Key Points
CH mutations persist through AML-directed therapy, suggesting their presence in preleukemic cells is not eliminated by chemotherapy.
DNMT3A, TET2, and ASXL1 mutations differentially shape the subsequent evolutionary trajectories of AML from diagnosis through relapse.
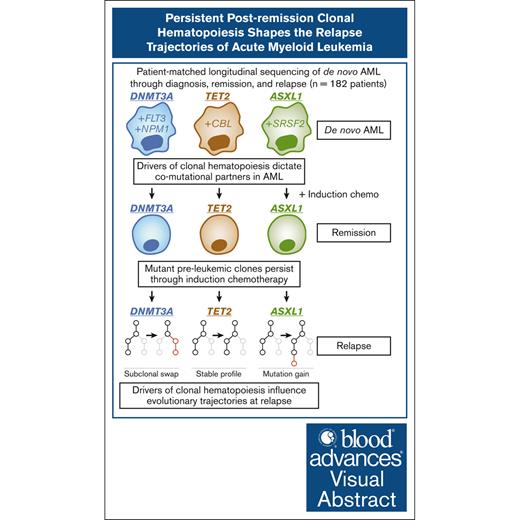
Visual Abstract
Mutations found in acute myeloid leukemia (AML) such as DNMT3A, TET2, and ASXL1 can be found in the peripheral blood of healthy adults, a phenomenon termed clonal hematopoiesis (CH). These mutations are thought to represent the earliest genetic events in the evolution of AML. Genomic studies on samples acquired at diagnosis, remission, and at relapse have demonstrated significant stability of CH mutations after induction chemotherapy. Meanwhile, later mutations in genes such as NPM1 and FLT3 have been shown to contract at remission, and in the case of FLT3 often are absent at relapse. We sought to understand how early CH mutations influence subsequent evolutionary trajectories throughout remission and relapse in response to induction chemotherapy. We assembled a retrospective cohort of patients diagnosed with de novo AML at our institution that underwent genomic sequencing at diagnosis, remission, and/or relapse (total N = 182 patients). FLT3 and NPM1 mutations were generally eliminated at complete remission but subsequently reemerged upon relapse, whereas DNMT3A, TET2, and ASXL1 mutations often persisted through remission. CH-related mutations exhibited distinct constellations of co-occurring genetic alterations, with NPM1 and FLT3 mutations enriched in DNMT3Amut AML, whereas CBL and SRSF2 mutations were enriched in TET2mut and ASXL1mut AML, respectively. In the case of NPM1 and FLT3 mutations, these differences vanished at the time of complete remission yet readily reemerged upon relapse, indicating the reproducible nature of these genetic interactions. Thus, CH-associated mutations that likely precede malignant transformation subsequently shape the evolutionary trajectories of AML through diagnosis, therapy, and relapse.
Introduction
Acute myeloid leukemia (AML) results from the accumulation of genetic alterations in hematopoietic stem/progenitor cells, leading to clonal expansion and impaired differentiation.1 Although common genomic aberrations in AML are well defined,2-6 many of these mutations are detected in older patients without hematologic malignancies.7-12 The acquisition of somatic mutations that result in clonal expansion, termed clonal hematopoiesis (CH), is associated with increased risk of developing not only hematologic malignancies but also a host of other diseases.7,8 Although only a fraction of patients with CH develop a hematologic malignancy, these somatic mutant clones likely represent preleukemic precursors primed for malignant transformation after further mutagenesis.13,14 CH-associated mutations can be detected in the peripheral blood of patients that have achieved complete remission (CR) from AML and have been identified in hematopoietic stem/progenitor cells that survive chemotherapy.15-19 Accordingly, CH mutations likely represent the earliest genetic events in AML and provide the substrate for further genomic evolution and transformation.
Extensive genomic profiling has been performed in de novo and secondary AML, highlighting trends of genomic evolution for FLT3- or NPM1-mutant disease.20-23 Other studies have described paired longitudinal sequencing of patient samples undergoing FLT3 tyrosine kinase inhibition treatment24,25 or induction chemotherapy.26,27 More recently, the advent of single-cell DNA sequencing28-30 and error corrected sequencing31-33 has improved the evaluation of mutation evolution during remission. These studies highlight the stability of CH mutations after therapy34,35 and the need to eradicate even the smallest of FLT3 and NPM1 mutant clones to control disease progression.36,37 To date, however, few cohorts have evaluated serial samples of patients from diagnosis through remission and subsequent relapse (REL), connecting genomic trajectories through several stages of disease.
Here, we assembled a cohort of patients diagnosed with de novo AML that underwent next-generation sequencing (NGS) at diagnosis and again at remission and/or REL. We find that DNMT3Amut, TET2mut, and ASXL1mut (DTA) AML exhibit distinct mutational profiles that persist throughout the disease course. In this manner, we demonstrate that early preleukemic drivers of CH can influence the subsequent evolutionary trajectories of AML.
Methods
Patient selection and data collection
Sequencing results, ancillary studies, and clinical information were collected in accordance with protocols approved by the institutional review board at the University of Pennsylvania. We searched internal pathology databases for patients with ≥2 NGS studies performed at least 30 days apart on blood or bone marrow specimens between 14 February 2013 and 31 June 2018 using our institution’s clinical targeted hematologic malignancies NGS panel. Patients with testing performed at initial diagnosis of de novo AML and subsequent testing performed at cytologic CR, REL, or disease refractory (REF) to initial therapy for de novo AML were included. Remission, REL, and REF states were determined from review of clinical notes from the electronic medical record and corresponding hematopathology studies performed on bone marrow specimens. CR was defined as having morphologic evidence on bone marrow biopsy of trilineage hematopoiesis and <5% blasts. Patients with diagnoses of therapy-related AML or AML with myelodysplasia-related changes were excluded. Cytogenetics, treatment history, and demographic details were also retrospectively recorded from the electronic medical record (supplemental Table 1).
Genomic sequencing
Samples from all patients were sequenced at the same institution on a clinically validated and Clinical Laboratory Improvement Amendments–certified customized NGS panel, which covers targeted coding regions and splicing junctions of genes that are commonly mutated in myeloid malignancies. All tests were ordered by treating physicians for clinical purposes.
DNA was extracted from fresh bone marrow aspirate or whole-blood samples, and targeted sequencing of hot spots in exomes of 33 genes (hemeV1 panel, 14 February 2013 to 21 April 2015) or 68 genes (hemeV2 panel, 22 April 2015 to 1 October 2020) was performed using an Illumina TruSeq Custom Amplicon assay. Genes included in each panel are noted in the supplemental Table 1. As CEBPA testing was performed only upon request, these data were excluded from the analysis because not all patients were routinely tested.
Across all samples included for final analysis, 83% (367/441) used the 68-gene panel (whereas the remaining 17% used the 33-gene panel). Among diagnosis samples, 76% used the 68-gene panel; for first CR (CR1) samples, 97% used the 68-gene panel; for first REL (REL1) samples, 76% used the 68-gene panel. The panel used for each sample is annotated in the supplemental Table 1.
Variant calling and annotation
NGS data were processed through a custom in-house bioinformatics pipeline that was clinically validated to call single-nucleotide variants at a frequency of 2% to 4% and small insertions and deletions (indels) at a frequency of 1%. The minimum mean coverage was 2500× across the entire panel and the minimum read depth for each amplicon was 250×. The lowest reportable variant allele frequency (VAF) was 2% for single-nucleotide variants in FLT3 and NPM1 and 4% for mutations in all other genes in the panel. Variants passing these criteria were included for analysis, regardless of pathogenicity classifications.
Data analysis
If multiple NGS studies were conducted at the same “stage” of disease (eg, CR1 and REL1), only the earliest NGS sample was retained for further analysis. To compare the mutation frequencies of genes between different time points, we used the Fisher's 2-sided exact test. For genetic interaction analyses, we compared the comutation frequencies for each gene pair using the Fisher's 2-sided exact test. For visual clarity in the figures, we omitted gene pairs that did not meet the nominal significance threshold of P <.05. To construct parsimonious phylogenies for the longitudinal NGS data, we used the CALDER algorithm.38 For each patient, we included all identified variants, at diagnosis, CR1, and/or REL1. We visualized the resulting phylogenies using clevRvis.39 If all mutations observed at diagnosis were observed at REL1 with no additional or lost mutations, the REL pattern was classified as “stable mutations.” If the REL1 mutation profile was the same as the diagnosis mutation profile but with the addition or loss of ≥1 mutations, these cases were classified as “mutation gain” or “mutation loss.” If the REL1 sample had acquired ≥1 new mutations while also losing ≥1 mutations that were originally present at diagnosis, we classified it as a “subclonal swap.”
Results
Charting the genomic evolution of de novo AML at diagnosis, remission, and REL
We retrospectively compiled patients diagnosed with de novo AML at our institution that had ≥2 NGS studies, performed at least 30 days apart (N = 182 patients). The average age at diagnosis was 58.06 ± 1.03 years (mean ± standard error for the mean), and 53.3% of patients were female. In total, 84.6% of patients received induction chemotherapy with combination anthracycline and nucleoside analog therapy (supplemental Figure 1A), and 41.8% (n = 76) underwent stem cell transplant during their treatment.
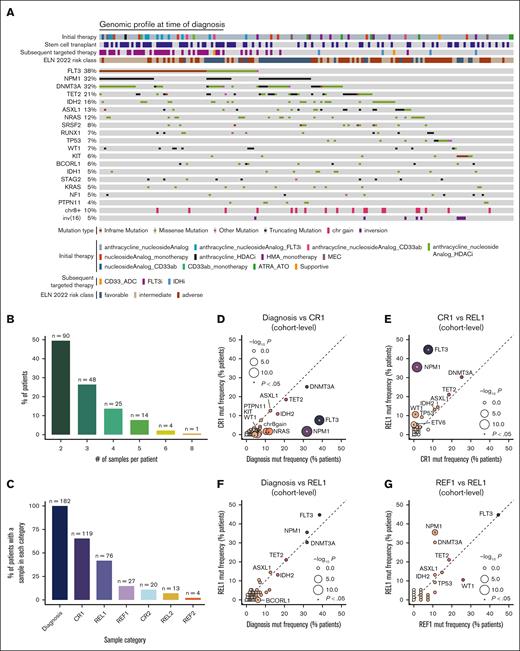
At the time of AML diagnosis, FLT3 (38%), NPM1 (32%), DNMT3A (32%), and TET2 (21%) were the most frequently mutated genes (Figure 1A), showing high correlation with the TCGA4 and BeatAML40 cohorts (supplemental Figure 1B-C). In total, 65.3% of patients (n = 119) had sequencing performed at the time of cytologic CR1 and 41.8% (n = 76) at the time of REL1 (Figure 1B-C).
Charting the genomic evolution of de novo AML at diagnosis, remission, and REL. (A) Frequently mutated genes and karyotype aberrations at time of diagnosis in the Penn AML cohort (total n = 182 patients). Patients are annotated by the treatments received throughout the course of the disease and by ELN 2022 risk classifications. (B) Distribution of the number of serial genomic profiles obtained for each patient, expressed as a percentage of the total cohort. All patients included in the cohort underwent genomic profiling at least twice, with more than half having ≥3 matched genomic samples. The number of patients in each category is annotated above. (C) Distribution of the number of patients with a genomic profile at each stage of AML disease progression, expressed as a percentage of the total cohort. The number of patients represented in each category is annotated above. (D-G) Comparison of cohort-level mutation (mut) frequencies across different disease time points; (D) diagnosis (n = 182) vs CR1 (n = 119); (E) CR1 vs REL1 (n = 76); (F) diagnosis vs REL1; and (G) REF1 (n = 27) vs REL1. Mut frequencies are calculated from all samples at each time point, irrespective of patient-level sample matching. Point sizes are scaled by statistical significance (Fisher's 2-sided exact test) and colored based on mut frequency. Asterisks indicate P < .05. Dashed lines denote equality between disease stages. ATO, arsenic trioxide; ATRA, all-trans retinoic acid; chr, chromosome; ELN, European LeukemiaNet; HDACi, histone deacetylase inhibitor; HMA, hypomethylating agent; IDHi, IDH inhibitor; MEC, mitoxantrone etoposide and cytarabine.
Charting the genomic evolution of de novo AML at diagnosis, remission, and REL. (A) Frequently mutated genes and karyotype aberrations at time of diagnosis in the Penn AML cohort (total n = 182 patients). Patients are annotated by the treatments received throughout the course of the disease and by ELN 2022 risk classifications. (B) Distribution of the number of serial genomic profiles obtained for each patient, expressed as a percentage of the total cohort. All patients included in the cohort underwent genomic profiling at least twice, with more than half having ≥3 matched genomic samples. The number of patients in each category is annotated above. (C) Distribution of the number of patients with a genomic profile at each stage of AML disease progression, expressed as a percentage of the total cohort. The number of patients represented in each category is annotated above. (D-G) Comparison of cohort-level mutation (mut) frequencies across different disease time points; (D) diagnosis (n = 182) vs CR1 (n = 119); (E) CR1 vs REL1 (n = 76); (F) diagnosis vs REL1; and (G) REF1 (n = 27) vs REL1. Mut frequencies are calculated from all samples at each time point, irrespective of patient-level sample matching. Point sizes are scaled by statistical significance (Fisher's 2-sided exact test) and colored based on mut frequency. Asterisks indicate P < .05. Dashed lines denote equality between disease stages. ATO, arsenic trioxide; ATRA, all-trans retinoic acid; chr, chromosome; ELN, European LeukemiaNet; HDACi, histone deacetylase inhibitor; HMA, hypomethylating agent; IDHi, IDH inhibitor; MEC, mitoxantrone etoposide and cytarabine.
To investigate how AML-directed therapy would affect the mutational landscape, we compared cohort-level gene mutation frequencies across disease stage. Comparing all samples taken at CR1 with those taken at diagnosis, irrespective of patient-level matching, we observed significant depletion of FLT3, NPM1, and NRAS mutations at the cohort level (Figure 1D). In contrast, mutation frequencies in CH-associated genes DNMT3A, TET2, and ASXL1 were unchanged between diagnosis and CR1.6,15-17 When patients relapsed after initial remission, the mutation frequencies of FLT3 and NPM1 returned to pretreatment levels (Figure 1E-F). Comparison of REL1 and primary REF (REF1) samples showed similar mutation frequencies across genes except for NPM1, which was enriched in REL1 samples (Figure 1G). These analyses illustrate the dynamics of driver mutations during AML progression, highlighting the consistency in driver mutation frequencies at the cohort level between diagnosis and REL, despite the selective pressure of chemotherapy.
DTA mutations persist at the time of CR
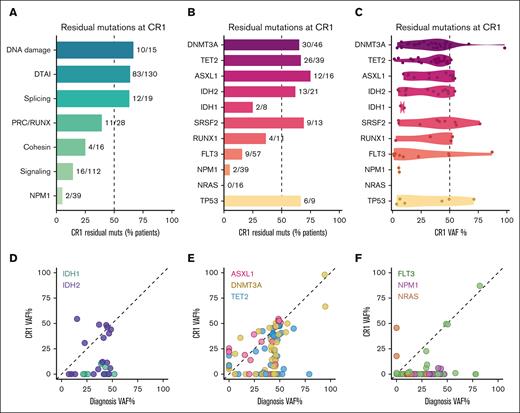
Next, we investigated mutation persistence at CR1. We grouped genes into biological categories and evaluated the frequency of detection in paired diagnosis and REL samples (Figure 2A). We observed persistence of mutations in genes related to DNA damage (10/15 [66.7%]), CH-associated DTAI factors (DNMT3A, TET2, ASXL1, and IDH1 and IDH2 combined: 83/130 [63.8%]), and splicing (12/19 [63.2%]), with less mutational persistence observed in genes associated with the Polycomb repressive complex and RUNX (PRC/RUNX: BCOR, BCORL1, RUNX1, EZH2: 11/28 [39.3%]) and cohesin complex (SMC1A, RAD21, STAG2: 4/16 [25%]). On an individual gene basis, we observed persistence of IDH2 mutations in 13 of 21 cases (61.9%), whereas a smaller fraction of IDH1 mutations persisted at remission (2/8 [25%]; Figure 2B). Accordingly, the VAFs for the 2 persistent IDH1 mutations were 6.9% and 10.3%, compared with a mean VAF of 30.3% ± 5.1% (standard error of the mean) for IDH2 (Figure 2C-D). Similar to IDH2, the mean VAFs for DNMT3A, TET2, and ASXL1 remained high at the time of remission (33.7% ± 3.6%, 33.8% ± 2.8%, and 34.7% ± 5.0%, respectively), with most patients retaining DTA mutations, often showing few differences between diagnosis and CR1 (Figure 2E). In addition, we found that variants in RUNX1, SRSF2, and TP53 were frequently identified at CR1 (supplemental Figure 2A).
Muts associated with CH are persistent at remission. (A) Bar plot depicting the percentage of muts identified in diagnosis that were also identified at CR1. Numbers to the right of each bar indicate the proportion of variants initially found at diagnosis that were subsequently detected at CR1. Genes are grouped into their relevant biological categories as follows: DNA damage (TP53, ATM); DTAI (DNMT3A, TET2, ASXL1, IDH2, and IDH1); splicing (SRSF2, U2AF1, and ZRSR2); PRC/RUNX (BCOR, BCORL1, RUNX1, and EZH2); cohesin (SMC1A, RAD21, and STAG2); and signaling (CSF1R, FLT3, NF1, KRAS, NRAS, BRAF, KIT, PTPN11, JAK2, CSF3R, and CBL). (B) As in panel A, but individual genes are shown. (C) Violin plot of VAFs for persistent variants at CR1. (D-F) Scatterplot detailing patient-matched VAFs at diagnosis (x-axis) and CR1 (y-axis) for (D) IDH1 and IDH2; (E) DNMT3A, TET2, and ASXL1; and (F) FLT3, NPM1, and NRAS. Each point represents 1 variant in a specific patient, matched across time. DTAI, DNMT3A, TET2, ASXL1, IDH1/2; PRC, polycomb repressive complex.
Muts associated with CH are persistent at remission. (A) Bar plot depicting the percentage of muts identified in diagnosis that were also identified at CR1. Numbers to the right of each bar indicate the proportion of variants initially found at diagnosis that were subsequently detected at CR1. Genes are grouped into their relevant biological categories as follows: DNA damage (TP53, ATM); DTAI (DNMT3A, TET2, ASXL1, IDH2, and IDH1); splicing (SRSF2, U2AF1, and ZRSR2); PRC/RUNX (BCOR, BCORL1, RUNX1, and EZH2); cohesin (SMC1A, RAD21, and STAG2); and signaling (CSF1R, FLT3, NF1, KRAS, NRAS, BRAF, KIT, PTPN11, JAK2, CSF3R, and CBL). (B) As in panel A, but individual genes are shown. (C) Violin plot of VAFs for persistent variants at CR1. (D-F) Scatterplot detailing patient-matched VAFs at diagnosis (x-axis) and CR1 (y-axis) for (D) IDH1 and IDH2; (E) DNMT3A, TET2, and ASXL1; and (F) FLT3, NPM1, and NRAS. Each point represents 1 variant in a specific patient, matched across time. DTAI, DNMT3A, TET2, ASXL1, IDH1/2; PRC, polycomb repressive complex.
Mutations in signaling genes (FLT3, NRAS, KRAS, and PTPN11) were rarely maintained at CR1 (16/112 [14.3%]), and only 2 instances of NPM1 mutations persisted at CR1 (2/39 [5.1%]; Figure 2A). Although we did not observe any persistent NRAS mutations at CR1 (0/16), several FLT3 variants were unexpectedly shared between diagnosis and CR1 in 9 of 57 patients (16%); of these, 4 were at similar VAFs between diagnosis and CR1 (Figure 2B,F). Two of these were FLT3 internal tandem duplications (E604-F605ins[11aa] and T582_E598dup), whereas the remaining 2 variants were missense mutations of uncertain significance (V214I and V795I). Similarly, both of the identified persistent NPM1 mutations were pathogenic W288Cfs∗12 variants. We further observed rare persistent or acquired variants in JAK2, TP53, U2AF1, NF1, SRSF2, ZRSR2, STAG2, and CBL at remission (supplemental Figure 2B-C).
Collectively, we observed that DNMT3A, TET2, and ASXL1 variants were less likely to be eliminated by chemotherapy than FLT3 or NPM1 (DTA combined vs FLT3, P = 9.3 × 10−11; DTA combined vs NPM1, P = 1.7× 10−12). Overall, these data indicate that CH-associated mutations frequently persist through chemotherapy at the time of remission in de novo AML, presumably because of their presence in a preleukemic cell compartment that remains intact despite effective AML-directed therapy.
FLT3 variants are dynamically acquired and eliminated between diagnosis and REL, whereas NPM1 mutations persist
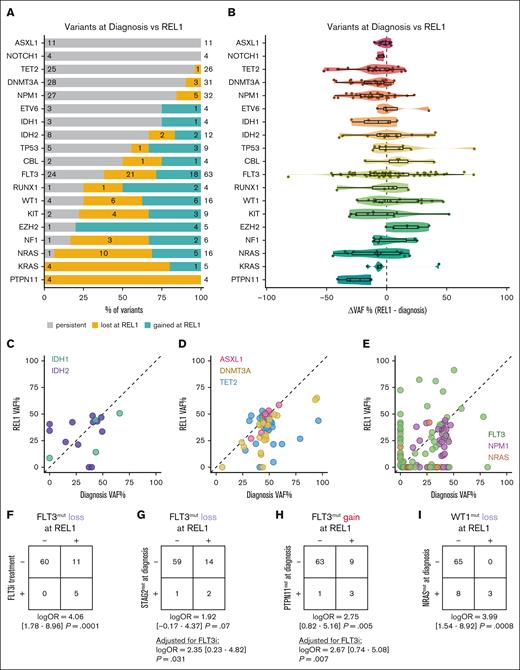
Next, we compared the presence of matched variants at diagnosis and REL (Figure 3A). We observed that all ASXL1 variants (11/11 [100%]) identified at diagnosis were present at REL. Similar results were evident with DNMT3A (28/31 [90.3%]), TET2 (25/26 [96.2%]), IDH2 (8/10 [80%]), and IDH1 (3/3 [100%]). NPM1 variants were similarly stable, with 27 of 32 (84.4%) shared between diagnosis and REL. In comparing VAFs between diagnosis and REL, we observed that most of the DTAI mutations reemerged to a similar VAF seen at diagnosis, (DNMT3A, 34.6% ± 3.0%; TET2, 35.8% ± 2.8%; ASXL1, 45.2% ± 2.7%; IDH1, 29.5% ± 10.6%; and IDH2, 31.4% ± 4.9%; Figure 3B-D). Similar results were evident for NPM1 (Figure 3E). We did not observe an instance of a new mutation in DNMT3A, TET2, or ASXL1 at time of REL1, whereas we did occasionally observe gain of IDH1 and IDH2 mutations (supplemental Figure 3A).
Signaling muts undergo dynamic losses and gains from diagnosis through REL. (A) Bar plot depicting the relative proportions of different mut trajectories between diagnosis and REL1 in individual patients (n = 76), filtered for genes with at least 4 variants identified across the cohort. Colors indicate whether the mut was stable (gray), lost (yellow), or gained (teal) from diagnosis through REL. The number to the right indicates the total number of variants identified among paired diagnosis and REL samples for the indicated gene; within each section of the bar plot, the numbers indicate the number of variants within each category. (B) Violin plot depicting the difference in VAFs (ΔVAF) between REL and diagnosis. Negative values indicate a lower VAF at REL, whereas positive values indicate a higher VAF. (C-E) Scatterplot indicating VAF at diagnosis (x-axis) and REL1 (y-axis) for (C) IDH1 and IDH2; (D) DNMT3A, TET2, and ASXL1; and (E) FLT3, NPM1, and NRAS. Each point represents 1 variant in a specific patient, matched across time. (F-I) Contingency tables evaluating the association between FLT3i treatment (F), STAG2 muts at diagnosis (G), PTPN11 muts at diagnosis (H), or NRAS muts at diagnosis (I) with FLT3 mut loss in panels F-G, FLT3 mut gain in panel H, or WT1 mut loss in panel I. In the event that a patient had multiple variants in the same gene, the variant with the largest change in VAF between diagnosis and REL was retained. Statistics were determined by Firth penalized logistic regression models; for panels G-H, FLT3i treatment status was included as a covariate in the model. OR, odds ratio.
Signaling muts undergo dynamic losses and gains from diagnosis through REL. (A) Bar plot depicting the relative proportions of different mut trajectories between diagnosis and REL1 in individual patients (n = 76), filtered for genes with at least 4 variants identified across the cohort. Colors indicate whether the mut was stable (gray), lost (yellow), or gained (teal) from diagnosis through REL. The number to the right indicates the total number of variants identified among paired diagnosis and REL samples for the indicated gene; within each section of the bar plot, the numbers indicate the number of variants within each category. (B) Violin plot depicting the difference in VAFs (ΔVAF) between REL and diagnosis. Negative values indicate a lower VAF at REL, whereas positive values indicate a higher VAF. (C-E) Scatterplot indicating VAF at diagnosis (x-axis) and REL1 (y-axis) for (C) IDH1 and IDH2; (D) DNMT3A, TET2, and ASXL1; and (E) FLT3, NPM1, and NRAS. Each point represents 1 variant in a specific patient, matched across time. (F-I) Contingency tables evaluating the association between FLT3i treatment (F), STAG2 muts at diagnosis (G), PTPN11 muts at diagnosis (H), or NRAS muts at diagnosis (I) with FLT3 mut loss in panels F-G, FLT3 mut gain in panel H, or WT1 mut loss in panel I. In the event that a patient had multiple variants in the same gene, the variant with the largest change in VAF between diagnosis and REL was retained. Statistics were determined by Firth penalized logistic regression models; for panels G-H, FLT3i treatment status was included as a covariate in the model. OR, odds ratio.
In contrast to the CH-associated DTAI genes, mutations in signaling genes were largely unstable, with many being lost between diagnosis and REL. These included PTPN11 (4/4), NRAS (10/11 [90.9%]), KRAS (4/4), and NF1 (3/4 [75%]; Figure 3A). FLT3 mutations showed a more dynamic pattern compared with other signaling mutations, with 24 of 45 (53.3%) persisting from diagnosis to REL. Of all identified FLT3 variants in patients with paired diagnosis and REL1 samples, 18 of 63 (29%) were newly acquired upon REL. Given the variation in the acquisition vs loss of FLT3 variants at REL, we first explored how much of this effect was explained by FLT3-targeted therapies. Of 76 patients with matched diagnosis and REL1 samples, 5 patients had received a FLT3 inhibitor (FLT3i) between diagnosis and REL1. All 5 of these patients (100%) demonstrated loss of the FLT3 mutation at REL1 (Figure 3F); in comparison, of 71 patients who did not receive a FLT3i before REL1 sample collection, only 11 patients (15%) exhibited FLT3 mutation loss (log odds ratio, 4.06 [95% confidence interval, 1.78-8.96]; P = .0001 for FLT3i treatment and subsequent FLT3 loss at time of REL1). To identify comutational partners at diagnosis that might predict this evolution other than FLT3 inhibition, we constructed Firth penalized regression models to determine the association between mutations at diagnosis and subsequent gain or loss of FLT3 mutations, focusing on the variant with the largest change in VAF for each gene in a given patient. Accounting for FLT3i treatment status, FLT3 mutation loss was more common in patients with a STAG2 mutation at diagnosis (P = .031; Figure 3G), whereas FLT3 mutation gain was enriched in patients with a PTPN11 mutation at diagnosis (P = .007; Figure 3H). Interestingly, PTPN11 mutations at diagnosis were often replaced by FLT3 mutations at diagnosis (supplemental Figure 3B-C). We observed additional mutations with evidence of dynamic gains/losses including EZH2, NRAS, RUNX1, TP53, and KIT (Figure 3A). Mutations in WT1 were particularly dynamic, with 6 lost variants at REL1, 6 acquired variants, and 4 persistent variants, mirroring the diverse range of outcomes present in FLT3-mutant disease. Loss of WT1 mutations were associated with NRAS mutations at diagnosis (P = .0008; Figure 3I). These results indicate that comutational partners are associated with distinct evolutionary outcomes after chemotherapy. Although FLT3 mutations are dynamically acquired and eliminated between diagnosis and REL, CH-related mutations and NPM1 mutations persist through CR into REL.
Comutation analyses reveal conserved and disease stage-specific genetic interactions in AML
To further explore comutational partners, we identified co-occurring and mutually exclusive mutation pairs at the time of diagnosis, CR1, and REL1 (supplemental Figure 4A-C). We identified 8 putative genetic interactions that were conserved between diagnosis and CR1 including: ASXL1-STAG2, ASXL1-SRSF2, CBL-TET2, chr5qdel-TP53, and IDH2-SRSF2. We further identified 10 putative genetic interactions that were shared between diagnosis and REL1 including, CBL-TET2, chr17loss-TP53, DNMT3A-NPM1, DNMT3A-FLT3, FLT3-NPM1, NOTCH2-U2AF2, NPM1-TET2, and mutual exclusivity of NPM1-TP53. Co-occurrence of the CBL-TET2 mutation pair was consistently identified across diagnosis, CR1, and REL1 disease stages. Most putative interactions identified at diagnosis or REL1 were unique to each disease stage, despite being sampled from the same patient cohort: 32 of 49 (65.3%) unique to diagnosis and 18 of 28 (64.3%) unique to REL1. One such interaction pair was chr8gain-TET2, which was only statistically significant at the time of diagnosis (supplemental Figure 4D-E). Our analyses therefore suggest that genetic interactions exhibit a degree of context-dependence, demonstrating the importance of interrogating the genomic features of AML across diverse disease stages.
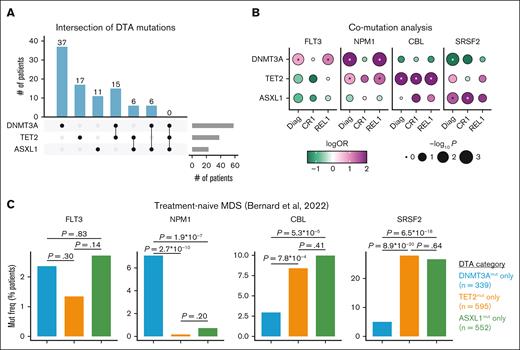
Early DNMT3A and TET2 mutations differentially shape the evolutionary trajectories of AML
Next, we sought to determine how early CH mutations influence downstream mutation stability at CR1 and loss/gain at REL1. We categorized patients by their mutational status in DNMT3A, TET2, and ASXL1 at the time of diagnosis (Figure 4A). Comparing pairs of DTA genes, we assessed cohort-level mutation frequencies at the time of diagnosis and REL (supplemental Figure 5A-F). Although FLT3 mutations were observed across patients with any of the DTA mutations, FLT3 mutations were enriched in DNMT3Amut cases at diagnosis (P = .01) and REL (P = .02; Figure 5B). Meanwhile, NPM1 mutations were enriched in DNMT3Amut cases at both diagnosis (P = 9.8 × 10−11) and REL (P = 5.5 × 10−7), as well as in TET2mut cases both at diagnosis (P = .03) and REL (P = .02). In contrast, CBL mutations were uniquely enriched in TET2mut samples at each stage: diagnosis (P = .009), remission (P = .03), and REL (P = .008). Notably, none of these mutations showed significant association with patients with ASXL1mut; rather these samples were enriched for SRSF2 at diagnosis (P = .02) and remission (P = .01; Figure 4B). Given the divergent genetic associations with distinct DTA mutations, and the relative enrichment of CBL and SRSF2 mutations in myelodysplastic syndrome (MDS), we wondered whether these findings generalized beyond our de novo AML cohort. In a cohort of untreated patients with MDS,41NPM1 mutations were enriched specifically in patients with DNMT3Amut, whereas CBL and SRSF2 mutations were associated with both TET2 and ASXL1 alterations (Figure 4C). FLT3 mutations were observed at similar frequencies between DNMT3Amut and ASXL1mut samples. These results indicate that early CH-related mutations show distinct mutational partners that persist at multiple stages of disease development and after therapy.
Early muts in DNMT3A, TET2, and ASXL1 differentially shape the subsequent evolution of AML from diagnosis (diag) through REL. (A) Upset plot indicating the number of patients at diag (n = 182) with muts in DNMT3A, TET2, and ASXL1. The number of patients per group is indicated above each bar. (B) Co-mut analysis of FLT3, NPM1, CBL, and SRSF2 in relation to DNMT3A, TET2, or ASXL1 across the entire cohort. Dots are color-coded by logORs and size-scaled by statistical significance (Fisher's 2-sided exact test). Asterisks denote P < .05. (C) Bar plot detailing the frequency of FLT3, NPM1, CBL, or SRSF2 muts in a cohort of patients with untreated MDS,41 stratified by DNMT3A, TET2, and ASXL1 mut status. Statistical significance was assessed by Fisher's 2-sided exact test. mut freq, mutation frequency; OR, odds ratio.
Early muts in DNMT3A, TET2, and ASXL1 differentially shape the subsequent evolution of AML from diagnosis (diag) through REL. (A) Upset plot indicating the number of patients at diag (n = 182) with muts in DNMT3A, TET2, and ASXL1. The number of patients per group is indicated above each bar. (B) Co-mut analysis of FLT3, NPM1, CBL, and SRSF2 in relation to DNMT3A, TET2, or ASXL1 across the entire cohort. Dots are color-coded by logORs and size-scaled by statistical significance (Fisher's 2-sided exact test). Asterisks denote P < .05. (C) Bar plot detailing the frequency of FLT3, NPM1, CBL, or SRSF2 muts in a cohort of patients with untreated MDS,41 stratified by DNMT3A, TET2, and ASXL1 mut status. Statistical significance was assessed by Fisher's 2-sided exact test. mut freq, mutation frequency; OR, odds ratio.
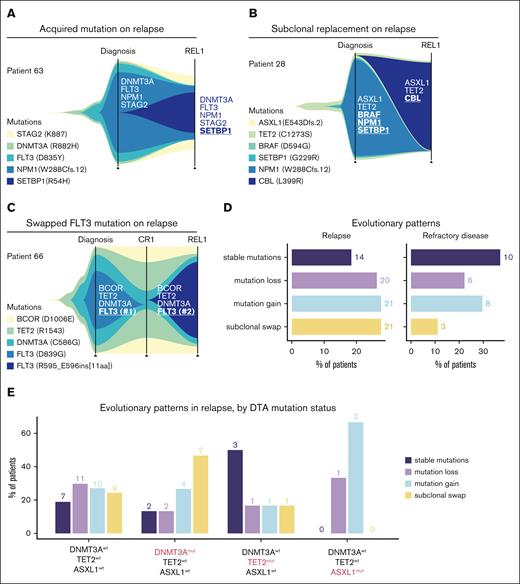
Patterns of AML genomic evolution from diag to REL. (A-C) Fish plots detailing the expansion and contraction of specific variants within individual patients from diag to REL. (D) Classification of evolutionary patterns at the time of REL (left) or REF disease (right) across the entire cohort. (E) Bar plot detailing the type of REL patterns observed in patients jointly stratified by DNMT3A, TET2, and ASXL1 mut status.
Patterns of AML genomic evolution from diag to REL. (A-C) Fish plots detailing the expansion and contraction of specific variants within individual patients from diag to REL. (D) Classification of evolutionary patterns at the time of REL (left) or REF disease (right) across the entire cohort. (E) Bar plot detailing the type of REL patterns observed in patients jointly stratified by DNMT3A, TET2, and ASXL1 mut status.
Patterns of AML genomic evolution from diagnosis to REL
Because the prior analyses were performed on the cohort level, comparing mutation frequencies in different cross-sections of the AML disease course, we sought to explore the characteristics of AML evolution within individual patients. We applied the CALDER algorithm38 to infer phylogenetic relationships from matched longitudinal AML sequencing data in individual patients.
Among the patterns of AML evolution from diagnosis to REL, we observed several cases in which, at the time of REL, an additional driver mutation had been acquired on top of the original mutations that were seen on diagnosis (Figure 5A). In such patients, it is likely that the initial AML clone observed at diagnosis subsequently reexpanded after incomplete elimination by induction chemotherapy, gaining additional mutations in the process. In other cases, we observed evidence of subclonal replacement, with reciprocal loss and gain of mutations (Figure 5B). Among these, we observed cases in which a FLT3 mutation seen at the time of diagnosis was subsequently replaced by a different FLT3 alteration on REL (Figure 5C). It is likely that chemotherapy had successfully eliminated the AML-driving FLT3mut clone, with subsequent disease REL being driven by the acquisition or expansion of a distinct FLT3mut clone.
Next, we analyzed the evolutionary trajectories for all patients profiled at both diagnosis and REL (n = 76) and classified them into 1 of 4 broad REL patterns. Patients were distributed across these 4 categories, with 14 of 76 (18.4%) of cases demonstrating stable mutational profiles, 21 of 76 (27.6%) acquiring a new mutation, 20 of 76 (26.3%) losing an initial mutation, and 21 of 76 (27.6%) exhibiting subclonal swaps (Figure 5D). In patients sequenced at the time of diagnosis and REF disease (n = 27), comparatively fewer patients (3/27 [11.1%]) underwent subclonal swaps, whereas stable mutational profiles were most common (10/27 [37%]). Subclonal swapping trended toward being more common in REL compared with REF disease (P = .11). Compared with stable mutational profiles, mutational swaps were more common in REL than in REF disease (P = .049). Finally, in an exploratory analysis, we asked how founding CH mutations in DNMT3A, TET2, or ASXL1 influenced evolutionary trajectories in REL. Although there were limited sample sizes for patients with only a single DTA mutation (DNMT3A, TET2, or ASXL1) and matched REL genomic profiles, we observed that DNMT3Amut (TET2wt and ASXL1wt) AML was more likely to REL through subclonal swaps (7/15 [46.7%]) than TET2mut (DNMT3Awt and ASXL1wt) AML (1/6 [16.7%]). In contrast, TET2mut (DNMT3Awt and ASXL1wt) AML more often REL with stable mutation profiles (3/6 [50%]) than DNMT3Amut (TET2wt and ASXL1wt) AML (2/15 [16%]; Figure 5E). These analyses showcase the utility of longitudinal genomic profiling to reveal recurrent evolutionary modes of AML REL across individual patients.
Discussion
Although other large patient cohorts have been analyzed with both exome sequencing and panel-based approaches, most include mixtures of AML evolved from a prior MDS, de novo AML, and REL/REF AML. Our retrospective study specifically focused on patients with no prior hematologic diagnoses or hematopoietic abnormalities. Similar studies have been performed retrospectively on clinical trial samples,27 including those specifically focused on FLT3 mutant24,25 or NPM1 mutant22,42 AML. These studies were largely executed in the research setting using exome-wide assays.43 Both a strength and limitation of our study was the use of a Clinical Laboratory Improvement Amendments–approved targeted panel; because our study is built on real-world data collected through routine clinical practice, our findings are directly relevant to clinicians and patients. We note that variants of unknown significance were included; thus, not all variants analyzed here are pathogenic variants. Furthermore, the technical limitations of our targeted NGS panel likely leads to underestimation of mutation evolutionary processes. In addition, our analysis pipeline allowed for a minimum 2% to 4% VAF cutoff for reporting variants. Error corrected sequencing approaches have demonstrated that VAFs as low as 10−5 can offer prognostic information for FLT3 and NPM1 mutations in the context of measurable residual disease detection at CR1.36,37 The paucity of NPM1 mutations detected at CR1, and their near uniform recurrence at REL, suggests that our data set is likely enriched for false negatives for NPM1, and potentially other genes, at remission.
Additionally, most of our cohort (82%) was treated with conventional “7+3” induction chemotherapy; in light of ongoing work evaluating venetoclax/azacitidine regimens,44 including a randomized trial vs conventional induction chemotherapy (ClinicalTrials.gov identifier: NCT04801797), future studies should explore whether the findings described here are conserved in patients treated with venetoclax/azacitidine. We anticipate that clonal evolution trends will diverge significantly between these 2 classes of therapy, given the collapse of RAS-family mutations after 7+3 in our cohort, and their emergence as a mechanism of resistance to venetoclax/azacitidine treatment.45,46 Similar trends are evident for FLT3 and targeted therapy, which are now emerging as frontline therapy alongside 7+3 induction.47 We anticipate that understanding the drivers of clonal evolution will unlock optimal therapy sequences.
As the co-occurring module of TET2, CBL, and SRSF2 mutations is highly prevalent in MDS,41 it is interesting that we were able to recapitulate this mutational pattern in our cohort of de novo AML (ie, patients without preexisting MDS). We further found that the co-occurring module of DNMT3A and NPM1 mutations observed in de novo AML were also seen in the MDS cohort. Although there were distinctions between the mutational archetypes seen in each cohort, these commonalities suggest that regardless of whether AML arises de novo or as a gradual progression from MDS, the underlying genetic interactions shaping their evolutionary trajectories appear to be conserved. These data are consistent with the European LeukemiaNet 2022 guidelines of NPM1 mutations being sufficient to diagnose patients with AML, that might otherwise fit histopathological descriptions of MDS.48 Our data support the notion that DNMT3Amut MDS likely encompasses a genomic comutational landscape that is reminiscent of comutational partners found in AML.
To our knowledge, no study, to date, has compared the genomic profiles of DNMT3Amut and TET2mut AML as the disease evolves from initial diagnosis through remission and subsequent REL. DNMT3A and TET2 are the most commonly mutated genes associated with CH,7-9 and both of these genes encode key regulators of DNA methylation.49 As DNMT3A and TET2 mutations are among the earliest genetic alterations in AML, dysregulation of DNA methylation is presumably an important predisposing factor for the subsequent pathogenesis of AML. DNMT3A and TET2 play diametrically opposing roles in DNA methylation: whereas DNMT3A catalyzes DNA methylation, TET2 demethylates DNA. It stands to reason, then, that the evolutionary fitness landscapes of malignancies arising from DNMT3Amut clones likely differ from those that derive from TET2mut clones. Our analyses illuminate the distinct genomic features of DNMT3Amut, TET2mut, and ASXL1mut AML. Although some of these associations have been previously reported on the basis of single time point genomic profiling,50 they have not been described longitudinally over the course of AML evolution through chemotherapy and REL. In an exploratory analysis, we also find preliminary evidence that the clonal dynamics of REL may vary depending on the underlying CH driver, with DNMT3Amut AML exhibiting a higher propensity for larger scale subclonal swapping at REL than TET2mut AML. Although these findings require validation in larger cohorts, we speculate that by inducing genome-wide hypomethylation,51DNMT3A mutations may engender epigenetic plasticity that can accommodate a broader range of comutational drivers compared with TET2 mutations. Thus, as DNMT3A, TET2, and ASXL1 mutations represent the earliest genetic events in the pathogenesis of AML, our findings demonstrate how “founding” preleukemic driver mutations can subsequently mold the evolutionary paths traversed during AML evolution.
Acknowledgments
R.L.B. was supported by National Cancer Institute grants R00CA248460 and UG1CA233332, the American Society of Hematology, and the Leukemia Research Foundation.
Authorship
Contribution: P.V. and J.M. conceived and designed the study; R.D.C. designed and executed the experimental analysis; R.D.C., P.V., S.D., J.M., A.Y., and N.S. performed experimental analysis and curated patient records and data; J.M. and R.L.B. supervised the study; R.D.C. and R.L.B. wrote the manuscript with significant revisions and critical feedback from P.V., M.P.C., S.M.L., and J.M.; and all authors reviewed and commented on the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert L. Bowman, University of Pennsylvania, 421 Curie Blvd, Room 753, Philadelphia, PA 19104; email: robert.bowman@pennmedicine.upenn.edu.
References
Author notes
R.D.C. and P.V. contributed equally to this study.
All mutation calls, clinical annotations, and code are publicly available on GitHub (https://github.com/rdchow/PennAML). All summarized data needed to replicate this study are included in the supplemental Data. Deidentified patient data are available upon reasonable request from the corresponding author, Robert L. Bowman (robert.bowman@pennmedicine.upenn.edu).
The full-text version of this article contains a data supplement.