Key Points
VEN plus HMA induces superior response rates in patients with R/R AML than conventional salvage therapy.
The VEN plus HMA combination allows for a safe and effective bridging to allo-HCT.
Visual Abstract
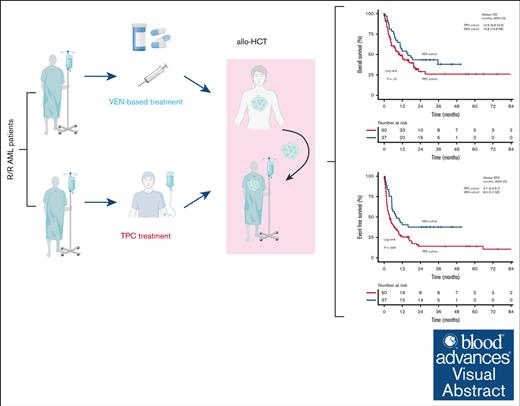
The B-cell lymphoma 2 inhibitor venetoclax (VEN) in combination with hypomethylating agents has been approved for first-line treatment of patients with acute myeloid leukemia (AML) ineligible for intensive treatment. VEN-containing treatment strategies may also be effective in relapsed/refractory (R/R) AML; however, comparative studies with conventional therapies for fit patients as a bridge-to-transplant strategy are limited. Using propensity score matching (PSM), we compared 37 patients with R/R AML, who received VEN-based salvage therapy as bridge to allogeneic hematopoietic stem cell transplantation (allo-HCT), with 90 patients from the German Study Alliance Leukemia AML registry, who were treated with non–VEN-containing salvage therapy according to their treating physician’s choice (TPC). The overall response rate among VEN patients was higher than the TPC control cohort (62% vs 42%; P = .049). Overall, 73% of VEN-treated patients vs 63% of TPC patients were bridged to allo-HCT (P = .41). After a median follow-up of 34.3 months for the VEN and 21.0 months for the TPC cohort, the median overall survival (OS) were 15.8 months (95% confidence interval [CI], 10.6 to not evaluable) and 10.5 months (95% CI, 6.8-19.6; P = .15), respectively. PSM revealed a trend toward improved OS for VEN patients (hazard ratio, 0.70; 95% CI, 0.41-1.22; P = .20). Median event-free survival was significantly longer in the VEN cohort (8.0 months) than the TPC cohort (3.7 months; P = .006). Our data suggest that VEN-based salvage therapy is a safe and effective bridge to allo-HCT for this difficult-to-treat AML patient population.
Introduction
The current standard of care for fit patients with newly diagnosed acute myeloid leukemia (AML) is standard induction chemotherapy for remission induction, followed by chemotherapy consolidation and/or allogeneic hematopoietic stem cell transplantation (allo-HCT) for postremission treatment.1 With this treatment strategy, disease remission can be achieved in up to 70% of patients with AML, depending on patients’ age and disease characteristics.2
With a 5-year overall survival (OS) of only 10%, the prognosis for patients with AML with primary refractory or relapsed (R/R) disease remains poor.3 Gilteritinib is approved in Europe and the United States for the treatment of R/R FMS-like tyrosine kinase 3 (FLT3)-mutated AML; however, for all other patients, there is currently no standard salvage therapy available. Historically, patients with R/R AML have been treated with a variety of approaches ranging from nonintensive to intensive salvage therapy strategies, all aiming to bridge patients to allo-HCT,4-6 the only potentially curative therapy. However, in most cases, these strategies have shown to be either inefficient or too toxic, thereby often restricting the path to allo-HCT.7-9
Venetoclax (VEN) is a potent inhibitor of B-cell lymphoma 2 (Bcl-2), a member of the Bcl-2 family of apoptosis regulating proteins.10,11 Based on the results of a randomized phase 3 clinical trial, VEN in combination with hypomethylating agents (HMAs) has been approved as first-line treatment for patients with AML ineligible for intensive treatment.12,13 In the United States, the label also includes the combination with low-dose cytarabine (LDAC).14
Treatment strategies that contain VEN are also increasingly being used as salvage treatment for patients with R/R AML,15-21 and these studies frequently include patients who are not candidates for intensive salvage therapy.15-21 However, studies comparing the efficacy of VEN-based therapy with conventional treatment strategies for medically fit patients with R/R AML as a bridge-to-transplant approach are still limited.21,22
Our retrospective analysis aimed to investigate the response and survival rates of patients with R/R AML considered to be eligible for intensive treatment, who received a VEN-containing salvage therapy as a bridge to allo-HCT in our institution, assuming reduced therapy-related toxicity before allo-HCT. In contrast to other publications, we performed a propensity score matching analysis to compare the results from our VEN-treated patients with a cohort of patients with R/R AML derived from the German Study Alliance Leukemia (SAL) AML registry (ClinicalTrials.gov identifier NCT03188874), who were treated with conventional non–VEN-containing treatment regimens at the discretion of their treating physicians, mirroring the treatment strategies of multiple AML centers in Germany.
Patients and methods
Patient cohorts
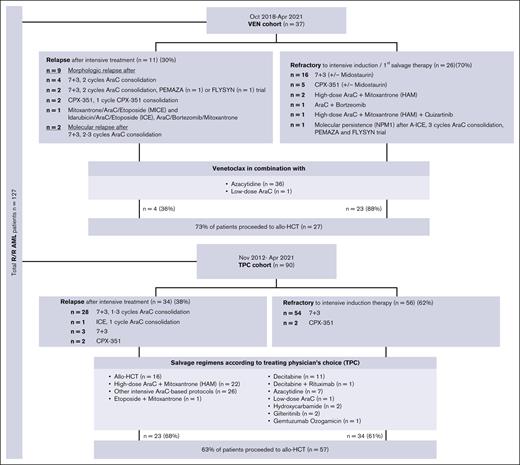
We included all patients aged ≥18 years who were refractory to or relapsed after at least 1 cycle of an intensive cytarabine- and anthracycline-containing induction chemotherapy and subsequently received a salvage treatment with VEN in combination with azacytidine (AZA) or LDAC at our institution between October 2018 and April 2021. All patients received the VEN-based regimens as their first- or second-line salvage therapy after relapse (n = 11; including 9 patients with morphologic and 2 patients with molecular relapse) or with refractory disease (n = 26; including 25 patients with morphologic and 1 with molecular disease persistence) (Figure 1). These patients were generally considered to be eligible for intensive salvage treatment regimens. However, they were deemed to have a higher risk of significant toxicity associated with intensive salvage therapy, and therefore, a less intensive VEN-containing treatment regime was chosen.
Flow diagram by treatment groups. AraC, cytarabine arabinoside; CPX-351, liposomal formulation of AraC and daunorubicin at a fixed 5:1 molar ratio; FLYSYN trial (NCT02789254), therapy with Fc-optimized FLT3 antibody FLYSYN; HAM, high-dose AraC + mitoxantrone; ICE, idarubicin/AraC/etoposide; MICE, mitoxantrone/AraC/etoposide; PEMAZA trial (NCT03769532), combinational therapy of pembrolizumab and azacitidine; 7+3, intensive induction therapy with daunorubicin on days 1 to 3 plus AraC on days 1 to 7.
Flow diagram by treatment groups. AraC, cytarabine arabinoside; CPX-351, liposomal formulation of AraC and daunorubicin at a fixed 5:1 molar ratio; FLYSYN trial (NCT02789254), therapy with Fc-optimized FLT3 antibody FLYSYN; HAM, high-dose AraC + mitoxantrone; ICE, idarubicin/AraC/etoposide; MICE, mitoxantrone/AraC/etoposide; PEMAZA trial (NCT03769532), combinational therapy of pembrolizumab and azacitidine; 7+3, intensive induction therapy with daunorubicin on days 1 to 3 plus AraC on days 1 to 7.
The matched cohort contained patients with R/R AML from the German SAL registry (ClinicalTrials.gov identifier NCT03188874), who received at least 1 cycle of a non–VEN-containing TPC salvage therapy between November 2012 and April 2021 (Figure 1) with the intention to be bridged to allo-HCT and for whom clinical and genetic data (eg, European LeukemiaNet [ELN] risk, karyotype, and molecular findings) were available. For this TPC cohort, overall response rates (ORRs) were calculated only for the first salvage therapy.
Informed consent
Informed consent was obtained from all patients included in the study following institutional guidelines and in accordance with the Declaration of Helsinki. The study was approved by the local ethics committee of the University Hospital Heidelberg, Germany, and the ethics committee of the University Hospital Dresden, Germany.
Cytogenetic and molecular genetic characterization
For cytogenetic evaluation, standard banding techniques were performed, and karyotypes were described according to the International System for Human Cytogenetic Nomenclature.23 Next-generation sequencing was used to detect AML-specific molecular genetic aberrations (Platform: Illumina; Analysesoftware: Variant Studio 3.0, V3.0.12 & Pindel v.1.1.1). Based on cytogenetic and molecular genetic findings, patients were classified as favorable, intermediate, or adverse risk according to the ELN 2017 risk stratification.24
Treatment modalities
Patients in the VEN cohort received VEN in combination with 7 days of AZA at 75 mg/m2 subcutaneously (n = 36). Only 1 patient was treated with 10 days of LDAC 40 mg absolute subcutaneously instead of AZA. VEN in combination with HMA was given orally at a dose of 400 mg for 28 days, with an initial dose ramp-up of 100 mg on day 1 and 200 mg on day 2 in cycle 1, or in combination with LDAC at 600 mg for 28 days, with an initial dose ramp-up of 100 mg on day 1, 200 mg on day 2, and 400 mg on day 3 in cycle 1.
Dose adjustments of VEN were performed based on concomitant azole therapy, tolerance, and cytopenia.25 Patients with concomitant posaconazole or voriconazole therapy received a daily VEN dose of 100 mg, and patients who achieved a blast clearance in the bone marrow (BM) but experienced prolonged cytopenia received dose-reduced cycles consisting of 5 days of AZA and 21 days of VEN. All patients were VEN naïve, and none received any concomitant targeted therapy.
Patients in the TPC cohort received up to a maximum of 6 different salvage therapy regimes (Figure 1). The majority of patients were treated with high-dose cytarabine in combination with mitoxantrone (high-dose cytarabine 3 g/m2 or 1 g/m2 at age >60 years26 and mitoxantrone 10 mg/m2; n = 22) or other intensive high/intermediate-dose cytarabine–containing therapy protocols (n = 26; Figure 1). One patient received etoposide in combination with mitoxantrone. Sixteen patients proceeded directly to allo-HCT without prior remission induction, whereas 22 patients received nonintensive salvage treatment (decitabine mono, n = 11; decitabine with rituximab, n = 1; AZA mono, n = 7; LDAC, n = 1; hydroxycarbamide, n = 2; Figure 1). Other nonintensive treatment protocols included gemtuzumab ozogamicin monotherapy (n = 1) and gilteritinib (n = 2).
For all patients included in this analysis, both in the VEN and in the TPC cohort, simultaneous preparation for allo-HCT was initiated.
Figure 1 provides detailed information on prior therapy regimes and the sequential steps of the treatment process.
Assessment of response and definition of survival end points
For the VEN cohort, response evaluation in the BM was performed between day 21 and day 28 of the first therapy cycle and subsequently after every cycle. Best response was determined after a maximum of 3 therapy cycles.
In the TPC cohort, response was assessed after the completion of first salvage chemotherapy and blood count recovery or day 42 in case of persisting cytopenia. Treatment response was assessed according to the recommendations of the ELN 2017.20 ORR was defined as morphologic complete remission (CR) or CR with incomplete hematologic recovery (CRi).
Results of measurable residual disease (MRD) assessment were available for the VEN cohort only. MRD monitoring was performed by flow cytometry using a leukemia-associated immunophenotypic profile–based approach and by polymerase chain reaction for patients with suitable molecular markers.27
OS was defined as the time from initiation of the salvage therapy (first salvage for TPC and VEN-based salvage for VEN patients) until death from any cause. Relapse-free survival (RFS) was defined as the time between first CR/CRi after salvage therapy for responding patients and relapse or death from any cause. Event-free survival (EFS) was defined as the time from initiation of the salvage therapy (first salvage for TPC and VEN-based salvage for VEN patients) to treatment failure (morphologic leukemia-free state [MLFS] and partial remission [PR] as response after salvage therapy not leading to an event), relapse, or death from any cause for all patients.
Statistical analysis
Descriptive statistics were performed to analyze the distributions and frequencies of patients’ characteristics. Differences between the 2 treatment groups were analyzed using Fisher exact test for categorical variables and Wilcoxon rank-order test for continuous variables. OS, EFS, and RFS were estimated using the Kaplan-Meier method and compared using the log-rank test. Furthermore, propensity score matching including age, white blood cell count, sex, ELN 2017 risk, and allo-HCT status as matching variables was used for OS comparison. In all analyses, a P value of <.05 was considered statistically significant. Statistical testing was performed using IBM SPSS (version 27) and R statistical software (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria).28
Results
Baseline characteristics and comparison between treatment groups
Baseline and treatment characteristics of patients from both cohorts are shown in Table 1.
Patient baseline and treatment characteristics
| Characteristics . | Total (N = 127), n (%) . | VEN cohort (n = 37), n (%) . | TPC cohort (n = 90), n (%) . | P value . |
|---|---|---|---|---|
| Sex | .695 | |||
| Male | 71 (56) | 22 (60) | 49 (54) | |
| Female | 56 (44) | 15 (40) | 41 (46) | |
| Age, median (range), y | 61 (22-79) | 62 (23-79) | 61 (22-79) | .661 |
| AML subtype | .271 | |||
| De novo | 93 (73) | 30 (81) | 63 (70) | |
| sAML | 34 (27) | 7 (19) | 27 (30) | |
| tAML | 13 (10) | 0 | 13 (16) | |
| Previous MDS, MPN, or MDS/MPN overlap | 21 (17) | 7 (19) | 14 (14) | |
| Status at start of salvage therapy | .422 | |||
| Refractory | 82 (65) | 26∗ (70) | 56 (62) | |
| Relapsed | 45 (35) | 11† (30) | 34 (38) | |
| Hematologic parameters at initial diagnosis | ||||
| WBC count, median (range), ×109/L (n = 125) | 17.1 (0.32-320.2) | 16.3 (0.32-226) | 18.4 (0.48-320.2) Missing n = 2 | .691 |
| Hemoglobin, median (range), g/dL (n = 124) | 9.3 (4.8-16.6) | 9.65 (5-13.4) Missing n = 1 | 9.1 (4.8-16.6) Missing n = 2 | .334 |
| PLT count, median (range), ×109/L (n = 124) | 48 (4-380) | 49 (4-253) Missing n = 1 | 48 (5-380) Missing n = 2 | .434 |
| Peripheral blood blasts, median (range), % (n = 112) | 34 (0-96) | 26 (0-95) Missing n = 5 | 42 (0-96) Missing n = 10 | .445 |
| Morphological BM blasts, median (range), % (n = 115) | 65 (8-98) | 60 (12-95) | 66 (8-98) Missing n = 12 | .512 |
| 2017 ELN risk stratification | .055 | |||
| Favorable | 32 (25) | 10 (27) | 22 (24) | .823 |
| Intermediate | 54 (43) | 10 (27) | 44 (49) | .030 |
| Adverse | 41 (32) | 17 (46) | 24 (27) | .039 |
| Cytogenetics | .552 | |||
| Aberrant | 43 (34) | 15 (40) | 28 (31) | |
| Complex aberrant | 13 (10) | 4 (11) | 9 (10) | |
| Normal | 71 (56) | 18 (49) | 53 (59) | |
| Genomic alteration | ||||
| NPM1 | 23 (18) | 8 (22) | 15 (17) | .613 |
| FLT3-ITD | 19 (15) | 6 (16) | 13 (14) | .789 |
| FLT3-TKD | 9 (7) | 1 (3) | 8 (9) | .282 |
| IDH1 | 14 (11) | 6 (16) | 8 (6) | .231 |
| IDH2 | 24 (19) | 9 (24) | 15 (17) | .327 |
| CEPBA (n = 122) | 20 (16) | 2 (5) | 18 (21) Missing n = 5 | .034 |
| TP53 (n = 94) | 12 (13) | 4 (11) Missing n = 2 | 8 (14) Missing n = 31 | 1.0 |
| ASXL1 (n = 94) | 13 (14) | 6 (17) Missing n = 2 | 7 (12) Missing n = 31 | .543 |
| RUNX1 (n = 94) | 17 (18) | 7 (20) Missing n = 2 | 10 (17) Missing n = 31 | .784 |
| TET2 (n = 94) | 27 (29) | 6 (17) Missing n = 2 | 21 (36) Missing n = 31 | .063 |
| Salvage setting | ||||
| Median no. of salvage therapy regimens (range) | 1 (1-6) | 1 (1-3) | 1 (1-6) | .180 |
| Patients proceeded to allo-HCT | 84 (66) | 27 (73) | 57 (63) | .409 |
| Conditioning regimens prior allo-HCT | .001 | |||
| Myeloablative | 47 (56) | 8 (30) | 39 (68) | |
| Nonmyeloablative and RIC | 37 (44) | 19 (70) | 18 (32) | |
| Previous therapy | ||||
| Prior allo-HCT for MDS | 2 (2) | 2 (5) | 0 (0) | |
| Prior intensive chemotherapy | 127 (100) | 37 (100) | 90 (100) |
| Characteristics . | Total (N = 127), n (%) . | VEN cohort (n = 37), n (%) . | TPC cohort (n = 90), n (%) . | P value . |
|---|---|---|---|---|
| Sex | .695 | |||
| Male | 71 (56) | 22 (60) | 49 (54) | |
| Female | 56 (44) | 15 (40) | 41 (46) | |
| Age, median (range), y | 61 (22-79) | 62 (23-79) | 61 (22-79) | .661 |
| AML subtype | .271 | |||
| De novo | 93 (73) | 30 (81) | 63 (70) | |
| sAML | 34 (27) | 7 (19) | 27 (30) | |
| tAML | 13 (10) | 0 | 13 (16) | |
| Previous MDS, MPN, or MDS/MPN overlap | 21 (17) | 7 (19) | 14 (14) | |
| Status at start of salvage therapy | .422 | |||
| Refractory | 82 (65) | 26∗ (70) | 56 (62) | |
| Relapsed | 45 (35) | 11† (30) | 34 (38) | |
| Hematologic parameters at initial diagnosis | ||||
| WBC count, median (range), ×109/L (n = 125) | 17.1 (0.32-320.2) | 16.3 (0.32-226) | 18.4 (0.48-320.2) Missing n = 2 | .691 |
| Hemoglobin, median (range), g/dL (n = 124) | 9.3 (4.8-16.6) | 9.65 (5-13.4) Missing n = 1 | 9.1 (4.8-16.6) Missing n = 2 | .334 |
| PLT count, median (range), ×109/L (n = 124) | 48 (4-380) | 49 (4-253) Missing n = 1 | 48 (5-380) Missing n = 2 | .434 |
| Peripheral blood blasts, median (range), % (n = 112) | 34 (0-96) | 26 (0-95) Missing n = 5 | 42 (0-96) Missing n = 10 | .445 |
| Morphological BM blasts, median (range), % (n = 115) | 65 (8-98) | 60 (12-95) | 66 (8-98) Missing n = 12 | .512 |
| 2017 ELN risk stratification | .055 | |||
| Favorable | 32 (25) | 10 (27) | 22 (24) | .823 |
| Intermediate | 54 (43) | 10 (27) | 44 (49) | .030 |
| Adverse | 41 (32) | 17 (46) | 24 (27) | .039 |
| Cytogenetics | .552 | |||
| Aberrant | 43 (34) | 15 (40) | 28 (31) | |
| Complex aberrant | 13 (10) | 4 (11) | 9 (10) | |
| Normal | 71 (56) | 18 (49) | 53 (59) | |
| Genomic alteration | ||||
| NPM1 | 23 (18) | 8 (22) | 15 (17) | .613 |
| FLT3-ITD | 19 (15) | 6 (16) | 13 (14) | .789 |
| FLT3-TKD | 9 (7) | 1 (3) | 8 (9) | .282 |
| IDH1 | 14 (11) | 6 (16) | 8 (6) | .231 |
| IDH2 | 24 (19) | 9 (24) | 15 (17) | .327 |
| CEPBA (n = 122) | 20 (16) | 2 (5) | 18 (21) Missing n = 5 | .034 |
| TP53 (n = 94) | 12 (13) | 4 (11) Missing n = 2 | 8 (14) Missing n = 31 | 1.0 |
| ASXL1 (n = 94) | 13 (14) | 6 (17) Missing n = 2 | 7 (12) Missing n = 31 | .543 |
| RUNX1 (n = 94) | 17 (18) | 7 (20) Missing n = 2 | 10 (17) Missing n = 31 | .784 |
| TET2 (n = 94) | 27 (29) | 6 (17) Missing n = 2 | 21 (36) Missing n = 31 | .063 |
| Salvage setting | ||||
| Median no. of salvage therapy regimens (range) | 1 (1-6) | 1 (1-3) | 1 (1-6) | .180 |
| Patients proceeded to allo-HCT | 84 (66) | 27 (73) | 57 (63) | .409 |
| Conditioning regimens prior allo-HCT | .001 | |||
| Myeloablative | 47 (56) | 8 (30) | 39 (68) | |
| Nonmyeloablative and RIC | 37 (44) | 19 (70) | 18 (32) | |
| Previous therapy | ||||
| Prior allo-HCT for MDS | 2 (2) | 2 (5) | 0 (0) | |
| Prior intensive chemotherapy | 127 (100) | 37 (100) | 90 (100) |
MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms; PLT, platelet; RIC, reduced-intensity conditioning; sAML, secondary AML; tAML, therapy-related AML; WBC, white blood cell.
P values < 0.05 are displayed in bold.
n = 26 (including 25 with refractory AML and 1 with AML in morphologic remission but with molecular disease persistence).
n = 11 (including 9 with morphologic relapse and 2 with molecular relapse).
The VEN cohort included a total of 37 patients (median age, 62 years [range, 23-79]), of whom 26 patients (70%) had been refractory to intensive induction chemotherapy (25 patients with morphologic and 1 patient with molecular persistent disease), and 11 patients (30%) had been diagnosed with morphologic (9 patients) or molecular relapse (2 patients). According to the 2017 ELN classification,24 10 patients (27%) had a favorable risk, 10 (27%) had an intermediate risk, and 17 (46%) had an adverse risk disease at the time of diagnosis.
A total of 90 patients (median age, 61 years [range, 22-79]) with hematologic R/R disease were included in the TPC cohort. Overall, 56 patients (62%) had been refractory to intensive induction therapy, and 34 patients (38%) had relapsed after intensive first-line treatment. Twenty-two (24%), 44 (49%), and 24 patients (27%) had a favorable-, intermediate-, and high-risk disease according to the 2017 ELN classification, respectively.
None of the other clinical factors including sex, age, reason for salvage treatment (refractory or relapsed), distribution of AML subtypes, and hematologic parameters at first diagnosis were different between the 2 treatment cohorts. We also compared the genetic characteristics of patients and found a higher percentage of ELN 2017 adverse-risk patients in the VEN cohort (46% vs 27%; P = .039), whereas the percentage of patients with intermediate-risk disease was lower in the VEN cohort (27% vs 49%; P = .030) than TPC patients. The distribution of favorable-risk characteristics was similar (27% vs 24%; P = .823). Although CCAAT/enhancer-binding protein-alpha (CEBPA) mutations were detectable less frequently in VEN patients (5% vs 21%; P = .034), no differences between the groups were notable for all other analyzed cytogenetic and molecular genetic aberrations.
Response to treatment and bridging to allo-HCT
Next, we analyzed the response to treatment for both patient cohorts. The ORR for all patients was 62% in the VEN cohort compared with 42% in the TPC cohort (P = .049), and it was 59% for the VEN and 42% for the TPC cohorts when patients with molecular relapse or persistence were excluded (P = .106). Upon further exclusion of 16 patients from the TPC cohort who underwent allo-HCT without prior salvage therapy, the ORR for the TPC cohort was 29%, which was significantly lower than that of the VEN cohort (P = .005; Table 2).
Clinical outcomes: antileukemic responses and survival
| . | VEN cohort (n = 37), n (%) . | TPC cohort (n = 90), n (%) . | P value . |
|---|---|---|---|
| Best response∗ | Missing n = 6 | ||
| CR | 12 (32) | 27 (32) | 1.0 |
| CRi | 11 (30) | 8 (10) | .012 |
| MLFS | 5 (13) | 0 (0) | .002 |
| w/o blast clearance (PR/PD) | 8 (22) | 39 (46) | .015 |
| Dead | 1 (3) | 10 (12) | .170 |
| Overall response | Missing n = 6 | ||
| ORR (CR/CRi) | 23 (62) | 35 (42) | .049 |
| ORR (CR/CRi)† | 20 (59) | 35 (42) | .106 |
| ORR (CR/CRi)‡ | 20 (59) | 20 (29) | .005 |
| Early mortality | |||
| 30-d mortality | 1 (3) | 7 (8) | .436 |
| 60-d mortality | 2 (5) | 15 (17) | .149 |
| Median follow-up (95% CI), mo | 34.3 (28.3-45.3) | 21.0 (18.0-54.4) | .300 |
| Bridging to allo-HCT | .409 | ||
| Transplant | 27 (73) | 57 (63) | |
| No transplant | 10 (27) | 33 (37) | |
| Remission status before allo-HCT, excluding 16 patients with direct allo-HCT (n = 65 [of 68]) | Missing n = 3 | .095 | |
| CR/CRi/MLFS/PR | 23 (85) | 25 (66) | |
| SD/PD | 4 (15) | 13 (34) | |
| Survival | |||
| Median OS for all patients from the start of salvage treatment (95% CI), mo | 15.8 (10.6 to NE) | 10.5 (6.8-19.6) | .15 |
| Median OS censored at allo-HCT (95% CI), mo | 6.7 (6.2 to NE) | 5.0 (4.6-16.6) | .70 |
| . | VEN cohort (n = 37), n (%) . | TPC cohort (n = 90), n (%) . | P value . |
|---|---|---|---|
| Best response∗ | Missing n = 6 | ||
| CR | 12 (32) | 27 (32) | 1.0 |
| CRi | 11 (30) | 8 (10) | .012 |
| MLFS | 5 (13) | 0 (0) | .002 |
| w/o blast clearance (PR/PD) | 8 (22) | 39 (46) | .015 |
| Dead | 1 (3) | 10 (12) | .170 |
| Overall response | Missing n = 6 | ||
| ORR (CR/CRi) | 23 (62) | 35 (42) | .049 |
| ORR (CR/CRi)† | 20 (59) | 35 (42) | .106 |
| ORR (CR/CRi)‡ | 20 (59) | 20 (29) | .005 |
| Early mortality | |||
| 30-d mortality | 1 (3) | 7 (8) | .436 |
| 60-d mortality | 2 (5) | 15 (17) | .149 |
| Median follow-up (95% CI), mo | 34.3 (28.3-45.3) | 21.0 (18.0-54.4) | .300 |
| Bridging to allo-HCT | .409 | ||
| Transplant | 27 (73) | 57 (63) | |
| No transplant | 10 (27) | 33 (37) | |
| Remission status before allo-HCT, excluding 16 patients with direct allo-HCT (n = 65 [of 68]) | Missing n = 3 | .095 | |
| CR/CRi/MLFS/PR | 23 (85) | 25 (66) | |
| SD/PD | 4 (15) | 13 (34) | |
| Survival | |||
| Median OS for all patients from the start of salvage treatment (95% CI), mo | 15.8 (10.6 to NE) | 10.5 (6.8-19.6) | .15 |
| Median OS censored at allo-HCT (95% CI), mo | 6.7 (6.2 to NE) | 5.0 (4.6-16.6) | .70 |
PD, progressive disease; PR, partial remission; SD, stable disease; w/o, without.
P values < 0.05 are displayed in bold.
Best response in VEN-treated patients after up to 3 treatment cycles and best response in TPC patients after first salvage therapy.
ORR (CR/CRi) excluding patients with molecular relapse/persistence.
ORR (CR/CRi) excluding patients with molecular relapse/persistence or direct allo-HCT.
The CR rate (32% vs 32%; P = 1.0) of all patients were similar between the 2 treatment groups: however, more patients in the VEN cohort achieved a CR with incomplete hematologic recovery (CRi; 30% vs 10%; P = .012; Table 2). Four out of 5 patients, who achieved an MLFS with VEN-containing salvage therapy proceeded to allo-HCT before hematologic reconstitution. One patient treated with VEN and 10 patients in the SAL group died before disease evaluation.
A total of 39 patients from the TPC cohort (46%) and 8 patients from the VEN cohort (22%) failed to achieve a BM blast clearance after the salvage therapy (first salvage for TPC and VEN-based salvage for VEN patients) (P = .015).
The 30- and 60-day mortality rates were 3% and 5% in the VEN group and 8% (P = .44) and 17% (P = .15) in the TPC cohort, respectively.
Because allo-HCT is the only potentially curative treatment for patients with R/R AML, we next analyzed the efficacy of both salvage therapies as bridge-to-transplant strategy. Overall, 27 patients (73%) in the VEN arm and 57 patients (63%) from the TPC cohort subsequently underwent allo-HCT (P = .41). For 16 patients (18%) in the TPC cohort, 6 with relapsed and 10 patients with refractory disease, allo-HCT was the first salvage treatment, and these patients did not receive any type of chemotherapy before allograft. With the exclusion of these patient, we observed a trend toward a higher proportion of patients who proceeded to allo-HCT in the VEN cohort (73% vs 55%; P = .098). In the TPC cohort, significantly more patients received a myeloablative conditioning regimen before allo-HCT than the VEN cohort (68% vs 30%; P = .001; Table 1), even when patients who proceeded directly to allo-HCT were excluded (66% vs 30%; P = .006).
Differences were also observed with respect to the remission status before allo-HCT. Whereas 85% of patients in the VEN-based cohort had no detectable blasts or at least a PR at the time of transplantation, this was the case in 66% of patients who received conventional salvage therapy (P = .095). Data on MRD assessment by flow cytometry before allo-HCT were available only for patients treated with VEN-based salvage therapy, and MRD analysis could be performed in 23 of 27 patients. MRD negativity before allo-HCT, assed by flow cytometry, was achieved in 17 patients (74%), and it was significantly associated with improved EFS and RFS and a trend toward superior OS (supplemental Figure 1A-C).
Survival analysis
The median follow-up for survival of the total cohort was 27.3 months (95% CI, 21.8-37.6). EFS (including MLFS and PR as response after salvage therapy not leading to an event) was significantly longer in the VEN-based cohort (median, 8.0 months; 95% CI, 5.1 to NE) than the TPC cohort (median, 3.7 months; 95% CI, 2.2-6.7; P = .006; Figure 2A). In addition, we observed a trend toward improved OS in the VEN-treated cohort compared with the TPC cohort, with a median OS of 15.8 months (95% CI, 10.6 to NE) for the VEN-treated patients vs 10.5 months (95% CI, 6.8-19.6; P = .15) for TPC patients and a 2-year survival rate of 43% (95% CI, 30-62.5) vs 29% (95% CI, 19-43), respectively (Figure 2B). In contrast, once a remission was achieved, no difference was evident for RFS (P = .47; Figure 2C).
Survival analysis for VEN-treated and TPC patients. (A-C) Kaplan- Meier estimates for EFS (A), OS (B), and RFS (C) in all patients were measured from starting of VEN-based salvage therapy in VEN cohort or starting of first conventional salvage therapy in TPC cohort.
Survival analysis for VEN-treated and TPC patients. (A-C) Kaplan- Meier estimates for EFS (A), OS (B), and RFS (C) in all patients were measured from starting of VEN-based salvage therapy in VEN cohort or starting of first conventional salvage therapy in TPC cohort.
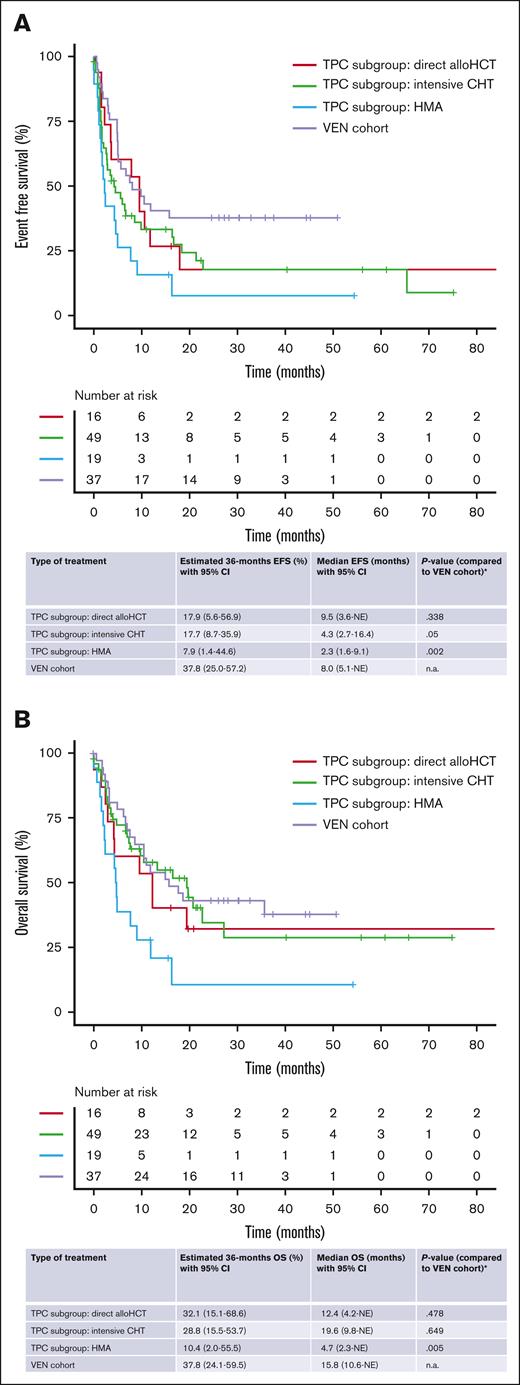
To take the heterogeneity of salvage therapies in the TPC cohort into account, we performed a subgroup analysis to compare survival end points of VEN-treated patients with TPC patients who received intensive chemotherapy or HMA as salvage therapy or who proceeded directly to allo-HCT. The median EFS for the VEN cohort was significantly longer than the TPC HMA cohort (2.3 months; 95% CI, 1.6-9.1 months; P = .002). There was also a trend toward improved EFS in comparison with the TPC intensive chemotherapy cohort (median, 4.3 months; 95% CI, 2.7-16.4 months; P = .05), whereas we did not observe a significant difference compared with TPC patients who proceeded to allo-HCT without prior treatment (median, 9.5 months; 95% CI, 3.6 months to NE; P = .34; Figure 3A). The median OS of VEN-treated patients was also signficiantly longer than TPC patients who were treated with HMA (median, 4.7 months; 95% CI, 2.3 months to NE; P = .005). On the contrary, no significant difference was observed between the VEN cohort and patients from the TPC intensive chemotherapy cohort (median, 19.6 months; 95% CI, 9.8 months to NE; P = .65) or the patients who proceeded straight to allo-HCT (median, 12.4 months; 95% CI, 4.2 months to NE; P = .48; Figure 3B).
Survival analysis in different subgroups. (A-B) Subgroup analysis; comparison of Kaplan-Meier estimates for EFS (A) and OS (B) between VEN-treated patients and patients from the TPC cohort who received different types of salvage therapy. OS and EFS were calculated from the initiation of VEN-based salvage therapy or the first conventional salvage therapy in the TPC cohort. ∗Due to the explorative nature of this analysis, no correction for multiple testing was performed. CHT, chemotherapy.
Survival analysis in different subgroups. (A-B) Subgroup analysis; comparison of Kaplan-Meier estimates for EFS (A) and OS (B) between VEN-treated patients and patients from the TPC cohort who received different types of salvage therapy. OS and EFS were calculated from the initiation of VEN-based salvage therapy or the first conventional salvage therapy in the TPC cohort. ∗Due to the explorative nature of this analysis, no correction for multiple testing was performed. CHT, chemotherapy.
To rule out conditioning intensity as a potential confounder for patients in both cohorts, we investigated the impact of myeloablative and nonmyeloablative/reduced-intensity conditioning on survival end points in the VEN and TPC patient cohorts.
EFS (median not reached vs 10.6 months; 95% CI, 5.7 months to NE; P = .22) and OS (median not reached vs 35.8 months; 95% CI, 15.8 months to NE; P = .3) were similar for VEN-treated patients who received myeloablative and non-myeloablative/reduced-intensity conditioning. Furthermore, the intensity of conditioning also did not affect EFS (median, 6.7 months; 95% CI, 3.7-11.7 months vs median, 10.6 months; 95% CI, 2.9 months to NE; P = .71) and OS (median, 19.5 months; 95% CI, 10.5 months to NE vs median, 19.9 months; 95% CI, 12.4 months to NE; P = .86) in the TPC patient cohort.
Finally, propensity score matching, including age, white blood cell count, sex, ELN 2017 risk, and allo-HCT status as matching variables, revealed, although not significant (P = .20), a favorable impact of VEN-based therapy with a hazard ratio of 0.70 (95% CI, 0.41-1.22) and thus suggests that the efficacy of our VEN-based approach was at least comparable with conventional salvage treatment.
Discussion
The treatment of patients with AML with relapsed and refractory disease remains challenging, and allo-HCT is still considered to be the only potentially curative treatment in these patients.29,30 Based on the results of a recently published phase 3 clinical trial, it has become a matter of debate whether remission induction before transplant improves the prognosis for patients with R/R AML.31 However, in patients considered eligible for allo-HCT, intensive chemotherapy is still a commonly used approach to induce a subsequent remission, even though responses rates are generally not satisfactory, and intensive regimens are frequently associated with significant treatment-associated toxicity.32 Further intensive reinduction therapy for R/R disease bears a substantial risk of significant morbidity that may decrease the likelyhood of patients to undergo allo-HCT. Thus, there is an urgent need for a bridge-to-transplant strategy that is associated with a lower risk of therpapy-related toxicity.
VEN has not only significantly improved the outcome of patients with AML unfit for intensive treatment,12,13 but recently published results suggest that VEN-containing treatment strategies are also effective in the R/R setting.15-22
Thus, we and others are hypothesizing that VEN combinations may effectively reduce or even elimintate the leukemic burden while preventing significant treatment-related toxicity before allo-HCT that may impair the patients’ performance and potentially jeopardize the patients' fitness for allo-HCT.
Thus, in our study, we analyzed response rates and outcome of patients with R/R AML treated in our institution who received a VEN-containing salvage therapy as a bridge-to–allo-HCT strategy. To put the analysis into perspective, we performed a propensity score matching analysis using patients with R/R AML from the SAL AML registry, who were treated with conventional non–VEN-containig treatment strategies, reflecting not only our but therapy concepts of multiple other health care providers in Germany. Of note, allo-HCT planning was initiated for all patients included in our study. This standard approach for all analyzed patients ensured the comparability of the 2 patient cohorts with respect to their comorbidity profile and overal performance status. So far, there is only 1 other retrospective study by Park et al comparing VEN treatment with standard salvage therapy in patients with R/R AML with a similar design.22 A second published study by Jamy et al did not explicitely specify whether all VEN-treated patients were initially considered to be eligible for allo-HCT,21 which could potentially hamper the comparability.
In general, patients' characteristcs were well balanced between the 2 treatment cohorts in our analysis, suggesting that it is feasible to retrospectively compare the treatment response and survival between the 2 groups. Of note, there was a higher proportion of patients with adverse-risk disease in the VEN cohort, which may reflect that conventional therapy was less likely to be considered a reasonable treatment option for adverse-risk patients in the SAL registry, whereas it has been shown that the ELN classification is less predictive for patients with AML receiving VEN combinations, at least in the first-line setting of unfit patients.33
With respect to treatment response, CR rates were similar between VEN and TPC patients. However, owed to the fact that treatment with BH3 mimetics such as VEN induces prolonged cytopenia despite effective clearance of BM blasts, CRs with incomplete hematologic recovery (CRi) and MLFS, which refers to blast clearance without adequate hematologic recovery, were more frequently observed in VEN-treated patients, leading to an improved ORR (defined as CR and CRi) among all patients and also when patients from the VEN cohort with molecular R/R disease and patients from the TPC cohort who proceeded directly to allo-HCT were excluded from the analysis. Of note, the ORR that we observed in VEN-treated patients may underestimate the actual treatment response because the ORR did not include patients with blast clearance and incomplete recovery of the entire hematopiesis (MLFS). The ORR in VEN-treated patients compares slightly more favorable with other studies that report CR/CRi of 35% to 59%,15-17,19-22 which is likely to be explained by the heterogeneity of the patient cohorts. However, the improved ORR for the VEN cohort highlights the potency of Bcl-2 inhibition in achieving a meaningful reduction of tumor burden in patients with R/R AML. The improved response rates for patients receiving VEN also translated into a more favorable remission status at the time of allo-HCT because more patients underwent allo transplant with no detectable BM blasts or at least in PR in the VEN cohort (85% vs 66% in TPC cohort, excluding 16 patients who directly proceeded to transplant). Because allo-HCT is the only potentially curative treatment for patients with R/R AML, the percentage of patients actually undergoing transplant after salvage therapy is a relevant indicator for its efficacy and also toxicity. We observed a trend toward a higher percentage of VEN-treated patients receiving their allo-HCT than TPC patients with conventional salvage therapy (excluding the 16 patients who directly proceeded to allo-HCT; 73% vs 55%; P = .098). In the study by Park et al, similar fractions of patients proceeded to allo-HCT in the VEN-treated and the intensive chemotherapy cohorts (68.5%). Furthermore, several studies have emphasized the importance of the remission status at the time of allo-HCT because transplantation with active disease is frequently associated with inferior survival rates.34-36
In addition to the encouraging response rates, we also observed improved EFS for patients treated with VEN (8.0 vs 3.7 months; P = .006) compared with conventionally treated patients in the TPC cohort. Furthermore, we also noticed a trend toward prolonged OS, even though this difference did not reach statistical significance (15.8 months [95% CI, 10.6 to NE] vs 10.5 months [95% CI, 6.8-19.6], respectively; P = .15). Thus, the survival of our VEN-treated patients compares favorably with other published studies that reported OS survival rates between 3.5 and 12.4 months.15-17,19-22 This may be due to the fact that all VEN patients in our cohort were deemed fit for allo-HCT at the time of the start of salvage therapy.
Our statistical analyses revealed that significantly more patients in the TPC cohort received a myeloablative conditioning regimen than the VEN cohort (68% vs 30%; P = .001), even when patients with direct allo-HCT as salvage treatment were excluded (66% vs 30%; P = .006). This difference may be attributed to the higher percentage of patients in the TPC cohort who received transplantation with active disease. However, the intensity of conditioning did not significantly affect survival neither in the TPC nor in the VEN cohort, suggesting that the intensity of conditioning does not explain the differences we observed between the 2 cohorts with respect to EFS and OS.
RFS was similar for VEN and TPC patients, suggesting that neither treatment approach was superior in preventing disease relapse once a remission was achieved.
Recently, we demonstrated that VEN response can be predicted by a flow cytometry–based assay.37 Thus, in case that either VEN-based therapy or intensive chemotherapy is considered in an R/R AML situation, it might be advisable to consider flow cytometry information for the best choice of treatment or the possibility to proceed to allo-HCT without preceeding therapy.
With respect to toxicity, treatment with VEN combinations was tolerable as indicated by the low 30-day and 60-day mortality. Even though early mortality was similar in both groups, data on treatment-associated toxicity were not available for the TPC cohort, rendering it impossible to directly compare therapy-related side effects. Furthermore, risk factors for potentially higher therapy-related toxicity in our VEN-based patient cohort could not be considered as variables in propensity score matching analysis. However, given that more than half of the VEN patients had an Eastern Cooperative Oncology Group performance status >1 at the time of treatment initiation and more VEN patients proceeded to allo-HCT, it is reasonable to conclude that VEN-based salvage therapy may allow for a reasonably toxic path toward curative allo-HCT. These results are in line with published studies that reported similarly low mortality rates for salvage treatment containing VEN.21,22
Park et al22 reported a relatively lower early mortality rate in their VEN- combined group as well (day 30, 0 vs 4.5%; day 60, 11.1% vs 16.9%), whereas Jamy et al21 presented day 30 mortality rates of 11% in the VEN-based cohort and 13% in the intensive chemotherapy cohort.
Our analysis has relevant limitations. First, the retrospective design of the study and the fact that patients in the TPC cohort received their treatment at different centers and during a time span of almost 9 years lead to a significant heterogeneity between the 2 patients cohorts but also within the TPC cohort. This was most apparent for the type of salvage therapy that included a variety of different intensive and nonintensive treatment regimes. Furthermore, the TPC cohort also included patients who proceeded straight to allo-HCT without prior salvage therapy. We acknowledge that the inclusion of these patients increases the heterogeneity, and the survival benefit of VEN-treated patients, particularly with respect to OS, is restricted to certain subgroups of TPC patients. However, we emphasize the importance of the control cohort to reflect the real-world situation as accurately as possible, highlighting the fact that there is no treatment strategy with a proven benefit available in this difficult-to-treat patient population.
Propensity score matching that we used to balance the treatment groups on confounding factors can reduce but not fully exclude this potential bias. Furthermore, the duration of follow-up was noticeably shorter for patients in the TPC cohort, potentially underestimating the survival difference between the 2 patient cohorts. Data on MRD as determined by flow cytometry using leukemia-associated immunophenotypic profile–based approach and/or by suitable molecular markers were available only for VEN-treated patients, which did not allow for us to directly compare the impact of MRD clearance and persistence on survival rates for VEN- and conventionally treated patients. However, in the VEN cohort, MRD positivity before allo-HCT, as detected by flow cytometry, was significantly associated with impaired outcomes, underscoring the importance of MRD for prognostication and guiding treatment decisions.
In summary, the results of our retrospective analysis suggest that VEN-containing salvage treatment is a feasible and effective bridging strategy to allogeneic stem cell transplantation for patients with R/R AML, with the potential to improve long-term survival for this difficult-to-treat patient population.
Acknowledgments
The authors thank all contributing physicians, laboratories, and nurses associated with the German Study Alliance Leukemia and especially participating patients and their families for their valuable contributions.
Authorship
Contribution: J.M.U. and T.S. conceived, designed, and performed the analysis, collected the data, and wrote the manuscript; R.F.S. performed and provided support for statistical analysis; E.K. collected the data and provided support with statistical analysis; S.Z. and M.K. provided support with statistical analysis; M.B., J.M.M., C.R., T.L., C.P., P.D., C.M.-T., S.W.K., S.K., H.E., J.K., and S.R. contributed data; and all authors provided critical feedback and helped shape the analysis and manuscript.
Conflict-of-interest disclosure: J.M.U. reports travel support from AbbVie and Jazz Pharmaceuticals. J.M.M. reports advisory board fees and honoraria from AbbVie. S.W.K. reports travel support from AbbVie. P.D. reports consultancy from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb (BMS), Miltenyi Biotec, Gilead Sciences, Novartis, Riemser, and Roche; speakers' bureau fees from AbbVie, AstraZeneca, BeiGene, BMS, Gilead Sciences, Novartis, Riemser, and Roche; and research support from Riemser. M.B. reports honoraria, lecture, and advisory board fees from Jazz Pharmaceuticals, and travel support from Merck Sharp and Dohme. C.M.-T. reports grants and/or provisions of Investigational Medicinal Products from Pfizer, Daiichi Sankyo, and BiolineRx; research support from Bayer; and is a data and safety monotoring board/scientific advisory board member of Pfizer and Janssen-Cilag. T.S. reports honoraria from Pfizer, Gilead Sciences, and Jazz Pharmaceuticals; advisory board/consultation fees from AbbVie, Takeda, Astellas Pharma, Amgen, BMS/Celgene, Ridgeline Discovery, Novartis, and Stemline Therapeutics; and travel support from AbbVie and Jazz Pharmaceuticals. C.R. declares institutional research funding by AbbVie, Astellas, Novartis, and Pfizer, and advisory honoraria from AbbVie, Astellas, BMS, Jazz Pharmaceuticals, Janssen, Novartis, Otsuka, Pfizer, and Servier. The remaining authors declare no competing financial interests.
A complete list of the hospitals that are members of the Study Alliance Leukemia Study Group appears in the supplemental Appendix.
Correspondence: Tim Sauer, Department of Hematology, Oncology and Rheumatology, University Hospital Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; email: tim.sauer@med.uni-heidelberg.de.
References
Author notes
The full-text version of this article contains a data supplement.