Key Points
Increasing total social vulnerability associated with markedly decreased surveillance and survival periods across 11 of 14 cancer types.
Housing, transportation, and socioeconomic factors, compared to racial-ethnic and household composition, were the largest contributors.
Visual Abstract
Although social determinants of health (SDoH) investigations have shown limited analyses of socioeconomic and race-ethnic status on certain hematologic malignancies, the impact of factors beyond those across a fuller scope of hematologic cancers remains unknown. The Social Vulnerability Index (SVI), a tool for assessing varied US census–derived sociodemographic factors, allows for the specific quantification of SDoH in dynamic, regional contexts for their associations with hematologic malignancy inequities. To assess the summative influence of varied SDoH factors on hematologic malignancy outcomes and discern which SDoH factors contributed the largest associations toward disparities, 796 005 adults with hematologic malignancies between 1975 to 2017 were identified for this retrospective cohort study. Vulnerability in 15 SDoH factors was measured using composite and subcategory SVI scores geographically matched to patients. Regressions between SVI factors and follow-up time after diagnosis and survival period were performed. Increasing overall SVI correlated with significantly decreased surveillance period in 11 of 14 hematologic malignancies, with decreases upward of 33.4% (39.0-26.0 months for acute lymphocytic leukemia). Increasing SVI significantly associated with decreased survival period across 11 of 14 hematologic malignancies, with decreases upward of 47.2% (89.5-47.3 months for Hodgkin lymphoma). Socioeconomic status and housing and transportation vulnerabilities showed the largest magnitude of contributions, followed by minority language and household composition. Significant decreases in hematologic malignancy prognosis associate with increasing overall SDoH vulnerability in varied sociodemographic contexts in the United States. Furthermore, there are quantifiable differences in which types of SDoH contribute more to trends per malignancy type. These findings demonstrate specific SDoH targets for further research and policy initiatives to combat hematologic malignancy disparity more effectively.
Introduction
Social determinants of health (SDoH) have demonstrated substantial impact on health outcomes across various diseases. In the field of hematologic malignancies, many cancers including acute lymphocytic leukemia (ALL),1-3 chronic myeloid leukemia,4 and non-Hodgkin lymphoma5,6 have been shown to be negatively affected by specific SDoH, such as lower socioeconomic status (SES) or race. There is, however, a limited scope for many of these studies on SDoH and hematologic malignancies, necessitating a more comprehensive method for clinicians to better understand how combinations of different SDoH factors affect hematologic malignancy outcomes. Addressing these knowledge gaps is essential for establishing actionable targets to provide the most benefit in addressing disparities observed and improving health outcomes.
The Centers for Disease Control and Prevention (CDC)–Social Vulnerability Index (SVI), originally developed to assess health resources during natural disasters,7 has been increasingly used in SDoH research in fields ranging from cardiovascular health8,9 to COVID-1910-13 to hepatocellular carcinoma14 among others. SVI provides an updated, US census–based tool featuring 15 social factors grouped into 4 SDoH-related theme categories that are relatively ranked across all US census tracts and counties, providing a wide-spanning, yet in-depth, index to evaluate SDoH. As seen in Figure 1, the 4 categories consist of SES, minority status and language, household composition and disability, and housing type and transportation. These are then further combined for an overall dynamically weighted total SVI assessment for a given defined area based on observed, regional sociodemographic contexts. In turn, the 4 categories and total SVI are used for SDoH impact assessment on clinical outcomes. Ultimately, this tool allows for greater precision and generalizability than other forms of SDoH analysis, as its wide-spanning yet in-depth scope can explore changes specifically related to the categories of SVI with real-world context and applicability, as demonstrated in our previous investigation with pediatric head and neck malignancies and preliminary investigations into singular types of hematologic malignancy.15-17
Schematic data workflow. ICD-O-3, ICD for Oncology, third edition; Pop, Population; Regs, Registries; Yo, Years old.
Schematic data workflow. ICD-O-3, ICD for Oncology, third edition; Pop, Population; Regs, Registries; Yo, Years old.
By analyzing adult hematologic malignancy outcomes with overall and theme category SVI assessment, this study seeks to explore the associations of SDoH on prognostic outcomes of adult hematologic malignancy across the United States and showcases how the SVI can evaluate the impact of a multitude of SDoH on adult hematologic cancers. We hypothesize that individuals from areas with increasing overall social vulnerability will correlate with detrimental outcomes across all hematologic malignancies in prognostic measures. Secondarily, we hypothesize that the SDoH themes, seen in the SVI categories, will have specific quantifiable differences in association with these detrimental trends for each hematologic malignancy and across outcomes.
Methods
This retrospective cohort study compared different levels of 15 SDoH factors represented in the SVI with the months surveyed and survival across the 14 hematologic malignancy types defined in the Surveillance, Epidemiology, and End Results (SEER) database. The databases consist of publicly available, deidentified data, exempting this study from Institutional Review Board (IRB) committee approval or waiver of informed consent as determined in the policies of the Northwestern University Institutional Review Board. This retrospective cohort study follows the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline.
Databases
The CDC-SVI was used to identify ranked scores among 15 census factors within 4 SDoH subcategories of SES (poverty, unemployment, income level, and high school diploma status), minority status and language (minority status and proficiency with English), household composition (household members aged 65+ years, household members aged ≤17 years, disabilities, and single-parent status), and housing and transportation (multiunit structure, mobile homes, crowding, no vehicle, and group quarters; Figure 1). Based on CDC-SVI documentation, SVI subscores are differentially weighed to formulate the total composite score and are assigned different weights based on sociodemographic census data of the designated area. Total and SVI subscores are based on relative social vulnerabilities of a particular census tract among all 72 158 US census tracts, ranging from 0 to 1, with 0 representing the lowest social vulnerability and 1 representing the highest (supplemental Figure 1).
The SEER database contains comprehensive national data sets of patient variables, pathological characteristics, treatment modalities, and outcomes of all cancers and was specifically queried for hematologic malignancies, organized into 14 types. SEER patient data were matched to abstracted SVI scores based on the county or census tract of residence at the time of diagnosis. Schematic workflow is provided in Figure 1.
Population and outcome definitions
SEER was queried for adult patients, defined as individuals aged ≥20 years based on divisions in the database, who were diagnosed with hematologic malignancies. Disease categories were determined by SEER-defined recoded variables derived from the International Classification of Disease (ICD) for Oncology third edition disease classes.
The surveillance time was measured in months from the diagnosis of the primary malignancy to the time of last known follow-up, regardless of whether the patient was alive/lost or suffered a mortal outcome on last follow-up. Months of survival were assessed as the time in months from the diagnosis of the primary malignancy to the time of last known follow-up with a confirmed mortal outcome; those who were alive/lost on the last follow-up were excluded. Ann Arbor lymphoma staging values were also extracted for non-Hodgkin/Hodgkin disease categories. Based on the design of this SEER data set, treatments received by any and all patients were considered indicated based on the standard practices at the time of diagnosis for the patient recorded.
Statistical methods
Months surveyed within each ICD disease class was analyzed using SVI overall scores and category subscores, given as a comparative percentile for census tracts across the United States in the SVI database. These were grouped into relative, equivalently sized quintiles based on their actual SVI percentile scores within each disease class to allow for adequate comparison of distribution across groups, ranging from the lowest to the highest vulnerability. The generated relative SVI quintiles were delineated by “<20,” “20 to 39.99,” “40 to 59.99,” “60 to 79.99,” and “80 to 99.99,” representing their relative percentiles per disease class (ie, patients with the lowest SVI scores are grouped into the “<20” quintile group for a specific disease class). Violin plots, which are density plot and box plot composite graphs, were used to display data distribution for months surveyed for each relative SVI quintile, while additionally displaying interquartile range and median using the inner box plot for comparison across quintiles. Means, standard deviations, and ranges for months surveyed per quintile were also calculated. The proportion of patients who were alive/lost or dead upon last follow-up was also calculated per quintile and depicted through bicolor heat map. Means, standard deviations, and ranges for overall SVI scores and SVI category subscores were calculated per relative quintile group included in supplemental Figure 2. Linear regression across relative SVI quintiles for months surveyed was analyzed for significance.
Months survival within disease classes were analyzed similarly as months surveyed. Groups were generated from the existing relative SVI quintiles by removing patients who were lost to follow-up to generate new SVI quintiles. These groups varied in number, so box plots, instead of violin plots, were used to compare months survival across these new groups. This can also be found in supplemental Figure 3. By analyzing months of survival independently through this approach alongside Kaplan-Meier survival analyses with log-ranked significance testing, potential confounders relating to follow-up difficulties can be accounted for to compare the effects SDoH have on disease survival directly.
Additionally, the most socially vulnerable quintile was compared against the least vulnerable quintile for relative percent decrease in patient month surveyed and survival for each hematologic malignancy to compare overall impacts of SVI across SEER-defined cancer types.
Advanced Ann Arbor lymphoma staging occurrence on preliminary diagnosis was also assessed for lymphoma disease categories. “Advanced”/comparator level was set to “stage III” or “stage IV” as designated in SEER, whereas “early”/reference was set to “stage I” or “stage II.” Reference group for ordinal variates (total SVI and its themes) were the lowest relative SVI quintile and ordinally increasing factors of sequentially more vulnerable quintiles as comparators.
Statistics and data quantification were generated using R (R Core Team [2021]; R: a language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria). Univariate regressions were elected to preserve the internal weighing of SVI subscores contributing to the total SVI score by design. Statistical significance was set as P value <.05, with 2-sided P values reported for all analyses.
Results
A total of 796 005 patients with hematologic malignancies were identified across the United States in the SEER database. The most represented demographics were 371 782 participants (46.7%) aged 65 to 84 years, 440 613 (55.4%) of male sex, and 597 621 of non-Hispanic White race (75.1%). The 14 hematologic malignancy disease class types included myeloid, lymphoid, and myeloma cancer types. Across the 14 disease class types reported in SEER, the most common cancer types were nodal non-Hodgkin lymphoma (n = 255 555 [32.1%]), myeloma (n = 123 693 [15.5%]), extranodal non-Hodgkin lymphoma (n = 120 841 [15.2%]), and chronic lymphocytic leukemia (n = 94 134 [11.8%]). Patient clinical characteristics and other malignancy types for these patients stratified by total SVI are reported in Table 1.
Clinicodemographic characteristics by SVI score
| Characteristic . | N . | Total SVI score . | ||||
|---|---|---|---|---|---|---|
| 0.000-0.199, n = 5329 (0.7%) . | 0.200-0.399, n = 180 407 (23%) . | 0.400-0.599, n = 403 072 (51%) . | 0.600-0.799, n = 194 591 (24%) . | 0.800-0.999, n = 12 606 (1.6%) . | ||
| Age, y | 796 005 | |||||
| 20-44 | 636 (12%) | 22 878 (13%) | 53 319 (13%) | 26 033 (13%) | 1 425 (11%) | |
| 45-64 | 1851 (35%) | 55 575 (31%) | 125 616 (31%) | 61 535 (32%) | 4 021 (32%) | |
| 65-84 | 2394 (45%) | 85 236 (47%) | 188 030 (47%) | 90 022 (46%) | 6 100 (48%) | |
| 85+ | 448 (8.4%) | 16 718 (9.3%) | 36 107 (9.0%) | 17 001 (8.7%) | 1 060 (8.4%) | |
| Sex | 796 005 | |||||
| Male | 3045 (57%) | 99 472 (55%) | 223 161 (55%) | 107 960 (55%) | 6 975 (55%) | |
| Female | 2284 (43%) | 80 935 (45%) | 179 911 (45%) | 86 631 (45%) | 5 631 (45%) | |
| Race | 796 005 | |||||
| White | 4751 (89%) | 161 205 (89%) | 299 379 (74%) | 124 389 (64%) | 7 897 (63%) | |
| Hispanic | 359 (6.7%) | 5 828 (3.2%) | 29 407 (7.3%) | 35 535 (18%) | 2 726 (22%) | |
| Black | 88 (1.7%) | 8 071 (4.5%) | 42 631 (11%) | 21 344 (11%) | 1 379 (11%) | |
| Asian or Pacific Islander | 49 (0.9%) | 3 731 (2.1%) | 27 394 (6.8%) | 10 423 (5.4%) | 132 (1.0%) | |
| Unknown | 50 (0.9%) | 1 217 (0.7%) | 3 053 (0.8%) | 2 000 (1.0%) | 77 (0.6%) | |
| Native American | 32 (0.6%) | 355 (0.2%) | 1 208 (0.3%) | 900 (0.5%) | 395 (3.1%) | |
| Region | 796 005 | |||||
| Midwest | 790 (15%) | 46 185 (26%) | 72 582 (18%) | 3 014 (1.5%) | 0 (0%) | |
| Northeast | 2216 (42%) | 62 736 (35%) | 56 077 (14%) | 10 727 (5.5%) | 0 (0%) | |
| South | 1367 (26%) | 17 355 (9.6%) | 71 345 (18%) | 41 211 (21%) | 4 502 (36%) | |
| West | 956 (18%) | 54 131 (30%) | 203 068 (50%) | 139 639 (72%) | 8 104 (64%) | |
| Disease class | 796 005 | |||||
| ALL | 82 (1.5%) | 2 530 (1.4%) | 6 192 (1.5%) | 4 028 (2.1%) | 276 (2.2%) | |
| Acute myeloid leukemia | 478 (9.0%) | 16 666 (9.2%) | 38 657 (9.6%) | 19 191 (9.9%) | 1 226 (9.7%) | |
| Aleukemic, subleukemic, and NOS | 36 (0.7%) | 1 632 (0.9%) | 3 744 (0.9%) | 1 995 (1.0%) | 168 (1.3%) | |
| Chronic lymphocytic leukemia | 786 (15%) | 23 594 (13%) | 47 246 (12%) | 21 088 (11%) | 1 420 (11%) | |
| Chronic myeloid leukemia | 219 (4.1%) | 7 779 (4.3%) | 17 596 (4.4%) | 8 581 (4.4%) | 609 (4.8%) | |
| Hodgkin, extranodal | 8 (0.2%) | 288 (0.2%) | 616 (0.2%) | 272 (0.1%) | 18 (0.1%) | |
| Hodgkin, nodal | 347 (6.5%) | 11 785 (6.5%) | 25 132 (6.2%) | 11 416 (5.9%) | 686 (5.4%) | |
| Myeloma | 763 (14%) | 26 562 (15%) | 63 002 (16%) | 31 103 (16%) | 2 263 (18%) | |
| Non-Hodgkin, extranodal | 779 (15%) | 26 003 (14%) | 62 066 (15%) | 30 214 (16%) | 1 779 (14%) | |
| Non-Hodgkin, nodal | 1701 (32%) | 58 775 (33%) | 128 745 (32%) | 62 469 (32%) | 3 865 (31%) | |
| Other acute leukemia | 42 (0.8%) | 1 650 (0.9%) | 3 480 (0.9%) | 1 477 (0.8%) | 97 (0.8%) | |
| Other lymphocytic leukemia | 65 (1.2%) | 2 249 (1.2%) | 4 513 (1.1%) | 1 958 (1.0%) | 134 (1.1%) | |
| Other myeloid/monocytic leukemia | 23 (0.4%) | 894 (0.5%) | 2 083 (0.5%) | 799 (0.4%) | 65 (0.5%) | |
| Vital status on last follow-up | 796 005 | |||||
| Alive | 2608 (49%) | 68 071 (38%) | 147 569 (37%) | 78 982 (41%) | 4 817 (38%) | |
| Dead | 2721 (51%) | 112 336 (62%) | 255 503 (63%) | 115 609 (59%) | 7 789 (62%) | |
| Characteristic . | N . | Total SVI score . | ||||
|---|---|---|---|---|---|---|
| 0.000-0.199, n = 5329 (0.7%) . | 0.200-0.399, n = 180 407 (23%) . | 0.400-0.599, n = 403 072 (51%) . | 0.600-0.799, n = 194 591 (24%) . | 0.800-0.999, n = 12 606 (1.6%) . | ||
| Age, y | 796 005 | |||||
| 20-44 | 636 (12%) | 22 878 (13%) | 53 319 (13%) | 26 033 (13%) | 1 425 (11%) | |
| 45-64 | 1851 (35%) | 55 575 (31%) | 125 616 (31%) | 61 535 (32%) | 4 021 (32%) | |
| 65-84 | 2394 (45%) | 85 236 (47%) | 188 030 (47%) | 90 022 (46%) | 6 100 (48%) | |
| 85+ | 448 (8.4%) | 16 718 (9.3%) | 36 107 (9.0%) | 17 001 (8.7%) | 1 060 (8.4%) | |
| Sex | 796 005 | |||||
| Male | 3045 (57%) | 99 472 (55%) | 223 161 (55%) | 107 960 (55%) | 6 975 (55%) | |
| Female | 2284 (43%) | 80 935 (45%) | 179 911 (45%) | 86 631 (45%) | 5 631 (45%) | |
| Race | 796 005 | |||||
| White | 4751 (89%) | 161 205 (89%) | 299 379 (74%) | 124 389 (64%) | 7 897 (63%) | |
| Hispanic | 359 (6.7%) | 5 828 (3.2%) | 29 407 (7.3%) | 35 535 (18%) | 2 726 (22%) | |
| Black | 88 (1.7%) | 8 071 (4.5%) | 42 631 (11%) | 21 344 (11%) | 1 379 (11%) | |
| Asian or Pacific Islander | 49 (0.9%) | 3 731 (2.1%) | 27 394 (6.8%) | 10 423 (5.4%) | 132 (1.0%) | |
| Unknown | 50 (0.9%) | 1 217 (0.7%) | 3 053 (0.8%) | 2 000 (1.0%) | 77 (0.6%) | |
| Native American | 32 (0.6%) | 355 (0.2%) | 1 208 (0.3%) | 900 (0.5%) | 395 (3.1%) | |
| Region | 796 005 | |||||
| Midwest | 790 (15%) | 46 185 (26%) | 72 582 (18%) | 3 014 (1.5%) | 0 (0%) | |
| Northeast | 2216 (42%) | 62 736 (35%) | 56 077 (14%) | 10 727 (5.5%) | 0 (0%) | |
| South | 1367 (26%) | 17 355 (9.6%) | 71 345 (18%) | 41 211 (21%) | 4 502 (36%) | |
| West | 956 (18%) | 54 131 (30%) | 203 068 (50%) | 139 639 (72%) | 8 104 (64%) | |
| Disease class | 796 005 | |||||
| ALL | 82 (1.5%) | 2 530 (1.4%) | 6 192 (1.5%) | 4 028 (2.1%) | 276 (2.2%) | |
| Acute myeloid leukemia | 478 (9.0%) | 16 666 (9.2%) | 38 657 (9.6%) | 19 191 (9.9%) | 1 226 (9.7%) | |
| Aleukemic, subleukemic, and NOS | 36 (0.7%) | 1 632 (0.9%) | 3 744 (0.9%) | 1 995 (1.0%) | 168 (1.3%) | |
| Chronic lymphocytic leukemia | 786 (15%) | 23 594 (13%) | 47 246 (12%) | 21 088 (11%) | 1 420 (11%) | |
| Chronic myeloid leukemia | 219 (4.1%) | 7 779 (4.3%) | 17 596 (4.4%) | 8 581 (4.4%) | 609 (4.8%) | |
| Hodgkin, extranodal | 8 (0.2%) | 288 (0.2%) | 616 (0.2%) | 272 (0.1%) | 18 (0.1%) | |
| Hodgkin, nodal | 347 (6.5%) | 11 785 (6.5%) | 25 132 (6.2%) | 11 416 (5.9%) | 686 (5.4%) | |
| Myeloma | 763 (14%) | 26 562 (15%) | 63 002 (16%) | 31 103 (16%) | 2 263 (18%) | |
| Non-Hodgkin, extranodal | 779 (15%) | 26 003 (14%) | 62 066 (15%) | 30 214 (16%) | 1 779 (14%) | |
| Non-Hodgkin, nodal | 1701 (32%) | 58 775 (33%) | 128 745 (32%) | 62 469 (32%) | 3 865 (31%) | |
| Other acute leukemia | 42 (0.8%) | 1 650 (0.9%) | 3 480 (0.9%) | 1 477 (0.8%) | 97 (0.8%) | |
| Other lymphocytic leukemia | 65 (1.2%) | 2 249 (1.2%) | 4 513 (1.1%) | 1 958 (1.0%) | 134 (1.1%) | |
| Other myeloid/monocytic leukemia | 23 (0.4%) | 894 (0.5%) | 2 083 (0.5%) | 799 (0.4%) | 65 (0.5%) | |
| Vital status on last follow-up | 796 005 | |||||
| Alive | 2608 (49%) | 68 071 (38%) | 147 569 (37%) | 78 982 (41%) | 4 817 (38%) | |
| Dead | 2721 (51%) | 112 336 (62%) | 255 503 (63%) | 115 609 (59%) | 7 789 (62%) | |
NOS, not otherwise specified.
Surveillance trends with increasing social vulnerability
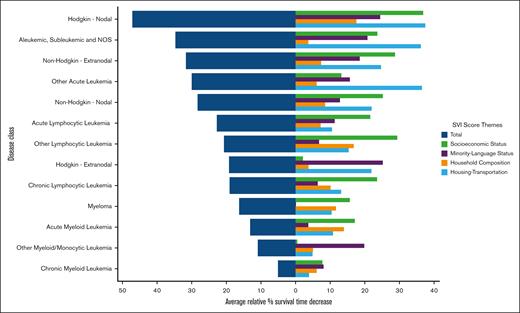
With increasing total SVI score (ie, overall social vulnerability), significant decreases in long-term surveillance after a primary cancer diagnosis across 11 of 14 hematologic malignancy types were observed (P < .008 for all; supplemental Figure 2). Mean surveillance period decreases between the lowest and highest total social vulnerability cohorts ranged from 33.4% (39.0-26.0 months) for ALL to as low as 0.7% (51.0-51.0 months) for chronic myeloid leukemia (Figure 2).
Relative decreases in mean long-term surveillance period with increasing social vulnerability. Percentage decreases from the lowest to highest SVI quintiles based on mean months of surveillance for total SVI score and subcomponent SVI theme subscores per category. NOS, not otherwise specified.
Relative decreases in mean long-term surveillance period with increasing social vulnerability. Percentage decreases from the lowest to highest SVI quintiles based on mean months of surveillance for total SVI score and subcomponent SVI theme subscores per category. NOS, not otherwise specified.
Comprising these overall social vulnerability trends, specific vulnerabilities in SES were most strongly associated by magnitude, followed closely by housing and transportation and household composition, and then lastly by minority language status (Figure 2).
Survival trends with increasing social vulnerability
Similarly, with increasing overall social vulnerability/total SVI score, significant decreases in survival period across 11 of 14 hematologic malignancy were observed (P < .043 all; supplemental Figure 3). Mean survival period decreases between the lowest and highest total social vulnerability cohorts ranged from 47.1% (89.5-47.3 months) for non-Hodgkin lymphoma to as low as 5.1% (33.2-31.5 months) for chronic lymphocytic leukemia (Figure 3). When taking the survival of all hematologic malignancies, Kaplan-Meier analyses revealed significant survival differences when comparing the lowest with the highest social vulnerability groups (supplemental Figure 4).
Relative decreases in mean survival period with increasing social vulnerability. Percentage decreases from the lowest to highest SVI quintiles based on mean months of survival for total SVI score and subcomponent SVI theme subscores per category. NOS, not otherwise specified.
Relative decreases in mean survival period with increasing social vulnerability. Percentage decreases from the lowest to highest SVI quintiles based on mean months of survival for total SVI score and subcomponent SVI theme subscores per category. NOS, not otherwise specified.
Comprising these overall social vulnerability trends, specific vulnerabilities in housing-transportation and SES were equivalent and strongly associated by magnitude, followed closely by minority language status and household composition (Figure 3).
Ann arbor staging trends with increasing social vulnerability
For lymphoma categories, increasing overall social vulnerability/total SVI score saw significantly increased odds of having advanced staging for Hodgkin (odds ratio, 1.06; 95% confidence interval, 1.04-1.07; P < .001) and extranodal non-Hodgkin (odds ratio, 1.03; 95% confidence interval, 1.02-1.04; P < .001; Table 2).
Advanced Ann Arbor staging on preliminary presentation for lymphomas
| Disease class∗ . | Characteristics . | OR . | 95% CI . | P value . |
|---|---|---|---|---|
| Hodgkin | Total | 1.06 | 1.04-1.07 | <.001 |
| SES | 1.04 | 1.02-1.05 | <.001 | |
| Minority language status | 1.06 | 1.04-1.08 | <.001 | |
| Household composition | 1.01 | 0.99-1.02 | .514 | |
| Housing and transportation | 1.05 | 1.04-1.07 | <.001 | |
| Non-Hodgkin, extranodal | Total | 1.03 | 1.02-1.04 | <.001 |
| SES | 1.03 | 1.02-1.04 | <.001 | |
| Minority language status | 0.98 | 0.97-0.99 | <.001 | |
| Household composition | 1.03 | 1.01-1.04 | <.001 | |
| Housing and transportation | 1.02 | 1.01-1.03 | .001 | |
| Non-Hodgkin, nodal | Total | 1.00 | 0.99-1.00 | .292 |
| SES | 0.98 | 0.98-0.99 | <.001 | |
| Minority language Status | 1.03 | 1.02-1.04 | <.001 | |
| Household composition | 0.96 | 0.96-0.97 | <.001 | |
| Housing and transportation | 1.01 | 1.00-1.01 | .019 |
| Disease class∗ . | Characteristics . | OR . | 95% CI . | P value . |
|---|---|---|---|---|
| Hodgkin | Total | 1.06 | 1.04-1.07 | <.001 |
| SES | 1.04 | 1.02-1.05 | <.001 | |
| Minority language status | 1.06 | 1.04-1.08 | <.001 | |
| Household composition | 1.01 | 0.99-1.02 | .514 | |
| Housing and transportation | 1.05 | 1.04-1.07 | <.001 | |
| Non-Hodgkin, extranodal | Total | 1.03 | 1.02-1.04 | <.001 |
| SES | 1.03 | 1.02-1.04 | <.001 | |
| Minority language status | 0.98 | 0.97-0.99 | <.001 | |
| Household composition | 1.03 | 1.01-1.04 | <.001 | |
| Housing and transportation | 1.02 | 1.01-1.03 | .001 | |
| Non-Hodgkin, nodal | Total | 1.00 | 0.99-1.00 | .292 |
| SES | 0.98 | 0.98-0.99 | <.001 | |
| Minority language Status | 1.03 | 1.02-1.04 | <.001 | |
| Household composition | 0.96 | 0.96-0.97 | <.001 | |
| Housing and transportation | 1.01 | 1.00-1.01 | .019 |
Univariate logistic regressions across SVI quintiles based on advanced staging on first presentation occurrence for increasing total SVI score and subcomponent SVI theme subscores per lymphoma category. Reference group for outcome, combining “early” staging/“stage I or II,” ; this was compared with the group comprised of “advanced”/“stage III-IV.” Reference group for ordinal variates (total SVI and its themes) were the lowest relative SVI quintile and ordinally increasing factors of sequentially more vulnerable quintiles as comparators.
By InteICD for Oncology, third edition (ICD-O-3).
Comprising these overall vulnerability trends in Hodgkin, specific vulnerabilities in minority language status were most strongly associated by magnitude, followed by housing and transportation and SES. For extranodal non-Hodgkin, specific vulnerabilities in household composition and SES were most strongly associated by magnitude, followed by housing and transportation (Table 2).
Discussion
This investigation represents, to our knowledge, the first comprehensive analysis on how SDoH interactively affects the care and prognosis of hematologic malignancies. Using the large, data-validated tool of the SVI, our results display how SDoH vulnerabilities, comprising themes of SES, minority language status, household composition, and housing and transportation, affect the long-term surveillance, survival, and staging of one of the largest sampled patient cohorts of hematologic malignancies. Furthermore, these findings also delineate how certain types of SDoH vulnerabilities confer the highest associations with outcome disparities while considering a wider array of SDoH factors.
Unlike prior SDoH investigations, which have solely investigated on single individual-level factors of SES or minoritized race/ethnicity, the SVI presents unique community-level contextualization and incorporation of a wide variety of SDoH factors to interpret SDoH associations with oncologic disparities. This strength is attributed to its unique formulation of the total and theme SVI scores that adjust for the dynamic, sociodemographic contexts across varied US geographic settings. Other accepted methods that analyze SDoH can provide useful information about specific themes such as SES,18 but they can fail to consider more detailed SDoH that still affects health disparities.19 Given that the range of SDoH is multifaceted, assessing the cumulative effect on determinants (ie, education and income) on health outcomes,15 rather than an individual determinant (ie, education or income), is likely to provide a more comprehensive and informative understanding of the overall impact of the determinants impact on health outcomes and health disparities.18 SVI not only allows for the assessment of the cumulative effect of determinants but also allows for the analysis of the individual impact of determinants, allowing for the identification of more actionable targets to address and ameliorate disparities, as our previous research has shown.15,20,21 To this end, the utility of the SVI’s specific independent themes allows for more comprehensive analysis to inform interventional studies and public health programming.19 For example, as seen in supplemental Figure 2, patients who had ALL in the lowest SVI quintile (least vulnerable) were found to have significantly improved months surveyed and months of survival than those in the highest quintile (most vulnerable). Through SDoH theme category analysis, it is noted that the statistical significance in survival is related to SES and housing/transportation but not to racial/ethnic minority status and language or household composition. From interpreting these findings, specific investments into supporting aspects of one’s community-level education, income, or insurance status, alongside providing subsidized housing and transportation services, would benefit patients with ALL, perhaps more so than investing into English-second language courses (regarding minority language status vulnerability) or other SDoH factors. This analysis can be extended to all 14 of the cancer categories analyzed here, moving toward a more evidence-based approach, which may be used when assessing SDoH.
The findings of SVI and Hodgkin lymphoma illustrate sizable detrimental trends in survival and surveillance of the 14 cancer types assessed, corroborating with prior literature,22 while also novelly depicting with increased advanced staging. Given the increasing national availability of diagnostic and treatment modalities for common hematologic malignancies such as Hodgkin lymphoma,23 our findings notably showcase that disparities remain persistent throughout the entire chronology from which such care access changes occurred. With this discordance in care availability but starkly apparent prognostic disparities, there lies a gap in understanding what social factors drive this gap of Hodgkin lymphoma disparity. Prior studies have attempted single-site or smaller-scaled assessments into nonclinical factors, such as implicit provider bias among others, toward explaining some of these care and prognostic gaps.24-26 However, none have attempted to contextualize a fuller extent of SDoH factors that encompass patients with Hodgkin lymphoma’s lived-in environment and, thus, have differential effects on their outcomes on a national level.
Seeing as our results and discussion of Hodgkin lymphoma provide nuanced contextualization, similar analytical approaches can be applied toward our study’s gamut of hematologic malignancy findings. Beyond inspiring future retrospective and prospective analyses, our results relay the landscape for which the larger movement of implementation work against health disparities can begin. Specifically, with the calls to action about using large-data findings to guide initiatives against cancer disparities as a whole,27-30 this study hopes to usher in a renewed focus toward how impactful real-world, modifiable SDoH vulnerabilities affect hematologic cancer, facilitating future consideration of efforts toward aspects of care outside of biological and clinical advancements. Especially as public health resources become more and more limited, this study’s approach of using large-data tools to compare a wide swathe of factors can allow for the identification of the most pertinent associative factors of hematologic-oncologic disparities and, in turn, directly inform where these limited public health resources would see the most benefit.
Strengths and limitations
Among its strengths, this study is, to our knowledge, the first to comprehensively apply the SVI and its many SDoH themes toward one of the largest patient cohorts of hematologic malignancy in the United States. With the benefits of the SVI formulation and its dynamic weighting of subcategories by geographic region, it provides per-sociodemographic contextualization across all geographic areas nationally. Furthermore, by accounting for a wide range of SDoH factors, it also provides quantitative comparisons of social vulnerability that can elucidate which specific factors associate most strongly with disparities.
However, this study must be considered within the context of certain limitations. The SEER grouping of hematologic malignancies is not fully equivalent to the standard World Health Organization (WHO) classifications. Grouping many cancers together with different molecular characteristic and prognostic features can potentially obscure nuances that make these types unique in management.31,32 The basic SEER data set used here also does not allow access to specific treatment strategies (such as not featuring specific types/ICD codes for chemotherapy or immunotherapy), as well as capturing patients beyond their primary diagnosis (ie, if they relapse, go to a non–National Cancer Institute-SEER–designated cancer center, etc). In addition, despite the SVI relaying a fuller representation of SDoH factors, it does not encompass the full extent of social determinant factors that may be of interest to public health and clinical investigators. There is also the inherent limitations of using geospatial approaches that cannot account for the full variability of sociodemographic factor distribution standardized geographic areas.33 This also includes the lack of information as to whether a patient would move from their primary residential address at the time of their primary diagnosis, which could make the SVI measures associated with said patients less accurate. In future investigations that are ongoing, increasing efforts to prospectively capture and use individual-level variates that are either self-reported by patients themselves or as standardized procedures for any patient intake would be less prone to such geospatial variations. Lastly, correlative investigations such as this cannot purport causality but can provide the basis for follow-up investigations to perform causal analyses.
Conclusion
In turn, this large-data application of the SVI and its SDoH factors, grouped into themes of SES, minority status, household composition, and housing/transportation categories, showed significant associations with detrimental trends in the prognosis and care of nearly all hematologic malignancies represented in the United States. Through both using amalgamated measures of social vulnerability and assessing individual component contributions of specific types of SDoH, this study lays the foundation for targeted, future interventions to strategically allocate resources toward the most vulnerable areas of need in improving disparities of hematologic malignancies nationally.
Acknowledgment
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention or the National Cancer Institute.
Authorship
Contribution: D.J.F.-Z., E.W., A.V.S., and J.P. designed the study; D.J.F.-Z., E.W., and A.V.S. contributed to data acquisition; all authors assisted with data interpretation and critical revision; D.J.F.-Z., E.W., and A.V.S. drafted the manuscript; D.J.F.-Z. and E.W. designed and generated data visualization; D.J.F.-Z. conducted the statistical analysis; S.M.A., M.W.L.-T., S.M.B., and J.P. oversaw the work; and all authors had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David J. Fei-Zhang, Northwestern University Feinberg School of Medicine, 420 E Superior St, Chicago, IL 60611; email: david.fei-zhang@northwestern.edu.
References
Author notes
D.J.F.-Z. and E.W. contributed equally to this study.
Public data sets are available for the Social Vulnerability Index (https://www.atsdr.cdc.gov/placeandhealth/svi/index.html) and upon request from the National Cancer Institute–Surveillance, Epidemiology, and End Results (SEER) data administrators (https://seer.cancer.gov/).
Formatted data may be found in a data supplement available with the online version of this article. The original data underlying this study are publicly available through the SEER database.
The full-text version of this article contains a data supplement.