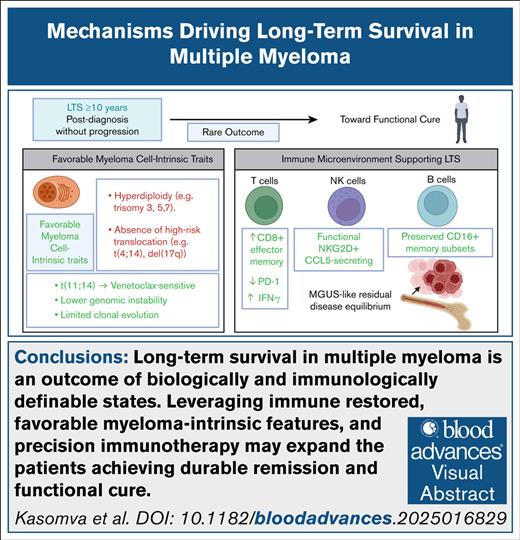
Visual Abstract
Long-term survival (LTS) in multiple myeloma (MM), defined as survival of ≥10 years after diagnosis following a single line of therapy, is an increasingly observed outcome due to significant therapeutic advancements. The introduction of proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies, autologous stem cell transplantation, and novel immunotherapies has transformed MM treatment. Importantly, only a subset of patients achieves long-term, durable disease control, suggesting that both myeloma-intrinsic and immune-mediated mechanisms play critical roles. Therapeutic advancements, including chimeric antigen receptor T-cell therapy and bispecific antibodies, have primarily benefited standard-risk patients. Beyond therapeutic interventions, LTS appears to be driven by distinct features of the immune bone marrow environment (IBME), in which enhanced T-cell function, increased natural killer cell cytotoxicity, and reduced immunosuppressive myeloid populations contribute to disease control. Understanding these immune adaptations in LTS MM provides a foundation for developing next-generation treatment strategies. Future research integrating genomic and immune profiling, along with IBME modulation, will be critical in shifting MM treatment paradigms from disease management to sustained remission and functional cures.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, with an estimated 36 110 new cases in the United States in 2025.1 Despite therapeutic advancements, MM remains incurable, characterized by cycles of remission and relapse.2 Over the past 2 decades, proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), monoclonal antibodies (mAbs), and autologous stem cell transplantation (ASCT) have extended survival.3,4 Median overall survival (OS) for standard-risk MM patients can now exceed 7 to 8 years. However, outcomes vary by cytogenetics, treatment response, and access to care.2 Identifying predictors of favorable vs poor responses remains a critical question.
A subset of MM patients achieves long-term survival (LTS), which we define as survival of ≥10 years after diagnosis, based upon prior studies identifying exceptional responders and functionally cured patients who remain progression-free or alive a decade or more after a single line of therapy.5-7 Importantly, the determinants of LTS remain unknown.4 The Connect MM Registry identifies common LTS traits; younger age, better baseline health, favorable cytogenetics, and use of ASCT and triplet therapies.8 One of the strongest predictors of LTS is a deep and sustained treatment response, with measurable residual disease (MRD) negativity.8 Yet, MRD negativity alone does not fully explain all cases of prolonged survival.4,8
Myeloma-intrinsic features alone do not fully account for LTS. Emerging evidence suggests that the immune bone marrow environment (IBME) plays a pivotal role in MM progression, therapeutic responses, and ultimately, LTS.9,10 The IBME fosters myeloma cell survival, immune evasion, and drug resistance through complex interactions between malignant plasma cells (PCs), stromal cells, immune cells, and other bone marrow components.11 Immune dysfunction–impaired T-cell and natural killer (NK) cell activity and expansion of myeloid-derived suppressor cells contribute to an immunosuppressive milieu.11-15
Novel immunotherapies have revolutionized MM treatment.2 Chimeric antigen receptor T-cell (CAR-T) therapies and bispecific antibodies (BsAbs) are capable of overcoming immune suppression and achieving prolonged remissions.3 These advancements raise the possibility of functional cures, defined as sustained disease control without progression despite the presence of residual disease.4 This review explores the latest advancements in MM treatment, including novel immunotherapy strategies and their impact on LTS, while dissecting the biological mechanisms that support disease control over prolonged periods, focusing on the role of immunotherapy and the IBME. This review does not comprehensively cover conventional treatment strategies such as novel PIs, chemotherapy regimens, or ASCT.
Current clinical treatment in MM
Although the primary focus of this review is on the molecular and immunological mechanisms supporting LTS in MM, it is important to summarize the current therapeutic landscape, which has rapidly evolved with over a dozen US Food and Drug Administration–approved agents in the last decade2 (Table 1; Figure 1).
Comparative overview of major clinical trials in MM
| Trial name . | Study population . | Treatment arm . | Control arm . | Primary end point . | PFS, mo . | OS, mo . | Key finding . | Reference . |
|---|---|---|---|---|---|---|---|---|
| CASSIOPEIA | NDMM, transplant-eligible | Dara-VTd | VTd | sCR rate | 57.6 | Not reached | Dara-VTd improved depth of response, increased stringent CR rate, and prolonged PFS over VTd. | 16 |
| GRIFFIN | NDMM, transplant-eligible | Dara-VRd | VRd | sCR Rate | 48.7 | Not reached | Dara-VRd significantly increased MRD-negative rates and improved PFS compared to VRd. | 17 |
| ICARIA-MM | R/R MM, lenalidomide-refractory | Isa-Pd | Pd | PFS | 11.5 | 17.4 | Isatuximab + Pd improved PFS and OS over Pd alone in lenalidomide-refractory MM. | 18 |
| POLLUX | R/R MM | Dara-Rd | Rd | PFS | 44.5 | Not reached | Dara-Rd significantly improved PFS and response rates, establishing Dara-Rd as a preferred regimen. | 19 |
| MagnetisMM-3 | R/R MM, triple-class refractory | Elranatamab (BsAb) | N/A | ORR | 17.2 | Not reached | Elranatamab, a BCMA/CD3 bispecific antibody, showed durable responses in heavily pretreated patients with R/R MM. | 20 |
| KarMMa-1 | R/R MM, triple-class refractory | Ide-cel (CAR-T) | N/A | ORR | 8.8 | 24.8 | Ide-cel demonstrated deep and durable responses in heavily pretreated patients with R/R MM. | 21 |
| CARTITUDE-1 | R/R MM, triple-class refractory | Cilta-cel (CAR-T) | N/A | ORR | 33 | Not reached | Cilta-cel achieved high ORR and deep responses, with superior survival benefits over standard care. | 22 |
| MajesTEC-1 | R/R MM, triple-class refractory | Teclistamab (BsAb) | N/A | ORR | 11.3 | Not reached | Teclistamab, a BCMA/CD3 bispecific antibody, showed promising efficacy in heavily pretreated patients with R/R MM. | 23 |
| Trial name . | Study population . | Treatment arm . | Control arm . | Primary end point . | PFS, mo . | OS, mo . | Key finding . | Reference . |
|---|---|---|---|---|---|---|---|---|
| CASSIOPEIA | NDMM, transplant-eligible | Dara-VTd | VTd | sCR rate | 57.6 | Not reached | Dara-VTd improved depth of response, increased stringent CR rate, and prolonged PFS over VTd. | 16 |
| GRIFFIN | NDMM, transplant-eligible | Dara-VRd | VRd | sCR Rate | 48.7 | Not reached | Dara-VRd significantly increased MRD-negative rates and improved PFS compared to VRd. | 17 |
| ICARIA-MM | R/R MM, lenalidomide-refractory | Isa-Pd | Pd | PFS | 11.5 | 17.4 | Isatuximab + Pd improved PFS and OS over Pd alone in lenalidomide-refractory MM. | 18 |
| POLLUX | R/R MM | Dara-Rd | Rd | PFS | 44.5 | Not reached | Dara-Rd significantly improved PFS and response rates, establishing Dara-Rd as a preferred regimen. | 19 |
| MagnetisMM-3 | R/R MM, triple-class refractory | Elranatamab (BsAb) | N/A | ORR | 17.2 | Not reached | Elranatamab, a BCMA/CD3 bispecific antibody, showed durable responses in heavily pretreated patients with R/R MM. | 20 |
| KarMMa-1 | R/R MM, triple-class refractory | Ide-cel (CAR-T) | N/A | ORR | 8.8 | 24.8 | Ide-cel demonstrated deep and durable responses in heavily pretreated patients with R/R MM. | 21 |
| CARTITUDE-1 | R/R MM, triple-class refractory | Cilta-cel (CAR-T) | N/A | ORR | 33 | Not reached | Cilta-cel achieved high ORR and deep responses, with superior survival benefits over standard care. | 22 |
| MajesTEC-1 | R/R MM, triple-class refractory | Teclistamab (BsAb) | N/A | ORR | 11.3 | Not reached | Teclistamab, a BCMA/CD3 bispecific antibody, showed promising efficacy in heavily pretreated patients with R/R MM. | 23 |
Cilta-cel (CAR-T), ciltacabtagene autoleucel; CR, complete response; Dara-VTd, daratumumab + VTd; Dara-VRd, daratumumab + VRd; Dara-Rd, daratumumab + lenalidomide, dexamethasone; Elranatamab (BsAb), BCMA/CD3 bispecific antibody; Isa-Pd, isatuximab + pomalidomide, dexamethasone; Ide-cel (CAR-T), idecabtagene vicleucel (CAR-T); N/A, not applicable; ORR, overall response rate; sCR, stringent complete response; Teclistamab (BsAb), BCMA/CD3 bispecific antibody; VTd, bortezomib, thalidomide, dexamethasone.
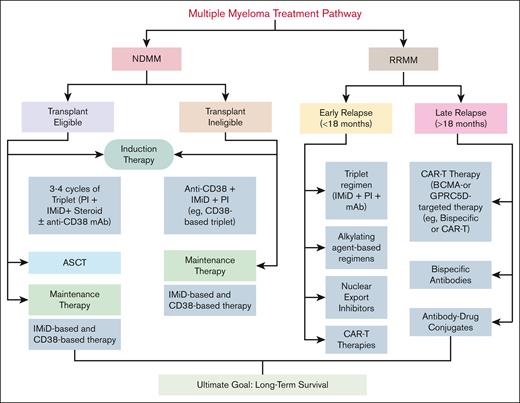
MM treatment pathway. Transplant-eligible patients with NDMM receive 3 to 4 cycles of induction therapy with a triplet regimen (PI + IMiD + steroid) or a quadruplet regimen that includes an anti-CD38 mAb, followed by ASCT and maintenance therapy. Transplant-ineligible patients are treated with CD38-based triplet regimens, combining an anti-CD38 mAb, an IMiD, and a PI, followed by maintenance therapy. The maintenance phase aims to prolong remission and typically includes IMiD-based or CD38-based regimens, selected according to individual risk stratification. R/R MM treatment is divided into early and late relapse. Early relapse is treated with triplet regimens, alkylating agents, nuclear export inhibitors, or CAR-T therapy. Late relapse is managed with BCMA- or GPRC5D-targeted therapies, including CAR T cells, BsAbs, or antibody-drug conjugates. The overall goal is to achieve long-term disease control and extend survival.
MM treatment pathway. Transplant-eligible patients with NDMM receive 3 to 4 cycles of induction therapy with a triplet regimen (PI + IMiD + steroid) or a quadruplet regimen that includes an anti-CD38 mAb, followed by ASCT and maintenance therapy. Transplant-ineligible patients are treated with CD38-based triplet regimens, combining an anti-CD38 mAb, an IMiD, and a PI, followed by maintenance therapy. The maintenance phase aims to prolong remission and typically includes IMiD-based or CD38-based regimens, selected according to individual risk stratification. R/R MM treatment is divided into early and late relapse. Early relapse is treated with triplet regimens, alkylating agents, nuclear export inhibitors, or CAR-T therapy. Late relapse is managed with BCMA- or GPRC5D-targeted therapies, including CAR T cells, BsAbs, or antibody-drug conjugates. The overall goal is to achieve long-term disease control and extend survival.
Treatment for transplant-eligible patients
In transplant-eligible patients (Figure 1), standard induction includes a quadruplet regimen: a PI, an IMiD, and a CD38-targeting mAb4 to enhance early disease control. Lenalidomide and pomalidomide enhance immune surveillance and exert antimyeloma effects by modulating T-cell and NK cell activity.8,9 Bortezomib and carfilzomib disrupt protein degradation in malignant PCs, leading to apoptosis, with carfilzomib being more potent.10,11 Anti-CD38 mAbs like daratumumab and isatuximab (Isa) promote direct apoptosis, NK cell–mediated antibody-dependent cellular cytotoxicity, and macrophage-mediated phagocytosis.17 Quadruplet regimens incorporating daratumumab have demonstrated improved stringent complete response and MRD negativity rates, which are strong predictors of prolonged progression-free survival (PFS).17 The GMMG-HD7 trial showed Isa plus VRd (bortezomib, lenalidomide, dexamethasone) (Isa-VRd) significantly increased MRD negativity over VRd alone, validating CD38 mAbs in early immune modulation.24 These findings support the growing adoption of quadruplet induction regimens to optimize early disease control and LTS.25
Treatment for transplant-ineligible patients
In transplant-ineligible patients (Figure 1), frontline regimens combining mAbs with standard therapies have improved PFS and OS.16,26 DRd (daratumumab, lenalidomide, dexamethasone) and VRd have shown superiority over Rd (lenalidomide and dexamethasone) alone.27 Recent studies extending the quadruplet approach indicate that adding daratumumab or Isa to VRd has improved MRD negativity rates and remission durability, highlighting the importance of early immune modulation.28,29 These regimens enhance T-cell and NK cell activity, suppress immunosuppressive subsets, and may support durable disease control.28,29 Although promising, managing toxicities, especially in older patients, remains critical to balancing safety and efficacy.30
Maintenance treatment
Maintenance therapy (Figure 1) prolongs remission and delays relapse by suppressing residual disease and sustaining immune surveillance. Lenalidomide remains the standard post-ASCT maintenance therapy, enhancing T and NK cell function while suppressing interleukin 6 (IL-6) to prolong PFS and OS.31-34 In high-risk patients, bortezomib-, carfilzomib-, or daratumumab-based regimens may offer added benefit, though superiority in t(4;14), del(17p), or gain(1q21) remains inconclusive. Recent trials like FORTE, ATLAS, and AURIGA support a role for anti-CD38 antibodies and PIs in sustaining immune surveillance and deep responses.35-38 Collectively, maintenance therapies sustain deep responses and reinforce immune-mediated disease control.
Treatment of relapsed MM
Management of relapsed MM (Figure 1) is guided by prior treatments, resistance patterns, and patient fitness. Patients with early relapse typically receive triplet combinations that introduce new drug classes.18,19,39-43 In triple-class refractory settings, novel immune-based therapies such as selinexor, B-cell maturation antigen (BCMA)-targeted CAR T cells (idecabtagene vicleucel, ciltacabtagene autoleucel), BsAbs (eg, teclistamab, talquetamab), and antibody-drug conjugates (eg, belantamab mafodotin) offer potent antimyeloma activity through direct cytotoxicity and immune system engagement.20-23,44-47 These agents activate T cells, enhance immune surveillance, or bypass resistance to prior therapies. Although most patients with relapsed/refractory MM (R/R MM) eventually relapse and progress, a subset achieves LTS. Data from the Connect MM Registry suggest that LTS is associated with early use of ASCT,8 lenalidomide maintenance, and deeper responses. Importantly, modern induction and salvage regimens incorporating CD38 mAbs and immune-enhancing agents have improved MRD negativity rates, which strongly correlate with durable remission.20-23,44-47 Although long-term outcome data from recent trials are still evolving, their ability to induce sustained immune control positions them as potential functional cures.4,8
Immunomodulatory effects of PIs
Beyond their direct cytotoxic effects on malignant PCs, PIs such as bortezomib and carfilzomib exert significant immunomodulatory effects. PIs stabilize MHC class I molecules, enhancing cytotoxic T lymphocytes (CTLs) recognition of myeloma cells.48-50 Preclinical studies have shown that bortezomib sensitizes myeloma cells to immune-mediated lysis and augments antimyeloma T-cell responses.51 Notably, bortezomib has been shown to induce immunogenic cell death in myeloma cells, a process that involves calreticulin exposure, dendritic cell–mediated phagocytosis, and activation of type I interferon (IFN) responses through the cyclic GMP-AMP synthase/stimulator of interferon genes pathway.52 However, at higher doses, PIs can impair T-cell proliferation, dampen dendritic cell function, and modulate cytokine production, which may contribute to immune suppression.53-55 Therefore, the immune effects of PIs are dose-dependent and context-specific.
Immunotherapy in MM
Immunotherapies like CAR-T therapy and BsAbs have transformed MM treatment by enhancing immune responses against myeloma cells.
CAR-T therapy: a breakthrough in MM treatment
CAR-T therapy represents a transformative approach in R/R MM, offering deep responses in patients with otherwise limited options. BCMA-targeted CAR-T products like idecabtagene vicleucel and ciltacabtagene autoleucel induce high MRD negativity and prolonged remissions.21,22 Beyond cytotoxicity, CAR T cells can restore antimyeloma immune surveillance, but their persistence is often limited by T-cell exhaustion, antigen escape, and manufacturing delays.21,22 Emerging strategies to address these challenges include “armored” CAR T cells that secrete IL-15 to promote persistence, dual-targeting constructs to overcome antigen loss, and off-the-shelf CAR-T platforms to expand accessibility.56 Maintenance approaches, such as lenalidomide or checkpoint blockade postinfusion, are also being explored to sustain T-cell activity and immune memory.57,58 CAR-T therapy is now under investigation in newly diagnosed MM (NDMM), reflecting its therapeutic potential.
BsAbs and T-cell engagers: redirecting immune responses
BsAbs represent a major advance in immune-directed therapy for R/R MM by simultaneously binding CD3 on T cells and myeloma-associated antigens such as BCMA,59,60 GPRC5D, or FcRH5 on myeloma cells.20,23,61 This redirection of cytotoxic T cells enables potent, antigen-specific killing independent of native TCR specificity. Agents like teclistamab and elranatamab (BCMA×CD3) and talquetamab (GPRC5D×CD3) have shown high response rates and deep remissions in heavily pretreated patients, including those refractory to prior T-cell–based therapies.20,23,59-61 BsAbs offer advantages over CAR T cells including immediate availability and combinatorial use with agents that enhance T-cell persistence.23 However, challenges such as cytokine release syndrome, neurotoxicity, infection risk,20 and antigen escape remain significant.59 The durability of BsAb-induced responses and their capacity to generate long-term immune memory are under active investigation.11,62 Early integration of BsAbs into frontline settings in which immune function is more intact may enhance their potential to support long-term disease control.62,63 Although LTS data are still emerging, the combination of rapid immune activation, MRD clearance, and treatment-free intervals suggests that BsAbs may become important in inducing sustained immune surveillance and contributing to LTS in MM.4,11
Molecular and immunological mechanisms promoting LTS in MM
LTS in MM is likely driven by intrinsic myeloma cell characteristics and extrinsic interactions within the IBME. Intrinsic mechanisms involve genetic and molecular alterations within malignant PCs, influencing their survival, proliferation, and resistance to therapy. Extrinsic mechanisms encompass interactions between malignant cells and the components of the IBME, including immune cells, stromal cells, and soluble factors, which shape disease progression and treatment response.64,65 Extensive preclinical work has helped elucidate these immune interactions with the bone marrow (Table 2). Together, these mechanisms dictate the disease trajectory and potential for LTS in some patients.
Key preclinical studies informing the role of the IBME in MM
| Preclinical model . | Key finding . | Relevance to IBME . | Reference . |
|---|---|---|---|
| Mouse models | Defined the concept of tumor immunoediting (elimination, equilibrium, escape) | Basis for understanding immune control and evasion in MM | 66 |
| Human MGUS patient samples + functional assays | Identified SOX2-specific T-cell immunity delaying MM progression | Highlights antigen-specific T-cell surveillance in early MM | 67 |
| In vitro studies on soluble MICA | Soluble MICA impairs NKG2D function on NK cells and T cells | Mechanism of immune escape via downregulating NK/T cytotoxicity | 68 |
| Mouse models + human samples | DNAM-1 (CD226) is critical for NK cell antimyeloma immunity | Reinforces importance of NK cytotoxic checkpoints | 69 |
| Mouse models of CAR-T resistance | Targeting cancer-associated fibroblasts restores CAR-T function | Highlights the role of BM niche in therapy resistance | 58 |
| CS1 CAR T cells + lenalidomide | Lenalidomide enhances CAR-T function | Shows immunomodulatory agents synergize with immune effectors | 57 |
| Humanized mouse models | Microenvironment supports premalignant and malignant PCs | Demonstrates early microenvironmental dependency in MM | 70 |
| Preclinical model . | Key finding . | Relevance to IBME . | Reference . |
|---|---|---|---|
| Mouse models | Defined the concept of tumor immunoediting (elimination, equilibrium, escape) | Basis for understanding immune control and evasion in MM | 66 |
| Human MGUS patient samples + functional assays | Identified SOX2-specific T-cell immunity delaying MM progression | Highlights antigen-specific T-cell surveillance in early MM | 67 |
| In vitro studies on soluble MICA | Soluble MICA impairs NKG2D function on NK cells and T cells | Mechanism of immune escape via downregulating NK/T cytotoxicity | 68 |
| Mouse models + human samples | DNAM-1 (CD226) is critical for NK cell antimyeloma immunity | Reinforces importance of NK cytotoxic checkpoints | 69 |
| Mouse models of CAR-T resistance | Targeting cancer-associated fibroblasts restores CAR-T function | Highlights the role of BM niche in therapy resistance | 58 |
| CS1 CAR T cells + lenalidomide | Lenalidomide enhances CAR-T function | Shows immunomodulatory agents synergize with immune effectors | 57 |
| Humanized mouse models | Microenvironment supports premalignant and malignant PCs | Demonstrates early microenvironmental dependency in MM | 70 |
BM, bone marrow.
Cell-intrinsic mechanisms in LTS in MM
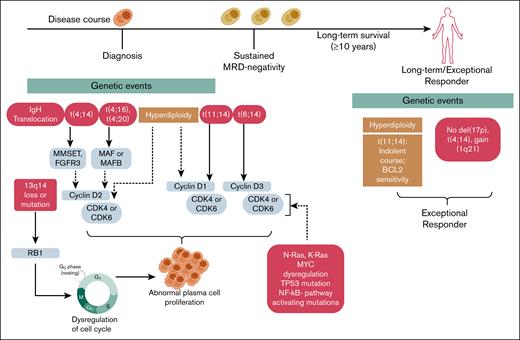
Emerging evidence suggests that myeloma cell-intrinsic mechanisms, such as favorable genomic and cytogenetic features like hyperdiploidy and the absence of high-risk chromosomal alterations, underlie LTS in a subset of patients71 (Figure 2).
Genetic features associated with LTS in MM. This schematic illustrates the evolution of MM from diagnosis through sustained MRD negativity to LTS (≥10 years), highlighting the genetic events that distinguish exceptional responders. Although disease progression often involves IgH translocations, chromosomal deletions (eg, del(13q14), del(17p)), and dysregulation of the cell cycle and oncogenic signaling, long-term survivors frequently exhibit more stable genomic profiles. Favorable features, such as hyperdiploidy and isolated t(11;14), which is associated with an indolent course and sensitivity to B-cell kymphoma 2 inhibition, are enriched in these patients, whereas high-risk lesions like t(4;14), gain(1q21), and del(17p) are typically absent. These intrinsic characteristics may contribute to reduced clonal evolution, sustained immune surveillance, and prolonged treatment-free remission. IgH, immunoglobulin H.
Genetic features associated with LTS in MM. This schematic illustrates the evolution of MM from diagnosis through sustained MRD negativity to LTS (≥10 years), highlighting the genetic events that distinguish exceptional responders. Although disease progression often involves IgH translocations, chromosomal deletions (eg, del(13q14), del(17p)), and dysregulation of the cell cycle and oncogenic signaling, long-term survivors frequently exhibit more stable genomic profiles. Favorable features, such as hyperdiploidy and isolated t(11;14), which is associated with an indolent course and sensitivity to B-cell kymphoma 2 inhibition, are enriched in these patients, whereas high-risk lesions like t(4;14), gain(1q21), and del(17p) are typically absent. These intrinsic characteristics may contribute to reduced clonal evolution, sustained immune surveillance, and prolonged treatment-free remission. IgH, immunoglobulin H.
Genomic instability, a hallmark of MM, drives myeloma progression, clonal diversity, and therapy resistance. It results from mitotic dysregulation, centrosome abnormalities, impaired DNA repair, and hypoxia-induced oxidative stress, leading to numerical and structural chromosomal alterations. Hyperdiploidy, present in ∼50% of cases, involves gains of odd-numbered chromosomes (eg, 3, 5, 7, 9, 11) and is associated with better prognosis. In monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM) hyperdiploidy is associated with slower progression.65 In contrast, immunoglobulin H translocations (∼40%) reposition oncogenes near the immunoglobulin H enhancer, driving abnormal gene expression.65 Common translocations include t(11;14), activating cyclin D1 (CCND1), and t(4;14), activating fibroblast growth factor receptor 3 and multiple myeloma su(var)3-9, enhancer-of-zeste, and trithorax, which confer a poor prognosis. Other translocations, such as t(14;16) and t(14;20), involve MAF and MAFB genes, which also drive myeloma aggression and therapy resistance. The t(6;14) translocation dysregulates CCND3. Although rare, it is generally considered a standard-risk abnormality and is not associated with poor prognosis in MM (Figure 2).72,73 Additional copy number alterations also promote genomic instability and can define high-risk MM.72,74
Hyperdiploidy is a feature of LTS. In a study of 33 exceptional responders to lenalidomide-based therapy, 19 of 24 patients (79%) with available cytogenetic data exhibited hyperdiploidy or trisomies, whereas none showed high-risk features such as del(17p), t(4;14), or t(14;16).73,75 The t(11;14) translocation was observed in 3 patients (12.5%), including 1 with concurrent trisomies. Although not universally associated with favorable outcomes, t(11;14) is linked to an indolent course and increased sensitivity to B-cell kymphoma 2 inhibitors like venetoclax and sonrotoclax, particularly in cases with high B-cell kymphoma 2 expression.76-78 This association is further supported by data from the Connect MM Registry, which reported a higher prevalence of t(11;14) among long-term survivors.8 Collectively, this suggests that the relative genomic stability seen in hyperdiploid and non–high-risk cytogenetic profiles may reduce evolutionary pressure and limit the emergence of resistant clones, supporting prolonged disease control.
Additional intrinsic mechanisms may contribute to LTS in MM, although direct evidence is limited. It is speculated that genomic stability in LTS MM may promote immune surveillance by reducing neoantigen load and clonal diversity. Stability in key regulatory pathways such as TP53 and NF-κB may also suppress inflammation and prevent immune-evasive clones. Mutations in NF-κB regulators (TRAF2, TRAF3, CYLD), common in advanced MM, impair antigen presentation, their absence in LTS MM may support sustained immune surveillance.76,79 Genomic instability also gives rise to mutational signatures reflecting specific DNA damage and repair mechanisms. The apoliprotein B mRNA editing enzyme (APOBEC) signature, caused by cytidine deaminases, is enriched in high-risk MM, particularly in cases with t(14;16).72,76 Another feature, kataegis, involves clusters of hypermutations mediated by APOBEC or adenosine deaminase enzymes, contributing to clonal evolution and immune escape.72,80 We speculate that reduced APOBEC activity in LTS MM may limit mutational burden and preserve immunogenicity.
Epigenetic stability may contribute to LTS. Progressive MM is marked by chromatin remodeling and aberrant methylation that promote immune evasion and transcriptional reprogramming.72,81 By contrast, MM cells in LTS may retain a more stable epigenetic landscape, thereby limiting phenotypic plasticity. In addition, LTS MM cells may exhibit reduced reliance on bone marrow stromal support, particularly through pathways such as CXCR4, alpha 4 beta 1 integrin, and the stromal cell-derived factor 1/CXCL12 axis.11,65,82 Although typical MM cells leverage the bone marrow microenvironment for survival, proliferation, and immune protection, reduced dependency on these cues in LTS MM may enhance their vulnerability to immune-mediated clearance and therapy-induced apoptosis. This diminished microenvironmental engagement may reflect a biologically indolent or less adaptive clone, contributing to more favorable outcomes.11,65,83 Lower metabolic plasticity may further reduce adaptability to oxidative stress and hypoxia, increasing vulnerability to therapy.72,79,81 Further studies integrating genomic, epigenomic, metabolic, and functional profiling will enhance treatment options and long-term disease control.
Cell-extrinsic mechanisms in LTS in MM
Immunoediting in MM is a dynamic process comprising immune system–mediated elimination, equilibrium, and escape, which describes the interaction between the immune system and malignant PCs (Figure 3).66,83-85
Immune surveillance and tumor progression in MM. The immune system plays a crucial role in MM progression through 3 distinct phases. Elimination phase: abnormal PCs are recognized and destroyed by CD8+ T cells and NK cells through the release of IFN-γ and perforin. This immune response helps eliminate early malignant PCs, maintaining tumor control. Equilibrium phase: during this phase, immune pressure from CD4+ T cells and IL-12 maintains tumor dormancy, keeping myeloma cells in check. However, some myeloma cells survive by gradually reducing their immunogenicity. Escape phase: myeloma cells evade immune destruction by upregulating MHC-I, recruiting immunosuppressive cells (including Tregs, TAMs, and MDSCs), and expressing immune checkpoint molecules (PD-1, CTLA-4, CD160, 2B4). Additionally, soluble inhibitory molecules (MICA, MICB) contribute to immune escape, enabling disease progression. MDSC, myeloid-derived suppressor cell; TAMs, tumor-associated macrophages.
Immune surveillance and tumor progression in MM. The immune system plays a crucial role in MM progression through 3 distinct phases. Elimination phase: abnormal PCs are recognized and destroyed by CD8+ T cells and NK cells through the release of IFN-γ and perforin. This immune response helps eliminate early malignant PCs, maintaining tumor control. Equilibrium phase: during this phase, immune pressure from CD4+ T cells and IL-12 maintains tumor dormancy, keeping myeloma cells in check. However, some myeloma cells survive by gradually reducing their immunogenicity. Escape phase: myeloma cells evade immune destruction by upregulating MHC-I, recruiting immunosuppressive cells (including Tregs, TAMs, and MDSCs), and expressing immune checkpoint molecules (PD-1, CTLA-4, CD160, 2B4). Additionally, soluble inhibitory molecules (MICA, MICB) contribute to immune escape, enabling disease progression. MDSC, myeloid-derived suppressor cell; TAMs, tumor-associated macrophages.
Elimination phase
In MGUS, SMM, and MM, innate and adaptive immune cells collaborate to eliminate malignant PCs that bypass intrinsic apoptotic controls. CD8+ T cells and NK cells eliminate myeloma cells via perforin, granzyme B, and IFN-γ secretion, and through stress-induced ligands that activate NK cell receptors. Key receptors like DNAM-1 (CD226) enhance cytotoxicity by interacting with overexpressed ligands such as pulmonary vascular resistance (CD155) and nectin-2 (CD112) on malignant PCs.68,69,83,85-87 NKG2D receptors bind MICA, MICB, and UL16-binding proteins to trigger immune activation. However, soluble MICA impairs NKG2D function, facilitating immune evasion. Patients with MGUS exhibit high anti-MICA antibodies, which counteract this inhibition and slow myeloma progression.68,69,83,85-87 The elimination phase depends on strong cytotoxic T-cell and NK cell responses supported by proinflammatory cytokines.
Equilibrium phase
The equilibrium phase represents a state of myeloma dormancy, maintained by immune surveillance during MGUS and SMM. During this phase, the immune system constrains myeloma progression through antigen-specific T-cell responses. These include responses against targets such as SOX2 (an embryonic stem cell antigen) and oral-facial-digital syndrome 1, which are associated with a reduced risk of progression to MM.67,70,83,85,88 SOX2-specific T cells are linked to lower progression risk.89 IMiDs, such as lenalidomide, delay SMM progression by enhancing NK/CD8+ T-cell function and tumor immunosurveillance.90 IMiDs also reduce cereblon levels, destabilizing transcription factors IKZF1 (Ikaros) and IKZF3 (Aiolos), which are critical for myeloma cell survival.85,90-92 Mouse studies support a role for IL-12, IFN-γ, and cytotoxic CD8+ T cells in maintaining equilibrium.66 However, accumulating genomic and epigenetic alterations, such as somatic mutations, chromosomal aberrations, and transcriptional dysregulation, can eventually disrupt this balance, enabling immune evasion and myeloma progression.72,79,84,85,93
Escape phase
In the escape phase, malignant PCs evade immune control through mechanisms such as downregulation of MHC class I, loss of tumor antigen expression, altered antigen processing, immune checkpoint upregulation, and secretion of immunosuppressive factors, all of which impair recognition and destruction by CTLs.72,79,84,85,93-95 Myeloma cells also modify tumor-associated epitopes to avoid immune detection93,94 or overexpress immune checkpoint molecules such as programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte associate protein 4 (CTLA-4), CD160, and 2B4 to inhibit T-cell function.93-96 MM reshapes the bone marrow microenvironment by expelling NK cells and recruiting myeloid-derived suppressor cells, tumor-associated macrophages, regulatory B cells, and regulatory T cells (Tregs), which release TGF-β, IL-10, and indoleamine 2,3-dioxygenase, further suppressing CTL/NK cell activity. Additionally, malignant PCs shed antigenic molecules (MICA/MICB), impairing NK-mediated cytotoxicity.68,85 These mechanisms allow myeloma progression despite immune surveillance.93-95
Immune surveillance and long-term immune regulation in LTS MM
LTS MM exhibits a distinct IBME that supports durable immune surveillance and disease control.11,83,97 Compared to NDMM, patients with LTS MM demonstrate enhanced cytotoxic responses, sustained inflammatory remodeling, and adaptive immune changes that contribute to prolonged remission.11,83 Key immune components, such as T cells, NK cells, and B cells are functionally adapted to support remission (Figure 4; Table 3).83,97,98
Immune mechanisms contributing to LTS in patients with MM. CD8+ and CD4+ T cells exhibit increased activation (↑CD57, CD45RA, OX-40) and produce IFN-γ, enhancing antimyeloma responses. NK cells, activated by IL-15 and ADCC, secrete CCL3, CCL4, and CCL5 to target myeloma cells. Naïve and memory B cells increase, whereas regulatory immune cells (MDSCs, TAMs, Tregs, and Bregs) show reduced suppressive activity (reduced IL-10 and PD-1 expression). Reducing the immunosuppressive environment and promoting immune restoration contribute to prolonged tumor growth control. ADCC, antibody-dependent cellular cytotoxicity; Bregs, regulatory B cells; MDSC, myeloid-derived suppressor cell; TAMs, tumor-associated macrophages.
Immune mechanisms contributing to LTS in patients with MM. CD8+ and CD4+ T cells exhibit increased activation (↑CD57, CD45RA, OX-40) and produce IFN-γ, enhancing antimyeloma responses. NK cells, activated by IL-15 and ADCC, secrete CCL3, CCL4, and CCL5 to target myeloma cells. Naïve and memory B cells increase, whereas regulatory immune cells (MDSCs, TAMs, Tregs, and Bregs) show reduced suppressive activity (reduced IL-10 and PD-1 expression). Reducing the immunosuppressive environment and promoting immune restoration contribute to prolonged tumor growth control. ADCC, antibody-dependent cellular cytotoxicity; Bregs, regulatory B cells; MDSC, myeloid-derived suppressor cell; TAMs, tumor-associated macrophages.
Roles of immune cells in LTS MM
| Immune cell type . | Role in immune surveillance . | Changes in LTS MM . | Therapeutic modulation . |
|---|---|---|---|
| T cells (CD8+) | Direct cytotoxicity against myeloma cells via perforin/granzyme release, cytokine production (IFN-γ, TNF-α) | Increased CD8+ T-cell counts, reduced Tregs, enhanced effector memory subsets, improved polyfunctional response | Pomalidomide enhances CD8+ T-cell activation, reduces Tregs, and promotes T-cell persistence. Checkpoint inhibitors (anti–PD-1/PD-L1) may further improve exhausted T cells |
| T cells (CD4+) | Helper function, cytokine release (IL-2, IL-21), support for CD8+ T cells and B-cell activation | Shift toward Th1/Th17 responses, reduced Tfh (follicular helper) support for myeloma-promoting B cells, functional Treg suppression | IL-2 agonists promote effector T-cell expansion while reducing suppressive Tregs. IMiDs (eg, lenalidomide, pomalidomide) modulate CD4+ T-cell function |
| NK cells | Cytotoxicity against myeloma cells via natural killing mechanisms, secretion of proinflammatory cytokines (IFN-γ, TNF-α) | Altered phenotype with increased CCL3/CCL4/CCL5 secretion, signs of exhaustion (elevated TIGIT, NKG2A) | IL-15 and mAbs targeting NK exhaustion markers (eg, anti-NKG2A) enhance NK function |
| B cells | Antigen presentation, production of myeloma-specific antibodies, regulatory functions | Partial recovery of naïve and memory B-cell subsets, reduced Bregs, which lowers immunosuppressive effects | Pomalidomide increases B1b cells, modulates Breg function, and enhances antigen presentation, contributing to an improved antimyeloma response |
| Immune cell type . | Role in immune surveillance . | Changes in LTS MM . | Therapeutic modulation . |
|---|---|---|---|
| T cells (CD8+) | Direct cytotoxicity against myeloma cells via perforin/granzyme release, cytokine production (IFN-γ, TNF-α) | Increased CD8+ T-cell counts, reduced Tregs, enhanced effector memory subsets, improved polyfunctional response | Pomalidomide enhances CD8+ T-cell activation, reduces Tregs, and promotes T-cell persistence. Checkpoint inhibitors (anti–PD-1/PD-L1) may further improve exhausted T cells |
| T cells (CD4+) | Helper function, cytokine release (IL-2, IL-21), support for CD8+ T cells and B-cell activation | Shift toward Th1/Th17 responses, reduced Tfh (follicular helper) support for myeloma-promoting B cells, functional Treg suppression | IL-2 agonists promote effector T-cell expansion while reducing suppressive Tregs. IMiDs (eg, lenalidomide, pomalidomide) modulate CD4+ T-cell function |
| NK cells | Cytotoxicity against myeloma cells via natural killing mechanisms, secretion of proinflammatory cytokines (IFN-γ, TNF-α) | Altered phenotype with increased CCL3/CCL4/CCL5 secretion, signs of exhaustion (elevated TIGIT, NKG2A) | IL-15 and mAbs targeting NK exhaustion markers (eg, anti-NKG2A) enhance NK function |
| B cells | Antigen presentation, production of myeloma-specific antibodies, regulatory functions | Partial recovery of naïve and memory B-cell subsets, reduced Bregs, which lowers immunosuppressive effects | Pomalidomide increases B1b cells, modulates Breg function, and enhances antigen presentation, contributing to an improved antimyeloma response |
Bregs, regulatory B cells; PD-L1, programmed cell death ligand 1; TNF-α, tumor necrosis factor-α.
T cells
Patients with LTS MM display higher frequencies of CD8+ cytotoxic T cells and a favorable Treg/Th17 balance, contributing to an antimyeloma immune environment (Figure 4; Table 3).97,98 CD8+ T cells in LTS MM maintain low exhaustion markers, sustained IFN-γ production, and CXCR3-driven bone marrow trafficking.11,97-99 These adaptations enhance immunosurveillance.97,98 Therapeutic agents such as IMiDs (lenalidomide, pomalidomide) help preserve T-cell functionality by downregulating PD-1 on CD4+ T cells, upregulating OX-40 on CD8+ T cells, and suppressing Tregs.100,101 Patients with LTS MM also show enrichment of effector memory CD8+ T cells (CD57+, CD45RA+), which are primed for rapid reactivation.102 These features support long-term immune memory and functional antimyeloma surveillance.
Longitudinal immune profiling in patients with MM on lenalidomide maintenance therapy reveals increased proportions of naïve and central memory CD4+ and CD8+ T cells, alongside reduced frequencies of exhausted and regulatory subsets. These immune profiles resemble those of healthy donors and occur irrespective of transplant status, suggesting that endogenous immune reconstitution may underpin long-term disease control.103 Immune normalization, defined as the restoration of bone marrow immune composition to a healthy-like state, has emerged as a potential biomarker of immune-mediated disease control. In high-risk SMM, posttherapy immune normalization is associated with prolonged PFS and is characterized by elevated GZMK+ CD8+ memory T cells, reduced inflammatory myeloid cells, and diminished expression of T-cell exhaustion markers. These signatures reflect restored immune surveillance and likely extend to LTS MM.104 Tregs are known to expand in MM bone marrow, suppressing cytotoxic T-cell activity and dendritic cell maturation, thereby promoting immune evasion.94 Their frequency correlates with progression from MGUS to MM.105,106 Although direct targeting of Tregs is not standard in MM, some agents such as low-dose cyclophosphamide and IMiDs (lenalidomide) can reduce Treg frequency or function.107,108 However, selective depletion remains challenging because of the risk of autoimmunity and disruption of immune homeostasis.109
An emerging concept in LTS MM is that some treated patients may enter a “MGUS-like” state, characterized by stable residual disease without progression. This likely reflects immune-mediated dormancy driven by restored immune surveillance, akin to that observed in MGUS and SMM, in which CD8+ T cells and other immune regulators constrain clonal PCs.67,70,83,85,88 Mechanisms such as IFN-γ signaling, memory T-cell maintenance, and immune checkpoint modulation may underlie this equilibrium and offer insight into durable, noneradicative disease control.70,83 Although checkpoint inhibitors (anti–PD-1, anti–CTLA-4) initially showed promise in MM, results from phase 3 trials were disappointing.83,94 Notably, the KEYNOTE-183110 and KEYNOTE-185111 trials, which combined PD-1 blockade with IMiDs, demonstrated no survival benefit and even a trend toward worse outcomes when pembrolizumab was combined with lenalidomide or pomalidomide in relapsed and NDMM, respectively.110,111 These findings prompted regulatory caution and have largely halted further checkpoint inhibitor development in myeloma.
Interestingly, similar features of immune surveillance are observed in patients with durable responses to CAR-T therapy. Both the T-cell phenotype at leukapheresis and the postinfusion immune environment are critical predictors of clinical outcomes.112,113 CAR-T products derived from earlier treatment stages often contain a higher proportion of CD8+CD45RO–CD27+ early memory T cells and exhibit higher CD4/CD8 ratios, features that are associated with improved expansion and antimyeloma activity.112 In vivo, greater CD8+ CAR-T expansion and effective lymphodepletion correlate with better clinical responses.113 Patients with prolonged PFS retain TCF1+CD27+ memory-like T cells and CLEC9A+ dendritic cells in the bone marrow, whereas early relapsers display exhausted CD8+ T cells and immunosuppressive BAFF+PD-L1+ myeloid cells.114 High peak CAR-T expansion, low baseline myeloma burden, and early depth of response are strong predictors of sustained remission.115 These findings underscore the importance of optimizing T-cell fitness during manufacturing, enriching for memory-like phenotypes, and employing maintenance strategies that preserve T-cell function. However, long-term follow-up data in true LTS post–CAR-T therapy remain limited, and defining the cellular correlates of durable response is essential to advancing functional cures in MM.
NK cells
NK cells in LTS MM retain plasticity, characterized by enhanced cytotoxic potential and inflammatory chemokine expression (CCL3, CCL4, CCL5), which facilitates immune surveillance within the bone marrow (Figure 4; Table 3).11,83,97,98 Unlike NDMM, in which NK cells downregulate activating receptors (NKG2D, NKp30, NKp46) and upregulate inhibitory ligands (HLA-C, HLA-E), LTS MM NK cells maintain strong NKG2D-mediated cytotoxicity, contributing to prolonged myeloma suppression.83,116-118
This sustained NK function may be influenced by IMiD therapy, as lenalidomide and pomalidomide restore NK cytotoxicity by enhancing IL-15 signaling and increasing antibody-dependent cellular cytotoxicity.119,120 In addition, mAbs such as daratumumab and elotuzumab further promote NK-mediated myeloma clearance by blocking immune evasion mechanisms.120,121 Clinically, higher NK cell counts correlate with prolonged PFS and OS in patients with LTS MM, particularly in those who have undergone allogeneic hematopoietic stem cell transplantation, highlighting the critical role of NK cells in long-term immune restoration.119,120
Nonmalignant B cells
Normal B cells in LTS MM play a key role in maintaining immune homeostasis and limiting progression (Figure 4; Table 3).98,122,123 Compared to NDMM, patients with LTS MM exhibit higher CD19+ B-cell counts, which correlate with improved OS.98,122 These findings suggest that B cells contribute to myeloma control through antigen presentation, immune-mediated cytotoxicity, and sustained immune surveillance.98 Interestingly, LTS MM B cells retain transcriptional imprints of chronic antigen exposure, indicating a functional adaptation that enhances immune memory persistence.11 Unlike NDMM, in which B-cell exhaustion contributes to disease progression, patients with LTS MM show preservation of memory B cells, supporting durable antimyeloma immunity.98 However, interactions with inflammatory monocytes and T cells, along with exposure to cytokines such as IL-1β and CXCL8, influence their functionality.11
Therapeutic interventions can modulate B-cell responses to support long-term remission. Pomalidomide reduces immunosuppressive regulatory B cells, lowers IL-10 production, and promotes the expansion of B1b cells, which are involved in innate-like cytotoxicity and enhanced vaccine responses.100,102 In addition, marginal zone B cells, crucial for early myeloma recognition, are better preserved in patients with LTS MM, suggesting a role in long-term immune stability.100,102 Understanding how to replicate these B-cell–mediated immune adaptations in standard-risk MM could inform novel therapeutic strategies to prolong survival.98
Conclusions and future directions
LTS in MM remains rare but demonstrates that myeloma-intrinsic and immune-mediated mechanisms contribute to prolonged disease control. LTS patients exhibit a more favorable IBME, characterized by robust CD8+ T-cell and NK cell function, fewer immunosuppressive cells, and persistent antimyeloma immune surveillance. Identifying and replicating these features in broader MM populations represent a promising strategy for extending survival and potentially achieving functional cures. Recent advancements in immunotherapy align with the immune profiles observed in LTS MM. CAR T cells, NK cell–based therapies, BsAbs, and IMiDs enhance myeloma immunity and may help sustain long-term disease control. Expanding CAR-T therapy to novel targets such as GPRC5D and FcRH5 could help overcome antigen escape and resistance.124-126 Furthermore, targeting the IBME by disrupting stromal support and modulating cytokine networks may help recreate an LTS-like microenvironment.127,128 Integrating immune and genetic profiling may further refine predictive models of long-term outcomes and enable more personalized treatment approaches.
To apply these insights effectively, advanced patient stratification using genomic and immune profiling is essential. Identifying patients with LTS-like immune characteristics could optimize immunotherapy combinations and improve response durability. Eliminating MRD remains a critical goal in the pursuit of long-term remission. High-sensitivity diagnostics, including next-generation sequencing and single-cell immune profiling, can guide treatment decisions, whereas immune-based strategies may help eradicate MRD and maintain remission.11,129 The future of MM treatment lies in a precision medicine approach that integrates immunotherapy, IBME modulation, and MRD-directed strategies. By leveraging key immune features observed in LTS patients, we can develop more effective therapies and move closer to functional cures, offering extended remission and improved survival for a larger proportion of patients with MM. Emerging molecular and immunological technologies, such as spatial transcriptomics, immune repertoire sequencing, and integrative multiomics, can enable deep characterization of LTS patients, revealing distinct patterns of immune activation, cellular composition, and clonal stability. These insights may inform the development of next-generation therapies designed to replicate or reinforce the protective immune features observed in long-term survivors.
Although LTS in MM has historically been viewed as rare, emerging evidence suggests that it may be more broadly achievable through intentional therapeutic design. Strategies to increase the proportion of long-term survivors include initiating immune-based therapy earlier in the disease course, tailoring treatment intensity based on real-time MRD dynamics, incorporating immune-supportive agents during maintenance, and preserving or enhancing T-cell fitness in the context of cellular therapies such as CAR-T. These approaches reflect a paradigm shift from passively identifying features of LTS to actively engineering durable immune control. Future research should prioritize prospective clinical trials and biomarker-driven strategies aimed at transforming LTS from an exception into a more routine outcome.
Acknowledgments
This work was supported by the Paula and Rodger Riney Foundation (B.D. and S.J.) and National Institutes of Health grants R01CA204231, P01HL149620, and R01AI183571 (S.R.).
Authorship
Contribution: K.K. and K.Y. wrote the manuscript; and S.J., B.D., and S.R. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sridhar Rao, Versiti Blood Research Institute, 8727 West Watertown Plank Rd, Milwaukee, WI 53226; email: sridhar.rao@versiti.org; and Binod Dhakal, Division of Hematology and Oncology, Medical College of Wisconsin, 9200 W Wisconsin Ave, Cancer Center, Milwaukee, WI 53226; email: bdhakal@mcw.edu.
References
Author notes
K.K. and K.Y. contributed equally to this study.
B.D. and S.R. are joint senior authors.