Key Points
MAC was no more effective than RIC in eradicating FLT3-ITD MRD.
NPM1 comutation was associated with the largest magnitude of relapse-free survival benefit from posttransplant gilteritinib.
Visual Abstract
We conducted a post hoc analysis of data from Blood and Marrow Transplant Clinical Trials Network 1506 (MORPHO), a randomized trial of gilteritinib vs placebo as posttransplantation maintenance for patients with FLT3-ITD–mutated acute myeloid leukemia (AML) undergoing allogeneic hematopoietic cell transplantation (HCT), focusing the interactions between conditioning regimen intensity, measurable residual disease (MRD), and NPM1 comutation status reported from diagnosis. Comparing FLT3-ITD MRD before and after conditioning, there was no difference between myeloablative conditioning (MAC) and reduced-intensity conditioning (RIC) in eradication or reduction of FLT3-ITD MRD. For participants who were FLT3-ITD MRD negative before HCT, there was no difference in the cumulative incidence of relapse during follow-up between those receiving MAC vs RIC. NPM1 comutation was associated with the largest magnitude of relapse-free survival benefit from post-HCT gilteritinib, and in these participants, post-HCT gilteritinib in the setting of RIC appeared to be as effective as MAC at preventing relapse. MAC appeared superior to RIC in preventing relapse only in participants who were NPM1 wild type at diagnosis and FLT3-ITD MRD positive before HCT. Our findings suggest that only a subset of patients with FLT3-ITD AML undergoing HCT may benefit from MAC and that, similar to AML therapy before HCT, the intensity of the HCT regimen should be adapted according to the molecular features of the disease. This trial was registered at www.clinicaltrials.gov as #NCT02997202.
Introduction
Patients with FLT3-ITD–mutated acute myeloid leukemia (AML) routinely undergo allogeneic hematopoietic cell transplantation (HCT) after achieving first remission to maximize their chances of survival.1 Although there are numerous disease- and patient-specific factors that can affect the prognosis for those undergoing HCT, recent studies have focused on measurable residual disease (MRD), conditioning regimens, the use of FLT3 inhibitors as post-HCT maintenance, and NPM1 comutation status.2-8 Myeloablative conditioning (MAC), in particular, has been suggested to be more effective than reduced intensity conditioning (RIC) for patients with AML and pre-HCT MRD, although the benefit of MAC specifically for patients with FLT3-ITD AML is still unclear.3,4,7
Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1506 (“MORPHO”; NCT02997202) was a global randomized study of patients with FLT3-ITD–mutated AML in first remission who had successfully undergone HCT and were randomized, in double-blinded fashion, to receive the FLT3 inhibitor gilteritinib or placebo as post-HCT maintenance.6 A statistically significant difference in relapse-free survival (RFS) between gilteritinib and placebo, which was the primary end point, was not detected (P = .0518). However, prespecified subgroup analysis revealed that post-HCT gilteritinib maintenance decreased the cumulative incidence of relapse and improved RFS for those participants with detectable pre- or post-HCT FLT3-ITD MRD. Participants were allowed to enroll irrespective of pre-HCT chemotherapy, HCT conditioning regimen, graft source, or graft-versus-host disease prophylaxis. NPM1 mutation status at AML diagnosis was reported for all participants. We conducted a post hoc subgroup analysis of the data set to identify any associations between MRD, the intensity of the conditioning regimen, FLT3 inhibitor maintenance, and NPM1 mutation status that could have influenced trial outcomes. Our findings suggest that there may be a potential benefit in “tailoring” the HCT regimen based on these disease characteristics.
Methods
The study population consisted of 356 adult participants aged 18 to 78 years enrolled between 17 August 2017 and 8 July 2020 at 122 centers in 16 different countries. BMT CTN 1506 enrolled patients diagnosed with FLT3-ITD–mutated AML who had achieved complete remission (CR) after no more than 1 or 2 courses of intensive chemotherapy and were proceeding to allogeneic HCT within 1 year of achieving remission. A total of 59.8% of participants had been treated with FLT3 inhibitors before HCT. At the time of HCT, participants were required to be in first CR. Any type of conditioning, graft source, and graft-versus-host disease prophylaxis were permitted during HCT. After confirmation of engraftment and a bone marrow (BM) examination showing no morphologic evidence of AML, conducted no sooner than 30 days and no later than 90 days after stem cell infusion, gilteritinib 120 mg per day or placebo was started in double-blinded fashion. BM aspirates were collected at defined intervals: (1) pre-HCT, within 30 days before the start of conditioning; (2) post-HCT, immediately before randomization; (3) at 3, 6, 12, 18, and 24 months after the start of gilteritinib/placebo; and (4) at the end of treatment. Relapses were centrally adjudicated by a blinded committee.
Conditioning
There were >30 unique conditioning regimens used in BMT CTN 1506. All conditioning regimens were reviewed by an adjudication committee, which used guidelines published by the Center for International Blood and Marrow Transplantation to classify conditioning regimens as MAC or RIC.9 MAC regimens were defined as regimens containing total body irradiation ≥5 Gy (single dose) or ≥8 Gy (fractionated), busulfan >8 mg/kg orally or 6.4 mg/kg IV (total dose), treosulfan >30 000 mg/m2 (or >30 g/m2), melphalan >150 mg/m2, or thiotepa ≥10 mg/kg. Any regimen that did not meet the criteria for MAC was classified as RIC.
FLT3-ITD and NPM1 mutation status at diagnosis
Both FLT3-ITD and NPM1 mutation status were determined by each participant’s local institution before enrollment. The results were entered into the trial database and reviewed by the study monitor.
MRD assay
In this post hoc study, only participants with a successful MRD result from a marrow aspirate in the peri-HCT period are included. The peri-HCT period is defined as before HCT without any further consolidation (the “pre-HCT BM biopsy”) up until randomization. Before randomization, participants were required to undergo a BM biopsy to confirm the ongoing morphologic absence of leukemia and to collect a sample for MRD. Therefore, there were 2 separate peri-HCT samples: one collected immediately before HCT and the other collected after engraftment but before randomization (which was required to take place between 30 and 90 days after stem cell infusion). The first 2-cm3 pull of marrow aspirate from any BM sample was designated for MRD analysis, which was performed in a reference laboratory (Invivoscribe, San Diego, CA) using an amplicon-based approach on DNA prepared from the marrow sample, as described previously.10 When multiple FLT3-ITD clones were detected in a single MRD sample, the sum of the individual variant allele frequencies was used as the final variant allele frequency for that sample. RNA was not prepared from these samples; therefore, MRD assessment by analysis of NPM1 transcripts by reverse-transcriptase quantitative polymerase chain reaction was not possible.11
Statistical analysis
Survival and time to relapse were calculated from the day of randomization. Overall survival, RFS, CR, and relapse were defined by standard criteria.12,13 Participants were stratified by conditioning regimen intensity (MAC vs RIC), time from transplantation to randomization (30-60 days vs 61-90 days), and the presence of FLT3-ITD MRD at a level of ≥1 × 10–4 (present vs absent/indeterminate) based on the pre-HCT BM aspirate. Survival was estimated using Kaplan-Meier analysis, and hazard ratios were estimated using Cox proportional hazard model. Competing risks end points (relapse and non-relapse mortality) were summarized using the cumulative incidence function and the subdistribution hazard ratios obtained using Fine-Gray model. SAS version 9.4 and GraphPad Prism 10 were used for graphing and statistical analysis. In the previously published intention-to-treat analysis of this clinical trial,6 participants for whom MRD was not performed before or after HCT were included as MRD negative. In this post hoc study, participants missing either or both peri-HCT samples were excluded from the analysis.
The study was conducted in compliance with the Declaration of Helsinki and approved by institutional review boards at each site. All participants provided informed consent.
Results
Participant characteristics
As reported in the primary manuscript for this study,6 of the 356 participants on BMT CTN 1506, marrow aspirates were successfully analyzed for FLT3-ITD MRD in 350 participants (98%) before HCT and 347 participants (97.5%) after HCT (before randomization). In 6 participants, the pre-HCT aspirate sample was not sent to the reference laboratory for MRD analysis, and the same was true for 9 completely different participants after HCT and before randomization. These 15 participants were therefore excluded from this post hoc study. Overall, peri-HCT (eg, both pre-HCT and post-HCT/prerandomization) FLT3-ITD MRD results were available for 341 of 356 participants (95.8%), constituting the population for this analysis. The characteristics of these 341 participants are displayed in Table 1. The characteristics of the 15 excluded participants were very similar to those of the other 341, with a median age of 56 years and a relapse incidence of 20% (compared with 53 years and 19.4%, respectively, for the 341 participants in the nonexcluded data set). A total of 206 of 341 participants (60.4%) were treated with FLT3 inhibitors (all in combination with chemotherapy) before HCT. Of these 206 participants, 191 (92.7%) received midostaurin, and only 4 (1.9%) received gilteritinib. Other FLT3 inhibitors used were sorafenib, ponatinib, quizartinib, and crenolanib (each in ≤5 participants).
Characteristics of the 341 participants from whom a pre- and post-HCT MRD specimen was successfully analyzed, according to the conditioning regimen received
| Characteristic . | All participants . | RIC . | MAC . |
|---|---|---|---|
| n (%) | N = 341 | 138 (40.5) | 203 (59.5) |
| Age, median, y | 53 | 61 | 46 |
| Time from pre-HCT MRD specimen collection to HCT, median, d | 18 | 19 | 18 |
| Time from HCT to collection of post-MRD specimen, median, d | 42 | 41 | 44 |
| Time from HCT to randomization (start of placebo or gilteritinib), median (interquartile range), d | 57 (43-76) | 54 (43-71) | 61 (44-79) |
| Geographic distribution, n (%) | |||
| North America | 146 (42.8) | 70 (50.7) | 76 (37.4) |
| Europe | 87 (25.5) | 42 (30.4) | 45 (22.2) |
| Asia/rest of world | 108 (31.7) | 26 (18.8) | 82 (40.4) |
| Characteristic . | All participants . | RIC . | MAC . |
|---|---|---|---|
| n (%) | N = 341 | 138 (40.5) | 203 (59.5) |
| Age, median, y | 53 | 61 | 46 |
| Time from pre-HCT MRD specimen collection to HCT, median, d | 18 | 19 | 18 |
| Time from HCT to collection of post-MRD specimen, median, d | 42 | 41 | 44 |
| Time from HCT to randomization (start of placebo or gilteritinib), median (interquartile range), d | 57 (43-76) | 54 (43-71) | 61 (44-79) |
| Geographic distribution, n (%) | |||
| North America | 146 (42.8) | 70 (50.7) | 76 (37.4) |
| Europe | 87 (25.5) | 42 (30.4) | 45 (22.2) |
| Asia/rest of world | 108 (31.7) | 26 (18.8) | 82 (40.4) |
Direct comparison of MRD results before and after HCT
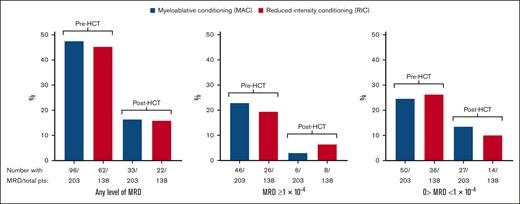
Because MRD results were obtained from most participants within a few weeks before and after conditioning, this provided an opportunity to examine the efficacy of HCT conditioning in lowering or eradicating MRD. Pre-HCT MRD specimens were collected a median of 18 days before stem cell infusion, and post-HCT (prerandomization) specimens were collected a median of 42 days after stem cell infusion (Table 1). Pre- and post-HCT MRD levels for all participants and by conditioning regimen are displayed in supplemental Table 1. Figure 1 graphically displays the percentage of participants with FLT3-ITD MRD before and after HCT. Although the percentage of participants with detectable FLT3-ITD MRD was consistently decreased after conditioning, the degree of lowering was similar between MAC and RIC. Because of prior reports that the inclusion of melphalan in an RIC regimen may lead to lower relapse rates,14,15 we repeated this analysis including melphalan-containing RIC regimens with MAC (supplemental Figure 1). Again, there was no discernible difference between MAC and RIC in eradicating MRD between the pre- and post-HCT time points.
Comparison of pre- and post-HCT MRD levels according to conditioning intensity. (A) Results for participants with any level of detectable FLT3-ITD MRD. (B) Results for participants with FLT3-ITD MRD VAF of ≥1 × 10–4. (C) Results for participants with detectable MRD but VAF <1 × 10–4. pts, patients; VAF, variant allele frequency.
Comparison of pre- and post-HCT MRD levels according to conditioning intensity. (A) Results for participants with any level of detectable FLT3-ITD MRD. (B) Results for participants with FLT3-ITD MRD VAF of ≥1 × 10–4. (C) Results for participants with detectable MRD but VAF <1 × 10–4. pts, patients; VAF, variant allele frequency.
Of the 341 participants in this data set, 16 were MRD negative before HCT but converted to MRD positive after HCT (supplemental Table 1). Of these 16, a total of 5 had received RIC, and 11 had received MAC.
Conditioning intensity and relapse
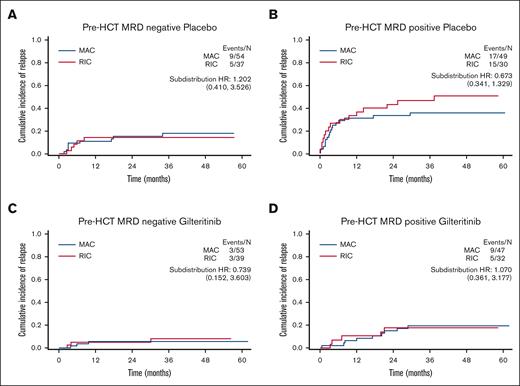
In BMT CTN 1506, the presence of detectable pre-HCT FLT3-ITD MRD was associated with increased risk of relapse,16 so we examined the impact of pre-HCT FLT3-ITD MRD on relapse according to the intensity of conditioning. In the study, gilteritinib decreased the relapse risk for participants with peri-HCT FLT3-ITD MRD. Therefore, to isolate the effect of conditioning intensity on MRD without the confounding effect of gilteritinib, we examined the placebo and gilteritinib arms separately (Figure 2). Consistent with previous reports,3,7 there was no difference in relapse risk between MAC and RIC for participants who had no detectable pre-HCT FLT3-ITD MRD (Figure 2A,C). There was a numerical improvement in relapse rate for MAC over RIC in participants who were pre-HCT MRD positive and received placebo (Figure 2B), although the small number of participants precludes a definitive conclusion. Interestingly, even this modest numerical advantage for MAC over RIC seems to have been abrogated by the addition of post-HCT gilteritinib (Figure 2D).
Cumulative incidence of relapse according to conditioning intensity, MRD, and randomization arm. (A) Pre-HCT FLT3-ITD MRD negative; placebo arm; MAC vs RIC. (B) Pre-HCT FLT3-ITD MRD positive; placebo arm; MAC vs RIC. (C) Pre-HCT FLT3-ITD MRD negative; gilteritinib arm; MAC vs RIC. (D) Pre-HCT FLT3-ITD MRD positive; gilteritinib arm; MAC vs RIC. HR, hazard ratio.
Cumulative incidence of relapse according to conditioning intensity, MRD, and randomization arm. (A) Pre-HCT FLT3-ITD MRD negative; placebo arm; MAC vs RIC. (B) Pre-HCT FLT3-ITD MRD positive; placebo arm; MAC vs RIC. (C) Pre-HCT FLT3-ITD MRD negative; gilteritinib arm; MAC vs RIC. (D) Pre-HCT FLT3-ITD MRD positive; gilteritinib arm; MAC vs RIC. HR, hazard ratio.
NPM1 comutations, gilteritinib, and conditioning intensity
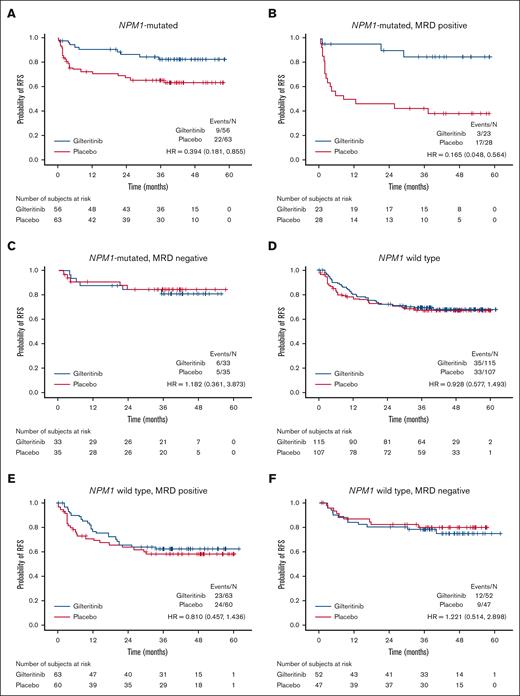
AML is not a single disease but one that can be categorized into different subtypes according to its mutation profile at diagnosis.13FLT3 mutations by themselves do not define a subtype because they occur across the spectrum of AML.17 However, NPM1-mutated AML constitutes a distinct subtype of AML, as defined by both the World Health Organization and the International Consensus Classification, and is a frequent comutation in patients with FLT3-ITD–mutated AML.18-20 Therefore, we first examined the impact of gilteritinib compared to placebo for participants with and without NPM1 mutations (Figure 3; supplemental Figures 2-7). Maintenance gilteritinib conferred a pronounced benefit for participants with an NPM1 comutation, driven entirely by those who were FLT3-ITD MRD positive in the peri-HCT period (Figure 3A-C). In contrast, those without an NPM1 comutation (Figure 3D-F) appear to derive minimal or no benefit from gilteritinib compared with placebo.
RFS for participants according to peri-HCT MRD, NPM1 comutation at initial diagnosis, and randomization arm. (A) NPM1 comutation; gilteritinib vs placebo. (B) NPM1 comutation; peri-HCT MRD positive; gilteritinib vs placebo. (C) NPM1 comutation; peri-HCT MRD negative; gilteritinib vs placebo. (D) NPM1 wild type; gilteritinib vs placebo. (E) NPM1 wild type; peri-HCT MRD positive; gilteritinib vs placebo. (F) NPM1 wild type; peri-HCT MRD negative; gilteritinib vs placebo. HR, hazard ratio.
RFS for participants according to peri-HCT MRD, NPM1 comutation at initial diagnosis, and randomization arm. (A) NPM1 comutation; gilteritinib vs placebo. (B) NPM1 comutation; peri-HCT MRD positive; gilteritinib vs placebo. (C) NPM1 comutation; peri-HCT MRD negative; gilteritinib vs placebo. (D) NPM1 wild type; gilteritinib vs placebo. (E) NPM1 wild type; peri-HCT MRD positive; gilteritinib vs placebo. (F) NPM1 wild type; peri-HCT MRD negative; gilteritinib vs placebo. HR, hazard ratio.
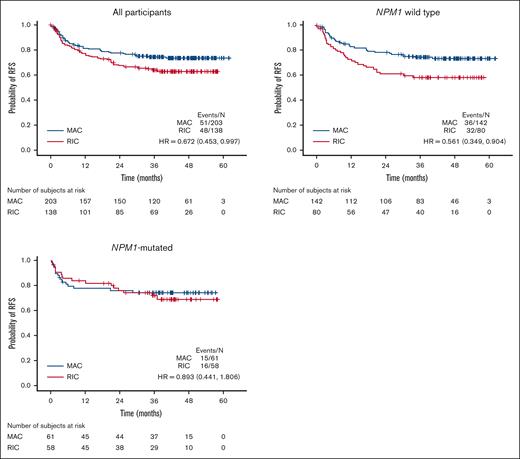
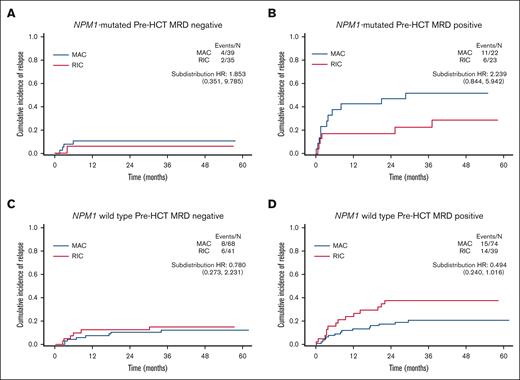
This dichotomy of response patterns to gilteritinib for NPM1-mutated vs NPM1 wild-type participants was paralleled by a similar dichotomy of response to conditioning intensity. With respect to conditioning intensity, participants without an NPM1 comutation displayed an improvement in RFS with MAC vs RIC, whereas those with an NPM1 comutation did not (Figure 4; supplemental Figures 8-10). Therefore, we examined the impact of MRD and conditioning intensity in the context of NPM1 comutation status (Figure 5). For both NPM1-mutated and NPM1 wild-type participants, among those who were FLT3-ITD MRD negative before HCT (Figure 5A,C), there is no indication that MAC decreased the relapse risk compared to RIC, consistent with previous reports on this issue.4,7 However, for FLT3-ITD MRD-positive participants, MAC was associated with a numerically lower relapse risk for those who were NPM1 wild type and yet seemingly had the opposite effect, at least numerically, for those with an NPM1 comutation. Breaking the groups down further according to gilteritinib randomization (supplemental Figure 11), for participants with an NPM1 comutation and detectable pre-HCT FLT3-ITD MRD, MAC did not appear to confer any benefit over RIC when maintenance gilteritinib was administered after HCT, although, again, no statistically firm conclusions can be drawn with such small numbers of participants.
RFS for participants according to NPM1 comutation status and conditioning intensity. HR, hazard ratio.
RFS for participants according to NPM1 comutation status and conditioning intensity. HR, hazard ratio.
Cumulative incidence of relapse for participants according to pre-HCT MRD status, NPM1 comutation status, and conditioning intensity. HR, hazard ratio.
Cumulative incidence of relapse for participants according to pre-HCT MRD status, NPM1 comutation status, and conditioning intensity. HR, hazard ratio.
Discussion
To summarize these findings, we did not observe any difference between MAC and RIC in eliminating or reducing FLT3-ITD MRD in marrow samples obtained at the pre- and post-HCT time points. In participants who were FLT3-ITD MRD negative before HCT, the relapse rate during follow-up was no different between those who had received MAC and those who received RIC. NPM1 comutation was associated with the largest magnitude of benefit from post-HCT gilteritinib. In these participants, post-HCT gilteritinib in the setting of RIC appeared to be as effective as MAC in preventing relapse. Only in participants who were NPM1 wild type at diagnosis and were FLT3-ITD MRD positive did MAC appear potentially superior to RIC in preventing relapse.
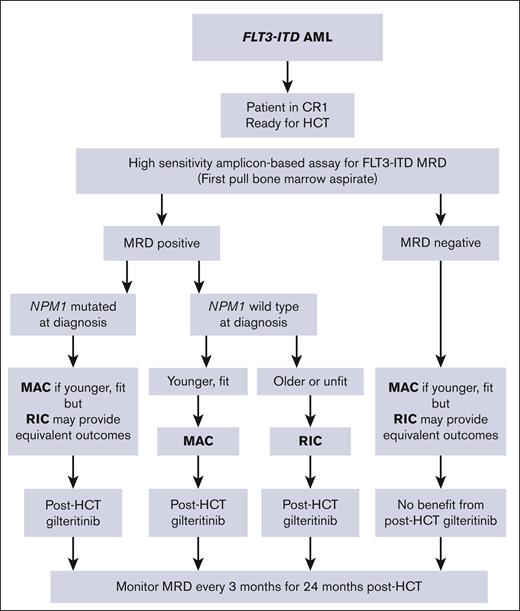
These hypothesis-generating data suggest that, for patients with FLT3-ITD AML undergoing HCT in first remission, the choice of conditioning intensity should be based not just on age and performance status but also potentially on disease biology. Figure 6 displays a flow diagram for suggested management of patients with FLT3-ITD AML undergoing HCT based on these data. From these data, there does not appear to be a compelling reason to use MAC over RIC for patients who are FLT3-ITD MRD negative before HCT, at least in terms of reducing relapse risk. For patients with an NPM1 comutation who are FLT3-ITD MRD positive before HCT, the intensity of the conditioning regimen is probably not as important as being able to start an FLT3 inhibitor as soon as possible after HCT. The data in Figure 5 suggest a paradoxically negative effect of MAC on relapse, although the numbers in that analysis are too small to draw a statistically firm conclusion. Nonetheless, there are potential explanations for why MAC could result in worse outcomes than RIC in that select population. First, it is well established from practical and published observations that when FLT3-ITD AML relapses after HCT, it typically does so very rapidly.16 The time required to recover from HCT and start an FLT3 inhibitor may be crucial, and participants in BMT CTN 1506 receiving MAC generally started gilteritinib later than those receiving RIC (Table 1). Second, there is indirect evidence that increasing the intensity of chemotherapy may actually promote relapse of FLT3-ITD AML by increasing FLT3 ligand levels systemically, which may promote leukemic cell survival and proliferation in the presence of FLT3-ITD mutations.21,22
Flow chart for suggested management of FLT3-ITD AML, incorporating MRD, NPM1 mutation status, and relative fitness of the patient. CR1, first remission.
Flow chart for suggested management of FLT3-ITD AML, incorporating MRD, NPM1 mutation status, and relative fitness of the patient. CR1, first remission.
The major drawback of this study is that the findings are derived from a post hoc subgroup analysis and are therefore subject to the expected statistical weaknesses of such an approach. In addition, patients were enrolled before HCT and randomized after HCT without any stipulation regarding the type of induction chemotherapy, duration of consolidation (<1 year), or selection for HCT or enrollment. Furthermore, we analyzed conditioning regimen intensity in a binary fashion of RIC vs MAC, whereas intensity in the real world is along a spectrum with much heterogeneity, especially with what is defined as RIC. However, the data presented in Figure 1 are difficult to refute by any statistical argument. MAC was no more effective than RIC in eliminating or reducing FLT3-ITD MRD by this direct measurement. Furthermore, our finding that post-HCT gilteritinib was associated with such a large magnitude of benefit in participants who were FLT3-ITD MRD positive and harbored NPM1 comutations at diagnosis is striking (Figure 3B) but not surprising in light of emerging data on this mutation combination in patients with AML treated with FLT3 inhibitors.23,24 Patients with FLT3-mutated AML harboring a concomitant NPM1 mutation display improved clinical responses to FLT3 inhibition compared to those with wild-type NPM1. NPM1 mutations have been shown to result in aberrant expression of HOX genes,25 with resultant upregulation of FLT3 expression via Meis1, resulting in a leukemia that is presumably addicted to mutant FLT3 signaling. Another drawback of this study is our inability to correlate these results with NPM1 MRD analysis using reverse transcription polymerase chain reaction.8,11,26 As noted earlier, RNA was not available for this analysis.
In BMT CTN 1506, the local investigator chose the conditioning regimen for participants before enrollment and without knowledge of the results of protocol measurements of MRD. The conditioning regimen was a stratification factor but not a randomization factor, and participants were fully engrafted at the time of randomization to gilteritinib or placebo. Therefore, survival between participants in BMT CTN 1506 receiving MAC vs RIC cannot be meaningfully compared, because there was, not unexpectedly, a 15-year difference in median age between the 2 groups. Furthermore, a survival comparison could not account for any difference in transplant-related mortality between MAC and RIC, some of which would already have taken place, and was 15.8% and 4.4% for MAC and RIC, respectively, in a recent study.27
Recent data indicate that patients with AML, without regard for molecularly defined subtypes, should undergo HCT with MAC when feasible,27 particularly if they have pre-HCT MRD.3 Although HCT is an integral part of AML therapy for many patients, AML itself is not a single disease, and our data suggest that this broad recommendation is not necessarily applicable to all patients with FLT3-ITD AML. Evidence is emerging that MRD might soon be used to determine which patients benefit most from HCT,8 but even when the decision has been made to proceed with HCT, the specific HCT regimen could still be modified according to the disease and the patient. Patients with acute promyelocytic leukemia, FLT3-mutated AML, core-binding factor AML, or TP53-mutated AML all technically have AML; yet they are all treated along different paradigms from diagnosis, each treatment regimen tailored according to the molecular lesions in the disease. With respect to choosing a regimen for HCT, our data, focusing on FLT3-ITD MRD and relapse risk, indicate that pre-HCT FLT3-ITD MRD does not by itself constitute a reason to choose MAC over RIC. In fact, it is quite possible that MAC should be avoided in patients with FLT3-ITD AML and an NPM1 comutation, in favor of emphasizing FLT3 inhibition. patients with wild-type NPM1, particularly those with MRD, would seem to be appropriate candidates for MAC, if eligible.
Acknowledgments
Support for this study was provided by grant numbers U10HL069294 and U24HL138660 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute, the National Cancer Institute, and by funding from Astellas Pharma Global Development.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the abovementioned parties.
Authorship
Contribution: M.J.L. contributed to study design, analyzed data, and wrote the manuscript; and all other authors contributed to study design, helped analyze data, and edited the manuscript.
Conflict-of-interest disclosure: M.J.L. reports consulting or advisory role fees from Daiichi-Sankyo, Amgen, Astellas Pharma, Bristol Myers Squibb (BMS), AbbVie/Genentech, GlaxoSmithKline, Syndax, and Takeda; expert testimony fees from Novartis; research funding from Astellas Pharma (to institution [Inst]); and travel, accommodation, and expenses from Astellas Pharma. M.H. received honoraria from Celgene; consulting or advisory role fees from Incyte, ADC Therapeutics, Puma Biotechnology, Verastem, Kite/Gilead, MorphoSys, Omeros, Novartis, Gamida Cell, Seagen, Genmab, Myeloid Therapeutics, BeiGene, AstraZeneca, Sanofi, BMS/Celgene, CRISPR Therapeutics, Caribou Biosciences, AbbVie, and Genentech; speakers' bureau fees from Genzyme, AstraZeneca, BeiGene, ADC Therapeutics, and Kite/Gilead; and research funding from Takeda, Spectrum Pharmaceuticals, Otsuka, Astellas Pharma, and Genzyme. M.R.L. received honoraria, speakers' bureau fees, and travel, accommodation, and expenses from BeiGene Shanghai and Amgen; received research funding from Amgen, Astellas Pharma, Actinium Pharmaceuticals, and Syndax; and reports other expenses with BioSight. J.R.W. reports consulting or advisory role fees from Shire, Celgene, Cidara Therapeutics, F2G, and ORCA Therapeutics. E.B.P. reports employment with Biogen, Exelixis, Regulus Therapeutics, Graviton Bioscience Corp, and EpiKast; leadership roles for Biogen, Exelixis, and Regulus Therapeutics; stock and other ownership interests with Biogen, Exelixis, Regulus Therapeutics, Apellis Pharmaceuticals, Leap Therapeutics, and Actio Biosciences Inc; consulting or advisory role fees from Actio Biosciences; research funding from AbbVie; and travel, accommodation, and expenses from Biogen, Exelixis, and Regulus Therapeutics. A.E.P. received honoraria from Astellas Pharma and Daiichi Sankyo; consulting or advisory role fees from Astellas Pharma, Actinium Pharmaceuticals, Daiichi Sankyo, AbbVie, FORMA Therapeutics, Sumitomo Dainippon, Celgene/BMS, Syndax, Genentech, BerGenBio, Immunogen, Foghorn Therapeutics, Rigel, and Curis; research funding from Astellas Pharma (Inst), Bayer (Inst), Daiichi Sankyo (Inst), Fujifilm (Inst), AbbVie (Inst), and Syndax (Inst); and travel, accommodation, and expenses from Daiichi Sankyo. R.J.S. reports leadership roles with Kiadis Pharma and Be the Match/National Marrow Donor Program; consulting or advisory role fees from Juno Therapeutics, Gilead Sciences, Rheos Medicines, Cugene, Jazz Pharmaceuticals, Precision Biosciences, Takeda, Jasper Therapeutics, Alexion Pharmaceuticals, Neovii, Vor Biopharma, Smart Immune, and Bluesphere Bio; expert testimony fees from Pfizer; and travel, accommodation, and expenses from Gilead Sciences. C.U. reports employment with Takeda and Blueprint Medicines; received honoraria from Novartis and Blueprint Medicines; and received speakers' bureau fees from Novartis. G.L.U. reports consulting or advisory role fees from Jazz Pharmaceuticals. E.K.W. reports leadership roles for Cambium Medical Technologies and Cambium Oncology; stock and other ownership interests with Cambium Medical Technologies, Cambium Oncology, Cerus, and Chimerix; honoraria from Novartis, Verastem, Kite (a Gilead Company), Pharmacyclics, Karyopharm Therapeutics, Sanofi, and Janssen Oncology; consulting or advisory role fees from Novartis, Verastem, Pharmacyclics, Karyopharm Therapeutics, Partners Healthcare, Kite (a Gilead Company), Cambium Medical Technologies, Alimera Sciences, and Sanofi; research funding from Novartis, Amgen, Juno Therapeutics, Verastem, Partners Healthcare, and Sanofi; royalties from patent on preparing platelet lysate that has been licensed to Cambium Medical Technologies; and travel, accommodation, and expenses from Janssen Oncology. S.V. reports consulting or advisory role fees from Omeros and Johnson & Johnson; and research funding from Sanofi (Inst). M. Stelljes received speakers' bureau fees from BMS, Amgen, Seagen, and GlaxoSmithKline; and research funding from Partner Therapeutics. A. Mendizabal received research funding from Novartis. L.S.M. reports stock and other ownership interests with Corvus Pharmaceuticals; honoraria from UpToDate; consulting or advisory role fees from Amgen, Medexus Pharmaceuticals, Astellas Pharma, Kite (a Gilead Company), and CTI BioPharma Corp; and research funding from Adaptive Biotechnologies, Astellas Pharma, Jasper Therapeutics, Kite (a Gilead Company), and BMS. H.-J.K. received honoraria from AbbVie, AML-Hub, BMS, Handok, Novartis, Aston Sci, Amgen, Takeda, Green Cross, AIM BioSciences, Astellas Pharma, Jazz Pharmaceuticals, Janssen, LG Chem, Pfizer, ViGen Cell, Ingenium, Sanofi, Meiji Seika Pharma, and MSD; consulting or advisory role fees from Jazz Pharmaceuticals, Novartis, AbbVie, Astellas Pharma, MSD, BMS, Takeda, Sanofi, Handok, and AML-Hub; and speakers' bureau fees from Jazz Pharmaceuticals, Takeda, and Novartis. M. Sohl reports consulting or advisory roles with Pfizer, MSD, BMS, Incyte, Takeda, Astellas, and Amgen; speakers’ bureau fees from Pfizer, Medac, MSD, Astellas, Jazz Pharmaceuticals, Amgen, Novartis, Gilead, Celgene, BMS, AbbVie, and Incyte; research funding from Pfizer; and travel support from Medac and Pfizer. Y.N. reports consulting or advisory role fees from Daiichi Sankyo/UCB Japan and Astellas Pharma; and speakers' bureau fees from Astellas Pharma, Daiichi Sankyo/UCB Japan, AbbVie, Amgen, BMS Japan, Chugai Pharmaceutical, CSL Behring, Janssen Pharmaceutical, Kyowa, Nippon Shinyaku, Novartis, Otsuka, Sumitomo Pharma Oncology, Takeda, MSD, and JCR Pharmaceuticals. M.O. received honoraria from Astellas Pharma; and speakers' bureau fees from Astellas Pharma, Daiichi Sankyo, Otsuka, and Novartis. C.C., N.H., M.R., J.E.H., S.C.G., and R.N. report employment with Astellas Pharma. S.M.D. reports leadership role with National Marrow Donor Program. M.M.H. reports consulting or advisory role fees from Medac (Inst); and research funding from Jazz Pharmaceuticals (Inst), Novartis (Inst), Sanofi (Inst), Astellas Pharma (Inst), Xenikos (Inst), and Gamida Cell (Inst). Y.-B.C. reports leadership role with ImmunoFree; stock and other ownership interests with ImmunoFree; consulting or advisory role fees from Magenta Therapeutics, Incyte, Novo Nordisk, Editas Medicine, Alexion Pharmaceuticals, Astellas Pharma, Takeda, Pharmacosmos, and Vor Biopharma. The remaining authors declare no competing financial interests.
Correspondence: Mark J. Levis, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, 1650 Orleans St, Room 2M44, Baltimore, MD 21287; email: levisma@jhmi.edu.
References
Author notes
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
The full-text version of this article contains a data supplement.