Key Points
Real-world data suggest that outcomes for patients with AML have improved since the approval of venetoclax, especially for NHB patients.
NHB patients, particularly those without TP53, RAS, or FLT3-ITD mutations, may derive greater benefit from venetoclax than NHW.
Visual Abstract
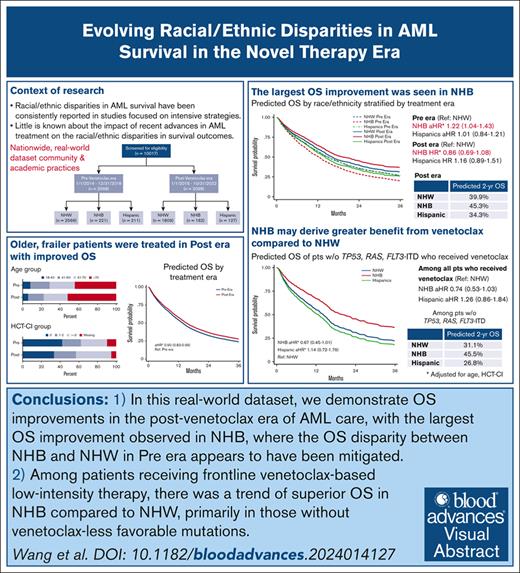
Little is known about the impact of recent advances in acute myeloid leukemia (AML) treatment on racial/ethnic disparities in survival outcomes. We performed a retrospective cohort study of patients with newly diagnosed AML using data from a nationwide electronic health record–derived deidentified database. Patients were categorized based on their diagnosis date relative to venetoclax approval, as pre–novel therapy era (Pre era; 2014-2018; n = 2998) or post–novel therapy era (Post era; 2019-2022; n = 2098). Patients in the Post era were older and had more comorbidities than Pre era. Non-Hispanic Black (NHB) and Hispanic patients were younger and more likely to have lower socioeconomic status than non-Hispanic White (NHW) patients, with no differences in the distributions of key disease features. After accounting for age and comorbidity, overall survival (OS) was higher in patients in Post era than Pre era (adjusted hazard ratio [aHR], 0.90; 95% confidence interval [CI], 0.83-0.96). In Pre era, NHB had a 22% higher hazard of death than NHW (aHR, 1.22; 95% CI, 1.04-1.43), whereas worse OS was not observed for NHB in Post era (aHR, 0.86; 95% CI, 0.69-1.08; predicted 2-year survival, 45.3% vs 39.9%). Utilization of novel therapeutics in frontline therapy did not differ by race/ethnicity. Among patients receiving venetoclax-based induction, particularly those without TP53, RAS, or FLT3-ITD mutations, results suggested higher OS for NHB than NHW patients (aHR, 0.67; 95% CI, 0.45-1.01). Additional studies are needed to elucidate factors contributing to these observed survival differences and to inform strategies to optimize outcomes for all patients with AML.
Introduction
Acute myeloid leukemia (AML) is an aggressive disease with considerable biological heterogeneity and poor outcomes.1 Racial and ethnic disparities in AML outcomes have been increasingly recognized.2 Studies focused on the overall improvement in AML long-term outcomes with intensive chemotherapy strategies have consistently reported a survival disadvantage for Black patients compared with White patients.3-9 Surveillance, Epidemiology, and End Results data demonstrate that non-Hispanic Black (NHB) and Hispanic patients have 12% and 6% higher 5-year mortality, respectively, despite younger ages at diagnosis and more favorable cytogenetics than non-Hispanic White (NHW) patients.10
Recent progress in the understanding of the molecular pathogenesis of AML has led to substantial advances in therapy.11-14 The approval of venetoclax/hypomethylating agent (HMA) frontline therapy in November 2018, based on results of the VIALE-A trial,15,16 marked a new era of AML care, shifting away from the historical intensive chemotherapy–based paradigm and providing a strategy for patients who were unfit for intensive chemotherapy. This rapidly evolving therapeutic landscape offers an unprecedented opportunity for patients to undergo AML therapy that is effective, better tolerated, and tailored to their individual needs. However, it also adds more complexity to their care.17,18 Appropriate testing, operational and financial means to access novel therapeutics, relevant toxicity monitoring and dosing adjustments, and a reliable social support system for outpatient care navigation are required in the modern era of AML care. These conditions are less accessible for historically marginalized racial/ethnic subpopulations of patients of lower socioeconomic status (SES) or under-resourced areas,19,20 threatening to further widen the disparities in AML care. The impact of racial/ethnic disparities on AML survival with respect to the recent advances in AML management remains largely unknown.
We performed a retrospective study in a large, real-world cohort to quantify differences in survival by race and ethnicity and identify potential targets for intervention to improve the outcomes of these vulnerable patient populations.
Methods
Study design and data sources
This report follows the Strengthening the Reporting of Observational Studies in Epidemiology Statement guidelines.21 We used a retrospective cohort study design. The study cohort included patients from the nationwide Flatiron Health electronic health record (EHR)–derived database. The Flatiron Health database is a longitudinal database, including patient-level structured and unstructured data, originated from ∼280 US cancer clinics (∼800 sites of care) and curated via technology-enabled and manual abstraction.22,23 The data are deidentified and subject to obligations to prevent reidentification and protect patient confidentiality.
Eligibility
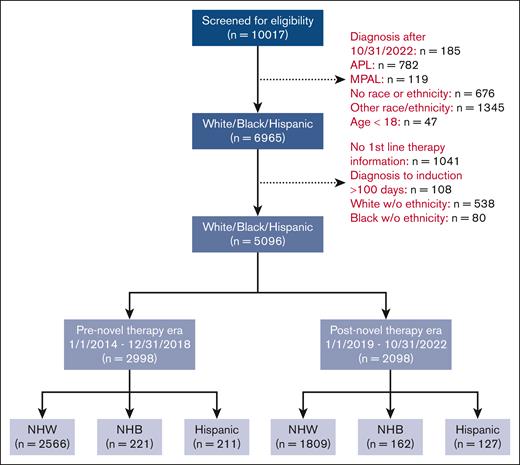
The study cohort included adult patients (aged ≥18 years) newly diagnosed with AML between 1 January 2014 and 31 October 2022 who initiated at least 1 line of antileukemic therapy and identified as NHW, NHB, or Hispanic. Patients with documented race/ethnicity other than these categories and those with undocumented race/ethnicity were excluded due to insufficient numbers, as were patients with acute promyelocytic leukemia, mixed phenotype, or ambiguous lineage acute leukemia, patients with missing information on first-line antileukemic therapy, and patients with a gap of >100 days between the date of diagnosis and the start of first documented therapy.
Exposures
The primary independent variable was EHR-documented race/ethnicity, categorized as NHW, NHB, and Hispanic. The timing of AML diagnosis relative to venetoclax approval (late 201816) was a secondary exposure of interest. Pre–novel therapy era (Pre era) was defined as January 2014 to December 2018; post–novel therapy era (Post era) was defined as January 2019 to October 2022 (the time of cohort cutoff).
Outcomes
The primary outcome was overall survival (OS), defined as the time from the initiation of antileukemic therapy to death from any cause.
Potential mediators
Based on our conceptual framework (supplemental Figure 1), we proposed the following variables as potential mediators of the survival disparity by race/ethnicity in modern AML care: (1) disease-related factors including (a) baseline genomic features (cytogenetics, molecular information, and the European LeukemiaNet 2017 (ELN2017) risk group24 derived based on available genomic data; supplemental Table 1) and (b) therapy-related AML/secondary AML (s-AML; history of myelodysplastic syndrome or myeloproliferative neoplasm); (2) patient-related factors including (a) SES index, computed based on 7 characteristics of census block group-level social determinants of health from the Census Bureau's American Community Survey (2015-2019), with the US population-weighted quintile for each patient provided in the database25; and (b) insurance status (Medicare, Medicaid, private, or none); and (3) management-related factors including (a) the type of treating facility (academic or community); (b) use of novel therapeutics, particularly venetoclax-based frontline therapy, including venetoclax/HMA and venetoclax/low-dose cytarabine; and (c) use of allogeneic hematopoietic stem cell transplantation at first remission.
Covariates
A minimally sufficient set of confounders was identified using a directed acyclic graph (supplemental Figure 2), informed by content area knowledge26 and our conceptual model (supplemental Figure 1), and included age and hematopoietic cell transplantation-specific comorbidity index (HCT-CI)27 (supplemental Table 2). Additional information on baseline covariates including sex, induction intensity (supplemental Table 3), and line of therapy was collected.
Statistical methods
Standard descriptive analyses were used to show the baseline characteristics of the 3 race/ethnicity groups. For continuous variables, means for symmetric distributions and medians for skewed distributions were reported. Frequencies and percentages for categorical or ordinal variables were reported. Median follow-up time was computed using reverse Kaplan-Meier method. OS was assessed using Kaplan-Meier curves. The log-rank test was used to assess survival differences among groups. Stratified analyses evaluated the heterogeneity of primary comparisons of interest by age group and sex. Unadjusted and adjusted Cox proportional hazards regressions were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between race/ethnicity and OS. Estimated survival curves and 2-year survival probabilities were computed based on these regression results.28,29 Proportional hazards assumptions were assessed using Schoenfeld residuals and log-log plot. A race/ethnicity × time (by years of follow-up) interaction term was added to the multivariate Cox regression model comparing OS by racial/ethnic groups, collapsed across treatment eras, because the proportional hazard assumption for this model was not met (supplemental Figure 3).
The component associations for each proposed mediator, namely the relationships between race/ethnicity and mediator, and the associations between mediator and the primary outcome were assessed. Causal mediation analyses with survival data using Cox proportional hazard model,30-32 which allows for the decomposition of the total effect of exposure into indirect (ie, through a defined mediator) and the direct components (ie, through other undefined paths),33-35 were planned (supplemental Figure 1) for proposed mediators in which the component associations were non-null; in such cases, HRs and corresponding 95% CIs for the indirect and direct associations and the proportions of the total effect of race/ethnicity on OS mediated through each of the proposed mediators would be calculated.
All tests were 2-sided, with a significance level of .05. All statistical analyses were performed in Stata 17.0.36
Exploratory analyses
After observing a near-null association between race/ethnicity and OS in the Post era in the primary analyses, post hoc exploratory analyses were performed to understand the mechanisms of the OS differences observed between the treatment eras across race/ethnicity. Secondary outcomes included the prevalence of novel therapeutics utilization in the frontline setting after corresponding US Food and Drug Administration approval, including (1) venetoclax-based low-intensity therapy overall and among those aged >70 years, (2) CPX-351 in patients with treatment-related AML or s-AML diagnosed after 03 August 2017,37 (3) midostaurin in patients with FLT3-mutated AML after 28 April 2017,38 and (4) gemtuzumab ozogamicin in patients with core-binding factor AML after 02 September 201739; and the OS of patients treated with different frontline novel therapeutics. Unadjusted and adjusted modified Poisson regressions were used to estimate prevalence ratios (PRs)40 and corresponding 95% CIs comparing the utilization of specific frontline therapies by race/ethnicity. OS, HRs of death, 95% CIs, and estimated survival curves were assessed as described above.
Sensitivity analyses
To assess the robustness of our results to less restrictive exclusion criteria, we repeated the primary survival analyses including the following: (1) Black and White patients with no ethnicity information in the NHB and NHW groups, respectively; (2) patients who had no documented antileukemic therapy; (3) patients who had >100-day gap between the time of AML diagnosis to the time of documented induction therapy; and (4) patients treated at community practices only. Similarly, we also repeated modified Poisson regression analyses comparing the prevalence of venetoclax-based low-intensity therapy including patients with documented Black or White race but no available ethnicity information. To assess whether modifications to standard AML management strategies implemented during the COVID-19 pandemic may have influenced our venetoclax utilization results, we performed subanalyzes including only patients diagnosed after the onset of the pandemic (March 2020). To assess whether the type of treating facility may affect the utilization of venetoclax frontline, we conducted subanalyzes including patients treated at community practices only. Lastly, we conducted a subanalysis assessing the utilization of venetoclax-based therapy among patients who were traditionally deemed ineligible for intensive induction therapy, by attempting to mimic the inclusion criteria used on VIALE-A trial16 to the best of our ability within the limitations of the real-world data set (supplemental Table 4).
Results
Study population and patient characteristics
A total of 2998 patients in the Pre era (2566 NHW, 221 NHB, 211 Hispanics) and 2098 patients in the Post era (1809 NHW, 162 NHB, 127 Hispanics) treated at 121 institutions (117 community and 4 academic institutions) were included (Figure 1; supplemental Figure 4). The median follow-up was 63.7 months and 22.2 months in the Pre era and Post era, respectively. The median follow-up times within each treatment era were similar across race/ethnicity (Pre era, NHW 63.3 months, NHB 69.8 months, and Hispanic 62.0 months; Post era, NHW 22.6 months, NHB 24.3 months, and Hispanic 18.9 months). Patients treated in the Post era were older at diagnosis (median, 71 years vs 69 years), had more comorbidities (HCT-CI ≥3, 40% vs 31%), and were more likely to have high-risk molecular features than those treated in the Pre era (presence of TP53, ASXL1, RUNX1, KRAS, or NRAS mutation[s], 42% vs 24%; Table 1).
CONSORT diagram of patient allocation. APL, acute promyelocytic leukemia; MPAL, mixed-phenotype acute leukemia; w/o, without.
CONSORT diagram of patient allocation. APL, acute promyelocytic leukemia; MPAL, mixed-phenotype acute leukemia; w/o, without.
Baseline demographic and clinical characteristics at diagnosis
| . | Pre era . | Post era . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total n = 2998 n (%) . | NHW n = 2566 n (%) . | NHB n = 221 n (%) . | Hispanic n = 211 n (%) . | Total n = 2098 n (%) . | NHW n = 1809 n (%) . | NHB n = 162 n (%) . | Hispanic n = 127 n (%) . | |
| Median age, y | 68.5 | 69.0 | 64.0 | 60.0 | 71.0 | 72.0 | 68.5 | 65.0 |
| Sex, M | 1734 (58) | 1518 (59) | 107 (48) | 109 (52) | 1187 (57) | 1061 (59) | 66 (41) | 60 (47) |
| Payer category | ||||||||
| Public | 653 (22) | 589 (23) | 28 (3) | 36 (17) | 520 (25) | 453 (25) | 43 (27) | 24 (19) |
| Private | 1413 (47) | 1234 (48) | 98 (44) | 81 (38) | 1063 (51) | 938 (52) | 76 (47) | 49 (39) |
| Other | 269 (9) | 221 (9) | 19 (9) | 29 (14) | 220 (11) | 183 (10) | 17 (11) | 20 (16) |
| No/unknown | 663 (22) | 522 (20) | 76 (34) | 65 (31) | 295 (14) | 235 (13) | 26 (16) | 34 (27) |
| Practice | n = 2992 | n = 2561 | n = 221 | n = 210 | n = 2096 | n = 1807 | n = 162 | n = 127 |
| Community | 1843 (62) | 1578 (62) | 130 (59) | 134 (64) | 1225 (58) | 1060 (59) | 91 (56) | 74 (58) |
| SES index∗,† | n = 2698 | n = 2315 | n = 196 | n = 187 | n = 1909 | n = 1646 | n = 147 | n = 117 |
| 1 | 331 (12) | 222 (10) | 67 (34) | 42 (23) | 244 (13) | 151 (9) | 53 (36) | 40 (34) |
| 2 | 512 (19) | 414 (18) | 48 (25) | 50 (27) | 322 (17) | 269 (16) | 34 (23) | 19 (16) |
| 3 | 606 (23) | 524 (23) | 34 (17) | 48 (26) | 419 (22) | 368 (22) | 23 (16) | 28 (24) |
| 4 | 653 (24) | 594 (26) | 27 (14) | 32 (17) | 472 (25) | 439 (27) | 18 (12) | 15 (13) |
| 5 | 596 (22) | 561 (24) | 20 (10) | 15 (8) | 452 (24) | 418 (25) | 19 (13) | 15 (13) |
| HCT-CI27† | n = 2704 | n = 2339 | n = 199 | n = 166 | n = 1988 | n = 1723 | n = 154 | n = 111 |
| 0 | 1268 (47) | 1085 (46) | 100 (50) | 83 (50) | 706 (36) | 599 (35) | 47 (31) | 60 (54) |
| 1-2 | 601 (22) | 523 (22) | 36 (18) | 42 (25) | 492 (25) | 440 (26) | 32 (21) | 20 (18) |
| ≥3 | 835 (31) | 731 (31) | 63 (32) | 41 (25) | 790 (40) | 684 (40) | 75 (49) | 31 (28) |
| ELN 2017† | n = 2505 | n = 2150 | n = 177 | n = 178 | n = 1781 | n = 1535 | n = 138 | n = 108 |
| Favorable | 338 (14) | 293 (14) | 20 (11) | 25 (14) | 243 (14) | 205 (13) | 20 (15) | 18 (17) |
| Intermediate | 1114 (45) | 959 (45) | 74 (42) | 81 (46) | 721 (41) | 608 (40) | 66 (48) | 47 (44) |
| Adverse | 1053 (42) | 898 (42) | 83 (47) | 72 (40) | 817 (46) | 722 (47) | 52 (38) | 43 (40) |
| s-AML, yes | 1039 (35) | 922 (36) | 64 (29) | 53 (25) | 770 (37) | 687 (38) | 45 (28) | 38 (30) |
| Treatment-related AML, yes | 251 (8) | 200 (8) | 31 (14) | 20 (10) | 217 (10) | 188 (10) | 22 (14) | 7 (6) |
| Cytogenetics‡ | n = 2523 | n = 2166 | n = 179 | n = 178 | n = 1825 | n = 1576 | n = 141 | n = 108 |
| t(8;21) | 125 (5) | 91 (4) | 16 (9) | 18 (10) | 82 (5) | 61 (4) | 13 (9) | 8 (7) |
| inv(16) | 131 (5) | 109 (5) | 13 (7) | 9 (5) | 70 (4) | 53 (3) | 11 (8) | 6 (6) |
| del(7) | 437 (17) | 383 (18) | 28 (16) | 26 (15) | 329 (18) | 301 (19) | 15 (11) | 13 (12) |
| del(17) | 191 (8) | 165 (8) | 17 (10) | 9 (5) | 134 (7) | 119 (8) | 10 (7) | 5 (5) |
| High-risk mutations‡ | n = 1991 | n = 1720 | n = 137 | n = 134 | n = 1740 | n = 1511 | n = 132 | n = 97 |
| TP53 | 133 (7) | 121 (7) | 4 (3) | 8 (6) | 243 (14) | 216 (14) | 16 (12) | 11 (11) |
| RUNX1 | 155 (8) | 130 (8) | 13 (10) | 12 (9) | 234 (13) | 207 (14) | 16 (12) | 11 (11) |
| ASXL1 | 149 (8) | 126 (7) | 12 (9) | 11 (8) | 232 (13) | 206 (14) | 15 (11) | 11 (11) |
| KRAS | 47 (2) | 38 (2) | 5 (4) | 4 (3) | 73 (4) | 65 (4) | 6 (5) | 2 (2) |
| NRAS | 124 (6) | 103 (6) | 14 (10) | 7 (5) | 171 (10) | 138 (9) | 18 (14) | 15 (16) |
| Induction intensity, high | 1628 (58) | 1341 (56) | 139 (67) | 148 (74) | 755 (37) | 618 (35) | 71 (45) | 66 (53) |
| . | Pre era . | Post era . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total n = 2998 n (%) . | NHW n = 2566 n (%) . | NHB n = 221 n (%) . | Hispanic n = 211 n (%) . | Total n = 2098 n (%) . | NHW n = 1809 n (%) . | NHB n = 162 n (%) . | Hispanic n = 127 n (%) . | |
| Median age, y | 68.5 | 69.0 | 64.0 | 60.0 | 71.0 | 72.0 | 68.5 | 65.0 |
| Sex, M | 1734 (58) | 1518 (59) | 107 (48) | 109 (52) | 1187 (57) | 1061 (59) | 66 (41) | 60 (47) |
| Payer category | ||||||||
| Public | 653 (22) | 589 (23) | 28 (3) | 36 (17) | 520 (25) | 453 (25) | 43 (27) | 24 (19) |
| Private | 1413 (47) | 1234 (48) | 98 (44) | 81 (38) | 1063 (51) | 938 (52) | 76 (47) | 49 (39) |
| Other | 269 (9) | 221 (9) | 19 (9) | 29 (14) | 220 (11) | 183 (10) | 17 (11) | 20 (16) |
| No/unknown | 663 (22) | 522 (20) | 76 (34) | 65 (31) | 295 (14) | 235 (13) | 26 (16) | 34 (27) |
| Practice | n = 2992 | n = 2561 | n = 221 | n = 210 | n = 2096 | n = 1807 | n = 162 | n = 127 |
| Community | 1843 (62) | 1578 (62) | 130 (59) | 134 (64) | 1225 (58) | 1060 (59) | 91 (56) | 74 (58) |
| SES index∗,† | n = 2698 | n = 2315 | n = 196 | n = 187 | n = 1909 | n = 1646 | n = 147 | n = 117 |
| 1 | 331 (12) | 222 (10) | 67 (34) | 42 (23) | 244 (13) | 151 (9) | 53 (36) | 40 (34) |
| 2 | 512 (19) | 414 (18) | 48 (25) | 50 (27) | 322 (17) | 269 (16) | 34 (23) | 19 (16) |
| 3 | 606 (23) | 524 (23) | 34 (17) | 48 (26) | 419 (22) | 368 (22) | 23 (16) | 28 (24) |
| 4 | 653 (24) | 594 (26) | 27 (14) | 32 (17) | 472 (25) | 439 (27) | 18 (12) | 15 (13) |
| 5 | 596 (22) | 561 (24) | 20 (10) | 15 (8) | 452 (24) | 418 (25) | 19 (13) | 15 (13) |
| HCT-CI27† | n = 2704 | n = 2339 | n = 199 | n = 166 | n = 1988 | n = 1723 | n = 154 | n = 111 |
| 0 | 1268 (47) | 1085 (46) | 100 (50) | 83 (50) | 706 (36) | 599 (35) | 47 (31) | 60 (54) |
| 1-2 | 601 (22) | 523 (22) | 36 (18) | 42 (25) | 492 (25) | 440 (26) | 32 (21) | 20 (18) |
| ≥3 | 835 (31) | 731 (31) | 63 (32) | 41 (25) | 790 (40) | 684 (40) | 75 (49) | 31 (28) |
| ELN 2017† | n = 2505 | n = 2150 | n = 177 | n = 178 | n = 1781 | n = 1535 | n = 138 | n = 108 |
| Favorable | 338 (14) | 293 (14) | 20 (11) | 25 (14) | 243 (14) | 205 (13) | 20 (15) | 18 (17) |
| Intermediate | 1114 (45) | 959 (45) | 74 (42) | 81 (46) | 721 (41) | 608 (40) | 66 (48) | 47 (44) |
| Adverse | 1053 (42) | 898 (42) | 83 (47) | 72 (40) | 817 (46) | 722 (47) | 52 (38) | 43 (40) |
| s-AML, yes | 1039 (35) | 922 (36) | 64 (29) | 53 (25) | 770 (37) | 687 (38) | 45 (28) | 38 (30) |
| Treatment-related AML, yes | 251 (8) | 200 (8) | 31 (14) | 20 (10) | 217 (10) | 188 (10) | 22 (14) | 7 (6) |
| Cytogenetics‡ | n = 2523 | n = 2166 | n = 179 | n = 178 | n = 1825 | n = 1576 | n = 141 | n = 108 |
| t(8;21) | 125 (5) | 91 (4) | 16 (9) | 18 (10) | 82 (5) | 61 (4) | 13 (9) | 8 (7) |
| inv(16) | 131 (5) | 109 (5) | 13 (7) | 9 (5) | 70 (4) | 53 (3) | 11 (8) | 6 (6) |
| del(7) | 437 (17) | 383 (18) | 28 (16) | 26 (15) | 329 (18) | 301 (19) | 15 (11) | 13 (12) |
| del(17) | 191 (8) | 165 (8) | 17 (10) | 9 (5) | 134 (7) | 119 (8) | 10 (7) | 5 (5) |
| High-risk mutations‡ | n = 1991 | n = 1720 | n = 137 | n = 134 | n = 1740 | n = 1511 | n = 132 | n = 97 |
| TP53 | 133 (7) | 121 (7) | 4 (3) | 8 (6) | 243 (14) | 216 (14) | 16 (12) | 11 (11) |
| RUNX1 | 155 (8) | 130 (8) | 13 (10) | 12 (9) | 234 (13) | 207 (14) | 16 (12) | 11 (11) |
| ASXL1 | 149 (8) | 126 (7) | 12 (9) | 11 (8) | 232 (13) | 206 (14) | 15 (11) | 11 (11) |
| KRAS | 47 (2) | 38 (2) | 5 (4) | 4 (3) | 73 (4) | 65 (4) | 6 (5) | 2 (2) |
| NRAS | 124 (6) | 103 (6) | 14 (10) | 7 (5) | 171 (10) | 138 (9) | 18 (14) | 15 (16) |
| Induction intensity, high | 1628 (58) | 1341 (56) | 139 (67) | 148 (74) | 755 (37) | 618 (35) | 71 (45) | 66 (53) |
HCT-CI, hematopoietic cell transplantation–specific comorbidity index; M, male.
SES index, Tier 1 being the lowest level and Tier 5 being the highest.
Among patients whose information was available.
Among patients tested.
NHB and Hispanic patients were younger at diagnosis than NHW patients in both eras (median ages for NHB, Hispanic, and NHW, 65, 62, and 70 years, respectively). NHB and Hispanic patients were more likely to have lower SES than NHW patients. There was higher prevalence of s-AML among NHW than NHB and Hispanic patients, whereas there was more core-binding factor AML among NHB and Hispanic patients than NHW patients. No difference was observed by race/ethnicity in the distribution of ELN201724 risk groups or high-risk mutations in either era (Table 1).
OS by treatment era
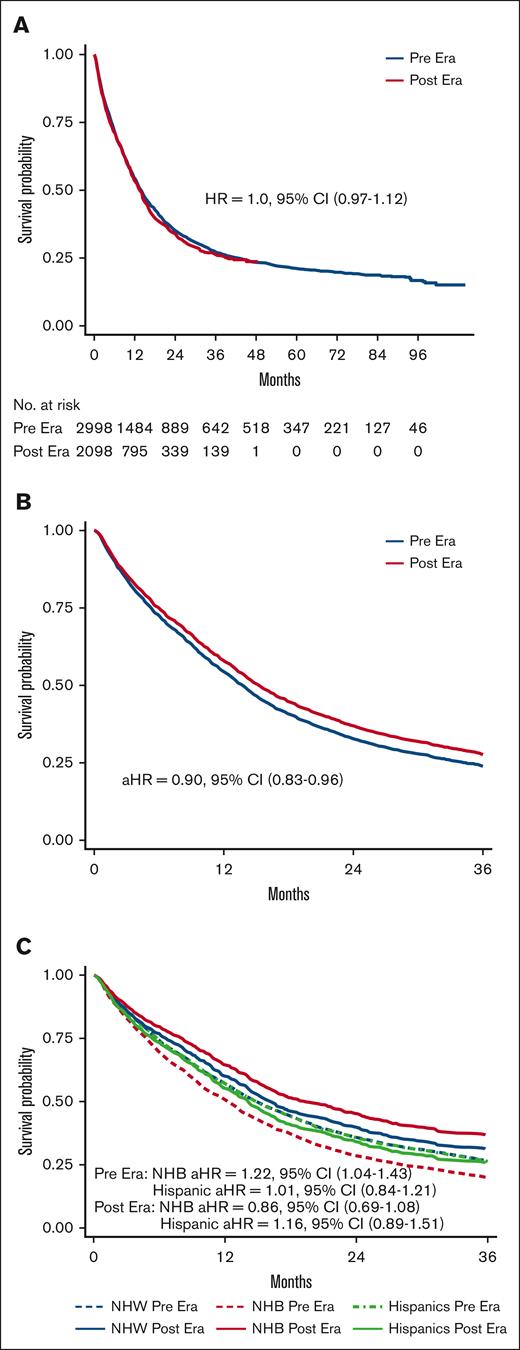
Unadjusted median OS was 13.9 months in Pre era and 13.3 months in Post era (HR, 1.04; 95% CI, 0.97-1.12; P = .28; Figure 2A). After adjusting for age at diagnosis and HCT-CI, patients treated in the Post era had a 10% lower hazard of death than those treated in the Pre era (adjusted HR [aHR], 0.90; 95% CI, 0.83-0.96), with predicted 2-year survival of 39.4% and 35.3%, respectively (Figure 2B). Among patients aged >70 years, those treated in the Post era had an 18% lower hazard of death relative to the Pre era (aHR, 0.82; 95% CI, 0.75-0.91; predicted 2-year survival, 25.8% vs 19.6%), whereas OS of patients aged ≤70 years was similar in the Pre and Post eras (aHR, 0.99; 95% CI, 0.88-1.11; predicted 2-year survival, 50.0% vs 49.7%; supplemental Figure 5). Similar changes were observed when we limit the study population to community practice only, with improved OS in patients treated in the Post era (aHR, 0.86; 95% CI, 0.79-0.95; predicted 2-year survival, 35.8% vs 30.5%), particularly in those aged >70 years (aHR, 0.81; 95% CI, 0.72-0.90; predicted 2-year survival, 23.4% vs 16.5%). Although no OS difference was found in those aged ≤70 years (aHR, 0.98; 95% CI, 0.83-1.15; predicted 2-year OS, 48.7% vs 48.0%). To further examine the heterogeneity of the impact of age on the treatment era–OS association on a ratio scale, we assessed the statistical interaction between age (dichotomized at 70 years) and treatment era. The P value for era × age category interaction was .04.
OS by treatment era across race/ethnicity. (A) Unadjusted Kaplan-Meier curves by treatment era (reference, Pre era). (B) Predicted OS curves by treatment era after adjusting for age and comorbidity (reference, Pre era). (C) Predicted OS curves by race/ethnicity between treatment eras after adjusting for age and comorbidity (reference, NHW patients).
OS by treatment era across race/ethnicity. (A) Unadjusted Kaplan-Meier curves by treatment era (reference, Pre era). (B) Predicted OS curves by treatment era after adjusting for age and comorbidity (reference, Pre era). (C) Predicted OS curves by race/ethnicity between treatment eras after adjusting for age and comorbidity (reference, NHW patients).
OS by race/ethnicity
Unadjusted median OS was higher for Hispanic patients than NHW patients (20.2 vs 13.2 months; HR, 0.72; 95% CI, 0.62-0.83), whereas unadjusted median OS for NHB and NHW patients was comparable (HR, 0.89; 95% CI, 0.79-1.06; supplemental Figure 6A). After controlling for age and HCT-CI and incorporating a race/ethnicity × time interaction, the aHR at 2 years for NHB compared with NHW was 1.21 (95% CI, 1.03-1.43), and the aHR at 2 years for Hispanic compared with NHW was 1.18 (95% CI, 0.98-1.42; supplemental Table 5).
Results of the multivariate Cox regressions comparing OS by racial/ethnic groups stratified by venetoclax treatment era are presented in Figure 2C. In the Pre era, the model-predicted 2-year survival was 28.6% for NWB and 35.8% for NHW patients (aHR, 1.22; 95% CI, 1.04-1.43); whereas, in the Post era, predicted 2-year survival was 45.3% and 39.9% for NHB and NHW, respectively (aHR, 0.86; 95% CI, 0.69-1.08). OS for Hispanic patients was comparable with that of NHW patients in the Pre era (aHR, 1.01; 95% CI, 0.84-1.21; predicted 2-year survival, 35.6% vs 35.8%), with no significant change in OS in the Post era (aHR, 1.16; 95% CI, 0.89-1.51; predicted 2-year survival, 34.3% vs 39.9%; Figure 2C; see supplemental Figure 6B for unadjusted Kaplan-Meier curves). When assessing the statistical interaction between race/ethnicity and treatment era on a ratio scale, the P values for NHB × era and Hispanic × era interaction terms were .026 and .50, respectively. Results of all sensitivity analyses were overall consistent with the primary analyses (supplemental Figure 7). When stratified by age group, OS improvements were observed in the Post era in all race/ethnicity among patients aged 40 to 70 years and those aged >70 years, except for Hispanic patients aged 40 to 70 years. Similar to our primary analyses, the most substantial OS improvements were observed among NHB patients (aged 40-70 years, predicted 2-year survival, NHW 46.0% vs 50.7%; NHB 38.6% vs 54.3%; and Hispanic 45.6% vs 44.8%; aged >70 years, predicted 2-year survival, NHW 18.3% vs 28.3%; NHB 13.6% vs 37.0%; Hispanic 17.3% vs 22.7%; supplemental Figure 8). The sample size and treatment options of novel therapeutics in adolescent and young adult (AYA) patients (aged 18-39 years) precluded meaningful analyses of the young adult population (supplemental Table 6). The changes in OS were not meaningfully different between sex groups (supplemental Table 7). Given the fact that near-null associations were observed between race/ethnicity and OS in the Post era, our preplanned mediation analyses to assess the component associations of each proposed mediator in the total effect of race/ethnicity on OS in the Post era were deemed unjustified. Post hoc analyses were performed instead, as described in the “Methods.”
Exploratory analyses
Utilization of novel therapeutics in the frontline setting
Overall, the prevalence of high-intensity induction therapy was lower for all race/ethnicity in the Post era than Pre era, but there were no differences by race/ethnicity after adjusting for age and HCT-CI (Table 2). Use of venetoclax-based low-intensity induction therapy among NHB and Hispanic patients was comparable with NHW in the entire study cohort (NHB adjusted PR [aPR], 0.93; 95% CI, 0.77-1.12; Hispanic aPR, 0.99; 95% CI, 0.80-1.22) and when restricted to patients aged >70 years (NHB aPR, 1.01; 95% CI, 0.83-1.23; Hispanic aPR, 1.09; 95% CI, 0.0.88-1.34; Table 2). Results of sensitivity analyses were consistent with the primary analyses (supplemental Figure 9).
Utilization of novel therapeutics in the frontline setting across race/ethnicity
| . | . | Pre era . | Post era . | ||||
|---|---|---|---|---|---|---|---|
| NHW . | NHB . | Hispanic . | NHW . | NHB . | Hispanic . | ||
| High intensity | |||||||
| All | Adjusted %∗ | 58 | 57 | 57 | 37 | 37 | 35 |
| aPR (95% CI) | Ref | 0.97 (0.88-1.08) | 0.98 (0.89-1.07) | Ref | 1.00 (0.85-1.17) | 0.94 (0.81-1.10) | |
| ≤70 y | Adjusted %∗ | 86 | 84 | 83 | 67 | 72 | 70 |
| aPR (95% CI) | Ref | 0.97 (0.91-1.04) | 0.96 (0.90-1.02) | Ref | 1.06 (0.94-1.20) | 1.04 (0.93-1.16) | |
| Venetoclax low intensity | |||||||
| All | Adjusted %∗ | 2 | 1 | 1 | 44 | 40 | 43 |
| aPR (95% CI) | Ref | 0.50 (0.12-2.03) | 0.30 (0.04-1.14) | Ref | 0.93 (0.77-1.12) | 0.99 (0.80-1.22) | |
| >70 y | Adjusted %∗ | Small event sizes | 61 | 61 | 66 | ||
| aPR (95% CI) | Ref | 1.01 (0.83-1.23) | 1.09 (0.88-1.34) | ||||
| CPX-351 in s-AML† | |||||||
| All | Adjusted %∗ | 24 | 26 | 32 | 15 | 20 | 10 |
| aPR (95% CI) | Ref | 1.07 (0.61-1.88) | 1.32 (0.70-2.50) | Ref | 1.33 (0.87-2.04) | 0.67 (0.31-1.48) | |
| ≤70 y | Adjusted %∗ | 40 | 41 | 55 | Small event sizes | ||
| aPR (95% CI) | Ref | 1.02 (0.58-1.80) | 1.39 (0.75-2.59) | ||||
| Midostaurin in FLT3-mutated AML† | |||||||
| All | Adjusted %∗ | 45 | 38 | 22 | 42 | 45 | 37 |
| aPR (95% CI) | Ref | 0.85 (0.36-2.00) | 0.52 (0.22-1.24) | Ref | 1.06 (0.69-1.61) | 0.87 (0.54-1.38) | |
| ≤70 y | Adjusted %∗ | Small event sizes | 56 | 62 | 54 | ||
| aPR (95% CI) | Ref | 1.10 (0.75-1.60) | 0.97 (0.62-1.51) | ||||
| . | . | Pre era . | Post era . | ||||
|---|---|---|---|---|---|---|---|
| NHW . | NHB . | Hispanic . | NHW . | NHB . | Hispanic . | ||
| High intensity | |||||||
| All | Adjusted %∗ | 58 | 57 | 57 | 37 | 37 | 35 |
| aPR (95% CI) | Ref | 0.97 (0.88-1.08) | 0.98 (0.89-1.07) | Ref | 1.00 (0.85-1.17) | 0.94 (0.81-1.10) | |
| ≤70 y | Adjusted %∗ | 86 | 84 | 83 | 67 | 72 | 70 |
| aPR (95% CI) | Ref | 0.97 (0.91-1.04) | 0.96 (0.90-1.02) | Ref | 1.06 (0.94-1.20) | 1.04 (0.93-1.16) | |
| Venetoclax low intensity | |||||||
| All | Adjusted %∗ | 2 | 1 | 1 | 44 | 40 | 43 |
| aPR (95% CI) | Ref | 0.50 (0.12-2.03) | 0.30 (0.04-1.14) | Ref | 0.93 (0.77-1.12) | 0.99 (0.80-1.22) | |
| >70 y | Adjusted %∗ | Small event sizes | 61 | 61 | 66 | ||
| aPR (95% CI) | Ref | 1.01 (0.83-1.23) | 1.09 (0.88-1.34) | ||||
| CPX-351 in s-AML† | |||||||
| All | Adjusted %∗ | 24 | 26 | 32 | 15 | 20 | 10 |
| aPR (95% CI) | Ref | 1.07 (0.61-1.88) | 1.32 (0.70-2.50) | Ref | 1.33 (0.87-2.04) | 0.67 (0.31-1.48) | |
| ≤70 y | Adjusted %∗ | 40 | 41 | 55 | Small event sizes | ||
| aPR (95% CI) | Ref | 1.02 (0.58-1.80) | 1.39 (0.75-2.59) | ||||
| Midostaurin in FLT3-mutated AML† | |||||||
| All | Adjusted %∗ | 45 | 38 | 22 | 42 | 45 | 37 |
| aPR (95% CI) | Ref | 0.85 (0.36-2.00) | 0.52 (0.22-1.24) | Ref | 1.06 (0.69-1.61) | 0.87 (0.54-1.38) | |
| ≤70 y | Adjusted %∗ | Small event sizes | 56 | 62 | 54 | ||
| aPR (95% CI) | Ref | 1.10 (0.75-1.60) | 0.97 (0.62-1.51) | ||||
Ref, reference.
Adjusted % represents adjusted prevalence, adjusted for age and HCT-CI.
Restricted to dates after US Food and Drug Administration approval only.
Among patients with documented s-AML or FLT3-mutated AML, no difference in race/ethnicity was observed in the frontline utilization of CPX-351 or midostaurin, respectively (Table 2).
Among patients who received induction with venetoclax-based therapy, there were no differences by race/ethnicity in age, ELN2017 risk groups, or the prevalence of the few mutations that are known to be associated with the outcomes of venetoclax-based induction therapy to date (favorable mutations include NPM1, IDH1, and IDH2; less favorable mutations include TP53, KRAS, NRAS, and FLT3-internal tandem duplication [FLT3-ITD]; Table 3).41-43 With respect to molecular features with available data, DNMT3A was the only baseline mutation that was differentially distributed by race/ethnicity (14.3% vs 28.6% vs 16.1% in NHW, NHB, and Hispanic patients, respectively; Table 3).
Baseline characteristics of patients receiving frontline venetoclax-based low-intensity therapy
| . | Total n = 976 n (%) . | NHW n = 870 n (%) . | NHB n = 61 n (%) . | Hispanic n = 45 n (%) . |
|---|---|---|---|---|
| Median age, y | 75.5 | 76.0 | 75.0 | 76.0 |
| Sex, male | 576 (59) | 531 (61) | 22 (36) | 23 (51) |
| ELN2017∗ | n = 817 | n = 733 | n = 48 | n = 36 |
| Favorable | 71 (9) | 62 (9) | 5 (10) | 4 (11) |
| Intermediate | 320 (39) | 283 (39) | 25 (52) | 12 (33) |
| Adverse | 426 (52) | 388 (53) | 18 (38) | 20 (56) |
| Venetoclax-favorable mutations∗ | n = 794 | n = 714 | n = 49 | n = 31 |
| NPM1 | 78 (10) | 71 (10) | 3 (6) | 4 (13) |
| IDH1 | 70 (9) | 64 (9) | 6 (12) | 0 (0) |
| IDH2 | 100 (13) | 85 (12) | 8 (16) | 7 (23) |
| Venetoclax-unfavorable mutations∗ | n = 794 | n = 714 | n = 49 | n = 31 |
| TP53 | 140 (18) | 127 (18) | 8 (16) | 5 (16) |
| KRAS | 27 (3) | 26 (4) | 1 (2) | 0 (0) |
| NRAS | 56 (7) | 48 (7) | 5 (10) | 3 (10) |
| FLT3-ITD | 65 (8) | 59 (8) | 3 (6) | 3 (10) |
| Others, DNMT3A | 121 (15) | 102 (14) | 14 (29) | 5 (16) |
| . | Total n = 976 n (%) . | NHW n = 870 n (%) . | NHB n = 61 n (%) . | Hispanic n = 45 n (%) . |
|---|---|---|---|---|
| Median age, y | 75.5 | 76.0 | 75.0 | 76.0 |
| Sex, male | 576 (59) | 531 (61) | 22 (36) | 23 (51) |
| ELN2017∗ | n = 817 | n = 733 | n = 48 | n = 36 |
| Favorable | 71 (9) | 62 (9) | 5 (10) | 4 (11) |
| Intermediate | 320 (39) | 283 (39) | 25 (52) | 12 (33) |
| Adverse | 426 (52) | 388 (53) | 18 (38) | 20 (56) |
| Venetoclax-favorable mutations∗ | n = 794 | n = 714 | n = 49 | n = 31 |
| NPM1 | 78 (10) | 71 (10) | 3 (6) | 4 (13) |
| IDH1 | 70 (9) | 64 (9) | 6 (12) | 0 (0) |
| IDH2 | 100 (13) | 85 (12) | 8 (16) | 7 (23) |
| Venetoclax-unfavorable mutations∗ | n = 794 | n = 714 | n = 49 | n = 31 |
| TP53 | 140 (18) | 127 (18) | 8 (16) | 5 (16) |
| KRAS | 27 (3) | 26 (4) | 1 (2) | 0 (0) |
| NRAS | 56 (7) | 48 (7) | 5 (10) | 3 (10) |
| FLT3-ITD | 65 (8) | 59 (8) | 3 (6) | 3 (10) |
| Others, DNMT3A | 121 (15) | 102 (14) | 14 (29) | 5 (16) |
Among patients whose information was available.
OS among patients receiving frontline novel therapy
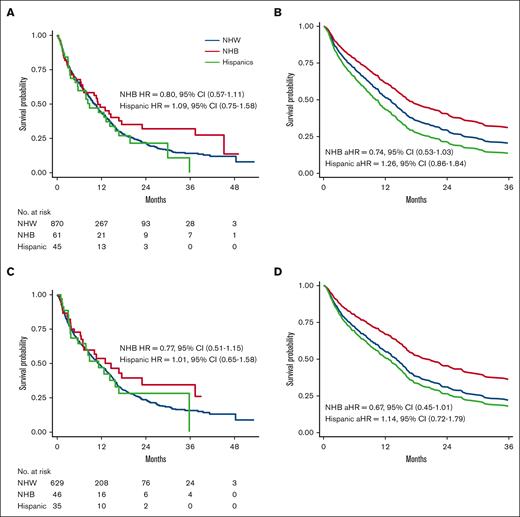
Among patients who received venetoclax-based low-intensity induction therapy, NHB patients had a 26% lower hazard of death than NHW (aHR, 0.74; 95% CI, 0.53-1.03; Figure 3A-B) and a predicted 2-year survival of 40.5% and 29.4%, respectively. Comparable OS was observed between Hispanic and NHW patients (Figure 3A-B). No differences were observed in the total lines of therapy or distribution of second-line regimens by race/ethnicity among patients who received induction with venetoclax-based low-intensity therapy (supplemental Table 8). Similar results were observed when restricting the analysis to patients treated at community practice only, with NHB patients having a 23% decreased hazard of death compared with NHW patients (aHR, 0.77; 95% CI, 0.51-1.15; predicted 2-year OS, 34.8% vs 26.7%).
OS of patients who received venetoclax-based low-intensity frontline therapy (reference, NHW patients). (A) Unadjusted Kaplan-Meier curves by race/ethnicity. (B) Predicted OS curves by race/ethnicity after adjusting for age and comorbidity. (C) Unadjusted Kaplan-Meier curves of patients without venetoclax-unfavorable mutations (TP53, KRAS, NRAS, and FLT3-ITD) by race/ethnicity. (D) Predicted OS curves of patients without venetoclax-unfavorable mutations (TP53, KRAS, NRAS, and FLT3-ITD) by race/ethnicity after adjusting for age and comorbidity.
OS of patients who received venetoclax-based low-intensity frontline therapy (reference, NHW patients). (A) Unadjusted Kaplan-Meier curves by race/ethnicity. (B) Predicted OS curves by race/ethnicity after adjusting for age and comorbidity. (C) Unadjusted Kaplan-Meier curves of patients without venetoclax-unfavorable mutations (TP53, KRAS, NRAS, and FLT3-ITD) by race/ethnicity. (D) Predicted OS curves of patients without venetoclax-unfavorable mutations (TP53, KRAS, NRAS, and FLT3-ITD) by race/ethnicity after adjusting for age and comorbidity.
Among patients with documentation of any of the generally accepted venetoclax–less favorable mutations (TP53, KRAS, NRAS, and FLT3-ITD), OS did not differ significantly by race/ethnicity (Figure 3C). Among patients who did not harbor any of such venetoclax–less favorable mutations, NHB patients had a 33% lower hazard of death than NHW patients (aHR, 0.67; 95% CI, 0.45-1.01; predicted 2-year survival, 45.5% vs 31.1%); whereas comparable OS was observed for Hispanic and NHW patients (Figure 3D).
Discussion
Research efforts in the past 10 to 15 years have dramatically improved our understanding of the molecular profiling11-13 and greatly expanded the therapeutic landscape of AML.13 Outside of the clinical trial setting, the real-world impact of novel therapeutic agents and modern AML care compared with historical standard treatments had not been well described. In this large, primarily community practice-based real-world cohort, we demonstrate that, since the approval of venetoclax, older patients and patients with more comorbidities were treated with an improvement in OS. The survival difference was driven by OS benefits observed in patients aged >70 years, a population for whom effective and safe therapeutic options had been limited previously.44 Still, the OS observed among patients aged >70 years in our study was inferior compared with data reported from major academic institutions,1,45 which may reflect the inherent heterogeneity in patients treated in different clinical settings or differences in AML care approaches between academic institutions/clinical trials and the broader real-world practice.
A growing number of studies have demonstrated survival disparities in AML for patients from racial minority groups, particularly Black patients, and have identified socioeconomic factors,46-48 structural racism,19 inequality in clinical trial enrollment,49,50 and differences in ancestry-based disease biology as potential drivers of the disparities.50,51 In this study, we explore racial/ethnic disparities in AML outcomes in the context of the rapidly expanding therapeutic landscape. We observed a survival disparity between NHB and NHW patients in the Pre era (Figure 2C), when high-intensity induction chemotherapy was the conventional treatment modality for AML, which aligns with the results of prior studies.8,10,51 Contrary to our expectation that racial/ethnic disparities would widen in the Post era, we found that NHB patients had the largest OS improvement in their survival disparity compared with NHW patients seemingly mitigated (Figure 2C). Compared with NHB and NHW patients, Hispanic patients did not experience similar OS improvement in the Post era. It is worth noting that Hispanic patients were younger than NHB and NHW patients in our cohort, for whom treatment modalities remained more consistent between the 2 eras, at least in the frontline setting (Table 1). Biological differences of the underlying disease cannot be excluded. In our study, similar OS changes were observed across age groups, except for AYA patients. The AYA group represents a unique patient population and a special focus in AML disparity research, who need to be further evaluated in future studies given the small sample sizes in the current cohort, particularly in the Post era (supplemental Table 6).
Consistent with recent publications,52-54 we found a shift in frontline AML treatment patterns in our study, in which intensive chemotherapy was less used in the Post era. Importantly, despite the disparities in baseline SES and insurance coverage status, the uptake of novel, targeted therapeutics, particularly venetoclax-based low-intensity therapy in the frontline setting, did not differ across race/ethnicity in this cohort. With the changing landscape of AML therapeutic strategies also comes a reconsideration of previously established AML risk stratification and the prognostic implications of certain genetic features.41,43 Long-term follow-up data from frontline venetoclax/HMA trials demonstrated that ELN risk groups,18,24 which were created based on data from patients receiving intensive chemotherapy, are less prognostic of clinical outcomes in this group of patients.41 In contrast, baseline mutational profile appears to be more closely correlated with responses and survival, with distinct clinical trajectories, including patients harboring the venetoclax-favorable mutations (NPM1, IDH1, and IDH2) and venetoclax–less favorable mutations (TP53, FLT3-ITD, KRAS, and NRAS).41-43 It is worth noting that the association between molecular features and survival outcomes has been generated in largely non-Black populations enrolled on frontline venetoclax clinical trials.16,55,56 Whether the same associations apply to Black patients remains unknown. In this study, although not statistically significant, our results favor a protective effect of venetoclax-based therapy among NHB relative to NHW patients, which was not driven by differences in the distribution of demographic features, ELN2017 groups, or venetoclax-favorable or –less favorable mutations but primarily by the patients who did not harbor any venetoclax–less favorable mutations. The small sample sizes in racial minority groups preclude our ability to assess the impact of individual molecular marker or perform more sophisticated molecular feature grouping. These results are post hoc and hypothesis generating in nature and should be explored further in larger, well-curated data sets, likely in multi-institutional or collaborative group setting. If confirmed in further studies, it may suggest the need for ancestry-inclusive considerations in AML risk stratification models and treatment strategies. Growing evidence suggests that NPM1 mutations do not seem to confer as favorable a prognostic impact on Black patients as on White patients treated with intensive chemotherapy8,51,57; therefore, the most effective regimen for Black patients with NPM1-mutated AML is unknown. Considering the potent efficacy of venetoclax in NPM1 clone elimination,42,58 it may be of particular interest to assess the outcomes of venetoclax-based therapy in the NPM1-mut group by race/ethnicity in future studies.
Our study is not without limitations. There is the possibility of exposure misclassification based on how race/ethnicity information were collected in EHRs. The small numbers of NHB and Hispanic patients affect the power of our subgroup analyses and introduce potential bias and unmeasured confounding. Due to limitations of the data source in capturing disease status assessment information and missingness, we were not able to reliably identify remission or relapse status, which prohibits our ability to accurately assess other relevant end points of interests such as relapse-free survival and the impact of subsequent lines of therapy, including allogeneic hematopoietic stem cell transplant. This should be addressed in manually curated EHR data or trial data in the future. Missingness is an inherited limitation in real-world data sets, which can be informative in disparity research and difficult to mitigate.59 In our data set, there was differential missingness by race/ethnicity, with Hispanic patients having a higher likelihood of missingness in HCT-CI. This is likely partially controlled by adjusting for age in our analyses. There was more missingness in baseline genomic features among patients treated in the Pre era. However, this was nondifferential across racial/ethnic groups. Lastly, despite being a broad nationwide database, the study cohort includes predominantly patients treated at community practices, which may limit the generalizability of our results.
With the evolution of AML care, we may be able to better serve the previously underrepresented, understudied patient populations with this challenging disease. Here, we present thought-provoking and hypothesis-generating results that demonstrate the encouraging progress we have made on the outcomes of NHB patients with AML, especially in the context of venetoclax-based induction therapy. Future studies are needed to validate and substantiate these findings and should focus on evaluating the mechanisms for the observed improvements in OS, and differentiating the effects of biological factors, such as AML genomics and pharmacodynamics of antileukemic therapy, and nonbiological factors, such as health care access and supportive care, to identify potential targets for intervention. Broader multi-institutional and multidisciplinary leveled collaborations are required to answer these important questions.
In this large, real-world data set, we demonstrate OS improvements in the post-venetoclax era of AML care, particularly among patients aged >70 years. Intriguingly, the largest OS improvement was observed in NHB patients, with the OS disparity between NHB and NHW patients in the Pre era appearing to have been mitigated. There were no differences in the utilization of novel AML therapeutics in the frontline setting across race/ethnicity. Among patients receiving frontline venetoclax-based low-intensity therapy, there was a trend toward superior OS in NHB compared with NHW patients, primarily in those without venetoclax–less favorable mutations at baseline. More studies are needed to better understand the biological and nonbiological factors contributing to these changes observed to identify potential targets for intervention, so we could further improve the outcomes for all patients with AML.
Acknowledgments
X.W. received funding support from an F32 Individual Postdoctoral Fellowship Award from the National Institutes of Health, National Institute of Minority Health and Health Disparities (1F32MD018944-01) and the Health Equity Research Pilot Award from the Division of Hematology and Oncology at the Perelman School of Medicine, University of Pennsylvania.
Authorship
Contribution: X.W., K.D.G., and C.L. developed the concept and designed the study; X.W. organized the data and conducted all analyses with the help of K.D.G. and P.A.G.; X.W. drafted the manuscript with contributions from K.D.G., C.L., and K.W.P.; and all authors read, edited, and approved the final manuscript.
Conflict-of-interest disclosure: S.M.L. received honoraria from Syros, Agios, Daiichi Sankyo, Jazz Pharmaceuticals, Brystol Myers Squibb (BMS), Acceleron, Astellas, and Pfizer, and research funding from Onconova, Celgene, Biosight, Hoffman-La Roche, and Kura. E.O.H. received research funding from Tmunity Therapeutics and Blueprint Medicines, and had membership on an entity's board of directors or advisory committees for Blueprint Medicines and PharmaEssentia. N.V.F. was a consultant for Sana Biotechnology, Kite Pharma, and Syndax Pharmaceuticals, and received research funding from Novartis. D.L.P. received honoraria from the American Society for Transplantation and Wiley and Sons Publishing; had membership on an entity's board of directors or advisory committees for American Society of Hematology, Decart, Incyte, Janssen, Kite/Gilead, National Marrow Donor Program, and Novartis; is a current equity holder in Genentech; and has patents and royalties with Unity. S.G. has licensed intellectual property with Novartis; received research funding from Novartis, Carisma Therapeutics, and Interius BioTherapeutics; and is a current holder of stock options in Carisma Therapeutics and Interius BioTherapeutics. I.M. received research funding from Genentech and Regeneron, and has reserved on an entity’s board of directors or advisory committees of Garuda Therapeutics. A.E.P. received research funding from Fujifilm, Daiichi Sankyo, Astellas, Arog, and AbbVie, and was a consultant for Daiichi Sankyo, Sumitomo Dainippon, Astellas, BMS/Celgene, Genentech, Loxo, Onconova, Syndax, Forma, Actinium, Roche, and AbbVie. K.W.P. has consulted for AbbVie, Agios, BMS, Astellas, and Novartis; received honoraria from AbbVie, BMS, Astellas, and Celgene; and received research funding from AbbVie, Astellas, and Millenium. C.L. served as a consultant for Taiho, Pfizer, Jazz, AbbVie, Rigel, BMS, Genentech, Novartis, Daiichi Sankyo, and Astellas, and has received research funding from Jazz. The remaining authors declare no competing financial interests.
Correspondence: Xin Wang, Leukemia Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 E 74th St, New York, NY 10021; email: wangx16@mskcc.org.
References
Author notes
K.D.G. and C.L. contributed equally to this study.
Flatiron data can be obtained through agreement; please contact academic-partnerships@flatiron.com. Original data are available on request from the corresponding author, Xin Wang (wangx16@mskcc.org).
The full-text version of this article contains a data supplement.