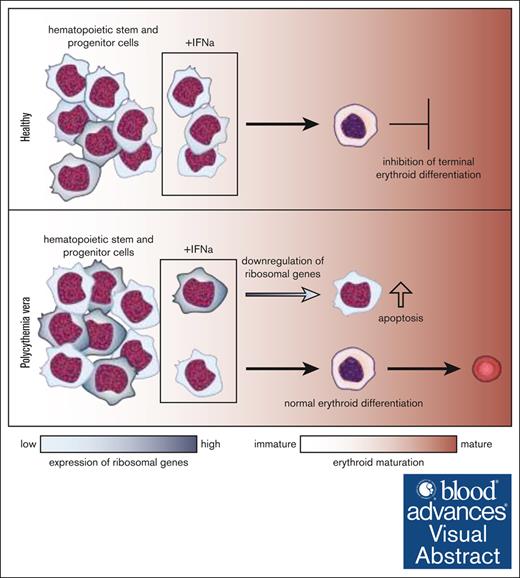
Key Points
IFN-α induced apoptosis in PV immature progenitors while erythroid differentiation was not inhibited.
IFN-α reduced ribosome genes in apoptotic cells; high ribosomal activity clones were most targeted hinting at vital ribosome-altering role.
Visual Abstract
Interferon alfa (IFN-α) is approved for the therapy of patients with polycythemia vera (PV), a subtype of myeloproliferative neoplasm (MPN). Some patients achieve molecular responses (MRs), but clonal factors sensitizing for MRs remain elusive. We integrated colony formation assays with single-cell RNA sequencing (scRNA-seq) and genotyping in PV-derived cells and healthy controls (HCs) to dissect how IFN-α targets diseased clones during erythroid differentiation. IFN-α significantly decreased colony growth in MPNs and HCs with variable transcriptional responses observed in individual colonies. scRNA-seq of colonies demonstrated more mature erythroid colonies in PV than HCs. JAK2V617F-mutant cells exhibited upregulated STAT5A, heme, and G2M checkpoint pathways compared with JAK2WT cells from the same patients. Subgroup analysis revealed that IFN-α significantly decreased immature erythrocytic cells in PV (basophilic erythroblasts P < .05; polychromatic erythroblasts P < .05) but not in HCs. CD71−/CD235a+ cells from HCs (P < .05) but not PV were inhibited by IFN-α, and the number of reticulocytes was less affected in PV. Robust IFN-α responses persisted throughout differentiation, leading to significant apoptosis in PV. Apoptotic cells displayed downregulation of ribosomal genes. This link between apoptosis and ribosomal genes was corroborated through the analysis of mitochondrial variants, demonstrating IFN-α–induced eradication of specific clones, characterized by elevated expression of ribosomal genes. Our findings indicate that PV-derived clones either undergo apoptosis or pass through differentiation, overall reducing the cycling mutant cells over long-term treatment. Furthermore, the significance of ribosomal genes and clonal prerequisites in IFN-α’s therapeutic mechanism is underscored, shedding light on the intricate dynamics of IFN-α treatment in PV.
Introduction
Myeloproliferative neoplasms (MPNs), a group of chronic hematologic malignancies, comprise polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).1 Patients with MPN have an increased risk to develop thrombosis and acute leukemia and the most prevalent driver mutation is JAK2V617F,2-5 which leads to the production of a constitutive active JAK2 protein.3
MPNs are clonal diseases, and the malignant clone reshapes normal hematopoiesis.6-8 The emergence of novel techniques like single-cell RNA sequencing (scRNA-seq), combined with TARGETseq9 or genotyping of transcripts,10 has allowed a much more refined description of clonal hierarchy and evolution, including the definition of transcriptomic differences between wild-type (WT) and mutated cells.9,11 In this manner, differentiation bias in mutated cells12-14 were identified, and the clonal differences in sensitivity toward different treatments were studied.15,16 Remarkably, malignant clones can exist decades before diagnosis and considerable evolution and subclones were observed, thereby expanding our view of MPNs as multiclonal diseases.14,17,18
Interferon alfa (IFN-α) is approved for the treatment of PV and proposedly has curative potential.19 Pegylated IFN-α exhibits an increased half-life and is applied less frequently than conventional forms, thereby leading to a reduction in side effects. Most of the patients show hematologic responses (HRs), defined by normalization of blood parameters and disease-associated symptoms.20-25 Furthermore, IFN-α can induce molecular responses (MRs), manifesting as decreases in the mutant variant allele frequency (VAF). This effect is especially pronounced in PV, with more heterogeneous responses in ET and PMF.22-25 The MR was shown to depend on driver and bystander mutations.26-28 In murine MPN models, IFN-α induced cycling and the subsequent differentiation of mutated hematopoietic stem cells.29 Recent studies have confirmed the reduction of quiescence in additional mouse models and in patients with a JAK2V617F mutation.16,28,30 This suggests that IFN-α treatment leads to a decrease in dormant mutant stem cells and, proposedly, an exhaustion of the disease-driving cells after long-term treatment. In addition, apoptosis was found to be induced by IFN-α in immature erythrocytic progenitors, and the VAF-reducing effect has been attributed to IFN-α–induced STAT1 and p38 activity.29,31-33
Previous investigations have centered around IFN-α’s impact within the stem cell compartment. Therefore, we investigated its effect in differentiating cells with special attention to clonal heterogeneity. Focusing on PV, we combined colony formation assays (CFU assays), in which colonies reflect the clonal landscape of patients, with scRNA-seq and single-cell genotyping14,34 to provide functional insights into the mode of action of IFN-α and heterogeneity of the IFN-α response at clonal level.
Methods
Cell culture and primary cells
Peripheral blood mononuclear cells (PBMCs) were isolated via density gradient centrifugation (Ficoll, Miltenyi; Pancoll, Pan Biotech) as summarized in supplemental Table 1. Samples were obtained after informed and written consent, as approved by the local ethics committee (EK099/14, EK127/12). CFU assays, colony genotyping, and calculation of the clonogenic VAF were performed as described previously.35 IFN-α2b (ImmunoTools), at a concentration of 500 U/mL, was directly added to the methylcellulose, which was supplemented with 50 ng/mL human stem cell factor, 10 ng/mL human interleukin-3, 10 ng/mL human granulocyte-macrophage colony-stimulating factor, and 14 ng/mL human erythropoietin (all ImmunoTools, Germany).
scRNA-seq
PBMCs were seeded in CFU assays with and without IFN-α, as described earlier, and harvested after 10 days of cultivation. Cells were counted and used for scRNA-seq. A detailed description of the procedure is given in the supplemental Methods.
Analysis of scRNA-seq results
We preprocessed data with the CellRanger pipeline (version 3.1.0) using the default parameters. Seurat (version 4.2.0) was used for integration and clustering (details can be found in the supplemental Methods). Annotation of the clusters was done with the help of previously published data sets.36-38
Single-cell genotyping
Cells were genotyped for JAK2V617F using VarTrix and MAEGATK41 for mitochondrial variants. We calculated the VAF per cell for all possible mitochondrial variants using the MAEGATK results and selected mitochondrial variants of interest using a thresholding approach.41 We developed SIGURD,42 an R package for this analysis, which is available on GitHub (https://github.com/CostaLab/sigurd) to enable easy adaptation and replication. The package integrates and analyzes single-cell genotyping (JAK2V617F and mitochondrial variants) and scRNA-seq data and store these within a Seurat object. The data are centrally organized in an input file, which tracks the location of the input files, such as the BAM files and the cell barcode files. We loaded the results of the genotyping from VarTrix and MAEGATK into R on a per-sample basis. For each cell, we retrieved the consensus and allele fraction values from VarTrix and calculated the corresponding values for MAEGATK. The results were then saved in a SummarizedExperiment object.
Ethics approval and consent to participate
All samples were obtained after informed and written consent of patients and HCs, as approved by the local ethics committees of the RWTH Aachen University (EK099/14 and EK127/12).
Results
CFU assays model the response of patients and elucidate clonal heterogeneity in transcriptional response to IFN-α
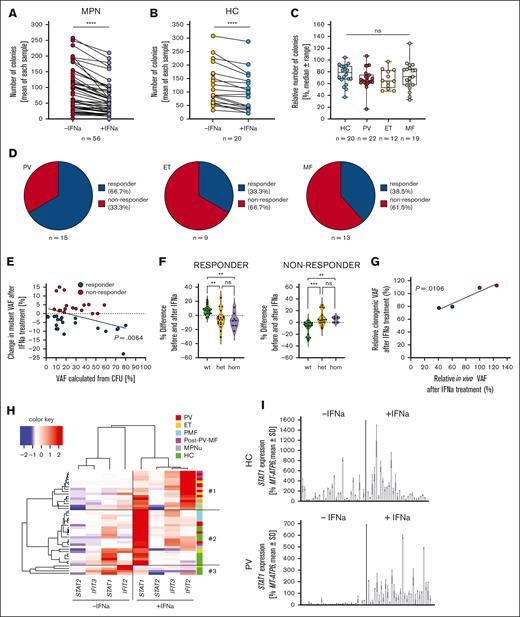
To improve our understanding of IFN-α responsiveness in JAK2V617F-mutated MPN, 22 PV, 12 ET, 16 PMF, 3 post–PV-MF, and 3 MPN-unclassifiable (MPNu) samples were investigated in CFU assays. Overall, a significant reduction in colony number was observed with in vitro IFN-α treatment in MPN and healthy control (HC) samples (Figure 1A-B), and this was accompanied by a decrease in colony size (supplemental Figure 1). There were no differences between the disease samples and HCs (Figure 1C).
CFU assays are a valuable model to study IFN-α hematologic and molecular responses in patients with MPN. (A) PBMCs of patients with MPN were seeded in CFU assays, supplemented with or without 500 U/mL IFN-α, and were counted after 10 to 14 days without discrimination between different types of colonies. Each data point represents an individual patient. The significance was analyzed using a Wilcoxon matched-pairs signed ranks test. ∗∗∗∗P < .0001. (B) HC samples were treated in the same manner as MPN samples in panel A. Significance was analyzed using a Wilcoxon matched-pairs signed ranks test. (C) The relative numbers of remaining colonies were calculated using untreated samples as control. Samples were grouped into HC and MPN subtypes. The data were analyzed using a Kruskal-Wallis test with Dunn multiple comparison after failing the Shapiro-Wilk test for normality. None of the comparisons reached significance. Blue indicates HC; Red indicates PV; yellow indicates ET; grey indicates MF; (D) DNA from 25 to 30 colonies per patient for each condition was isolated and screened for the presence of the JAK2V617F mutation via genotyping PCR. The ratio of MPN WT, MPN heterozygous, and MPN homozygous colonies was used to calculate the mutant VAF. The mutant VAF was compared between the untreated and treated condition for each patient. Patients who showed a decrease in mutant VAF upon treatment were grouped as molecular responders, patients who showed no change or an increase in VAF were termed non-responders. The ratios of molecular responders to nonresponders in the CFU model were analyzed in the different MPN subtypes. MF included patients with PMF and those with post–PV-MF. (E) Change in the mutant VAF upon in vitro IFN-α treatment was correlated with the mutant VAF calculated from the untreated CFU sample. Correlation was analyzed using Pearson r after passing the Shapiro-Wilk test for normality. (F) Differences in the fraction of colonies in the untreated and treated condition in responders (left) and non-responders (right) were analyzed. WT, het, and hom colonies were compared. Statistical analysis was performed using a Kruskal-Wallis test, followed by Dunn multiple comparison after failing the Shapiro-Wilk test for normal distribution. ∗∗P < .01; ∗∗∗P < .001. (G) The change in VAF was analyzed in patients treated with IFN-α in the clinics and compared with the change in VAF in the CFU assay. Data were calculated relative to the initial VAF. (H) RNA was isolated from individual colonies. Of each patient or HC, a minimum of 2 colonies was used. Complimentary DNA was generated, and the expression of STAT1, STAT2, IFIT2, and IFIT3 was measured using RT-qPCR with normalization to MT-ATP6. The mean of all measured colonies per patient and condition was calculated and the values were subjected to an unsupervised clustering. (I) PBMCs were seeded, with or without 500 U/mL IFN-α, and colonies were picked after 10 days of cultivation. STAT1 expression was analyzed using RT-qPCR in 30 untreated and treated colonies of an HC (top) and PV sample (bottom; PV14) (supplemental Table 1). Each bar represents a single colony. Expression was normalized to MT-ATP6.
CFU assays are a valuable model to study IFN-α hematologic and molecular responses in patients with MPN. (A) PBMCs of patients with MPN were seeded in CFU assays, supplemented with or without 500 U/mL IFN-α, and were counted after 10 to 14 days without discrimination between different types of colonies. Each data point represents an individual patient. The significance was analyzed using a Wilcoxon matched-pairs signed ranks test. ∗∗∗∗P < .0001. (B) HC samples were treated in the same manner as MPN samples in panel A. Significance was analyzed using a Wilcoxon matched-pairs signed ranks test. (C) The relative numbers of remaining colonies were calculated using untreated samples as control. Samples were grouped into HC and MPN subtypes. The data were analyzed using a Kruskal-Wallis test with Dunn multiple comparison after failing the Shapiro-Wilk test for normality. None of the comparisons reached significance. Blue indicates HC; Red indicates PV; yellow indicates ET; grey indicates MF; (D) DNA from 25 to 30 colonies per patient for each condition was isolated and screened for the presence of the JAK2V617F mutation via genotyping PCR. The ratio of MPN WT, MPN heterozygous, and MPN homozygous colonies was used to calculate the mutant VAF. The mutant VAF was compared between the untreated and treated condition for each patient. Patients who showed a decrease in mutant VAF upon treatment were grouped as molecular responders, patients who showed no change or an increase in VAF were termed non-responders. The ratios of molecular responders to nonresponders in the CFU model were analyzed in the different MPN subtypes. MF included patients with PMF and those with post–PV-MF. (E) Change in the mutant VAF upon in vitro IFN-α treatment was correlated with the mutant VAF calculated from the untreated CFU sample. Correlation was analyzed using Pearson r after passing the Shapiro-Wilk test for normality. (F) Differences in the fraction of colonies in the untreated and treated condition in responders (left) and non-responders (right) were analyzed. WT, het, and hom colonies were compared. Statistical analysis was performed using a Kruskal-Wallis test, followed by Dunn multiple comparison after failing the Shapiro-Wilk test for normal distribution. ∗∗P < .01; ∗∗∗P < .001. (G) The change in VAF was analyzed in patients treated with IFN-α in the clinics and compared with the change in VAF in the CFU assay. Data were calculated relative to the initial VAF. (H) RNA was isolated from individual colonies. Of each patient or HC, a minimum of 2 colonies was used. Complimentary DNA was generated, and the expression of STAT1, STAT2, IFIT2, and IFIT3 was measured using RT-qPCR with normalization to MT-ATP6. The mean of all measured colonies per patient and condition was calculated and the values were subjected to an unsupervised clustering. (I) PBMCs were seeded, with or without 500 U/mL IFN-α, and colonies were picked after 10 days of cultivation. STAT1 expression was analyzed using RT-qPCR in 30 untreated and treated colonies of an HC (top) and PV sample (bottom; PV14) (supplemental Table 1). Each bar represents a single colony. Expression was normalized to MT-ATP6.
The mutational status of JAK2 was determined for each colony, and the mutant VAF was calculated as described previously.35 The patients were grouped by their in vitro MR into those with a decrease in VAF (molecular responders) or those with no change or an increase in VAF (molecular nonresponders). Patients with PV showed the highest fraction of molecular responders (66.7%) when compared with those with ET (33.3%) or MF (PMF and post–PV-MF, 38.5%) (Figure 1D), reflecting patterns observed in clinical settings.43 Furthermore, patients’ VAF and their MR correlated significantly (Figure 1E). We compared the fractions of heterozygous (het) and homozygous (hom) colonies in the treated and untreated condition. In molecular responders, both the het and hom colonies were significantly decreased, whereas there was no preferential effect on hom colonies (Figure 1F). Conversely, an increase in mutated colonies (het and hom) was observed in the molecular non-responders (Figure 1F). To investigate if our model correlated with the molecular response of patients treated with IFN-α in the clinics, we analyzed the relative VAF after IFN-α treatment in colonies and in the peripheral blood (PB) of 4 patients. Intriguingly, we found a significant correlation between the in vitro and the in vivo response (Figure 1G). This indicates that our model is able to predict clinical responses.
Basal and induced STAT1, STAT2, IFIT2, and IFIT3 expression in 2 to 6 colonies per patient were analyzed using reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR). Only basal IFIT2 showed weak correlation with the VAF change (r = 0.3782). No clear correlation was found, and there was high interpatient variability (supplemental Figure 2). Expression values were then subjected to unsupervised clustering (Figure 1H). Three main clusters emerged. Cluster 1 comprised mainly patients with PV, ET, and post–PV-MF, and it was marked by IFIT2 being the highest expressed gene after stimulation. Cluster 2 comprised primarily HCs and patients with PMF. This cluster was characterized by STAT1 as the highest expressed gene after IFN-α stimulation. The third cluster comprised HCs and patients with ET, lacked transcriptional response after IFN-α stimulation, and was underrepresented overall, indicating that most of the analyzed samples showed an IFN-α response. The clustering revealed a closer relationship in gene expression patterns between PV and ET when compared with PMF.
The data earlier showed differences between patients but did not give an insight into the clonal heterogeneity of the IFN-α response. Therefore, colonies from HCs and patients with PV were analyzed for STAT1 expression (Figure 1I; supplemental Figure 3A-D). We observed high variability in the basal and IFN-α –stimulated STAT1 expression levels in colonies from patients with PV and HCs, indicating a pronounced clonal heterogeneity that also affected the IFN-α response.
Nonetheless, this analysis did not provide an answer to whether all clones were IFN-α responsive. Therefore, after further cultivation and stimulation, STAT1, IFIT2, and IFIT3 expression levels were assessed in single clones. Expression of the analyzed genes was induced in all HC and PV colonies (supplemental Figure 4A), but the intensities varied between colonies. The fold change (FC) for each gene and clone were subjected to an unsupervised clustering (supplemental Figure 4B) and distinct clusters were identified, but no correlation between these and individuals or disease states was observed, further underlining the clonal complexity. Furthermore, we analyzed the FC for all 3 genes based on the genotype of the colonies. We found that the induction of gene expression for STAT1 and IFIT3 was significantly stronger in WT than in mutated colonies, whereas it was comparable for IFIT2 (supplemental Figure 4C).
PV-derived JAK2WT cells have a distinct transcriptional signature
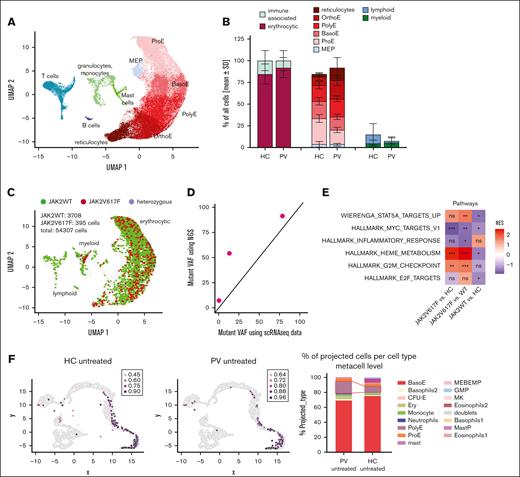
CFU assays were conducted for 3 patients with PV (PV01, 7%; PV02, 54%; and PV03, 91% JAK2V617F; none received cytoreductive therapy) and 3 HCs with and without 500 U/mL IFN-α, and the colonies were analyzed using scRNA-seq after 10 days of cultivation. This led to a total of 54,307 high quality cells with an average of 5395 reads per cell (supplemental Figure 5). Most of the cells belonged to the erythrocytic lineage, and different erythrocytic maturation stages, from megakaryocytic-erythrocytic progenitors (MEPs) to reticulocytes, were identified (Figure 2A). Furthermore, 2 myeloid (granulocytes/monocytes and mast cell progenitors) and 2 lymphoid (T cells and B cells) clusters were found. We compared differences in the untreated HC and PV samples and, numerically, more erythrocytic cells were found in PV, whereas the immune cells, especially lymphoid cells, were decreased (Figure 2B).
Healthy and diseased cells displayed distinct phenotype and signaling signatures. (A) PBMCs from 3 patients with PV and 3 HCs, untreated or treated with 500 U/mL IFN-α, were cultivated in CFU assays for 10 days. The cells were detached and used for scRNA-seq. The uniform manifold approximation and projection for dimension reduction (UMAP) is shown, which depicts the different cell types identified. (B) The relative amounts of different cell types per sample were analyzed. The distribution of cells in untreated HC and untreated PV samples is shown. None of the comparisons reached significance. Left: erythrocytic vs immune associated cells. Middle: different maturation stages of erythrocytic cells. Right: lymphocytic vs myeloid cells. (C) Genotyping of transcripts using locus-specific amplification of the JAK2V617F locus in the scRNA-seq samples. Mutated (hom), het, and WT cells are highlighted in the UMAP plot. (D) Comparison of mutant VAF as calculated from scRNAseq data with next-generation sequencing VAF from peripheral blood from the same patients (E) Comparison of the activity in the indicated pathways. All pathways were previously shown to be differentially expressed in JAK2V617F vs JAK2WT vs HC cells. (F) Integration of the scRNA-seq data in an additional differentiation trajectory model.44 Left: location of the analyzed samples in the metacell model. Right: statistical analysis of the differences in cell types. ns, not significant, ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Healthy and diseased cells displayed distinct phenotype and signaling signatures. (A) PBMCs from 3 patients with PV and 3 HCs, untreated or treated with 500 U/mL IFN-α, were cultivated in CFU assays for 10 days. The cells were detached and used for scRNA-seq. The uniform manifold approximation and projection for dimension reduction (UMAP) is shown, which depicts the different cell types identified. (B) The relative amounts of different cell types per sample were analyzed. The distribution of cells in untreated HC and untreated PV samples is shown. None of the comparisons reached significance. Left: erythrocytic vs immune associated cells. Middle: different maturation stages of erythrocytic cells. Right: lymphocytic vs myeloid cells. (C) Genotyping of transcripts using locus-specific amplification of the JAK2V617F locus in the scRNA-seq samples. Mutated (hom), het, and WT cells are highlighted in the UMAP plot. (D) Comparison of mutant VAF as calculated from scRNAseq data with next-generation sequencing VAF from peripheral blood from the same patients (E) Comparison of the activity in the indicated pathways. All pathways were previously shown to be differentially expressed in JAK2V617F vs JAK2WT vs HC cells. (F) Integration of the scRNA-seq data in an additional differentiation trajectory model.44 Left: location of the analyzed samples in the metacell model. Right: statistical analysis of the differences in cell types. ns, not significant, ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
We used a JAK2 locus-specific amplification to identify JAK2WT and mutated cells.34 This improved the coverage of the JAK2V617F locus from 1 to 9 reads per cell (supplemental Figure 6A). All mutated cells were part of the erythroid or myeloid lineage (Figure 2C). We compared the VAF based on the scRNA-seq data with that of the next-generation sequencing VAF of whole blood (Figure 2D) and observed an agreement of estimates. The number of genotyped cells decreased with erythroid maturation (supplemental Figure 6B), most likely because of a reduction in JAK2 expression (supplemental Figure 6C). We analyzed HBM expression to exclude a general decline in sequencing quality. HBM expression increased during differentiation, peaking in the final quarter of maturation, which indicated that sequencing quality remained consistent (supplemental Figure 6D). In addition, we analyzed 4 publicly available data sets45-48 (supplemental Figure 7) and found a reduction in JAK2 expression during the late stages of erythroid differentiation, corroborating our findings.
Signaling differences between HCs and JAK2WT MPN-derived cells were previously reported.9,16 We found that STAT5 target genes were significantly upregulated in JAK2V617F PV-derived cells when compared with JAK2WT PV-derived cells, whereas they were downregulated in JAK2WT PV-derived cells when compared with HC cells (Figure 2E). Intriguingly, MYC targets v1 were downregulated in PV overall, most markedly in JAK2V617F cells. In addition, inflammatory signaling was generally downregulated in PV-derived cells, whereas heme metabolism and the G2M checkpoint pathways were significantly upregulated in JAK2V617F cells when compared with JAK2WT and HC cells. Meanwhile, E2F targets were only significantly downregulated in JAK2WT when compared with HC cells. These results confirmed the distinct transcriptomic signature of JAK2WT PV-derived cells and that they differ from JAK2V617F and HC cells.
In addition, our data were integrated into an in vitro differentiation model established by Shlush et al.44 Here, it was discovered that PV-derived cells located with more mature erythroid cells than those of HCs (Figure 2F, left) and the most mature erythroid cells, polyE, were significantly enriched in PV (P < 2.8 × 10−4; Figure 2F, right), underlining a higher grade of erythroid maturation in PV.
IFN-α primarily targets immature erythrocytic cells in PV
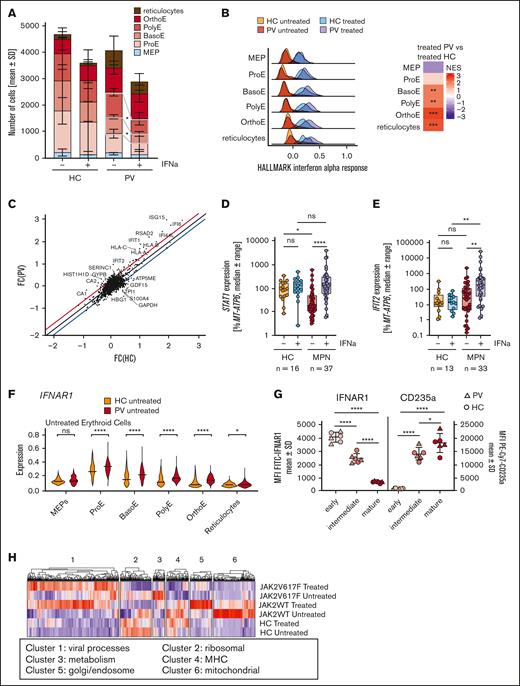
We next aimed to identify the cell type specifically targeted by IFN-α. In the PV-derived immature erythrocytic clusters (BasoE and PolyE), there was a significant reduction following addition of IFN-α (Figure 3A), whereas there was no significant decrease in specific populations in HCs. A clear induction of the hallmark IFN-α response49,50 was observed in all HC and PV erythrocytic clusters (Figure 3B). Throughout erythroid maturation, IFN-α response was stronger in PV-derived cells than in HC cells, leading to a higher IFN-α response in all PV-derived erythrocytic cells, except for MEPs and ProEs.
Immature erythrocytic PV-derived clusters decrease following IFN-α treatment, and differences between PV and HC cannot be attributed to IFNAR expression. (A) The absolute numbers of cells in the different erythrocytic maturation stages divided into treated and untreated HC or PV samples. The differences were analyzed using paired t tests after passing the Shapiro-Wilk test for normal distribution. (B) Ridge plots for the hallmark IFN-α response in the erythrocytic clusters, divided into 4 groups, namely HC untreated (orange), HC IFN-α treated (teal), PV untreated (red), and PV IFN-α treated (violet). The heat map on the right displays the differences in the IFN-α response between the treated PV and treated HC cells. The colors indicate the normalized enrichment score (NES). (C) FC following IFN-α treatment was calculated for DEGs in PV- and HC-derived erythrocytic cells. The FC in HC cells is shown on the x-axis and PV on y-axis. Genes that did not show a similar FC deviate from the diagonal. All genes below the blue line are more strongly regulated in HC, whereas genes above the red line show stronger regulation in PV cells. (D) STAT1 expression is shown in for patients with MPN and HCs. RNA was isolated from at least 2 colonies per patient and condition after the CFU assay. Complimentary DNA was generated, and the gene expression analyzed using qPCR. The mean STAT1 expression per patient and condition is shown. Statistical analysis was conducted using the Kruskal-Wallis test, followed by Dunn test for multiple comparisons. (E) IFIT2 expression as percentage of MT-ATP6 is shown for patients with MPN and HCs. RNA was isolated from at least 2 colonies per patient and per condition and analyzed using qPCR. The mean IFIT2 expression per patient and condition is shown. Statistical analysis was conducted using the Kruskal-Wallis test, followed by Dunn test for multiple comparisons. (F) The expression of IFNAR1 from scRNA-seq data was analyzed. Violin plots show the expression in PV cells and HC cells per cell type. Statistical analyses were conducted using the raw values, and MAGIC imputation was used for visualization. (G) Surface expression of IFNAR1 analyzed by flow cytometry. Blood after incomplete lysis of erythrocytes was stained for CD45, CD71, and CD235a to identify the different maturation stages. The gating strategy is shown in supplemental Figure 4. In a second panel, IFNAR1 was stained together with CD45 and CD235a. The mean fluorescence intensity of the different populations was calculated. Three patients with PV and 3 HCs were analyzed. Statistical analyses were conducted using a row-matched 1-way analysis of variance, followed by Tukey multiple comparisons tests. (H) Heat map of DEGs (P < .05; FC < or > 0.25) in JAK2V617F, JAK2WT, and HC cells following IFN-α stimulation. The gene ontology analysis was subsequently done, and the derived pathways are indicated. ns, not significant; ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Immature erythrocytic PV-derived clusters decrease following IFN-α treatment, and differences between PV and HC cannot be attributed to IFNAR expression. (A) The absolute numbers of cells in the different erythrocytic maturation stages divided into treated and untreated HC or PV samples. The differences were analyzed using paired t tests after passing the Shapiro-Wilk test for normal distribution. (B) Ridge plots for the hallmark IFN-α response in the erythrocytic clusters, divided into 4 groups, namely HC untreated (orange), HC IFN-α treated (teal), PV untreated (red), and PV IFN-α treated (violet). The heat map on the right displays the differences in the IFN-α response between the treated PV and treated HC cells. The colors indicate the normalized enrichment score (NES). (C) FC following IFN-α treatment was calculated for DEGs in PV- and HC-derived erythrocytic cells. The FC in HC cells is shown on the x-axis and PV on y-axis. Genes that did not show a similar FC deviate from the diagonal. All genes below the blue line are more strongly regulated in HC, whereas genes above the red line show stronger regulation in PV cells. (D) STAT1 expression is shown in for patients with MPN and HCs. RNA was isolated from at least 2 colonies per patient and condition after the CFU assay. Complimentary DNA was generated, and the gene expression analyzed using qPCR. The mean STAT1 expression per patient and condition is shown. Statistical analysis was conducted using the Kruskal-Wallis test, followed by Dunn test for multiple comparisons. (E) IFIT2 expression as percentage of MT-ATP6 is shown for patients with MPN and HCs. RNA was isolated from at least 2 colonies per patient and per condition and analyzed using qPCR. The mean IFIT2 expression per patient and condition is shown. Statistical analysis was conducted using the Kruskal-Wallis test, followed by Dunn test for multiple comparisons. (F) The expression of IFNAR1 from scRNA-seq data was analyzed. Violin plots show the expression in PV cells and HC cells per cell type. Statistical analyses were conducted using the raw values, and MAGIC imputation was used for visualization. (G) Surface expression of IFNAR1 analyzed by flow cytometry. Blood after incomplete lysis of erythrocytes was stained for CD45, CD71, and CD235a to identify the different maturation stages. The gating strategy is shown in supplemental Figure 4. In a second panel, IFNAR1 was stained together with CD45 and CD235a. The mean fluorescence intensity of the different populations was calculated. Three patients with PV and 3 HCs were analyzed. Statistical analyses were conducted using a row-matched 1-way analysis of variance, followed by Tukey multiple comparisons tests. (H) Heat map of DEGs (P < .05; FC < or > 0.25) in JAK2V617F, JAK2WT, and HC cells following IFN-α stimulation. The gene ontology analysis was subsequently done, and the derived pathways are indicated. ns, not significant; ∗P < .05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Furthermore, we analyzed the FC in all genes following IFN-α stimulation (Figure 3C), which showed that induction of mostly IFN-α pathway-associated genes was especially pronounced in PV. STAT1 expression, measured by RT-qPCR, was significantly lower in the untreated colonies from MPN samples when compared with HC samples (Figure 3D), and a significant induction following IFN-α stimulation was only detected in MPN-derived colonies. Consistently, when analyzing IFIT2 expression, a significant induction was only observed in MPN (Figure 3E). To exclude the possibility that the observed differences stem from variations in maturation, we analyzed STAT1 and IFIT2 throughout erythroid differentiation in our scRNA-seq data (supplemental Figure 8). STAT1 expression showed an overall decrease during erythroid differentiation in HC and PV (supplemental Figure 8A). PV samples exhibited a drastic decrease in STAT1 expression during late-stage maturation, which was less pronounced in HC, confirming a PV-specific downregulation. IFIT2 showed similar expression levels in untreated and treated HC samples and in untreated PV samples, whereas a distinct induction of IFIT2 expression in IFN-α–treated PV-derived erythrocytic cells was observed during late stages of differentiation. Given previous reports of differential STAT1 protein activation in ET,51 we assessed STAT1 expression in colonies from patients with different diagnoses (supplemental Figure 9A). In patients with ET, no changes in STAT1 messenger RNA (mRNA) expression were noted, supported by previously published CD34+ gene array data52 (supplemental Figure 9B) in which no differences were found between disease subtypes. We therefore concluded that differences in STAT1 activity could not be attributed to changes in STAT1 mRNA expression.
The IFN-α receptor consists of 2 subunits, which are both necessary for signaling.53 From MEP to ProE, IFNAR1 and IFNAR2 mRNA expression increased, followed by a downregulation during erythrocytic differentiation (Figure 3F; supplemental Figure 10A). PV samples showed elevated IFNAR1 expression when compared with HC samples in all erythroid clusters except MEPs. We examined IFNAR1 and IFNAR2 surface expression via flow cytometry, categorizing primary blood cells into immature, intermediate, and mature erythrocytes based on CD45, CD71, and CD235a surface levels (supplemental Figure 10A-C). Both receptor subunits declined as erythroid maturation progressed (Figure 3G; supplemental Figure 10B). No differences in surface expression were observed between HC and PV, showing that the higher mRNA expression in PV did not translate into higher surface localization.
To investigate whether habituation or defective negative feedback signaling in PV might underlie the overall stronger IFN-α response in PV, we analyzed the expression of known negative regulators in our scRNA-seq data as 1 gene set (supplemental Figure 12A). The expression of this gene set generally increased during erythroid differentiation, but we found no differences in HC or PV samples. The induction of the individual genes was comparable in HC and PV, therefore it did not hint to stronger negative feedback signaling in HC (supplemental Figure 12B-I).
We further investigated JAK2V617F vs JAK2WT vs HC cells (Figure 3H) and found a pronounced induction of viral response genes in JAK2V617F and JAK2WT cells when compared with HC cells (cluster 1). Ribosomal genes were most downregulated following IFN-α stimulation in JAK2V617F and JAK2WT cells when compared with HC cells (cluster 2). Metabolism genes (cluster 3) were increased in untreated JAK2V617F-mutated cells and downregulated following IFN-α treatment. Genes involved in the major histocompatibility complex (cluster 4) were upregulated in HC cells, but downregulated in JAK2WT cells following IFN-α treatment. JAK2V617F-mutated cells showed low expression, regardless of treatment. Cluster 5, encompassing the Golgi apparatus and endosome genes, demonstrated induction in JAK2WT cells upon treatment. The expression of mitochondrial genes was enriched in untreated JAK2WT cells and was downregulated following IFN-α treatment (cluster 6). This cluster exhibited low expression in both JAK2V617F and HC-derived cells, regardless of IFN-α treatment.
IFN-α induces different cellular processes in HC and PV cells
To investigate whether the decrease in immature erythrocytic clusters following IFN-α treatment was because of accelerated differentiation, we established a differentiation protocol using primary CD34+ cells. During the first 12 to 15 days, CD45−/CD235a+ erythrocytic cells increased in HC and PV, differentiating at comparable rates (supplemental Figure 13A-B). These cells reflect differentiating erythroid cells, but not necessarily terminally differentiated cells because they lose CD45 surface expression between the ProE and OrthoE stage. Upon addition of IFN-α, no alteration in differentiation was observed. We therefore concluded that IFN-α did not change the kinetics of early erythrocytic differentiation in PV.
Next, we analyzed cell cycle regulation using the scRNA-seq data (Figure 4A). In MEPs, about half of the cells were in G1 in both untreated HC and PV, whereas most of the HC and PV derived ProE, BasoE, PolyE, and OrthoE were in G2M and S. IFN-α elicited a subtle overall shift toward G1. The effect was comparable in all analyzed clusters of PV and HC, except for reticulocytes. Reticulocytes represented the most mature erythroid cells in our analysis, characterized by their inability to proliferate. Therefore, all cells that were classified as reticulocytes but that were not in G1 phase were not considered true reticulocytes. We observed a specific reduction in reticulocytes in G1 phase following IFN-α stimulation in HC, whereas the absolute number of OrthoE remained comparable in the IFN-α–treated and untreated conditions. At the same time, the absolute numbers of all other maturation stages were decreased following IFN-α stimulation. This indicates a specific accumulation of cells at the OrthoE stage. Hence, we hypothesized that the transition from OrthoE to reticulocytes was inhibited in both HC and PV. However, this effect seemed to be more pronounced in HC because mature reticulocytes (in G1) were nearly completely abolished.
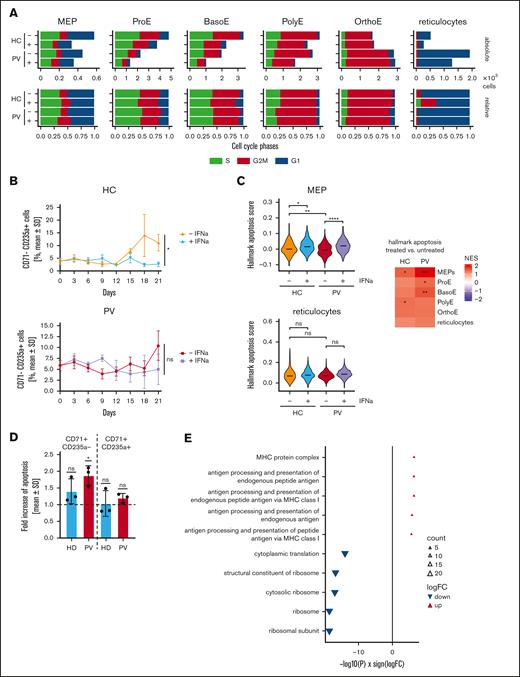
IFN-α only inhibits terminal differentiation in HC-derived cells and induces apoptosis in PV-derived cells. (A) Analysis of cell cycle regulation from scRNA-seq data. The absolute (top) and relative (bottom) numbers of cells are shown in the different erythroid maturation stages. Blue indicates G1 phase; red indicates G2M phase; green indicates S phase. Samples were grouped by treatment and diagnosis. (B) CD34+ cells isolated from blood were used in an in vitro differentiation model. Maturation was analyzed using flow cytometry. Erythrocytic cells that no longer expressed CD71 but still expressed CD235a were counted as mature erythrocytes. Statistical analysis was done using paired t tests after passing the Shapiro-Wilk test for normality. (C) The hallmark apoptosis pathway was analyzed from the scRNA-seq data in different cell types grouped by treatment and PV or HC. Two examples of violin plots in MEPs and reticulocytes are shown on the left. The heat map on the right summarizes the results for all cell types. (D) PBMCs were seeded in CFU assays and stimulated with 1000 U/mL IFN-α on day 8 to 12. Cells were grouped by erythroid maturation stages by first excluding all CD45+ cells and subsequently grouping the cells into CD71+CD235a+ (immature) and CD71−CD2354a+ (mature) populations. The FC in apoptosis was compared among the different groups, and induction was statistically analyzed using a one-sample t test. (E) Most and least apoptotic quantile of cells (PV-derived MEPs, ProE, and BasoE combined) from the scRNA-seq data were compared and DEGs were derived. The gene ontology terms were derived from DEGs, and the 5 most up- or downregulated pathways are shown. ns, not significant; ∗P <. 05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
IFN-α only inhibits terminal differentiation in HC-derived cells and induces apoptosis in PV-derived cells. (A) Analysis of cell cycle regulation from scRNA-seq data. The absolute (top) and relative (bottom) numbers of cells are shown in the different erythroid maturation stages. Blue indicates G1 phase; red indicates G2M phase; green indicates S phase. Samples were grouped by treatment and diagnosis. (B) CD34+ cells isolated from blood were used in an in vitro differentiation model. Maturation was analyzed using flow cytometry. Erythrocytic cells that no longer expressed CD71 but still expressed CD235a were counted as mature erythrocytes. Statistical analysis was done using paired t tests after passing the Shapiro-Wilk test for normality. (C) The hallmark apoptosis pathway was analyzed from the scRNA-seq data in different cell types grouped by treatment and PV or HC. Two examples of violin plots in MEPs and reticulocytes are shown on the left. The heat map on the right summarizes the results for all cell types. (D) PBMCs were seeded in CFU assays and stimulated with 1000 U/mL IFN-α on day 8 to 12. Cells were grouped by erythroid maturation stages by first excluding all CD45+ cells and subsequently grouping the cells into CD71+CD235a+ (immature) and CD71−CD2354a+ (mature) populations. The FC in apoptosis was compared among the different groups, and induction was statistically analyzed using a one-sample t test. (E) Most and least apoptotic quantile of cells (PV-derived MEPs, ProE, and BasoE combined) from the scRNA-seq data were compared and DEGs were derived. The gene ontology terms were derived from DEGs, and the 5 most up- or downregulated pathways are shown. ns, not significant; ∗P <. 05; ∗∗ P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Therefore, we analyzed the terminally differentiated CD71−/CD235a+ cells in our differentiation model (gating strategy shown in supplemental Figure 13A), and only HC, but not PV, demonstrated a significant inhibition of terminal differentiation on day 21 when treated with IFN-α (Figure 4B). Taken together, this implies that IFN-α inhibits terminal differentiation of erythrocytes in HC but not in PV. Furthermore, these data indicate that the increased fraction of more mature erythroid cells we observed in our scRNA-seq data is not a consequence of accelerated maturation of erythroid cells but instead is a consequence of increased survival of the erythroid progenitors, because PBMCs (and not more fractionated cells) were used in the colony formation assays. Analysis of the apoptosis pathways revealed significant downregulation of apoptosis genes in PV-derived MEPs, indicating increased survival with no changes in mature erythroid cells (Figure 4C). In addition, IFN-α treatment induced apoptosis in PV-derived MEPs, ProE, and BasoE (supplemental Figure 14), whereas apoptosis was only mildly induced in HC-derived MEPs and PolyE. The induction of apoptosis was highest in PV-derived MEPs (Figure 4C, heat map on the right). These results are consistent with the suppression of apoptosis in PV MEPs and the restoration of apoptosis by IFN-α. We evaluated apoptosis induction via flow cytometry by culturing PV and HC-derived PBMCs in colony assays for 8 days, followed by treatment with 1000 U/mL IFN-α for 4 days. The cells were detached and analyzed for erythroid maturation markers (CD45, CD71, CD235a; supplemental Figure 15A). Early erythroid (CD45−/CD71+/CD235a−) PV cells showed significant apoptosis induction, unlike late erythroid cells (CD45−/CD71+/CD235a+; Figure 4D; supplemental Figure 15B). Untreated, immature PV cells showed reduced apoptosis when compared with HC cells, although this was not significant because of the limited sample size (supplemental Figure 15).
We compared the differentially expressed genes (DEGs) between the highest and lowest quantiles by apoptosis hallmark expression among PV-derived MEPs, ProE, and BasoE (Figure 4E). In apoptotic cells, pathways involved in antigen processing were significantly upregulated, whereas ribosomal pathways were downregulated (Figure 4E).
Next, mutant and WT cells were analyzed in detail, but there was no significant enrichment in WT cells upon IFN-α stimulation (data not shown) and no differential cell cycle regulation (data not shown). Analysis of the IFN-α response showed that the basal levels were lower in mutated cells but reached comparable levels following stimulation (supplemental Figure 16A). Furthermore, WT and mutated cells showed no differences in apoptosis levels after stimulation (supplemental Figure 16B). STAT1 and IFIT2 expression were analyzed, and both were similarly regulated upon IFN-α stimulation in WT and mutated colonies (supplemental Figure 16C-D). Overall, PV-derived WT and mutated cells showed similar behavior following IFN-α treatment.
High ribosomal activity sensitizes PV clones to eradication by IFN-α
Identification of clones that were most susceptible to eradication by IFN-α proved to be difficult. We therefore aimed to identify clones in our scRNA-seq data. During replication, variants are introduced into DNA and can serve as natural barcodes of clones.41 The determination of mitochondrial variants of interest and their combination was used to analyze common ancestors and to track clones upon treatment (Figure 5A; supplemental Figure 17). We observed a numerical decrease in clones (effective number of species) in PV, likely not significant because of the variance between samples (Figure 5B). Treatment led to a significant reduction in the overall number of clones, highlighting its particularly strong effect on targeting specific clones. Several clones for PV02 and PV03 were reduced, and we isolated the DEGs from vanishing clones in comparison with all other cells. Next, we compared the DEGs and found a set of intersecting DEGs (Figure 5C). The gene ontology analysis of the upregulated DEGs showed an enrichment in ribosomal activity, whereas genes in erythroid pathways were downregulated (Figure 5D; supplemental Figure 18). Given the differential regulation of ribosomal genes in our earlier analyses, we determined the FC between treated and untreated erythroid cells and compared the isolated DEGs in HC and PV. Downregulation of all DEGs was stronger in PV than in HC following IFN-α stimulation (Figures 5E).
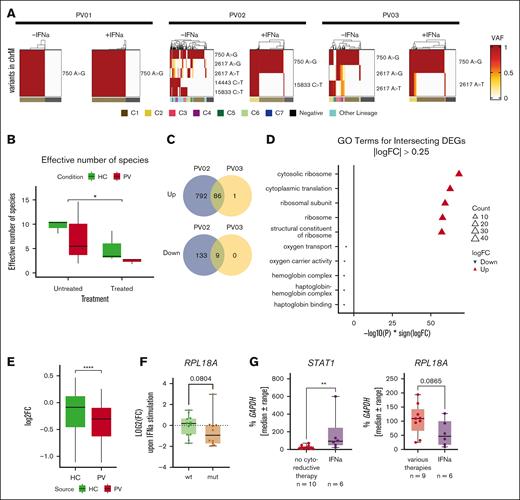
IFN-α treatment-responsive clones display high ribosomal activity. (A) Mitochondrial variants were used to analyze clonality from the scRNA-seq data. For each included sample, the profile of mitochondrial variants of interest and their combination to identify clones is shown. (B) The number of identified clones (effective number of species) was compared between PV and HC samples. Differences were analyzed using the Kruskal-Wallis rank sum test. (C) Identified clones in the untreated and treated condition of PV samples were compared. In 2 patients with PV, clones were identified in which the cell number was strongly decreased, termed vanishing clones, and the DEGs of these were analyzed. The Venn diagram depicts the number of DEGs in both patients. (D) Gene ontology (GO) terms were derived from the DEGs that were identified in both PV samples. The 5 most up- or downregulated pathways are shown. (E) Differential regulation of the genes derived from the DEGs was compared between the HC and PV samples. For each gene and group, the FC was calculated and directly compared between the HC and PV samples. Statistical analysis was done using a Wilcoxon rank sum test. ∗∗∗∗P < .0001. (F) Single clones (from supplemental Figure 4) were stimulated with IFN-α, and the FC was calculated following IFN-α treatment. Differences were analyzed using Wilcoxon rank sum tests. (G) The PBMC fraction from patients with PV were harvested and used for RNA isolation. Patients were grouped according to therapy into IFN-α only, which included IFN-treated patients, and all others were grouped together as various therapies. The STAT1 and RPL18A expression was analyzed using RT-qPCR. Statistical analysis was done using Mann-Whitney tests. ∗∗P < .01.
IFN-α treatment-responsive clones display high ribosomal activity. (A) Mitochondrial variants were used to analyze clonality from the scRNA-seq data. For each included sample, the profile of mitochondrial variants of interest and their combination to identify clones is shown. (B) The number of identified clones (effective number of species) was compared between PV and HC samples. Differences were analyzed using the Kruskal-Wallis rank sum test. (C) Identified clones in the untreated and treated condition of PV samples were compared. In 2 patients with PV, clones were identified in which the cell number was strongly decreased, termed vanishing clones, and the DEGs of these were analyzed. The Venn diagram depicts the number of DEGs in both patients. (D) Gene ontology (GO) terms were derived from the DEGs that were identified in both PV samples. The 5 most up- or downregulated pathways are shown. (E) Differential regulation of the genes derived from the DEGs was compared between the HC and PV samples. For each gene and group, the FC was calculated and directly compared between the HC and PV samples. Statistical analysis was done using a Wilcoxon rank sum test. ∗∗∗∗P < .0001. (F) Single clones (from supplemental Figure 4) were stimulated with IFN-α, and the FC was calculated following IFN-α treatment. Differences were analyzed using Wilcoxon rank sum tests. (G) The PBMC fraction from patients with PV were harvested and used for RNA isolation. Patients were grouped according to therapy into IFN-α only, which included IFN-treated patients, and all others were grouped together as various therapies. The STAT1 and RPL18A expression was analyzed using RT-qPCR. Statistical analysis was done using Mann-Whitney tests. ∗∗P < .01.
We analyzed RPL18A expression in short-term treated clones (from supplemental Figure 4) and observed a more pronounced reduction in RPL18A expression following IFN-α stimulation in mutated clones with a trend toward a stronger downregulation (Figure 5F). Because only in vitro IFN-α treatment was analyzed so far, we harvested PBMCs from patients with PV and compared the gene expression between IFN-α treated patients and those who received other therapies. In agreement with our data, STAT1 expression was significantly increased in IFN-α–treated patients. Furthermore, we found a trend toward reduced RPL18A expression in IFN-α–treated patients (Figure 5G). Overall, this indicates that differential regulation of ribosomal genes was not solely a culture-specific effect and may contribute to the targeting of malignant cells by IFN-α.
Discussion
IFN-α is approved for the treatment of PV based on its known antiproliferative and antitumor properties. The understanding of which patients benefit from IFN-α treatment is still fragmentary, and little is known about clonal differences in the response. We performed a detailed analysis of the responses of MPN vs HC at the single-cell level with a focus on clonal heterogeneity.
We employed CFU assays, and the addition of IFN-α reduced clonogenic growth. This phenomenon manifested across nearly all samples, aligning with most of the patients who experience HR in the clinics.24,25 Similarly, HC samples showed a reduction in the number of colonies, an anticipated finding because IFN-α is part of the innate immune response and a growth-reducing effect has already been described.54 An increased ratio of MR in the clinical setting was reported for PV when compared with ET and PMF and is reflected in our model.26,28 We observed a correlation between VAF and MR, although we did not find a stronger reduction of homozygous colonies. This contradicts previous clinical studies in which stronger effects were demonstrated on homozygous clones, most likely because of differences in the treatment regimen, for example, in vivo vs in vitro or continuous treatment vs one-time stimulation.28,55
Gene expression analysis in CFU-derived cells enabled the identification of distinct clusters. The first cluster was characterized by high IFIT2 expression after stimulation, primarily encompassing PV, ET, and post–PV-MF. IFIT2 has been linked to the induction of apoptosis, whereas IFIT3 attenuates the pro-apoptotic effects of IFIT2 by forming IFIT2-IFIT3 heterodimers.56,57 The specific upregulation of IFIT2 in PV and ET might indicate a more efficient induction of apoptosis within these samples.
Our scRNA-seq analyses revealed that most of the cells belonged to the erythrocytic lineage and that this lineage was numerically increased in PV, mimicking the PV phenotype.1 Using an additional differentiation trajectory model, Shlush et al identified a significant shift toward more mature erythroid cells in PV when compared with HCs. This was not recapitulated during our in vitro differentiation. We hypothesize that PV-derived cells do not exhibit accelerated differentiation. Instead, erythrocytic progenitors may be increased in number within the PBMC fraction of PV or their survival may be enhanced as suggested by the observed downregulation of apoptosis in PV-derived MEPs. Alternatively, diseased cells might retain their growth potential longer, thereby leading to the increased growth of rather mature cells.
A locus-specific amplification approach34 was used to identify WT and mutated cells. We found JAK2V617F-mutated cells in the erythroid and myeloid clusters but not in the lymphoid cluster. Small numbers of lymphoid cells carrying the JAK2V617F mutation have been reported previously.14,58 We may have missed mutated lymphoid cells because of the overall low number and the even lower number of genotyped lymphocytes in our assay. PV-derived JAK2WT cells were transcriptionally different from HC and JAK2V617F-mutated cells, aligning with earlier reports.9,16 We found that G2M targets and heme metabolism pathways were specifically upregulated in JAK2V617F-mutated cells, thereby directly linking them to the PV phenotype. Surprisingly, MYC target v1 genes50 were downregulated in PV, and the inflammatory response was not significantly upregulated. This is in contrast with former studies.9,16 Differences may be attributed to the analyzed cell type or in vitro culture conditions.
The fraction of genotyped cells was relatively low and depended on JAK2 expression. Therefore, it is possible that only high JAK2 expressing cells were analyzed. We used HC cells to adjust our threshold after unique molecular identifier (UMI) collapsing to 51% VAF per cell because of the partially very low number of reads per UMI and cell. Consequently, we might have lost heterozygous clones, evident in the lower VAF determined through scRNA-seq when compared with next-generation sequencing.
IFN-α significantly reduced the immature erythroid cells in PV but not in HC. This heightened response in PV was not linked to receptor expression or feedback signaling, although delayed analysis might have missed feedback effects. Paracrine cytokine signaling could explain the increased IFN-α sensitivity.
We report downregulation of IFNAR expression during erythrocytic maturation at the mRNA and protein level. Therefore, we hypothesize that mainly early erythrocytic progenitors were stimulated by IFN-α, as previously published,59 and that this signature was remarkably persistent throughout differentiation. Increased activation of STAT1 has previously been reported in ET,51 which was not reflected by increased STAT1 mRNA expression in colonies or CD34+ cells.
Reduced differentiation of reticulocytes was observed upon treatment with IFN-α, which was more pronounced in HCs than in PV. This block in differentiation has been reported previously in nonmutated cells.60 PV-derived cells are intrinsically prone to erythrocytic differentiation,61 which might surpass the inhibitory effect of IFN-α. Conversely, this indicates that PV cells undergo normal differentiation until their proliferation ceases, whereas healthy cells still retain a degree of growth potential. In addition, apoptosis was consistently induced in immature erythrocytic PV-derived cells, whereas apoptosis was only slightly induced in HC MEPs and PolyEs. This shows the higher sensitivity of PV-derived cells in primary patient-derived cells, previously only reported in cell lines.31 When analyzing various parameters (cell cycle, apoptosis, STAT1 and IFIT2 expression, fraction of cells) based on mutational status, we found that IFN-α induced similar effects in both groups. By decreasing the absolute number of mutated cells in the blood of a patient, secondary effects conveyed by the mutant cells may be decreased upon IFN-α therapy, which, in turn, reduce their suppressive effects on healthy cells. This may create an environment that does not favor the mutant cells anymore, thereby contributing to the long-term reduction of mutant clones.
To identify factors that sensitize cells for IFN-α–induced eradication, we used variants in mitochondrial RNA to distinguish clones. The number of clones in PV was numerically decreased when compared with HC, likely reflecting the expansion of diseased clones.7 Clones were found that vanished in the treated condition, indicating that these were particularly sensitive to IFN-α treatment. Increased expression of ribosomal genes and decreased hemoglobin expression in these clones were observed, indicating that immature cells, which display high protein translation activity, were most sensitive to IFN-α.
The downregulation of ribosomal genes is a crucial process in the IFN-α response,62,63 and differential regulation of ribosomal genes was indicated across various analyses. To our knowledge, the activation of ribosomal genes within MPN remains largely unexplored. Augmented ribosomal activity has been reported in numerous cancers and has been linked directly to the increased proliferation in these cells.62,63 Induced ribosomal gene downregulation likely impacts mutated cells more than WT cells. RPL18A showed more consistent suppression in mutated clones after 2 days of IFN-α. Similarly, PBMCs from patients on IFN-α therapy showed a trend toward ribosomal gene downregulation, although not significant, likely because of the limited sample size. Nonetheless, these findings suggest that the downregulation of ribosomal genes was not an assay-specific effect and may play a role in targeting mutated cells.
Substantial heterogeneity in the IFN-α response was evident in HC- and in PV-derived colonies. Recent studies on clonal evolution14,18 shed light on the clonal heterogeneity and underscore the need to understand the trajectory of single clones. We did not identify any clones in HC or PV that lacked an IFN-α response. This aligns with studies that showed that the cellular IFN-α response is stochastically activated and all cells of a cell type respond to IFN-α.64 We were able to expand on this and revealed that the extent of activation across different genes within individual clones varied. However, no distinct associations with disease or individuals emerged in the cohort. Although our results do not predict which clones are susceptible to IFN-α eradication, they highlight the significance of ribosomal genes in shaping these responses. Future analyses that incorporate mitochondrial barcoding and JAK2-locus specific amplification during clinical treatment in patients with PV will offer a chance to validate our findings in clinical scenarios.
Acknowledgments
The authors thank Kristin Sere for kindly providing the IFNAR1 antibody and Cheng Mingbo for providing his adaptation of the archR code. This work was supported, in part, by the Flow Cytometry Facility and the Genomics Facility of the Interdisciplinary Center for Clinical Research Aachen within the Faculty of Medicine at RWTH Aachen University. Biomaterial samples were provided by the RWTH centralized Biomaterial Bank Aachen, Germany, in accordance with the regulations of the biomaterial bank and the approval of the ethics committee of the medical faculty, RWTH Aachen.
This study was, in part, funded by grants to S.K. from the German Research Foundation (Deutsche Forschungsgemeinschaft; KO 2155/6-1) and by funds from the German Research Foundation as part of the Clinical Research Unit CRU 344 to S.K., N.C., I.G.C., and T.H.B. (KO2155/7-1, CH1509/1-1, GE2811/4-1, and BR1782/5-1).
Part of this work was generated within the doctoral thesis works of M.K. and M.G.
Authorship
Contribution: M.K. designed the study, performed experiments, collected and analyzed data, and prepared the manuscript; M.G. designed the study, analyzed data, and prepared the manuscript; T.M. analyzed data and prepared the manuscript; K.P. designed the study, performed experiments, and collected data; M.A.S.T. and K.O. designed the study and collected data; M.V., R.L., J.L., B.J., and J.B. performed experiments and collected data; A.G. and T.H.B. collected and analyzed data; J.S., K.K., M.S., N.C.I., D.B., E.C., M.C., and L.S. managed and analyzed data; R.W. and M.Z. designed experiments; N.C., I.G.C., and S.K. designed the study, analyzed data, and prepared the manuscript; and all authors revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.K. reports receiving a research grant or funding from Geron, Janssen, AOP Pharma, and Novartis; consulting fees from Pfizer, Incyte, Ariad, Novartis, AOP Pharma, Bristol Myers Squibb, Celgene, Geron, Janssen, CTI BioPharma, Roche, Bayer, GlaxoSmithKline (GSK), Protagonist, MPN Hub, Bedrock, and PharmaEssentia; payments or honoraria from Novartis, Bristol Myers Squibb/Celgene, Pfizer, Incyte, AOP Orphan, GSK, AbbVie, MPN Hub, Bedrock, iOMEDICO; travel or accommodation support from Alexion, Novartis, Bristol Myers Squibb, Incyte, AOP Pharma, CTI BioPharma, Pfizer, Celgene, Janssen, Geron, Roche, AbbVie, GSK, Sierra Oncology, Kartos, Imago Biosciences, MSD, and iOMEDICO; having a patent issued for a bromodomain and extra-terminal (BET) motif protein inhibitor at RWTH Aachen University; and participating on advisory boards for Pfizer, Incyte, Ariad, Novartis, AOP Pharma, Bristol Myers Squibb, Celgene, Geron, Janssen, CTI BioPharma, Roche, Bayer, GSK, Sierra Oncology, AbbVie, Protagonist, and PharmaEssentia. T.H.B. reports serving as a consultant or speaker for Gilead, Janssen, Merck, Novartis, and Pfizer; and receiving research support from Novartis and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Steffen Koschmieder, Department of Hematology, Oncology, Hemostaseology, and Stem Cell Transplantation, Faculty of Medicine, RWTH Aachen University, Pauwelsstr 30, D-52074 Aachen, Germany; email: skoschmieder@ukaachen.de.
References
Author notes
M.K. and M.G. are joint first authors.
N.C., I.G.C., and S.K. are joint senior authors.
Preprocessed single-cell RNA sequencing data have been deposited in Zenodo (https://doi.org/10.5281/zenodo.14204623). Raw sequencing files will be deposited into the European Genome Archive.
The full-text version of this article contains a data supplement.