Key Points
Consensus guidelines aim to harmonize key components of conduct and reporting of AML CAR T-cell clinical trials.
The objective is to allow meaningful intertrial comparisons to inform the next generation of clinical studies.
Visual Abstract
Early clinical experience with the use of chimeric antigen receptor (CAR) T-cell therapies for patients with acute myeloid leukemia (AML) has been beset by high rates of toxicities and low rates of response. We convened an international workshop with the goal of bringing investigators in the field of AML-directed CAR T-cell therapy together to facilitate discussion of challenges and to brainstorm potential solutions. Based on discussions at the workshop, it was evident that (1) treating and targeting AML with CAR T cells is associated with unique clinical challenges, and (2) variability in clinical trial design, definitions of toxicities, correlative data collection, and reporting methods hinders the field’s ability to compare study outcomes and to develop best practices. Further, details of fundamental CAR T-cell attributes and key correlates of efficacy and toxicity were not uniformly reported in published studies, limiting understanding of barriers to success. These observations led to a concerted team effort in which experts in basic/translational science and clinical investigation from pediatric and adult centers worked together to streamline key attributes of clinical trial design and reporting. Consensus criteria were discussed and agreed upon, leading to the creation of a white paper. These guidelines aim to bolster the overall quality of AML-directed CAR T-cell research, allow for comparisons across trials, and inform the next phase of development of AML-directed CAR T-cell therapies that we hope will improve patient outcomes.
Introduction
The adoptive transfer of chimeric antigen receptor (CAR)T cells has emerged as a promising treatment option for patients with hematologic malignancies. Their potent activity has resulted in US Food and Drug Administration/European Medicines Agency approval of CAR T-cell products directed against relapsed/refractory B-cell acute lymphoblastic leukemia, mature B-cell lymphoma, and multiple myeloma.1-12
In contrast, clinical experience with CAR T cells for patients with acute myeloid leukemia (AML) has been beset by low response rates.13,14 Several challenges have impacted the efficacy of CAR T-cell therapy for patients with AML.14-20 One major issue has been the identification of optimal antigen targets, namely cell surface antigens that are highly expressed on all malignant cells, but not on healthy tissues. CD33, CD123, or CLL-1 (CLEC12A) are the most common antigens targeted to date with antibody-based and cellular immunotherapies given their relatively broad expression across pediatric and adult AML subtypes. Because these antigens are also present on normal myeloid cells, there are concerns for potential on-target/off-tumor hematologic toxicity.21-27 Therefore, other targets have been or are actively being explored, including CD38, CD44v6, CD70, CD117 (c-KIT), CD276 (B7-H3), CD327 (Siglec-6), FLT3, FOLR1, FRβ, GRP78, LILRB3, LILRB4, and TIM3.28-43 Further, most phase 1 clinical trials to date have accrued only small numbers of patients, have closed early because of limited clinical efficacy, and/or have not been published.44-47 Collectively, the underlying biologic heterogeneity of AML in children and adults, coupled with limited data regarding CAR T-cell product attributes and possible deleterious disease-specific factors, have hampered robust conclusions and impeded progress in development of successful cellular immunotherapy approaches in this field.
Methods
Given the limited published data, we convened 2 AML CAR T-cell workshops attended by international investigators involved in the development and conduct of trials for AML-directed CAR T-cell therapy.
Purpose and scope
The goals of the workshops were to (1) foster national and international collaborations across the pediatric-to-adult age spectrum, (2) review and discuss clinical trial experience, (3) identify unique challenges and potential solutions, and (4) harmonize standards and develop consensus on the conduct, design, and reporting of data in future AML CAR T-cell therapy clinical trials.
Proceedings of the workshops
Before the first in-person meeting, all participants completed an online survey to submit feedback regarding the challenges and barriers to successful implementation of CAR T-cell therapy for AML (supplemental Methods; supplemental Figure 1).
Participating investigators presented data from recently completed, actively recruiting, and planned clinical trials of CAR T cells targeting CD123, CD33, CLL-1/CD371, NKG2D ligands, B7H3, CD70, FOLR1, or CD38 for adults and/or children with relapsed/refractory AML (supplemental Table 1). We found substantial differences in trial design, definition of dose-limiting toxicity (DLT), correlative data collection, and reporting content. The limited number of trials, small patient cohorts, and absence of a strong efficacy signal made intertrial comparisons challenging, and it was difficult to draw conclusions regarding barriers to success.
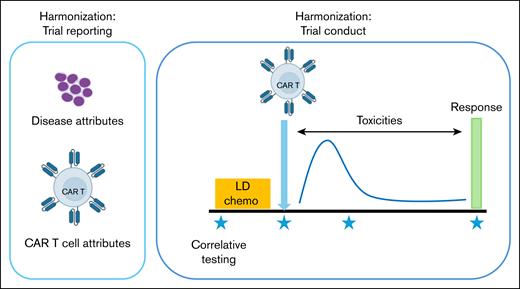
Subgroups comprising basic/translational scientists and clinical investigators from multiple centers were created to streamline and harmonize attributes of clinical trial design and reporting, namely (1) AML disease attributes, (2) CAR T-cell attributes (3) toxicity monitoring, (4) determination of response criteria, (5) lymphodepletion/bridging chemotherapy considerations, and (6) correlative biology studies (Figure 1).
Outline of key recommendations for conduct and reporting of AML CAR clinical trials.
Outline of key recommendations for conduct and reporting of AML CAR clinical trials.
Consensus criteria from individual working groups were collectively reviewed and discussed by all members at multiple follow-up meetings and agreed upon by the majority, leading to the creation of this white paper that was approved by all participants. Herein our collaborative group, the International Multi-center Partnership for AML CAR-T Cell Therapy (IMPACT), provides consensus guidance for the conduct and reporting of CAR T-cell therapy for AML.
Results and discussion
Harmonization of clinical trials
Lacking evidence-based criteria, we used best-practice guidelines and expert opinions of IMPACT consortium members to develop standards and a tiered approach toward harmonization. Tier 1 recommendations should be followed in all trials, tier 2 suggestions should be included where possible, and tier 3 are defined as optional (Table 1).
Definitions of tier system used
| Tier 1 . | Tier 2 . | Tier 3 . |
|---|---|---|
| Recommendation Should be included as part of AML CAR T-cell clinical trial | Suggestion To include whenever possible | Optional Not required, but could include if available |
| Tier 1 . | Tier 2 . | Tier 3 . |
|---|---|---|
| Recommendation Should be included as part of AML CAR T-cell clinical trial | Suggestion To include whenever possible | Optional Not required, but could include if available |
All tiers are based on best practice/expert opinion.
A. Harmonizing the reporting of clinical trials
Disease attributes
Considerations
Given that AML biology and disease attributes in patients receiving CAR T-cell therapies are heterogeneous, detailed descriptions of disease- and patient-specific attributes would be informative. Inclusion of these data in clinical trial reports will enable interpretation and comparison of results across trials.
Guidance for reporting of disease attributes
General AML- and patient-specific attributes
In the absence of established disease characteristics and prognostic factors for immunotherapy response, we recommend using prognostic features relevant to “conventional” AML therapies as in the most recent International Consensus Classification, World Health Organization, or European LeukemiaNet (ELN) classifications (tier 1).48 This categorization ensures allocation of patients enrolled in AML CAR T-cell clinical trials to defined disease and genetic risk categories48,49 (Table 2; supplemental Table 2). In addition, demographics, including age, sex, and prior treatment details should be provided and tabulated for each patient (tier 1) (Table 2; supplemental Table 2).
Recommendation for reporting of disease attributes
| Tier . | AML and patient-specific attributes . |
|---|---|
| Tier 1 | Patient-specific attributes: age, sex, number of lines of prior therapy, prior HSCT(s), bridging therapy received Disease-specific attributes∗: AML subtype, cytogenetic and molecular abnormalities at time of CAR T-cell therapy |
| Tier 2 | Percentage of blasts expressing target antigen |
| Tier 3 | MFI of target antigen or number of molecules/cells of target antigen |
| Tier . | AML and patient-specific attributes . |
|---|---|
| Tier 1 | Patient-specific attributes: age, sex, number of lines of prior therapy, prior HSCT(s), bridging therapy received Disease-specific attributes∗: AML subtype, cytogenetic and molecular abnormalities at time of CAR T-cell therapy |
| Tier 2 | Percentage of blasts expressing target antigen |
| Tier 3 | MFI of target antigen or number of molecules/cells of target antigen |
MFI, mean fluorescent intensity.
Based on internationally accepted criteria.
Immunotherapy-related attributes
CAR T-cell clinical trials require demonstration of the presence of the targeted antigen on malignant cells. Given that heterogeneous expression of the target antigen may result in rapid antigen loss or antigen-dim relapse (as observed in B-cell–targeted therapies),50 the percentage of AML blasts expressing the target antigen should be reported for each patient using clinically validated assays where available (tier 2). Because the quantitative expression level of the target antigen may correlate with CAR T-cell therapeutic efficacy, the number of molecules (or at least a surrogate measure such as the relative median fluorescence intensity) should be tabulated if available (tier 3). These assays should be performed at baseline (study entry) and at each protocol-defined disease assessment time point.
Overall, we recommend providing disease-specific and patient-level attributes summarized in Table 2 and supplemental Table 2 to measure and report in AML CAR T-cell trials.
CAR T-cell attributes
Considerations
Comprehensive and consistent reporting of CAR T-cell characteristics and manufacturing methodology will enable accurate comparisons across trials and analyses of the mechanisms of treatment success and failure. These data could inform preclinical development to advance therapeutic design.
A review of data from currently published AML CAR T-cell clinical trials is summarized in supplemental Table 3. There is considerable heterogeneity in the data provided in these trials, highlighting the need for harmonization to achieve meaningful comparisons. Given the limited data reported on AML CAR T-cell clinical trials thus far, the guidance below (summarized in Table 3) is primarily extrapolated from non-AML CAR clinical trials. Consideration of available preclinical data describing relevant AML CAR T-cell characteristics is included.
Recommendation for reporting of CAR T-cell attributes
| Tier . | CAR T-cell attributes . |
|---|---|
| Tier 1 | Cell source: autologous or allogeneic CAR design: vector used (eg, lentivirus/retrovirus, nonviral), antigen recognition domain (eg, scFv, murine/human), hinge/transmembrane domain, signaling domain, schematic representation of CAR construct used Manufacturing methods: PBMC or immune cell subset Product characteristics: fresh or frozen Cell dose: definition (flat dose, per kg or BSA), dosed on total cells, or CAR-positive cells |
| Tier 2 | Manufacturing methods: activation method, culture medium, cytokine/other supplementation, duration of culture, manufacturing technology, genome edits Product characteristics: transduction efficiency, vector copy number |
| Tier 3 | CAR design: additional genetic modification, construct sequence to be included in the manuscript or cross-referenced (eg, prior publication, patent filing) Manufacturing methods: activation method, cell selection, cytokines used, culture methods, supplementation with pharmacologic agents Product characteristics: immunophenotype (extended), CyToF, scRNA-seq |
| Tier . | CAR T-cell attributes . |
|---|---|
| Tier 1 | Cell source: autologous or allogeneic CAR design: vector used (eg, lentivirus/retrovirus, nonviral), antigen recognition domain (eg, scFv, murine/human), hinge/transmembrane domain, signaling domain, schematic representation of CAR construct used Manufacturing methods: PBMC or immune cell subset Product characteristics: fresh or frozen Cell dose: definition (flat dose, per kg or BSA), dosed on total cells, or CAR-positive cells |
| Tier 2 | Manufacturing methods: activation method, culture medium, cytokine/other supplementation, duration of culture, manufacturing technology, genome edits Product characteristics: transduction efficiency, vector copy number |
| Tier 3 | CAR design: additional genetic modification, construct sequence to be included in the manuscript or cross-referenced (eg, prior publication, patent filing) Manufacturing methods: activation method, cell selection, cytokines used, culture methods, supplementation with pharmacologic agents Product characteristics: immunophenotype (extended), CyToF, scRNA-seq |
BSA, body surface area; CyToF, cytometry by time of flight; PBMC, peripheral blood mononuclear cell; scFv, single-chain variable fragment; scRNA-seq, single-cell RNA sequencing.
Guidance for reporting CAR T-cell characteristics and product manufacturing details
Cell source
Characteristics of the starting cell source, such as CD4+/CD8+ ratio, T-cell differentiation, T-cell exhaustion or fitness, among other factors, may impact the functionality of CAR T-cell products.51,52 Description of the cell source must include if the product is autologous or allogeneic (tier 1). If allogeneic products are used, it is important to highlight if these are donor-derived from a prior transplant donor (eg, an HLA-identical relative, partially HLA matched donor, etc.) or derived from a third-party “universal donor” source. The starting material for CAR T-cell manufacturing should be specified (eg, peripheral blood mononuclear cells from whole blood/leukapheresis) and whether the starting material was enriched for T cells (eg, CD4+/CD8+ or CD3+ selection) (tier 1).53,54
CAR construct/vector design
Specific characteristics of CAR constructs and vectors influence CAR T-cell functionality, persistence, and patient outcomes. Beyond data describing the targeted antigen and CAR structure (antigen recognition domain, linker, hinge/transmembrane domain, signaling domain[s]), the type of vector used for manufacture should be reported (tier 1). We suggest providing an annotated schema of the construct as minimum information along with a citation of its preclinical development if available (tier 1). Additional details including further genetic modifications to the cells (eg, safety switch, cytokine transgene) can be reported where compatible with intellectual property considerations (tier 3). When possible, the construct sequence should be included in the manuscript or cross-referenced (eg, prior publication, patent filing) (tier 3).
Manufacturing methods
If possible and compatible with intellectual property considerations, details of manufacture that can influence clinical outcomes should be provided (tier 2). These include activation method (beads, antibodies, etc),55 culture medium,56 cytokine or other supplementation (eg, kinase inhibitors dasatinib or ibrutinib),57-59 approximate duration of culture,60 reagent sources, and the manufacturing technology used (eg, G-Rex, Prodigy).61 Any genome edits, if present, should be described alongside with the methodology and editing efficiency in final cell products.
Product characteristics
Details of the final infusion product characteristics must be provided, namely whether products were infused fresh or thawed from frozen storage62,63 (tier 1), the median and range of transduction efficiency, and the vector copy number range of the product64 (tier 2). Additional details could be helpful, including the phenotype of infused product as defined by flow cytometry (CD4/CD8 ratio, immunophenotype, and T-cell differentiation status)65,66 (tier 2) and, if available, cytometry by time of flight and/or single-cell RNA sequencing51,67 (tier 3). If technical or financial constraints preclude performance of these assays, we recommend whenever possible to seriously consider storage of samples for future centralized analyses.
Cell dose
We recommend specific reporting of infused cell doses, including total and CAR-positive cells infused (tier 1). Additional details should include whether flat, weight-based, or body surface area–based dosing was used and whether the dose was calculated per CAR-positive or total number of cells infused.68
Most tier 1 and 2 recommendations listed above are requirements from regulatory agencies that are a component of the Investigational New Drug (IND) approval for each trial and should be available at all sites. Providing these in a systematic manner in all AML CAR T-cell clinical trial manuscripts will improve understanding of the current challenges of immune effector therapies for AML.
B. Harmonizing the conduct of clinical trials
Toxicity criteria
Considerations
CAR T cell–associated toxicities can lead to morbidity and mortality.69,70 Consensus guidelines have been developed for the definition, monitoring, and management of the most common toxicities after CD19- and BCMA-directed CAR T-cell therapy, namely cytokine release syndrome (CRS),71 immune effector cell–associated neurotoxicity syndrome (ICANS),71 immune effector cell hemophagocytic lymphohistiocytosis-like syndrome,72 and immune effector cell–associated hematotoxicity.73 However, in early-phase studies of AML-directed CAR T-cell therapies, specific toxicities may need to be further defined and described. For instance, although CRS and ICANS definitions using American Society for Transplantation and Cellular Therapy (ASTCT) criteria should be adhered to, cytopenias may result not only from inflammatory cytokines, but also from on-target/off-tumor toxicity, particularly in the case of myeloid-lineage antigen targets such as CD123 or CD33. Hence, toxicity criteria for AML CAR T-cell studies that relate to cytopenias should account for the resultant correlation between on-target efficacy and off-target toxicity when the targeted antigen is also expressed on hematopoietic progenitor cells.
We reviewed all trials that were discussed at the workshop for protocol-specified toxicity criteria because these had been developed on the basis of individual discussions between investigators and regulatory authorities. We noted that despite the targeting of similar AML-associated antigens, there was significant heterogeneity in the definitions of DLTs across trials. These included differences in definitions of accepted CAR T-cell toxicities, including CRS, ICANS, infusion reactions, hematologic toxicities, tumor lysis syndrome, sinusoidal obstructive syndrome, and graft-versus-host disease.
Guidance for toxicity definitions
Nonhematologic toxicities
To achieve harmonization across AML CAR T-cell therapy trials, we recommend that the following DLT definitions be used (tier 1) for nonhematologic toxicities (Table 4).
Definition of DLTs
| Adverse event . | Definitions . |
|---|---|
| CRS | Grade ≥4 or grade 3 >7 days |
| ICANS | Grade ≥4 or grade 3 >72 hours |
| Infusion reaction | Grade ≥4 |
| Nonhematologic organ toxicities | Grade ≥3 involving vital organs that fail to resolve to grade ≤2 within 7 days if not preexisting and not attributable to underlying disease |
| Adverse event . | Definitions . |
|---|---|
| CRS | Grade ≥4 or grade 3 >7 days |
| ICANS | Grade ≥4 or grade 3 >72 hours |
| Infusion reaction | Grade ≥4 |
| Nonhematologic organ toxicities | Grade ≥3 involving vital organs that fail to resolve to grade ≤2 within 7 days if not preexisting and not attributable to underlying disease |
These include the presence of grade 4 CRS/ICANS (as defined by ASTCT criteria71) or grade 3 CRS lasting >7 days, grade 3 ICANS (as defined by ASTCT criteria) lasting >72 hours, grade 4 infusion reaction, and grade ≥3 adverse events (AEs) involving vital organs that fail to resolve to grade ≤2 within 7 days. These events would be considered DLTs only if not pre-existing or related to the underlying disease. In addition, we recommend that grade ≥3 nonhematologic/nonclinically significant laboratory abnormalities that resolve within 7 days be exempt from DLT definitions (tier 1).
Relevant potential on-target/off-tumor toxicities outside of the hematopoietic compartment (eg, development of sinusoidal obstructive syndrome with CD33 CAR T-cell immunotherapy given the expression of CD33 on normal hepatic Kupffer cells) should be carefully considered and defined by individual trials.
Hematologic toxicities
Navigating DLT criteria in the context of expected hematologic toxicities was particularly challenging because there was significant heterogeneity in the definitions incorporated in the trials evaluated. In some studies, hematologic AEs were not included as DLT criteria because they were considered to be expected on-target/off-tumor toxicities, whereas others incorporated implications of hematologic toxicities as DLT. Most studies included some definitions of hematologic AEs.
We recommend 1 of the following 2 options for defining hematologic toxicities (tier 1):
Option 1: For CAR T cells targeting myeloid-lineage antigens expected to cause myelosuppression, we advocate that neutropenia or thrombocytopenia should not be considered a DLT. The justification for option 1 is that (i) these cytopenias are expected on-target/off-tumor toxicities, and in many studies, patients proceed to hematopoietic stem cell transplantation (HSCT); and (ii) the consequences of severe cytopenias that result in severe toxicity will be captured as nonhematologic DLT.
Option 2: For CAR T cells targeting non–myeloid-lineage antigens, new-onset grade 4 neutropenia or thrombocytopenia not present at baseline and persisting at day 42, together with aplastic bone marrow (<5% bone marrow cellularity) in the absence of residual AML, should be considered a DLT. This option is justified because most patients referred to early-phase AML CAR T-cell therapy trials have had several lines of cytotoxic therapy and might have delayed count recovery. In such cases, we recommend obtaining a bone marrow biopsy at day 42.
Of note, we recommend that all DLTs be assessed before or by day 28 (tier 1) after CAR T-cell infusion, with the exception of hematologic toxicity if using option 2, which should be assessed at day 42.
Other considerations
Not all toxicities are dose-related, and some are expected, as with standard-of-care approaches, and should not be considered as dose-limiting for the investigational agent under study. Severe infections can occur in the setting of prolonged cytopenias, common in patients with relapsed/refractory AML. These infections should be documented according to Common Terminology Criteria for Adverse Events (CTCAE) criteria. Consequences of severe infections will be accounted for in the DLT definitions; thus, we recommend excluding infection itself as a DLT (tier 1). We also recommend that high-grade AEs that are clearly attributed to lymphodepleting (LD) chemotherapy (ie, onset before CAR T-cell infusion) be excluded from DLT definitions (given that such events do not inform dosing of AML CAR T cells) (tier 1).
Response criteria
Considerations
Bone marrow aspirate and biopsy-based morphological assessment performed upon hematologic recovery after chemotherapy are the accepted gold standard and are usually mandated by regulatory agencies for response assessment in AML clinical trials.74 Despite this standard, considerable heterogeneity in defining end points exists. Definitions of hematopoietic recovery for complete response have varied among clinical trials, with some defining complete response as complete blood count recovery to an absolute neutrophil count of 1000 cells/μL and platelet count of 100 000/μL,74 whereas other studies have used thresholds of an absolute neutrophil count of 500 cells/μL and platelet count of 50 000/μL.74-76
Flow cytometric measurable residual disease (MRD) analysis has been validated as a sensitive and specific assessment of AML response.77,78 MRD status informs clinical decision-making for HSCT and predicts outcomes.79,80 Molecular MRD monitoring is also highly sensitive, although its predictive value in terms of clinical outcomes is still evolving.77,81 However, most AML clinical trials have been allowed by regulatory agencies to use rates of MRD-negative remission only as secondary or even exploratory end points.49 In the setting of anticipated delayed count recovery, the optimal strategy to determine best response, whether based on morphology or MRD, has not yet been defined for CAR T-cell therapy for AML.
Furthermore, the most appropriate time point for response assessment in CAR T-cell therapy for AML is unknown. Later response assessment timing (eg, day 42 or later depending on hematologic recovery) may be more biologically appropriate/accurate. However, earlier response assessment may facilitate clinical decision-making when alternative or next-line therapies are needed.
Finally, unlike in B-cell acute lymphoblastic leukemia (B-ALL), where CAR T cells may be delivered as a stand-alone therapy, current AML CAR T-cell clinical trials are largely intended to eliminate disease and facilitate consolidative HSCT. Given the low response rates observed in trials to date, the heterogeneity in antigen expression, and the unknown long-term persistence potential of AML CAR T cells, use as a stand-alone therapy is unlikely in AML. The ability to proceed to allogeneic HSCT remains a meaningful clinical end point for patients with AML and is therefore a useful secondary/exploratory end point in clinical trials, although event-free survival remains the critical outcome measure in this patient population.
Guidance for response assessment after AML CAR T-cell therapy
We recommend following internationally accepted criteria to assess response to CAR T-cell therapy (tier 1), such as those in the adult AML European LeukemiaNet/International Working Group (ELN/IWG) guidelines49 (supplemental Table 4). These end points were developed specifically for use in early-phase AML-directed trials and are relevant to the AML CAR T-cell context (tier 1). Pediatric-specific AML ELN-type international consensus guidelines have also been developed and include additional response considerations for children with AML, which may also be relevant for CAR T-cell and other early-phase clinical trials (S. K. Tasian, written communication, manuscript submitted).
Response should be assessed at approximately day 28 after CAR T-cell therapy (tier 1). Both bone marrow aspirate and biopsy should be performed when possible for best measurement of cellularity and assessment of hematologic toxicities (tier 1). If incomplete hematologic recovery is observed (as is common in patients with relapsed/refractory AML), bone marrow evaluation may be repeated after a short interval. Additional time points for disease assessment, such as day 14 and day 42, can be considered to provide additional information (tier 3).
Standard response criteria as per IWG/ELN recommendations include reporting morphology and MRD quantification. Generally, MRD measurement is done by multiparameter flow cytometry (MFC) using the “different from normal” or leukemia-associated immunophenotype methodologies. MFC should be performed with a qualified assay based on guidelines for rare event detection.82 Molecular MRD analysis of known leukemia-associated abnormalities may also be performed.83
We recommend reporting assay sensitivity, sample source, and time point at which MRD was evaluated (tier 1). Although useful to monitor when possible, the potential impact of mutational or immunophenotypic subclones identified via MFC or molecular MRD assays remains currently unknown (tier 3), and we recommend banking of relevant posttreatment samples where possible.
Lymphodepleting and bridging chemotherapy
Lymphodepleting therapy: considerations and guidance
A critical component of CAR T-cell therapy is the use of LD chemotherapy administered before infusion of CAR T cells. Similar to non-AML CAR T-cell therapy trials, the most frequently used LD chemotherapy in AML cell therapy trials has been fludarabine (typically 25-30 mg/m2 per day) and cyclophosphamide (typically 300-750 mg/m2 per day) administered over 1 to 4 days.84,85 Whether fludarabine/cyclophosphamide-based LD chemotherapy can achieve the optimal environment for CAR T-cell expansion and efficacy in patients with AML or whether this regimen should be modified remains unknown and requires further study, particularly as the microenvironment in AML may differ from other hematologic malignancies.19,86 Alternative LD regimens such as cytarabine/etoposide or cyclophosphamide/etoposide have also been used, but data regarding their potential efficacy are inconclusive at this time.87 Supplemental Table 5 summarizes the LD regimens used in AML CAR T-cell clinical trials.
Until efficacy data become available, no clear recommendations regarding optimal LD therapy can be made (no recommendation). More rigorous evaluations of optimal LD regimens for AML CAR T-cell expansion and therapeutic efficacy are required and should be guided by correlative studies evaluating the microenvironment and cytokine milieu.
Bridging chemotherapy: considerations and guidance
Bridging chemotherapy is often necessary to limit disease progression and to reduce disease burden to mitigate toxicity risk (eg, CRS) while awaiting CAR T-cell manufacture and/or product availability.
Given the heterogeneity among patients with AML, including differences in disease burden, prior therapy, and individual tolerance, selecting a bridging regimen is particularly challenging. We did not find evidence for preferential use of specific bridging chemotherapy regimens, but clinical trial experience highlights the necessity of reasonable AML stabilization before CAR T-cell infusion to optimize clinical safety. There are limited published data regarding bridging therapy used in patients before AML CAR T-cell therapy (summarized in supplemental Table 6).
In general, we suggest avoiding intensive myelosuppressive AML regimens because of risk of toxicity unless necessary to control disease before CAR T-cell administration. For pediatric patients, we recommend evaluating cerebrospinal fluid and administering intrathecal therapy if central nervous system (CNS) disease is identified during the bridging phase.
In summary, given the insufficient data regarding bridging chemotherapy regimens in the context of CAR T-cell therapy for AML, we cannot provide specific guidance (no recommendation). Future studies are needed to define optimal combinations.
CAR T-cell depletion before HSCT: considerations and guidance
Given that several trials are designed as “bridge-to-transplant,” additional consideration might be required for CAR T-cell products targeting panmyeloid antigens to ensure elimination of infused CAR T cells before infusion of the hematopoietic stem cell (HSC) graft. Whether standard chemotherapy/radiation-based conditioning regimens can eliminate residual CAR T cells is unknown. Additional strategies can be used to ensure elimination of CAR T cells, such as the use of “safety switches”88 and/or modification of the conditioning regimen with the use of in vivo T-cell depleting agents, such as antithymocyte globulin or alemtuzumab.
Correlative testing
Considerations
Correlative testing is needed to monitor for toxicity end points and response. Early-phase studies should include biological correlatives to maximize understanding of infused CAR T-cell product expansion, persistence, and mechanisms of success or failure. We include guidance for correlative monitoring before and after CAR T-cell infusion (Table 5; supplemental Table 7). We recognize that some of these correlative tests may not be feasible at all centers and highlight the optional nature of these as tier 3 recommendation. However, we recommend wherever possible to strongly consider storage of samples relevant for such correlative studies (tier 1).
Recommendations for conduct and reporting of correlative testing
| Tier . | Correlative testing recommendations . |
|---|---|
| Tier 1 | Clinical laboratory tests: before and after LD chemotherapy and at serial time points after infusion CAR T-cell monitoring (flow cytometry– or qPCR-based): at serial time points after infusion |
| Tier 2 | Cytokine analysis: before and after LD chemotherapy and at serial time points after infusion |
| Tier 3 | Detailed CAR T-cell monitoring (eg, high-dimensional flow cytometry–based or transcriptomics-based): before and after LD chemotherapy and at serial time points after infusion Detailed non–CAR T-cell immune monitoring (eg, high-dimensional flow cytometry–based or transcriptomics-based): before and after LD chemotherapy and at serial time points after infusion Tumor microenvironment milieu bone marrow evaluation (eg, high-dimensional flow cytometry–based or transcriptomics-based): before LD chemotherapy and at disease response evaluation Pharmacokinetic monitoring of agents used in LD chemotherapy |
| Tier . | Correlative testing recommendations . |
|---|---|
| Tier 1 | Clinical laboratory tests: before and after LD chemotherapy and at serial time points after infusion CAR T-cell monitoring (flow cytometry– or qPCR-based): at serial time points after infusion |
| Tier 2 | Cytokine analysis: before and after LD chemotherapy and at serial time points after infusion |
| Tier 3 | Detailed CAR T-cell monitoring (eg, high-dimensional flow cytometry–based or transcriptomics-based): before and after LD chemotherapy and at serial time points after infusion Detailed non–CAR T-cell immune monitoring (eg, high-dimensional flow cytometry–based or transcriptomics-based): before and after LD chemotherapy and at serial time points after infusion Tumor microenvironment milieu bone marrow evaluation (eg, high-dimensional flow cytometry–based or transcriptomics-based): before LD chemotherapy and at disease response evaluation Pharmacokinetic monitoring of agents used in LD chemotherapy |
qPCR, quantitative polymerase chain reaction.
Guidance for correlative testing
Clinical laboratory testing
For end points of inflammatory toxicities, clinical and laboratory monitoring is recommended for all patients treated with CAR T cells. In addition to routine hematology and chemistry laboratory tests, C-reactive protein, ferritin, and measures of coagulation are of use in the monitoring of patients developing inflammatory toxicities following CAR T-cell infusion (tier 1).89 A suggested table for monitoring time points is included (supplemental Table 7).
In addition to the above-mentioned clinical laboratory testing, a well-delineated list of correlative assays can provide biological insight. Thus, we suggest assessing for the following.
CAR T-cell monitoring
Although a range of methods have been used, there are no standardized clinical assays to monitor CAR T-cell expansion and persistence. We recommend monitoring with MFC (if specific antibodies are available)90-92 and/or polymerase chain reaction92,93 assays for transgene frequency in blood and biopsies longitudinally (tier 1), with the suggested time points for analysis included in supplemental Table 7. These tests provide data to track CAR T-cell kinetics, including detection of peak CAR T-cell expansion and persistence. Other methods including high-dimensional flow cytometry or transcriptomics can provide more granularity and may be included when possible (tier 3).67
Cytokine profiling
Measurement of cytokines, chemokines, and other soluble factors can help characterize T-cell responses and detect inflammatory toxicities.89,94,95 Real-time cytokine measurement is typically not feasible, whereas batched analyses cannot be used (and, formally, should not be used) to guide clinical decision-making. However, useful information can be gleaned from these assays to guide future management and/or CAR design.96 We suggest that cytokines be measured serially, before and after LD therapy and through the first month following CAR T-cell infusion, with greater frequency in the first 2 weeks (tier 2). Suggested time points are included in supplemental Table 7. Where possible, brief methodology of plasma/serum collection, processing, and analysis (eg, via Luminex or Olink platforms) should be noted to facilitate comparison of data across trials.
Non–CAR T-cell immune monitoring
Monitoring of non–CAR T-cell–based immune cell subset compositions after infusion, either by flow cytometry or transcriptomics on a research basis, can be informative and could be provided when available (tier 3). Analyses of CD19-directed CAR T-cell therapies have shown that immune subset composition before and after LD therapy may predict toxicities and response.65,97,98 We therefore suggest obtaining samples for non–CAR T-cell therapy immune cell characterization before and after LD therapy and serially after CAR T-cell infusion when possible (tier 3). Additional assays include evaluation of the bone marrow microenvironment before and after CAR T-cell therapy using high-dimensional MFC or transcriptomics, if available (tier 3). Suggested time points for these assays are outlined in supplemental Table 7.
Pharmacokinetic monitoring of LD chemotherapy
Given the limitations in standardization of agent choice and dosing for LD therapy for AML CAR T-cell trials, as discussed above, we recommend diagnostic interventions that will provide additional comparative data to inform future treatment.
In recent studies of patients with relapsed/refractory B-cell malignancies treated with CD19 CAR T-cell products, optimal fludarabine exposure was suggested to be a predictor of leukemia-free survival, B-cell aplasia, and CD19+ relapse.99,100 Optimizing fludarabine pharmacokinetics could influence outcomes following AML CAR T-cell therapy. We therefore suggest whenever possible to collaborate with centers performing pharmacokinetic analyses for LD therapies (tier 3).
Conclusions
Most children and adults with relapsed/refractory AML have unfortunately not benefited from CAR T-cell therapies to the extent expected from promising preclinical data. However, biological activity, manifested as CRS in many and disease responses in some patients, has been reported. In the absence of high-quality data from adequately powered studies, biological and clinical insights can be compiled from smaller studies if those studies contain similarly comprehensive and detailed information presented in a homogeneous manner. The recommendations outlined in this consensus report are intended to facilitate harmonization of key components of AML CAR T-cell therapy clinical trials and meaningful intertrial comparisons that will inform the next generation of clinical studies. Our IMPACT consortium will continue to review available and emerging clinical data and provide updated recommendations in the future as appropriate. These efforts will be instrumental in promoting improved outcomes of patients treated with AML CAR T-cell immunotherapies.
Acknowledgments
The authors acknowledge Aksana Vasilyeva, Amber Smith, Jola Dowdy, and Jessica Hickey for their support and help in organizing the workshops. The workshops were supported by the Center of Excellence for Pediatric Immuno-Oncology at St. Jude Children’s Research Hospital and the American Lebanese Syrian Associated Charities.
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH). The contributions of the NIH author(s) were made as part of their official duties as NIH federal employees, are in compliance with agency policy requirements, and are considered Works of the US Government. However, the findings and conclusions presented in this paper are those of the author(s) and do not necessarily reflect the views of the NIH or the US Department of Health and Human Services.
Authorship
Contribution: All authors attended the workshops, developed the guidelines, contributed to the development of the first and subsequent drafts, and approved the final submission of the manuscript.
Conflict-of-interest disclosure: S.H.C.B. reports that their spouse is a former employee of Takeda Pharmaceuticals and Alexion Pharmaceuticals; and has received salary and stock/stock options unrelated to this work. C.L.B. reports holding pending patents in the field of engineered cell therapies. L.E.B. serves as a consultant for Genentech, MustangBio, Roche, AstraZeneca, Amgen, Merck, and ADC Therapeutics. K.J.C. reports consultancy for Novartis; board membership and equity in Turn Bio and PromiCell; and research funding from Novartis, Cellectis, and Atara Biotherapeutics. A.F.D. reports enlistment as an inventor on multiple patents related to chimeric antigen receptor (CAR) T-cell therapy; possible eligibility to receive a portion of royalties paid to Memorial Sloan Kettering (MSK) by Caribou Biosciences Inc, Tigen Pharma SA, and PromiCell Therapeutics Inc; financial interests as the chief scientific officer of PromiCell Therapeutics Inc; and advisory consultancy for Shoreline Biosciences Inc. R.A.G. reports patents related to CAR T-cell technologies licensed to Bristol Myers Squibb (BMS). S. Ghorashian reports affiliations with Autolus Ltd and related patents; membership on the data safety monitoring board of Immatics; scientific advisory board membership for Be Biopharma; consultancy for Cargo Therapeutics within the last 12 months; and patents and patent applications in the fields of T-cell and/or gene therapy for cancer. L.C.H. reports being on the advisory board of March Biosciences; membership in the speakers bureau of Kite/Gilead; and honoraria from Sanofi. F.L. reports honoraria from and/or membership in the speakers bureau for Novartis, Jazz Pharmaceuticals, Sanofi, Sobi, Miltenyi Biotec, bluebird bio, Inc, Amgen, and BMS; and has participated in an advisory board for Amgen and Vertex. M.M. reports being a cofounder with equity and a director at March Biosciences; patent fees from Fate Therapeutics, Beam Therapeutics, and March Biosciences; research support from Fate Therapeutics and March Biosciences; and advisory board membership for NKILT Therapeutics and March Biosciences. B.O. reports patents in cell therapy unrelated to this study. J.H.P received consulting fees from Adaptive Biotechnologies, AffyImmune Therapeutics, Allogene Therapeutics, Amgen, Artiva Biotherapeutics, Ascentage Pharma, Autolus, BeiGene, Bright Pharmaceutical Services, Inc, Caribou Biosciences, Curocell, Kite Pharma, Galapagos, Iovance Biotherapeutics, Jazz Pharmaceuticals, Medpace, Pfizer, Servier, Sobi, Synthekine, and Takeda. C.Q. reports patent applications in the field of CAR T cells. D.A.S. reports consultancy for AbbVie, Agios, Debiopharm, Janssen Pharmaceuticals, Johnson & Johnson, Molecular Partners, and Novartis; advisory board membership for AbbVie, Agios, AvenCell, Astellas Pharma, bluebird bio, BMS, Dark Blue Therapeutics, Geron, Novartis, Shattuck Labs, Servier, Syndax, Syros, and Taiho Oncology; and research funding from Aprea Therapeutics and Jazz Pharmaceuticals. N.N.S. reports research funding from Lentigen, Vor Bio, and Cargo Therapeutics; attending advisory board meetings (no honoraria) for Vor Bio, ImmunoACT, and Sobi; royalties from Cargo Therapeutics; and was supported in part by the Intramural Research Program, Center for Cancer Research, National Cancer Institute and National Institutes of Health Clinical Center, National Institutes of Health (ZIA BC 011927). M.S. receives industry research support from Amgen, BMS/Celgene, Gilead/Kite, Johnson & Johnson, Miltenyi Biotec, Novartis, Roche, Seattle Genetics, and Takeda; serves as a consultant/advisor to AbbVie, Crossbow Therapeutics, Debiopharm, Gilead/Kite, Interius, Johnson & Johnson, Molecular Partners, Novartis, and Otsuka; and serves on the speakers bureau at Amgen, BMS/Celgene, Gilead/Kite, Miltenyi Biotec, Novartis, Roche, and Takeda. S.K.T. reports research funding from Beam Therapeutics, Incyte Corporation, and Kura Oncology; serves or has served on scientific advisory boards for Aleta Biotherapeutics, AstraZeneca, Jazz Pharmaceuticals, Kestrel Therapeutics, Kura Oncology, Novartis, Syndax Pharmaceuticals, and Wugen, Inc; and has received travel support from Amgen and Jazz Pharmaceuticals. S.K.T was supported by the Andrew McDonough B+ Foundation for Childhood Cancer Research; is a Scholar of the Leukemia & Lymphoma Society; and holds the Joshua Kahan Endowed Chair in Pediatric Leukemia Research at the Children’s Hospital of Philadelphia. M.P.V. has patent applications in the field of T-cell and/or gene therapy for cancer; and is a member of the Rally! Foundation medical advisory board. S.I.G. reports being a named inventor on intellectual property related to CAR T cells in AML, including patents licensed to Novartis; is a scientific cofounder and holds equity in Interius Biotherapeutics and Carisma Therapeutics; and reports being a scientific advisor to Carisma Therapeutics, Cartography Biosciences, Currus, Interius, Kite, NKILT Therapeutics, and Mission Bio. The remaining authors declare no competing financial interests.
Correspondence: Swati Naik, Department of Bone Marrow Transplantation and Cellular Therapy, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105; email: swati.naik@stjude.org.
References
Author notes
M.P.V. and S.I.G. contributed equally to this study.
Publication-related data are available on request to the corresponding author, Swati Naik (swati.naik@stjude.org).
The full-text version of this article contains a data supplement.