemental Key Resources Table) to generate single-cell–derived HSPC colonies.8 DNA extraction and digital droplet polymerase chain reaction to assess colony genotypes showed that, whereas HSC-derived colonies closely mirrored total BM VAFs, SF3B1K666N unexpectedly dominated multipotent progenitor–derived colony-forming units (Patient 1: month 23: 67 K666N/71 total [P < .0001]; Patient 1: month 57: 8/8 [not significant]; Patient 2: 105/121 [P < .0001]; Figure 1D-E; supplemental Table 2). Wild-type (WT) HSC/multipotent progenitor colony-forming units were near-absent and instead overrepresented in committed progenitors, likely because of SF3B1mt differentiation defects.
Colony whole-genome sequencing (supplemental Methods) allowed us to reconstruct clonal hierarchies for each patient (Figure 1F-G). Although no candidate driver mutations were identified, most co-occurring mutations were present at an early age in both patients, and SF3B1mt mutational rates were generally higher than reference WT data (supplemental Figure 4). Importantly, comparing the age intervals for SF3B1mt acquisition (Patient 1, 0.5-50 years; Patient 2, 6-63 years) to age at diagnosis indicates that SF3B1mt acquisition predated MDS onset by a minimum of 14 and 20 years, respectively. These MDS findings thus provide a direct parallel to the long clonal histories in myeloproliferative neoplasm development.3,4
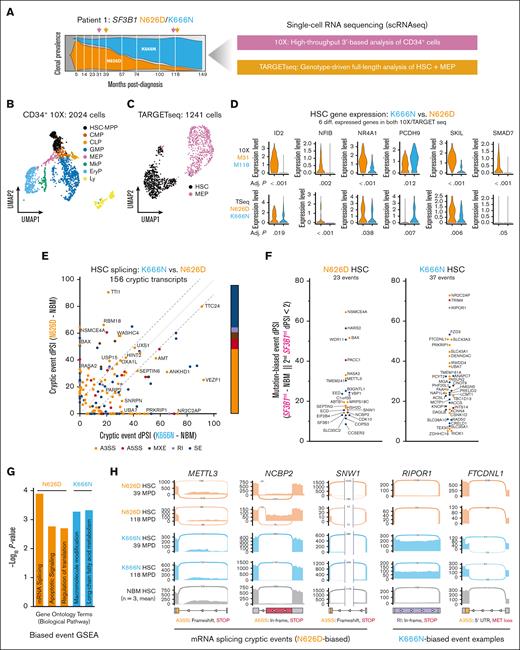
Finally, the effect of SF3B1mt on Patient 1’s HSPC compartment was evaluated using two single-cell RNA-sequencing techniques (Figure 2A; supplemental Methods): a pan-CD34 10x Genomics 3′ analysis capturing all HSPC compartments (Figure 2B; supplemental Figure 5) and genotype–targeted full-length TARGET sequencing (TARGET-seq)11 directed to HSC/MEP (Figure 2C; supplemental Figure 6). The median TARGET-seq splice junction coverage was ∼6500 splice junctions per cell (supplemental Figure 7A), enabling genotype-informed RNA splicing analyses. Despite obvious clonal outgrowth of SF3B1K666N, differential gene expression analysis of both data sets to identify shared patterns over time (10X) or mutation (TARGET-seq) identified only 6 matching differentially expressed genes, of which 4 were transcriptional regulators (Figure 2D).
SF3B1mt clone-intrinsic dominance is associated with differential transcript splicing but not differential gene expression. (A) Distribution of scRNA-seq sampling times and experimental methods. (B-C) Uniform manifold approximation and projection (UMAP) of 10x Genomics-sequenced HSPCs (B) and TARGET-seq sequenced HSCs/MEPs (C). 10x HSPC subsets were annotated by comparing marker gene expression with existing literature data. The TARGET-seq annotation shown reflects the ground truth cell identification obtained from fluorescence-activated cell sorting purification. (D) Violin plots of common differentially expressed genes comparing month 118 and month 31 visits in the 10x HSC/MPP subset and between SF3B1 K666N and N626D-genotyped HSC in TARGET-seq. Bonferroni-adjusted P values for the respective comparison are shown below each violin plot. (E) dPSI values comparing SF3B1K666N and SF3B1N626D HSC PSIs against control PSIs (NBM, n = 3). Event types are annotated per color. The diagonal lines highlight events with absolute clone/clone dPSI of <10. (F) PSI values of mutation-biased cryptic splicing events in SF3B1N626D (left) and SF3B1K666N HSC (right). A cryptic event was considered mutation-biased if the other mutant clone’s dPSI was <2%. (G) GO enrichment analysis of mutation-biased cryptic spliced genes in SF3B1K666N and SF3B1N626D HSC. (H) Sashimi plots of cryptic splicing events in METTL3, NCBP2, and SNW1, 3 of 4 genes associated with RNA splicing in the GO enrichment analysis; and RIPOR1 and FTCDNL1, examples of K666N-biased splicing events. The predicted molecular consequences of the cryptic sites are annotated below the corresponding sashimi plots. A3SS, alternative 3′ splice site; A5SS, alternative 5′ splice site; CLP, common lymphoid progenitors; dPSI, percent spliced-in difference; EryP: erythroid progenitors; GO, Gene Ontology; Ly, lymphoid; MkP, megakaryocyte progenitors; MPP, multipotent progenitor; MPD, months post diagnosis; MXE, mutually exclusive isoform usage; NBM, normal BM; RI, intron retention; scRNA-seq, single-cell RNA sequencing; SE, exon skipping.
SF3B1mt clone-intrinsic dominance is associated with differential transcript splicing but not differential gene expression. (A) Distribution of scRNA-seq sampling times and experimental methods. (B-C) Uniform manifold approximation and projection (UMAP) of 10x Genomics-sequenced HSPCs (B) and TARGET-seq sequenced HSCs/MEPs (C). 10x HSPC subsets were annotated by comparing marker gene expression with existing literature data. The TARGET-seq annotation shown reflects the ground truth cell identification obtained from fluorescence-activated cell sorting purification. (D) Violin plots of common differentially expressed genes comparing month 118 and month 31 visits in the 10x HSC/MPP subset and between SF3B1 K666N and N626D-genotyped HSC in TARGET-seq. Bonferroni-adjusted P values for the respective comparison are shown below each violin plot. (E) dPSI values comparing SF3B1K666N and SF3B1N626D HSC PSIs against control PSIs (NBM, n = 3). Event types are annotated per color. The diagonal lines highlight events with absolute clone/clone dPSI of <10. (F) PSI values of mutation-biased cryptic splicing events in SF3B1N626D (left) and SF3B1K666N HSC (right). A cryptic event was considered mutation-biased if the other mutant clone’s dPSI was <2%. (G) GO enrichment analysis of mutation-biased cryptic spliced genes in SF3B1K666N and SF3B1N626D HSC. (H) Sashimi plots of cryptic splicing events in METTL3, NCBP2, and SNW1, 3 of 4 genes associated with RNA splicing in the GO enrichment analysis; and RIPOR1 and FTCDNL1, examples of K666N-biased splicing events. The predicted molecular consequences of the cryptic sites are annotated below the corresponding sashimi plots. A3SS, alternative 3′ splice site; A5SS, alternative 5′ splice site; CLP, common lymphoid progenitors; dPSI, percent spliced-in difference; EryP: erythroid progenitors; GO, Gene Ontology; Ly, lymphoid; MkP, megakaryocyte progenitors; MPP, multipotent progenitor; MPD, months post diagnosis; MXE, mutually exclusive isoform usage; NBM, normal BM; RI, intron retention; scRNA-seq, single-cell RNA sequencing; SE, exon skipping.
These restricted differences in gene expression (similar to previous reports)7 and the key splicing factor role of SF3B1 led us to focus on RNA splicing patterns. Each SF3B1mt clone was first compared against normal BM to delineate true RNA mis-splicing, primarily comprising alternative 3′ splice site usage and exon skipping events in both HSCs/MEPs (Figure 2E; supplemental Figure 7B-D; supplemental Table 3). Next, SF3B1mt clones were compared with identify RNA mis-splicing biases. Several known SF3B1mt mis-splicing events were indeed detected as common to HSCs/MEPs from both clones (eg, OXA1L and SEPTIN6; supplemental Figure 7E).12,13 Functional investigation of common HSC events (N626D/K666N, percent spliced-in difference of >2) identified several chromatin remodeling genes, including 2 critical to lymphoid development (INO80D14 and CHD215); as well as R-loop formation elements16 (ERCC3 and TCEA2; new: LEO1).
Mutation-specific filtering (second clone, percent spliced-in difference of <2) identified 60 exclusive mis-splicing events (Figure 2F). This included significant SF3B1N626D–unique truncating events affecting RNA splicing–associated genes (Figure 2G-H; per Gene Ontology enrichment, METTL3, NCBP2, SNW1, and SF3B1).17-19 Despite lacking a clear functional pattern, K666N events affected critical transcriptional regulators (eg, Myc pathway–associated MGA [not shown in figure] and PHF20L1; supplemental Figure 7E)20,21 with similar coding truncation (Figure 2H). Although the longitudinal study of only 1 patient limits the general inferences that can be made, our results lead us to hypothesize that (1) SF3B1mt may induce epigenetic dysregulation in HSC via differential gene expression and mis-splicing of transcriptional regulators; and (2) “benign” SF3B1 mutations (N626D/K700E) are partially so because of increased mis-splicing of splicing effectors, as has been indicated for the K700E hot spot.22 In contrast, the lack of this effect in SF3B1K666N would increase relative fitness and the long-term likelihood of leukemic transformation.
Beyond the mechanisms explored here, other SF3B1mt effects may also differ between mutations, such as R-loop accumulation and DNA damage,23 or protein changes downstream of aberrant RNA expression/splicing. However, our targeted investigation of the HSC compartment demonstrates that RNA mis-splicing is prevalent in the cell of disease origin, in which outgrowth capacity is most relevant.8 In conclusion, this work provides insight into long-term clonal competition patterns and enables a model for the relative advantages of SF3B1mt/WT and SF3B1K666N/SF3B1mt,10 highlighting the need for further investigation of the HSC compartment.
Acknowledgments: The authors thank the patients and their families, and the healthy donors for their willingness to participate in this research. The authors acknowledge support from the Karolinska Institutet MedH Flow Cytometry Core Facility for assistance with fluorescence-activated cell sorting experiments; the Karolinska Institutet Bioinformatics and Expression Analysis Facility for assistance with 10X single-cell RNA sequencing; the Karolinska Institutet Research Data Office for assistance with data management and deposition; and support from the National Genomics Infrastructure in Stockholm funded by Science for Life Laboratory, the Knut and Alice Wallenberg Foundation, and the Swedish Research Council, and Swedish National Infrastructure for Computing/Uppsala Monitoring center for Advanced Computational Science for assistance with massively parallel sequencing and access to the Uppsala Multidisciplinary Center for Advanced Computational Science computational infrastructure.
This project received grant support from Cancerfonden/Swedish Cancer Society (grant numbers 21 0340 [P.L.M.] and 19 0200 [E.H.-L.]); the Myelodysplastic Syndromes Foundation, Inc. (grant number 1142079 [P.L.M.]); Vetenskapsrådet/Swedish Research Council (grant numbers 538-2013-8995 [S.E.W.J.] and 211133 [E.H.-L.]); Knut and Alice Wallenberg Foundation (grant numbers 2016.0105 [S.E.W.J.] and 2017.0359 [E.H.-L.]); Torsten Söderberg Foundation (S.E.W.J.); Center for Innovative Medicine Karolinska Institutet (grant number 613/06 [S.E.W.J.]); Bloodwise (grant number 17017 [S.E.W.J.]); and Medical Research Council (grant number MC_UU_12009/5 [S.E.W.J.]).
Contribution: P.L.M., I.J.H., P.S.W., S.E.W.J., S.O., and E.H.-L. conceptualized the study; P.L.M., Y.N., A.A., I.J.H., T.M.B., R.S., P.S.W., S.E.W.J., S.O., and E.H.-L. performed formal analysis; P.L.M., Y.N., A.A., I.J.H., T.M.B., T.Y., R.S., and M.M.N. were responsible for data curation; P.S.W., S.E.W.J., S.O., and E.H.-L. supervised, acquired funding, and managed resources; P.L.M., Y.N., A.A., I.J.H., T.M.B., T.Y., R.S., M.M.N., M.C., A.-C.B., G.W., I.B., M.J., F.G., and E.M.E. conducted investigation; P.L.M., Y.N., A.A., I.J.H., T.M.B., and P.S.W. developed the methodology; E.H.-L. was responsible for project administration; P.L.M., Y.N., A.A., T.Y., R.S., and M.M.N. were responsible for software; P.L.M., Y.N., A.A., I.J.H., T.M.B., T.Y., and R.S. were responsible for validation; P.L.M., Y.N., A.A., and I.J.H. were responsible for visualization; P.L.M. and E.H.-L. wrote the original draft; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eva Hellström-Lindberg, Division of Hematology, Department of Medicine, Karolinska Institutet, Karolinska University Hospital, SE-141 86, Huddinge, Sweden; email: eva.hellstrom-lindberg@ki.se; Seishi Ogawa, Department of Pathology and Tumor Biology, Kyoto University, Yoshida-honmachi, Sakyo-ku, Kyoto 160-8582, Japan; email: sogawa-tky@umin.ac.jp; and Sten Eirik W. Jacobsen, Karolinska Institutet, Center for Hematology and Regenerative Medicine, Department of Medicine Huddinge, Karolinska University Hospital, Huddinge SE-141 86, Sweden; email: sten.eirik.jacobsen@ki.se.
References
Author notes
P.L.M., Y.N., A.A., and I.J.H. are joint first authors, and contributed equally to this study.
Deidentified raw data for all high-throughput sequencing data sets, corresponding metadata, intermediate files, and analysis-ready files have been deposited on the Swedish National Data Service’s research data repository (online data descriptions are available in both English and Swedish) and are accessible upon request to the Swedish National Data Service through the following DOI links: SND-ID 2023-222: 10X single-cell RNA-sequencing data of hematopoietic stem and progenitor cells from a dual SF3B1–mutant MDS-RS patient and a healthy donor, available at https://doi.org/10.48723/tt0e-eq82; SND-ID 2023-223: TARGET-seq genotyped single-cell RNA-sequencing data of hematopoietic stem cells and megakaryocyte-erythroid progenitor cells from a dual SF3B1-mutant MDS-RS patient and 3 healthy donors, available at https://doi.org/10.48723/d7s9-6336; SND-ID 2023-227: Colony whole-genome sequencing data of hematopoietic stem and progenitor cell–derived colonies from 2 dual SF3B1-mutant MDS-RS patients, available at https://doi.org/10.48723/n8wy-bb08.
Other raw data are available on request from the corresponding authors, Eva Hellström-Lindberg (eva.hellstrom-lindberg@ki.se), Seishi Ogawa (sogawa-tky@umin.ac.jp), and Sten Eirik W. Jacobsen (sten.eirik.jacobsen@ki.se).
The online version of this article contains a data supplement.