Key Points
Loss of PC and PS in zebrafish results in discrepant spontaneous thrombotic phenotypes.
PC has genetic interactions with a novel G-protein–coupled receptor.
Visual Abstract
Venous thrombosis is a leading cause of morbidity/mortality and associated with deficiencies of the anticoagulant protein C (PC; PROC) and its cofactor, protein S (PS; PROS1). Heterozygous mutations increase the risk of adult-onset thrombosis, whereas homozygous mutations result in pre/neonatal lethal thrombosis. Phenotypes of patients with PC and PS deficiency are generally considered clinically indistinguishable. Here, we generate proc (zebrafish PROC ortholog) and pros1 knockouts through genome editing in zebrafish and uncover partially discordant phenotypes. proc−/− mutants exhibited ∼70% lethality at 1 year of age, whereas pros1−/− survival was unaffected. Induced venous endothelial injury in both mutants revealed reduced occlusive thrombus formation. This is consistent with the consumptive coagulopathy of zebrafish antithrombin 3 knockouts, which also results in spontaneous venous thrombosis. However, proc and pros1 mutants revealed a discrepancy. Although both mutants demonstrated spontaneous thrombosis, proc−/− was localized to the cardiac and venous systems, whereas pros1−/− was intracardiac. Aside from coagulation, PC has been shown to have PS-independent roles in inflammation. proc mutants displayed altered inflammatory markers and defects in neutrophil migration independent of pros1. Transcriptomic analysis and gene knockdown identified novel proc genetic interactions with adgrf7, a G protein-coupled receptor (GPCR) not previously known to be involved in coagulation. In summary, our data reveal differences between PC- and PS-deficient thrombosis, with cardiovascular tissue–specific phenotypes and survival differences, suggesting the possibility of underlying clinical differences in affected patients. This model of complete proc−/− deficiency in an accessible organism will facilitate further in vivo study of these distinctions, as well as PS-dependent and -independent functions of PC.
Introduction
Protein C (PC; PROC gene), together with its cofactor protein S (PS; PROS1 gene) are critical anticoagulation components of the coagulation cascade.1 Upon clotting initiation, activated PC is produced by the thrombin–thrombomodulin complex and with PS inhibits activated factors V (FVa) and VIII (FVIIIa).2,3 Complete PC or PS deficiency are rare disorders caused by homozygous or compound heterozygous mutations in PROC and PROS1, respectively, and lead to pre/perinatal lethality due to purpura fulminans (intravascular thrombosis resulting in skin infarction).1,4 The more common form of human deficiency is due to heterozygous mutations, and typically does not manifest until the third or fourth decades of life unless triggered by stimuli such as immobilization, infection, surgery, or pregnancy.5 Sequalae include deep vein thrombosis, pulmonary embolism, and stroke,6 and clinically these deficiencies are thought to be indistinguishable. Although both proteins have been extensively studied, our knowledge of how to manage asymptomatic deficient patients is limited, because the majority never manifest episodes of thrombosis, or do so later in life with unpredictable timing.
In addition to coagulation, PC has been found to be an important modulator of inflammation.7 In studies of sepsis, abnormally low levels of PC have been reported, which may result in thrombosis and disseminated intravascular coagulation (DIC).8 However, potent anticoagulants such as heparin are not effective in treating severe sepsis,9,10 suggesting that PC may have anti-inflammatory functions independent of the coagulation cascade.11 PC has been shown to regulate leukocyte trafficking and infiltration.12
Studying complete PC and PS deficiency in animal models has been limited because of pre/neonatal death of mouse homozygous knockouts.4,13,14 The zebrafish model has distinctive features that include external fertilization, rapid development, high fecundity, and optical transparency. Zebrafish have all key coagulation factors present in humans, which develop functionally in the first week of life.15-17 Transparent larvae enable visualization of blood circulation and fluid dynamics in real time. We have previously used genome editing in zebrafish and discovered striking similarities of multiple procoagulant and anticoagulant factors to that of human,18-23 including FV21 and FVIII.24 There are also differences, including lack of factor XI and the contact pathway.
The zebrafish ortholog of PROC (proc) is duplicated in tandem. Using genome editing with multiple CRISPR single guide RNAs (sgRNAs) we show the consequences of complete loss of proc as well as pros1. Our results indicate that zebrafish tolerate loss of these factors and survive into adulthood. Although both mutants have a thrombophilic coagulopathy in the embryonic/larval period, they have some differences in site-specific manifestations. We also show that loss of PC results in inflammatory deficits that are independent of PS and thrombin generation, and discover new interactions with a factor previously, to our knowledge, not known to be involved in coagulation.
Methods
Zebrafish
All fish were maintained on a hybrid background of wild-type strains (ABxTL) to minimize strain specific effects and enhance fecundity.25 Zebrafish were analyzed at various developmental stages, embryo, larvae, juvenile and adult, defined by 0 to 2, 3 to 29, 30 to 89, and >90 days postfertilization (dpf), respectively.26 Zebrafish used in this study were housed at 28.5°C under light-control (14/10 hours on/off) rooms, and all care and usage were approved by the institutional animal care and use committee at the University of Michigan. The green fluorescent protein (GFP)–tagged fibrinogen transgenic (Tgfgb-eGFP), which labels fibrinogen with green fluorescence,27 and the prothrombin (f2) mutant allele23 have been described previously.
CRISPR-mediated genome editing
The loci for zebrafish proc, pros1, adgrf7, and serpina10b were edited using CRISPR/Cas9. sgRNAs targeted to pros1 exons 1 and 11 and proc exons 4 and 10 sequence (supplemental Table 1) were synthesized by polymerase chain reaction (PCR) using 2 60-mer oligonucleotides containing the T7 promoter sequence, 18 to 20 nucleotides of target sites, and sgRNA scaffold; followed by in vitro transcription using HiScribe T7 Quick High Yield RNA Synthesis kit (New England BioLabs).28 Injection mixture containing 200 mM potassium chloride, 8.3 mM HEPES (N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid; Corning), 200 to 250 ng/μL total sgRNAs and 2.8 to 3.5 μM Cas9 protein (New England BioLabs, EnGen Spy Cas9 NLS) was incubated at 37°C for 5 minutes, and 1 to 2 nL were injected into 1-cell–stage embryos. Mutant lines were generated by outcrossing chimeric founders with confirmed germ line transmission. For knockdown studies, sgRNAs were synthesized (Synthego), complexed with Cas9, and injected as above. Fish were genotyped by PCR as previously described29 using primers shown in supplemental Table 2.
Laser-induced endothelial injury
Anesthetized zebrafish larvae were immobilized in 0.8% low-melting agarose on a glass coverslip and visualized on an inverted microscope (Olympus IX73) with an attached pulsed-nitrogen dye laser system (MicroPoint, Andor Technology). Venous thrombi were generated by laser-induced injury to the endothelium of the posterior cardinal vein (PCV) at the fifth somite distal to the anal pore at 3 dpf.30,31 After injury, the time to occlusion (TTO) was monitored for 2 minutes by a blinded observer followed by genotyping.
Quantification of spontaneous thrombosis
Mutant zebrafish lines were bred into the Tgfgb-eGFP background; fibrin accumulation visualized by green fluorescence; and quantified by counting thrombi, binning into 4 groups. Zebrafish larvae (5 dpf) were anesthetized in tricaine and embedded in 0.8% low-melt agarose on glass coverslips and visualized on an inverted microscope. An observer blinded to genotype performed semiquantitative analysis of fluorescence along the PCV and in the heart. Larvae were retrieved from agarose for genotyping.
Tail transection studies and phenotyping
Embryos were collected and grown in sterile E3 medium (5 mM sodium chloride, 0.17 mM potassium chloride, 0.33 mM calcium chloride, and 0.33 mM magnesium sulfate) until 3 dpf. Larvae were transected at the tail tip,32 Sudan Black B was used to stain neutrophils after 1 hour (peak abundance33). diaminofluorescein-FM (DAF-FM) diacetate (Sigma-Aldrich) was used to detect the presence of nitric oxide.34 Zebrafish larvae at 3 dpf were incubated in E3 medium containing 5 μM DAF-FM diacetate for 2 hours in the dark at 28°C. After incubation, larvae were briefly rinsed with E3 medium and anesthetized before imaging.
Transgene expression
Rescue plasmids were constructed by inserting proc complementary DNAs (cDNAs; proc1 or proc2) under control of ubi (ubiquitin) promoter followed by a cryaa promoter–driven mCherry, all flanked by Tol2 repeats. Plasmid (20-25 pg) was injected into proc+/− incrosses. Larvae positive for mCherry in the lens were selected for laser injury at 3 dpf. Spontaneous thrombosis was evaluated at 5 dpf. All data were recorded by a blinded observer before genotyping.
Statistical analysis
Data were analyzed using a 2-tailed Student t test (reverse transcription PCR and neutrophil counts), Mann-Whitney U test (TTO) and log-rank/Mantel-Cox test (survival), and Fisher's exact test (thrombosis).
Results
Zebrafish proc is duplicated in tandem
Gene duplication has been an important process for evolutionary adaptation. Teleost fish have undergone a whole-genome duplication over 400 million years ago resulting in 30% to 40% of genes remaining duplicated, so called “ohnologs.”35 Analysis of the genomic sequence of the proc locus (GRCz11/danRer11, chromosome 2:5,456,541-5,475,910) revealed a tandem duplication of the proc gene within 19.3 kilobases (kb; Figure 1A), with large portions of the genomic sequence nearly identical. Both proc cDNAs were amplified and cloned (referred to as proc1 and proc2; Figure 1B). Sequencing revealed 92% identity and 89% similarity at the amino acid level. Of randomly selected and sequenced clones, 79 of 96 (82.3%) were proc1 and 17 of 96 (17.7%) proc2. Interestingly, they share 93% identity at the nucleotide level, suggesting this is not a typical teleost ohnolog but rather a more recent duplication event.36,37 BLAT searches of the teleosts fugu, medaka, and tetraodon genomes with proc cDNA indicate that these only have 1 proc copy. Translated PC1 and PC2 are predicted to be 434 and 402 amino acids, and share 63% and 59% amino acid identity with human PC, respectively (supplemental Figure 1).
Complete loss of proc results in late onset lethality. (A) proc is duplicated in tandem, with near complete identify at the nucleotide and amino acid sequence level. Two sgRNAs (arrowheads) targeting identical sequences in both copies of exons 4 and 10 produced a 17.3-kb gene deletion resulting in nearly complete ablation of both proc copies. The locus is displayed in 5′ to 3′ orientation, but the actual genomic orientation is in reverse, and thus the copies are named according to the latter nomenclature. (B) proc1 is the predominant expressed copy of proc. Qualitative reverse transcription PCR with primers that match identical sequences in both copies reveals 2 bands that correspond to proc1 and proc2 predicted sizes. (C) DNA gel electrophoresis using a single primer pair that amplifies both copies showed the truncated genomic proc PCR product (0.3 kb) after CRISPR-mediated deletion. (D) qPCR (quantitative PCR) data of total proc expression revealed a 63% decrease in the heterozygous mutant embryos, and 99.8% decrease in homozygous mutants, (P < .0001 by t testing). (E) Survival curves indicated significant mortality in proc−/− mutants by 1 year of age using log-rank (Mantel-Cox) testing (P < .0001). Chr, chromosome.
Complete loss of proc results in late onset lethality. (A) proc is duplicated in tandem, with near complete identify at the nucleotide and amino acid sequence level. Two sgRNAs (arrowheads) targeting identical sequences in both copies of exons 4 and 10 produced a 17.3-kb gene deletion resulting in nearly complete ablation of both proc copies. The locus is displayed in 5′ to 3′ orientation, but the actual genomic orientation is in reverse, and thus the copies are named according to the latter nomenclature. (B) proc1 is the predominant expressed copy of proc. Qualitative reverse transcription PCR with primers that match identical sequences in both copies reveals 2 bands that correspond to proc1 and proc2 predicted sizes. (C) DNA gel electrophoresis using a single primer pair that amplifies both copies showed the truncated genomic proc PCR product (0.3 kb) after CRISPR-mediated deletion. (D) qPCR (quantitative PCR) data of total proc expression revealed a 63% decrease in the heterozygous mutant embryos, and 99.8% decrease in homozygous mutants, (P < .0001 by t testing). (E) Survival curves indicated significant mortality in proc−/− mutants by 1 year of age using log-rank (Mantel-Cox) testing (P < .0001). Chr, chromosome.
Generation of proc-null zebrafish
We used CRISPR-mediated genome editing to generate a complete knockout of the proc locus. Two sgRNAs were designed to recognize identical regions in both copies of exon 4 and 10 (Figure 1A). Injection induced a 17.3-kb deletion that resulted in nearly complete removal of the exonic sequence of both alleles (Figure 1C). mRNA levels were compared among genotypes, and we observed a 60% reduction of total proc RNA expression in heterozygotes and essentially no detectable transcript in homozygous mutants (Figure 1D). These analyses strongly suggest that this is a complete knockout of both proc copies.
Loss of proc results in adult lethality
Loss of PC in humans and mice results in pre/neonatal lethality due to severe widespread thrombosis with purpura fulminans.1,38 Tracking survival of homozygous proc knockouts revealed the surprising finding of extensive survival into adulthood, 50% survival at 6 months of age, and 30% by 1 year (Figure 1E). These results are similar to previous findings with loss of antithrombin 3 (At3) in zebrafish.18 Minor die-off was observed in heterozygous mutants, although this was not statistically significant.
proc-mutant larvae exhibit highly penetrant spontaneous thrombosis
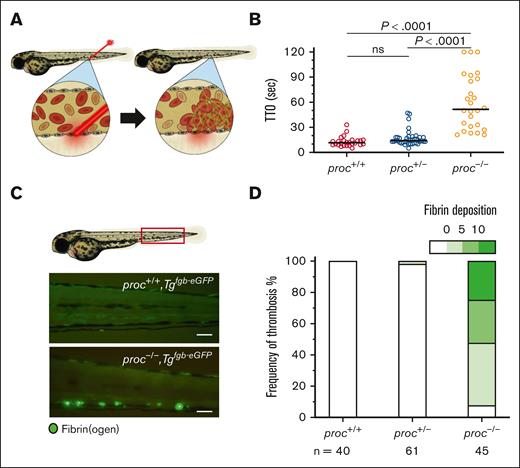
Despite adult lethality, blood flow in proc-mutant larvae appeared normal with no evidence for thrombosis. We induced thrombosis using laser-mediated endothelial injury, expecting rapid occlusion compared with wild-type siblings. Instead, we found a significantly prolonged TTO (Figure 2A-B), which is similar but less severe than previous observations with loss of At3.18 In at3 mutants, increased TTO was due to DIC and resulted in spontaneous larval venous thrombosis. To test this, we bred proc−/− into the Tgfgb-eGFP transgenic background.27 By 5 dpf, we observed fluorescent deposits along the PCV, indicating fibrin accumulation (Figure 2C) but normal blood flow (supplemental Videos 1-3). Only proc−/− mutants were observed to have a significant amount of spontaneous thrombosis compared with their siblings (Figure 2D). Thrombosis was highly penetrant and present in 92.5% of larvae.
proc mutants develop spontaneous thrombosis with secondary coagulopathy. (A) Schematic of laser-mediated endothelial injury that was performed to evaluate for intravascular thrombus formation. The laser injures the endothelium of the PCV, and TTO was measured for up to 2 minutes. (B) Homozygous proc mutants demonstrated a partial defect in hemostasis manifested by a significant prolonged TTO compared to wild-type and heterozygous siblings. n = 26, 41, 26 in proc+/+, proc+/−, and proc−/−, respectively. Bars indicate median TTO. Values of P were calculated by Mann-Whitney U test. (C) The proc mutation was bred into the Tgfgb-eGFP transgenic background so that spontaneous thrombosis could be visually observed and scored through green fluorescence. A representative image of spontaneous PCV thrombosis in proc−/− larvae, which was not observed in wild-type and heterozygous siblings. Scale bar 100 μm. (D) Quantification by a blinded observer demonstrated significant spontaneous thrombosis only in the homozygous proc mutants. P <.0001 for comparison of both wild-type and homozygous siblings, and between heterozygous and homozygous siblings (Fishers exact test). The fibrin deposition scale indicates the number of thrombi counted in each larva, binned into 4 groups. ns, not significant.
proc mutants develop spontaneous thrombosis with secondary coagulopathy. (A) Schematic of laser-mediated endothelial injury that was performed to evaluate for intravascular thrombus formation. The laser injures the endothelium of the PCV, and TTO was measured for up to 2 minutes. (B) Homozygous proc mutants demonstrated a partial defect in hemostasis manifested by a significant prolonged TTO compared to wild-type and heterozygous siblings. n = 26, 41, 26 in proc+/+, proc+/−, and proc−/−, respectively. Bars indicate median TTO. Values of P were calculated by Mann-Whitney U test. (C) The proc mutation was bred into the Tgfgb-eGFP transgenic background so that spontaneous thrombosis could be visually observed and scored through green fluorescence. A representative image of spontaneous PCV thrombosis in proc−/− larvae, which was not observed in wild-type and heterozygous siblings. Scale bar 100 μm. (D) Quantification by a blinded observer demonstrated significant spontaneous thrombosis only in the homozygous proc mutants. P <.0001 for comparison of both wild-type and homozygous siblings, and between heterozygous and homozygous siblings (Fishers exact test). The fibrin deposition scale indicates the number of thrombi counted in each larva, binned into 4 groups. ns, not significant.
proc1 encodes the majority of PC function in zebrafish
To explore whether the 2 copies of proc are both functional, we expressed proc1 and proc2 in proc−/− embryos under control of the ubi promoter (Figure 3A). Plasmids were injected into embryos from proc+/− incrosses, and those with red fluorescent lens selected for analysis (Figure 3A). Expression of proc1 rescued both the TTO after endothelial injury and spontaneous thrombosis, whereas proc2 showed minimal partial rescue (Figure 3B-C). There was no difference with combined expression of both proc1 and proc2. Based on those results, as well as the relative expression (Figure 1), we conclude that proc1 encodes the vast majority of PC function in zebrafish, with proc2 having minimal effects.
proc1 rescues the loss of PC function. (A) Plasmid constructed for proc rescue experiments. proc1 or proc2 were cloned separately under regulation of the ubi promoter. The mCherry gene driven by cryaa was used as an indicator of successful injection and expression of the plasmid construct. Outline of procedure for injection of proc expressing constructs and analysis. One-cell–stage embryos from proc+/− incrosses were injected with proc1, proc2, or a mixture of both. Injected embryos were raised to 3 dpf and screened for mCherry lens expression (scale bar, 250 μm), indicating successful injection and expression. mCherry-positive fish were selected for laser-mediated endothelial injury at 3 dpf or spontaneous thrombosis at 5 dpf in the Tgfgb-eGFP background. Scale bar, 250 μm. (B) Expression of proc1, but not proc2, is sufficient to rescue occlusion after endothelial injury in proc homozygous mutants at 3 dpf. (C) Overexpression of proc1 resolves spontaneous thrombosis in proc−/− fish at 5 dpf. The mock group was injected with vehicle only (P < .0001 in mock vs proc1; P < .0001 in mock vs proc1 + proc2; P < .01 in mock vs proc2; by χ2 testing). All studies were performed by an observer blinded to genotype and treatment. The fibrin deposition scale indicates the number of thrombi counted in each larva, binned into 4 groups. Ctl, control.
proc1 rescues the loss of PC function. (A) Plasmid constructed for proc rescue experiments. proc1 or proc2 were cloned separately under regulation of the ubi promoter. The mCherry gene driven by cryaa was used as an indicator of successful injection and expression of the plasmid construct. Outline of procedure for injection of proc expressing constructs and analysis. One-cell–stage embryos from proc+/− incrosses were injected with proc1, proc2, or a mixture of both. Injected embryos were raised to 3 dpf and screened for mCherry lens expression (scale bar, 250 μm), indicating successful injection and expression. mCherry-positive fish were selected for laser-mediated endothelial injury at 3 dpf or spontaneous thrombosis at 5 dpf in the Tgfgb-eGFP background. Scale bar, 250 μm. (B) Expression of proc1, but not proc2, is sufficient to rescue occlusion after endothelial injury in proc homozygous mutants at 3 dpf. (C) Overexpression of proc1 resolves spontaneous thrombosis in proc−/− fish at 5 dpf. The mock group was injected with vehicle only (P < .0001 in mock vs proc1; P < .0001 in mock vs proc1 + proc2; P < .01 in mock vs proc2; by χ2 testing). All studies were performed by an observer blinded to genotype and treatment. The fibrin deposition scale indicates the number of thrombi counted in each larva, binned into 4 groups. Ctl, control.
pros1 null larvae exhibit thrombosis
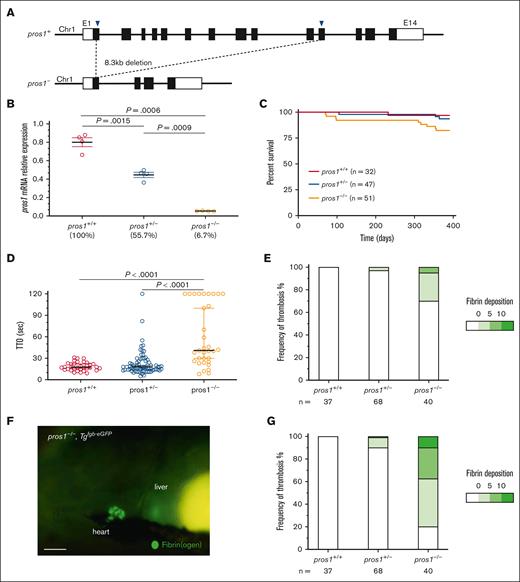
Because mammalian PS is a necessary cofactor for PC anticoagulant activity, we generated a pros1 knockout in zebrafish in a similar fashion as the proc knockout. Genome examination identified a conserved single copy39 (supplemental Figure 2). Two sgRNAs were designed to target exons 1 and 11, which resulted in a large deletion (Figure 4A). mRNA expression of pros1 in heterozygotes was reduced by 55% compared with wild-type siblings, and to 6.7% in homozygous mutants, suggestive of nonsense-mediated decay (Figure 4B). Loss of PS did not show any significant survival defect over the first year of life, although there was suggestive loss of homozygotes (Figure 4C). As with proc, pros1−/− larvae demonstrated a significant delay in occlusion after endothelial injury, which was also partially penetrant (Figure 4D). In the Tgfgb-eGFP background, thrombosis was observed in the PCV, but this was far less penetrant than proc, only in 30% of homozygous mutants (Figure 4E), with normal blood flow (supplemental Videos 4-6). Instead, substantial thrombosis was detected in the heart (Figure 4F-G).
Loss of pros1 partially phenocopies the proc mutation. (A) Two sgRNAs were selected to target exons 1 and 11 (indicated by arrowheads) and resulted in an 8.3-kb deletion. (B) qPCR of pros1 expression demonstrated 45% and 93% reduction in heterozygous and homozygous mutants, respectively; n = 4 in each group. Data were analyzed by unpaired 2-tailed t test and plotted as mean ± standard error of the mean. (C) Mortality tracking showed no survival defect in pros1-null fish. Log-rank (Mantel-Cox) testing revealed no significant difference between each group. (D) Laser-mediated endothelial injury revealed a significant coagulation defect in pros1 mutants (P < .0001 by Mann-Whitney U testing). Bars indicate median TTO. (E) pros1 mutants demonstrate significant spontaneous venous thrombosis (Fishers exact test P = .001 between pros1+/+ and pros1−/−) but to a far lesser degree than proc mutants. (F) Representative light microscopic image reveals spontaneous thrombosis in the heart of a pros1−/− mutant (scale bar, 100 μm), which was found in the majority of this group upon quantification (G). Fishers exact test P < .0001 between pros1+/+ and pros1−/−. All observations were performed by an observer blinded to genotype or condition. The fibrin deposition scale indicates the number of thrombi counted in each larva, binned into 4 groups.
Loss of pros1 partially phenocopies the proc mutation. (A) Two sgRNAs were selected to target exons 1 and 11 (indicated by arrowheads) and resulted in an 8.3-kb deletion. (B) qPCR of pros1 expression demonstrated 45% and 93% reduction in heterozygous and homozygous mutants, respectively; n = 4 in each group. Data were analyzed by unpaired 2-tailed t test and plotted as mean ± standard error of the mean. (C) Mortality tracking showed no survival defect in pros1-null fish. Log-rank (Mantel-Cox) testing revealed no significant difference between each group. (D) Laser-mediated endothelial injury revealed a significant coagulation defect in pros1 mutants (P < .0001 by Mann-Whitney U testing). Bars indicate median TTO. (E) pros1 mutants demonstrate significant spontaneous venous thrombosis (Fishers exact test P = .001 between pros1+/+ and pros1−/−) but to a far lesser degree than proc mutants. (F) Representative light microscopic image reveals spontaneous thrombosis in the heart of a pros1−/− mutant (scale bar, 100 μm), which was found in the majority of this group upon quantification (G). Fishers exact test P < .0001 between pros1+/+ and pros1−/−. All observations were performed by an observer blinded to genotype or condition. The fibrin deposition scale indicates the number of thrombi counted in each larva, binned into 4 groups.
PC and PS display additive and independent anticoagulant activities
We intercrossed the proc and pros1 mutants to determine whether PS activity is completely dependent on PC. Loss of pros1 in addition to proc did not improve or further deteriorate survival outcomes (Figure 5A). The prolonged TTO observed in the proc−/− venous system increased in a dose-dependent fashion with loss of pros1 alleles, and vice versa (Figure 5B). Complete loss of PC and PS resulted in the inability to form an occlusive thrombus after endothelial injury. These data demonstrate that each protein has independent anticoagulant activity in vivo, although the proc mutation is more severe than pros1. In contrast, evaluation of spontaneous venous thrombosis in proc and pros1 double mutants did not show a significant difference from proc−/− alone, confirming that the primary determinant is loss of proc (Figure 5C). However, among proc heterozygotes, there was a slight dose-dependent effect for loss of pros1 alleles. In the heart, individual loss of PC and PS resulted in similar levels of thrombosis but were additive in the double mutant (Figure 5D).
Additive effects of proc paralogs and pros1. For all experiments, proc and pros1 mutants were interbred to produce double heterozygotes, and then incrossed. (A) Mortality tracking showed no survival advantage or detriment in proc;pros1 double knockout fish in comparison with proc-null mutants. Log-rank (Mantel-Cox) testing revealed no significant differences between those groups. The results demonstrate nearly 100% proc+/−;pros1+/− survival, consistent with wild-type. (B) Laser-mediated endothelial injury was performed on the PCV at 3 dpf, followed by genotyping. Homozygous proc mutants demonstrated a higher TTO compared with pros1. Increasing TTO was dose dependent on the number of mutant alleles, and occlusion was completely absent in the double homozygotes. Statistical significance was determined by Mann-Whitney U testing. (C) In contrast to TTO, there was not much of an additive effect between proc and pros1 on spontaneous thrombosis. Larvae were scored for thrombosis along the PCV based on GFP intensity at 5 dpf, followed by genotyping. Fishers exact test P value <.0001 in the following groups: proc+/+;pros1+/+ vs proc−/−;pros1+/+, proc+/+;pros1+/− vs proc−/−;pros1+/−, proc+/+;pros1−/− vs proc−/−;pros1−/−, and proc+/−;pros1−/− vs proc−/−;pros1−/−. P value <.001 between proc+/−;pros1+/− vs proc−/−;pros1+/−. P value <.01 between proc+/−;pros1+/− vs proc+/−;pros1−/−. Other comparisons were not statistically significant. (D) Larvae were scored for intracardiac thrombosis based on GFP intensity at 5 dpf, followed by genotyping. Fishers exact test P value <.001 in the following groups: proc+/+;pros1+/+ vs proc−/−;pros1+/+, proc+/−;pros1+/+ vs proc−/−;pros1+/+ and proc+/+;pros1+/− vs proc−/−;pros1+/−. P value <.05 in the following group: proc+/+;pros1−/− vs proc−/−;pros1−/−, and proc−/−;pros+/+ vs proc−/−;pros1−/−. Other comparisons were not statistically significant. The fibrin deposition scale indicates the number of thrombi counted in each larva, binned into 4 groups. ns, not significant.
Additive effects of proc paralogs and pros1. For all experiments, proc and pros1 mutants were interbred to produce double heterozygotes, and then incrossed. (A) Mortality tracking showed no survival advantage or detriment in proc;pros1 double knockout fish in comparison with proc-null mutants. Log-rank (Mantel-Cox) testing revealed no significant differences between those groups. The results demonstrate nearly 100% proc+/−;pros1+/− survival, consistent with wild-type. (B) Laser-mediated endothelial injury was performed on the PCV at 3 dpf, followed by genotyping. Homozygous proc mutants demonstrated a higher TTO compared with pros1. Increasing TTO was dose dependent on the number of mutant alleles, and occlusion was completely absent in the double homozygotes. Statistical significance was determined by Mann-Whitney U testing. (C) In contrast to TTO, there was not much of an additive effect between proc and pros1 on spontaneous thrombosis. Larvae were scored for thrombosis along the PCV based on GFP intensity at 5 dpf, followed by genotyping. Fishers exact test P value <.0001 in the following groups: proc+/+;pros1+/+ vs proc−/−;pros1+/+, proc+/+;pros1+/− vs proc−/−;pros1+/−, proc+/+;pros1−/− vs proc−/−;pros1−/−, and proc+/−;pros1−/− vs proc−/−;pros1−/−. P value <.001 between proc+/−;pros1+/− vs proc−/−;pros1+/−. P value <.01 between proc+/−;pros1+/− vs proc+/−;pros1−/−. Other comparisons were not statistically significant. (D) Larvae were scored for intracardiac thrombosis based on GFP intensity at 5 dpf, followed by genotyping. Fishers exact test P value <.001 in the following groups: proc+/+;pros1+/+ vs proc−/−;pros1+/+, proc+/−;pros1+/+ vs proc−/−;pros1+/+ and proc+/+;pros1+/− vs proc−/−;pros1+/−. P value <.05 in the following group: proc+/+;pros1−/− vs proc−/−;pros1−/−, and proc−/−;pros+/+ vs proc−/−;pros1−/−. Other comparisons were not statistically significant. The fibrin deposition scale indicates the number of thrombi counted in each larva, binned into 4 groups. ns, not significant.
PC modulates inflammatory responses in zebrafish
It is well known that PC has functions outside of the coagulation cascade, such as regulating inflammation.40 We examined whether these functions are conserved in zebrafish. Tail transection injury was used to induce local inflammation (Figure 6A). Among many inflammatory cytokines, interleukin-1β is known as a key mediator of the inflammatory response.41,42 Relatively higher il1b expression was observed at 2 hours after tail injury in both wild-type and proc-mutant larvae, and nearly returned to baseline 6 hours after injury (Figure 6B). Although proc mutants had a lower baseline level, the upregulation of il1b was significantly higher than in wild-type siblings, suggesting an inflammatory modulating role. Additional inflammatory markers evaluated through RNA sequencing (RNA-seq) found only cxcl8b.1 altered, with decreased levels (see below; supplemental Figure 3). Next, we evaluated nitric oxide responses. We observed a baseline level of nitric oxide in wild-type uninjured larvae, and a clear increase upon injury (Figure 6C-D). In proc-mutant siblings, the production of nitric oxide was low in uninjured larvae and failed to respond to tail injury.
PC plays a role in inflammation and neutrophil migration after tail transection. (A) Tail transection injury distal to the notochord and vasculature was performed to induce injury-triggered inflammation at 3 dpf. (B) qPCR of total cDNA before and after transection revealed upregulation of il1b mRNA in both wild-type and proc−/− mutants but was more pronounced for the latter; n = 6 in each group. ∗P < .001 by t test (C) DAF-FM diacetate staining revealed that nitric oxide accumulation after injury was absent in proc−/− fish. Scale bar, 500 μm. All studies were performed by an observer blinded to genotype and treatment. (D) Fluorescence quantification using CTCF demonstrated a significant decrease of nitric oxide in injured proc−/− compared to injured wild-type (t test P < .05, n = 7-8 for each group). (E) Sudan Black staining revealed neutrophils in caudal hematopoietic tissue (CHT), boxed area (i), as well as at the tail transection site, boxed areas (ii-iii). Representative images demonstrate less neutrophils at the tail transection site in proc−/− in panel Eiii in comparison with wild-type siblings in panel Eii. Scale bar, 100 μm. (F) The unchanged neutrophil CHT counts reveal that proc mutation does not affect the production of neutrophils prior to injury (P = .09). Significant reduction of neutrophil numbers in CHT were observed 1 hour after tail transection in wild-type and heterozygous larvae, suggesting neutrophil migration in response to injury. No such change was detected in the proc homozygous group. (G) The absence of PC results in a reduction in neutrophil recruitment. (H) Loss of PS does not affect neutrophil recruitment. (I) Prothrombin (f2) deficiency does not affect neutrophil migration upon tail transection. P values were calculated by unpaired t test. All studies were performed by an observer blinded to genotype and treatment. DAF-FM, diaminofluorescein-FM; CTCF, corrected total cell fluorescence; hpi, hour post injury; ns, not significant.
PC plays a role in inflammation and neutrophil migration after tail transection. (A) Tail transection injury distal to the notochord and vasculature was performed to induce injury-triggered inflammation at 3 dpf. (B) qPCR of total cDNA before and after transection revealed upregulation of il1b mRNA in both wild-type and proc−/− mutants but was more pronounced for the latter; n = 6 in each group. ∗P < .001 by t test (C) DAF-FM diacetate staining revealed that nitric oxide accumulation after injury was absent in proc−/− fish. Scale bar, 500 μm. All studies were performed by an observer blinded to genotype and treatment. (D) Fluorescence quantification using CTCF demonstrated a significant decrease of nitric oxide in injured proc−/− compared to injured wild-type (t test P < .05, n = 7-8 for each group). (E) Sudan Black staining revealed neutrophils in caudal hematopoietic tissue (CHT), boxed area (i), as well as at the tail transection site, boxed areas (ii-iii). Representative images demonstrate less neutrophils at the tail transection site in proc−/− in panel Eiii in comparison with wild-type siblings in panel Eii. Scale bar, 100 μm. (F) The unchanged neutrophil CHT counts reveal that proc mutation does not affect the production of neutrophils prior to injury (P = .09). Significant reduction of neutrophil numbers in CHT were observed 1 hour after tail transection in wild-type and heterozygous larvae, suggesting neutrophil migration in response to injury. No such change was detected in the proc homozygous group. (G) The absence of PC results in a reduction in neutrophil recruitment. (H) Loss of PS does not affect neutrophil recruitment. (I) Prothrombin (f2) deficiency does not affect neutrophil migration upon tail transection. P values were calculated by unpaired t test. All studies were performed by an observer blinded to genotype and treatment. DAF-FM, diaminofluorescein-FM; CTCF, corrected total cell fluorescence; hpi, hour post injury; ns, not significant.
Because of the critical role of neutrophils in the early stages of inflammation, we examined their activity after tail transection. Chemical staining was used to label neutrophils in both the caudal hematopoietic tissue and at the tail injury site (Figure 6E). Compared with siblings, notably less neutrophils were recruited to the tail in proc-mutant fish (Figure 6G). However, no significant deficiency in neutrophils was observed in the proc-mutant caudal hematopoietic tissue, indicating that the phenotype was not due to decreased production (Figure 6F). There was a slight but significant reduction of neutrophils in wild-type and proc+/− larvae after tail injury, but not homozygotes, presumably because of the reduced number mobilized and recruited to the tail injury site. This same pattern of altered recruitment was not observed in pros1 or prothrombin (f2) mutants (Figure 6H-I). These data support an impaired response mechanism in the absence of proc, which is independent of PS and prothrombin, and thus likely independent of the coagulation cascade.
RNA-seq reveals distinctive gene expression patterns in proc−/− mutants
We performed tail transection at 3 dpf and collected mRNA from wild-type and mutant larvae before and after injury for transcriptomic analysis. Differential expression and principal component analysis revealed distinct patterns of expression under all 4 conditions (Figure 7A-B, wild-type vs mutant, with and without injury). The most dramatic difference in the number of genes upregulated and downregulated was between wild-type and proc−/− samples in both the uninjured and tail transection groups (Figure 7C-D). After filtering the data, we proceeded to functionally validate 2 top hits, adgrf7 (ENSDARG00000086445, upregulated) and si:ch1073-416d2.3 (ENSDARG00000036968, downregulated; Figure 7E-F; supplemental Figure 4) from the wild-type vs mutant comparison. adgrf7 is a predicted adhesion G protein–coupled receptor, and si:ch1073-416d2.3 is predicted to be 1 of 2 zebrafish orthologs to protein Z–dependent protease inhibitor (ZPI; serpina10b). We used CRISPR-mediated genome editing to perform transient knockdown of adgrf7 and serpina10b, followed by laser-mediated endothelial injury (Figure 7E-F). Knockdown of adgrf7 and serpina10b in a wild-type background demonstrated slight, but significantly prolonged TTOs. Reduction of adgrf7 in the context of PC deficiency revealed an additive effect on TTO, whereas reduction of serpina10b showed no effect. (Figure 7E-F).
RNA-seq reveals distinct changes in gene expression in the proc mutant. (A) Cluster analysis of gene expression in wild-type and proc−/− mutants at baseline and upon tail transection injury at 3 dpf. Color scale indicates log10 values. (B) Principal component analysis (PCA) 3-dimensional plot. Dots indicate biological replicates of each condition. (C) Venn diagram of the number of unique genes expressed in each condition. (D) Number of genes upregulated or downregulated between wild-type and proc−/− at baseline and after injury. (E-F) TTO assays in adgrf7 and serpina10b knockdowns. (E) sgRNAs targeting adgrf7 (arrowheads) were injected in wild-type and proc mutants, followed by laser-mediated venous endothelial injury; n = 34, 27, 35, and 33. (F) The delayed TTO in the serpina10b knockdown was only observed in wild-type larvae; n = 28, 50, 38, and 27. P values were calculated by Mann-Whitney U testing. All studies were performed by an observer blinded to genotype and treatment.
RNA-seq reveals distinct changes in gene expression in the proc mutant. (A) Cluster analysis of gene expression in wild-type and proc−/− mutants at baseline and upon tail transection injury at 3 dpf. Color scale indicates log10 values. (B) Principal component analysis (PCA) 3-dimensional plot. Dots indicate biological replicates of each condition. (C) Venn diagram of the number of unique genes expressed in each condition. (D) Number of genes upregulated or downregulated between wild-type and proc−/− at baseline and after injury. (E-F) TTO assays in adgrf7 and serpina10b knockdowns. (E) sgRNAs targeting adgrf7 (arrowheads) were injected in wild-type and proc mutants, followed by laser-mediated venous endothelial injury; n = 34, 27, 35, and 33. (F) The delayed TTO in the serpina10b knockdown was only observed in wild-type larvae; n = 28, 50, 38, and 27. P values were calculated by Mann-Whitney U testing. All studies were performed by an observer blinded to genotype and treatment.
Discussion
PC and PS are well known to form a complex that exerts an anticoagulant effect through inactivation of activated FV and FVIII. Human heterozygous null mutations of each result in increased susceptibility to thrombosis, usually manifesting in the third or fourth decade, whereas homozygous loss results in lethal pre/neonatal purpura fulminans.1 Mouse in vivo studies of anticoagulant (and many procoagulant) protein deficiencies have been complicated by in utero or neonatal lethality.13,14,43,44 We have found that many of these knockouts in zebrafish survive through the embryonic/larval period and into early adulthood, which has allowed greater flexibility in studying loss of coagulation factor function in vivo.22 An additional level of complexity was found in the zebrafish proc locus, which is duplicated in cis with 93% nucleotide and amino acid identity. Ohnologs are typically duplicated in syntenic blocks in trans.35 Our examination of 2 ohnologs from the zebrafish coagulation cascade, f3a/b and f9a/b reveal amino acid identities/similarities of 45/64% and 50/67%, respectively, but neither have any significant nucleotide similarity.45 Therefore, we conclude that the proc locus duplication is a much more recent event. This is supported by examination of other teleosts, including fugu, tetraodon, medaka, and stickleback genomic sequences, which suggest that this is a zebrafish-specific duplication. proc1 and proc2 are duplicated in tandem, with nearly identical nucleotide sequences (93%). This level of identity also suggests that the duplication occurred much more recently, and thus is not a classic ohnolog.36,37 Additionally, our data show that proc2 is expressed at much lower levels than proc1 and is not functional when overexpressed in a rescue experiment. Therefore, we hypothesize that proc2 is an expressed pseudogene without function.
Here, we used CRISPR-mediated genome editing to produce a large deletion that eliminated nearly the entirety of both proc loci. This strategy prevented the possibility of residual functions of the targeted gene due to alternative splicing, which we have previously observed with a single deletion in the prothrombin gene.23 It also ensures that there is no transcriptional adaptation that results in genetic compensation.46 Given the proximity of proc1 and proc2 and extremely high nucleotide sequence identify, we only required 2 sgRNAs that each had its target sequence in both genes. Our original strategy was intended to produce single deletions of each locus, but the sgRNAs were so effective that we were only able to recover the complete deletion. However, we were able to perform rescue experiments to determine single-copy effects.
proc−/− and/or pros1−/− larvae were found to have surprising findings of significantly impaired thrombus formation after endothelial injury but also spontaneous venous thrombosis. This seemingly contradictory result is consistent with our previous study of at3 mutation in zebrafish,18 in which the knockout developed a complete loss of occlusion after endothelial injury, and highly penetrant spontaneous thrombosis. We showed that this was due to a consumptive coagulopathy consistent with DIC. However, the results in this study are relatively mild in comparison with at3 mutants. The majority of at3 mutant fish died by 6 months of age, whereas 30% of proc−/− and nearly 80% of pros1−/− mutants survived to 1 year of age. The TTO and spontaneous thrombosis phenotypes were also less severe when compared with at3 homozygotes. The relative severity of these proc and pros1 phenotypes compared with at3 is consistent with human patients as well as mouse knockouts.1 However, it is curious that pros1 mutants presented with spontaneous thrombosis primarily affecting the heart rather than the venous circulation and had minimal adult lethality. This could be because of PS antithrombotic activity in the absence of PC,47 and/or interactions with tissue factor pathway inhibitor, loss of which is associated with intracardiac thrombosis in mice.48 Limited human studies suggest that deficiency of PC and PS results in differential phenotypes, although in clinical practice they are treated similarly. A case report demonstrated PS deficiency associated with infective endocarditis.49 In a moderately sized study of patients with Budd-Chriari syndrome, PC but not PS deficiency exhibited a higher prevalence.50 The accessibility and survival of proc and pros1 zebrafish larvae is a major advantage over mouse mutants, which are embryonic/neonatal lethal. Moreover, the fact that adults survive at relatively high levels in the first year of life will enable prototypical zebrafish studies, for example small molecule and genetic mutagenesis experiments51 on homozygous mutant embryos and larvae. Outcomes could determine whether there are key differences that should be investigated further in patients.
In addition to regulating coagulation, PC is known to be an important regulator of inflammation.40 We observed altered expression of il1b mRNA after tail transection, suggesting zebrafish PC modulates acute inflammation. Given the pivotal role of il1b,41,42 this may be a compensatory response to diminished neutrophil function. In the absence of PC, there was diminished response of nitric oxide to tail injury. Nitric oxide is secreted by neutrophils and macrophages and is considered anti-inflammatory in physiological conditions, but proinflammatory when being overproduced.52,53 This result was correlated with our finding that fewer neutrophils were recruited to the site of injury in proc mutants, which was independent of other coagulation factors, including PS and thrombin. Taken together, these data confirm that the involvement of human PC in inflammation is conserved in zebrafish. We probed this further with RNA-seq, comparing tail transection in the context of proc wild-type and mutant larvae. Surprisingly, principal component analysis indicated that, relatively speaking, gene expression did not change much with injury in the wild-type fish. Instead, the major differences were between wild-type and proc mutants, as well as within the proc−/− genotype after injury. Activated PC was initially approved for treatment of sepsis in intensive care unit patients but abandoned because the initial findings were not replicated in subsequent trials.54 Further study in this model might provide insight into why it failed and allow optimization of recombinant PC for improved therapeutic potential.
Our results demonstrate that PC can still provide some anticoagulant function in vivo, although less effectively, in the absence of PS, suggesting that zebrafish PS is an anticoagulant cofactor. Deficiency of PS results in spontaneous thrombosis but in the heart rather than the venous circulation, and it did not have additive effects on venous thrombosis in the proc−/− background. However, its loss also causes a consumptive coagulopathy on its own and displays an additive effect in double pros1 and proc mutants. These data are consistent with some literature that suggests PS might have anticoagulant functions independent of PC,47,50 but further study is needed to determine the PC/PS relationship in zebrafish. Other remaining questions include the roles of thrombomodulin; protease-activated receptors; and consequences of apparent absence of factor XI, contact pathway, and endothelial PC receptor.39
Through transcriptomic analysis, we have found 2 new potential genetic interactions with proc. One appears to be an ortholog of ZPI (SERPINA10), which complexes with protein Z to inhibit coagulation factors IX, X, and XI.55-57,serpina10 is duplicated in the zebrafish genome (ohnologs serpina10a and b). adgrf7 codes for a putative G-protein–coupled receptor and homology analysis suggests that human ADGRF5 is the most likely ortholog. Publicly available zebrafish transcriptomic data sets indicate that these are both expressed at low levels, with no clear tissue specificity. Knock down of both genes resulted in a slight increase in TTO in wild-type larvae, which was shown for proc/pros1 and previously18 could be an indication of either anticoagulant or procoagulant19-21,23 effects. ZPI inhibits factor X, as does AT3, so knockdown could cause a mild consumptive coagulopathy. The knockdown data in the proc−/− background suggest additive effects for adgrf7, implying that it has an anticoagulant role. We speculate that Adgrf7 exerts its effects at the vascular endothelium. ADGRF5 has not previously been associated with coagulation but has been shown to modulate vascular growth in diabetic retinopathy.58 It also facilitates cancer metastasis, through enabling cell migration and invasiveness.59,60 Further studies are needed to examine whether Adgrf7 or ADGRF5 have direct or indirect interactions with PC and/or PS.
In this study, we have demonstrated that proc and pros1 zebrafish mutants present with highly penetrant but different forms of thrombosis. These data indicate a high degree of conservation of the anticoagulant functions of PC and PS and suggest that there could be clinical differences unrecognized in patients. The lack of early lethality may be because of several factors: the artificial laboratory environment, low-pressure vasculature, or species-specific divergence in coagulation. The latter is most intriguing because discovery of novel protective factors could lead to new therapeutics. The robust survival of these mutants, even in the context of highly penetrant thrombotic disease that is lethal in mammals, will facilitate further studies of these disorders not previously possible in an in vivo model.
Acknowledgments
The authors thank the University of Michigan Advanced Genomics Core for services.
This work was supported by National Institutes of Health (grants R35 HL150784 [J.A.S.], T32 HL007853 [J.G.D.], T32 GM007863, and T32 HL125242 [S.J.G.]) and an American Heart Association Predoctoral Fellowship Award (S.J.G.).
Q.Y.Z. was supported by a Hemostasis and Thrombosis Research Society Student Research Award. J.A.S. is the Henry and Mala Dorfman Family professor of pediatric hematology/oncology.
Authorship
Contribution: C.-J.K. and X.Y. designed and performed research, analyzed data, and wrote the manuscript; Q.Y.Z., S.J.G., and J.G.D. designed and performed research and analyzed data; A.C.F. performed research; J.A.S. designed, performed, and supervised research, analyzed data, and wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: J.A.S. has been a consultant for Sanofi, Takeda, Pfizer, Genentech, CSL Behring, Medexus, and HEMA Biologics. The remaining authors declare no competing financial interests.
Correspondence: Jordan A. Shavit, Department of Pediatrics, University of Michigan, Room 8301, Medical Science Research Building III, 1150 West Medical Center Dr, Ann Arbor, MI 48109; email: jshavit@umich.edu.
References
Author notes
C.-J.K. and X.Y. contributed equally to this study.
RNA-sequencing raw data were deposited in the Sequence Read Archive at the National Center for Biotechnology Information (accession number PRJNA1077954).
The full-text version of this article contains a data supplement.