Key Points
G6PD-deficient mice maintain higher exercise speeds without increased red blood cell hemolysis, questioning assumed risks.
Increased cardiac output, sarcomere length, mitochondrial content, and morphology highlight adaptive benefits in G6PD-deficient mice.
Visual Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency affects 500 million people globally, affecting red blood cell (RBC) antioxidant pathways and increasing susceptibility to hemolysis under oxidative stress. Despite the systemic generation of reactive oxygen species during exercise, the effects of exercise on individuals with G6PD deficiency remain poorly understood This study used humanized mouse models expressing the G6PD Mediterranean variant (S188F, with 10% enzymatic activity) to investigate exercise performance and molecular outcomes. Surprisingly, despite decreased enzyme activity, G6PD-deficient mice have faster critical speed than mice expressing human canonical G6PD. After exercise, deficient mice did not exhibit differences in RBC morphology or hemolysis, but had improved cardiac function, including cardiac output, stroke volume, sarcomere length, and mitochondrial content. Proteomics analyses of cardiac and skeletal muscles (gastrocnemius, soleus) from G6PD-deficient compared with sufficient mice revealed improvements in mitochondrial function and increased protein turnover via ubiquitination, especially for mitochondrial and structural myofibrillar proteins. Mass spectrometry–based metabolomics revealed alterations in energy metabolism and fatty acid oxidation. These findings challenge the traditional assumptions regarding hemolytic risk during exercise in G6PD deficiency, suggesting a potential metabolic advantage in exercise performance for individuals carrying noncanonical G6PD variants.
Introduction
Glucose 6-phosphate dehydrogenase (G6PD) deficiency (G6PDd) is the most common enzymatic deficiency in humans, affecting ∼500 million people around the world.1 Because G6PD is encoded by a gene on chromosome X, deficiency is most prevalent in hemizygous males, including 1 in 10 men of African descent in the United States.2 G6PD is the rate-limiting enzyme of the pentose phosphate pathway (PPP), which is responsible for the generation of the majority of the reduced form of NAD phosphate (NADPH), vital for antioxidant reactions within human cells.3 NADPH-dependent reactions regulate recycling of oxidized glutathione and, directly or indirectly, NADPH/NADP+ ratios preserve the activity of virtually all antioxidant systems. As such, G6PD activity plays a critical role, especially in red blood cells (RBCs), the most numerous cell in the human body (∼83% of the total), which are exposed to severe oxidant stress during their 120 day circulatory life span; at the same time, mature RBCs lack mitochondria and, as such, are devoid of most of the enzymes that catalyze alternative NADPH-generating reactions.4 Therefore, the PPP critically shields RBCs against oxidative stress–induced hemolysis, both intra- or extravascular, via splenic or hepatic sequestration and erythrophagocytosis by the reticuloendothelial system.5-8
Exercise tolerance and cardiorespiratory fitness are powerful predictors of mortality,9 in which higher exercise capacity inversely correlates with early mortality rates.10,11 Similarly, G6PD overexpression is associated with life span extension in mice.12 Although acknowledging caveats related to exercise duration and intensity,13-15 consensus in the literature indicates an increase in the production of reactive oxygen species in response to strenuous exercise.16,17 Such stress arises upon mechanical or metabolic stress, damaging both cells with mitochondria18 and mitochondria-deficient mature RBCs, whose membrane rheological properties such as integrity and deformability are negatively affected by intense exercise activity in recreational and elite professional athletes.19,20 In contrast, regular moderate exercise strengthens antioxidant defense systems, enhancing RBC resilience against oxidative damage.21 Thus, whereas acute, intense exercise exacerbates oxidative stress and RBC damage, consistent moderate exercise may offer a protective adaptive response, balancing reactive oxygen species production with antioxidant capacity, a sort of “good stress” (also termed “eustress”22).
Hemolytic anemia upon oxidant challenge is a common, clinically relevant complication in patients with G6PDd,23 a complication that occurs even simply upon consumption of fava beans, sulfa drugs, or quinone antimalarials. Additionally, higher incidences of hypertension and idiopathic cardiomyopathies have been observed in patients with G6PDd.24,25 Although encouraged to maintain an active lifestyle to promote longevity and overall health,13 individuals with G6PDd are currently recommended to refrain from strenuous exercise because they are deemed to be at increased risk for hemolytic events upon physical activity.26 Despite these guidelines, there is a paucity of clinical data supporting these recommendations, and knowledge is limited regarding the molecular impacts underlying exercise capacity in the context of G6PDd.27 In light of the above, the overarching hypothesis underlying the rationale for this study posits that individuals with G6PD deficiency might be characterized by reduced exercise capacity because of increased susceptibility to exercise-induced oxidant stress and hemolysis. Although literature is limited to preliminary observational studies,13 this hypothesis has not been tested mechanistically in humans with G6PDd, owing to ethical and technical limitations. As a result, here, we leveraged recently developed28 humanized mouse models expressing either the human canonical (ie, nonmutant, normal activity) G6PD or 1 of the most common hypomorphic G6PD variants, the Mediterranean variant (S188F, <10% residual activity).7,28 The present model differs from previously described models in that mutations were introduced upon the whole murine endogenous coding region, that is, excluding also murine exons 1 to 2, not just the S188F on exon 5 as in previous models.7 The full murine G6PD coding region was genetically manipulated as to replace it with the human canonical variant, without interference to the synthetic antisense coding regions on chromosome X.28 Recently, we have shown that RBCs from these mice are susceptible to hemolysis upon oxidant stress, such as that arising upon incubation with dapsone hydroxylamine.28 G6PD-sufficient and -deficient mice were then tested for their responses to critical speed (CS) tests, which allowed us to define the highest work intensity at which a mouse can maintain a physiological steady state,29 while also monitoring for systemic and tissue-specific metabolic responses, including hemolysis, upon repeated stress by exercise to exhaustion.
Materials and methods
G6PDMed− mouse model
The humanized mouse models, G6PD canonical nondeficient and Mediterranean S188F (henceforth, G6PDND and G6PDMed−, respectively), have been previously described in detail.7 These mice were further engineered to not have the 13 residual base pairs from causes recombination event excision of mouse G6PD, as described.28 All experiments were approved by the University of Colorado Anschutz Medical Campus institutional animal care and use committee (protocol no. 218). Mice were 4 to 5 months old at the time of the study. G6PD activity was quantitated using the G6PD reagent set (Pointe Scientific, catalog no. G7583180) following the manufacturer’s protocol, unless noted otherwise.
Treadmill exercise and constant speed tests
Before the determination of CS, mice completed a treadmill familiarization phase, which consisted of four ∼5-minute runs on a motor-driven rodent treadmill (Exer 3/6, Columbus Instruments, Columbus, OH). For the first several runs, the treadmill speed was maintained at 10 to 15 m/min (up a 5% grade, which was maintained throughout all treadmill tests). For the last several runs, the speed of the treadmill increased progressively over the last minute to ∼30 to 35 m/min to familiarize the mice with high-speed running. Animals were encouraged to run with intermittent bursts of compressed room air aimed at the hind limbs from directly above the animal (so as not to push the mouse up the treadmill). All treadmill testing protocols were designed and conducted by experienced staff and strictly followed the guidelines set by the American Physiological Society’s resource book for the design of animal exercise protocols. Methods for exercise tolerance testing are further detailed in supplemental Methods.29-33 After CS tests, blood was collected immediately after the final run (after up to 5 days of strenuous activity) and organs were obtained 3 days after the animals’ final CS run.
Hemodynamics
After CS testing, mice underwent terminal open chest right ventricular and left ventricular function measurements with a 1.2F, FTE-1212B-4018 pressure volume catheter (Transonic Systems Inc, Ithaca, NY) as detailed in supplemental Methods.30
Mass spectrometry–based proteomics
Mass spectrometry–based proteomics were performed as previously described31 and detailed in supplemental Methods.
Mass spectrometry–based metabolomics and lipidomics
Electron microscopy
Cardiac tissue samples were prepared transition electron microscopy (TEM) and scanning electron microscopy (SEM) according to standard procedures,34 detailed in supplemental Methods.
mQTL
We implemented an metabolite quantitative trait loci (mQTL) workflow consistent with prior work35 using genotype array data generated within the RBC Omics cohort.5 Briefly, genotypes were measured with a custom transfusion medicine microarray36 and phasing performed with Shape-IT37 and then imputed using Impute238 against the 1000 Genomes Project phase 3 reference panel. Associations between genotypes and metabolite levels was performed using the R package saigeGDS,39 implementing a multivariate linear regression adjusting for sex, additive solution, age, blood donation frequency in the prior 2 years, and the top 10 genetic principal components. Only genetic variants at a minor allele frequency of >1% were considered. Significantly associated single-nucleotide polymorphisms (SNPs; P < 5 × 10−8) were annotated using the ENSEMBL Variant Effect Predictor.40
Statistics
Data are represented as means ± standard error of the mean. Statistical tests were performed with GraphPad Prism version 10.0 (San Diego, CA), OmicsNet, and an in-house–developed R code (version 4.1.0; 2023-06-16 ucrt). Multivariate and pathway analyses were performed using MetaboAnalyst 6.0.41 A 2-tailed Student t test was used for comparisons of continuous variables between 2 groups, and P values were calculated using the log-rank test.
Results
Circulating levels of the CS marker succinate correlate with polymorphisms in the coding region for G6PD in 13 091 blood donor volunteers
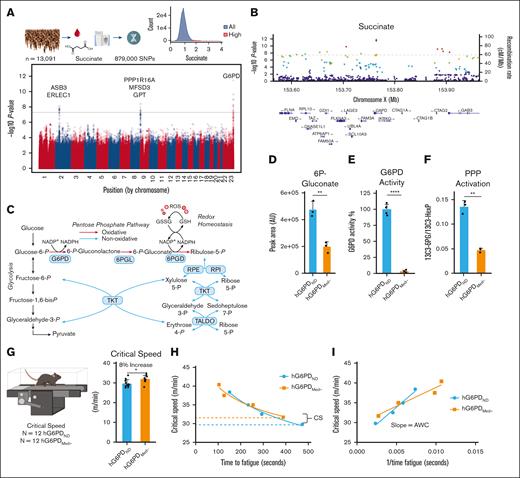
First, we investigated the distribution of succinate measurements in blood from 13 091 donor volunteers enrolled in the Recipient Epidemiology and Donor Evaluation study, RBC Omics project. We focused on succinate, based on its correlation to CS in other models.39,42,43 This analysis identified a subset of donors (∼1.7% of 13 091 volunteers) with up to fourfold higher basal succinate compared with the median of the total donor population (Figure 1A). We then sought to investigate the genetic underpinnings of elevated succinate levels in the blood from these volunteers, which was feasible because donors in the Recipient Epidemiology and Donor Evaluation RBC Omics study were also genotyped for 879 000 SNPs.5 Here, we performed a mQTL analysis, linking succinate levels to genetic traits. This analysis identified a significant association between succinate levels and a region coding for G6PD on chromosome X (top SNP including the S188F rs5030868 Mediterranean variant and the V68M rs1050828 SNP for the African variant; Figure 1B).
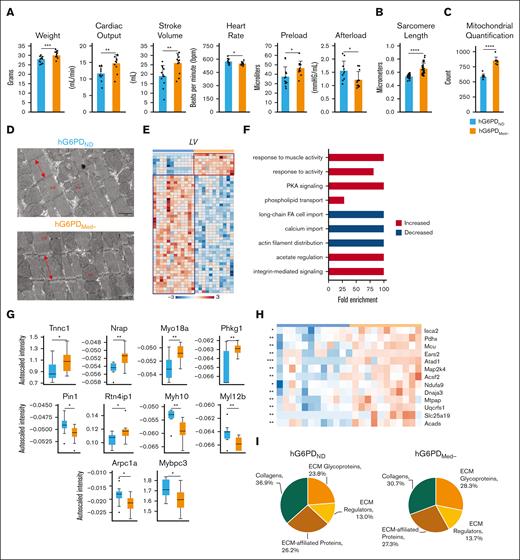
Characterization of G6PDMed− mice. (A) mQTL analysis of samples from 13 091 blood donors highlights correlation of succinate levels with polymorphic G6PD. (B) Locus zoom identifying G6PD as a target of interest. (C) An overview of glucose metabolism in redox homeostasis in RBCs. (D) Total abundance of 6-phosphogluconate (6P-gluconate) from metabolic tracing (sum of labeled and unlabeled) present in genotypes. (E) G6PD activity assay; (F) PPP activation as judged by relative levels of labeled 6P-gluconate to hexose phosphate. (G-H) CS testing of mice (n = 12) showed that G6PDMed− mice maintained a significantly faster CS (8% increase). Dashed lines indicate CS. (I) G6PDMed− mice have higher anaerobic work capacity (AWC) as measured by the slope, hG6PDND = 1715 and hG6PDMED- = 945.4 (significance, ∗P < .05, ∗∗P < .01, ∗∗∗∗P < .0001).
Characterization of G6PDMed− mice. (A) mQTL analysis of samples from 13 091 blood donors highlights correlation of succinate levels with polymorphic G6PD. (B) Locus zoom identifying G6PD as a target of interest. (C) An overview of glucose metabolism in redox homeostasis in RBCs. (D) Total abundance of 6-phosphogluconate (6P-gluconate) from metabolic tracing (sum of labeled and unlabeled) present in genotypes. (E) G6PD activity assay; (F) PPP activation as judged by relative levels of labeled 6P-gluconate to hexose phosphate. (G-H) CS testing of mice (n = 12) showed that G6PDMed− mice maintained a significantly faster CS (8% increase). Dashed lines indicate CS. (I) G6PDMed− mice have higher anaerobic work capacity (AWC) as measured by the slope, hG6PDND = 1715 and hG6PDMED- = 945.4 (significance, ∗P < .05, ∗∗P < .01, ∗∗∗∗P < .0001).
G6PDMed− mice maintain higher CS
To understand how G6PDd alters exercise capacity, we leveraged our recently developed mouse models. We characterized G6PD-deficient mice at baseline by profiling blood glucose metabolism with a specific focus on the PPP. Branching from glycolysis, G6PD catalyzes the first and rate-limiting step of the oxidative phase of the PPP, generating 6-phosphogluconolactone and then 6-phosphogluconate from catabolism of glucose 6-phosphate (Figure 1C). To test the impact of G6PDd, we leveraged a recently described28 humanized mouse model coding for the S188F G6PD variant. These mice display deficient G6PD activity, as gleaned by significantly lower levels of the metabolic product 6-phosphogluconate at steady state (Figure 1D; supplemental Figure 1A) and an overall reduced G6PD activity, as determined by standard enzymatic assays (Figure 1E). Additionally, evaluation of the flux into the PPP via tracing of 1,2,3-13C3-glucose confirmed significantly lower 13C3-6-phosphogluconate-to-13C3-hexose phosphate ratios (Figure 1F). Next, CS of hG6PDND and hG6PDMed− mice were measured to determine exercise tolerance (Figure 1G). Unexpectedly, G6PDMed− mice showed ∼8% faster CS than mice carrying the canonical human G6PD (P < .02, respectively; Figure 1H; supplemental Figure 1B). The G6PDMed− mice also showed a decrease in their anaerobic work capacity, highlighting their ability to work closer to their maximum volume of oxygen (Figure 1I).
Preexercise and postexercise blood analysis
Based on the unexpected CS finding, we challenged our hypothesis that RBCs from G6PDMed− mice were more susceptible to hemolysis after exercise-induced oxidant stress. Combined metabolomics and proteomics analyses of RBCs and plasma from G6PDND vs G6PDMed− mice were performed to determine the impact of exercise. First, postexercise/preexercise fold-change ratios of metabolites highlighted differences in RBCs with respect to G6PD status. Fatty acids (FAs) decreased and nonoxidative PPP/oxylipin/acylcarnitine intermediates increased in RBCs from G6PDMed− mice compared with hG6PDND, respectively (Figure 2A; supplemental Figure 2A-F). Plasma metabolites show increases in the postexercise-to-preexercise ratio of acylcarnitine pools in G6PDMed− compared with hG6PDND. Furthermore, the postexercise-to-preexercise ratios for glucose and tryptophan metabolites (indole 3-acetate and picolinic acid) decreased in G6PDMed− mice (Figure 2B; and details elaborated in supplemental Figure 3A-E). Of note, plasma glucose consumption was significantly higher in G6PDMed− mice than in hG6PDND, which was accompanied by higher lactate generation in RBCs but not plasma and less marked depletion of 2,3-bisphosphoglycerate (BPG; Figure 2C), an allosteric regulator of hemoglobin that, by promoting the stabilization of the tense deoxygenated state, favors oxygen offloading during hypoxia.44 Consistent with the G6PD-deficient state, baseline levels of PPP metabolites were lower in hG6PDMed− mice, although a significant postexercise drop in PPP end-product pentose phosphate was observed in hG6PDND compared with hG6PDMed−, suggestive of a preference toward glycolytic fluxes rather than the PPP in response to exercise. Finally, RBC levels of long-chain FAs (16:0 and 18:0) decreased in hG6PDND and were unchanged in hG6PDMed−.
Metabolic alterations detected in the blood before and after exercise. (A) RBC and (B) plasma heat map with hierarchical clustering of the top 25 metabolites significantly different (analysis of variance [ANOVA]) between hG6PDND and hG6PDMed− taken from the before/after exercise fold change. Autoscaled metabolite abundances were compared before and after exercise between hG6PDND and hG6PDMed− genotypes. (C) Glycolysis, PPP, and long-chain FA intermediates before and after CS tests. Metabolite name is color coded according to fraction with plasma (blue) and RBC (red) and genotype is indicated by hG6PDND (blue) and hG6PDMed− (orange). (D) Succinate accumulation after exercise in the plasma and RBCs. (E) Mean corpuscular volumes (MCV) as a function of exercise in RBCs. (F) SEM images of RBC morphology before and after run to exhaustion. (G) Echinocyte-to-discocyte ratios before and after exercise expressed as relative percentages. (H) Before to after changes in markers of hemolysis in plasma (significance ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001).
Metabolic alterations detected in the blood before and after exercise. (A) RBC and (B) plasma heat map with hierarchical clustering of the top 25 metabolites significantly different (analysis of variance [ANOVA]) between hG6PDND and hG6PDMed− taken from the before/after exercise fold change. Autoscaled metabolite abundances were compared before and after exercise between hG6PDND and hG6PDMed− genotypes. (C) Glycolysis, PPP, and long-chain FA intermediates before and after CS tests. Metabolite name is color coded according to fraction with plasma (blue) and RBC (red) and genotype is indicated by hG6PDND (blue) and hG6PDMed− (orange). (D) Succinate accumulation after exercise in the plasma and RBCs. (E) Mean corpuscular volumes (MCV) as a function of exercise in RBCs. (F) SEM images of RBC morphology before and after run to exhaustion. (G) Echinocyte-to-discocyte ratios before and after exercise expressed as relative percentages. (H) Before to after changes in markers of hemolysis in plasma (significance ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001).
As a marker of energy metabolism and mitochondrial function in nonerythroid tissues, succinate-to-fumarate ratios in plasma significantly increased after exercise in hG6PDND and hG6PDMed− mice. This relationship was mirrored in RBCs (influx of these dicarboxylates can occur via monocarboxylate transporters at low pH45), which also had higher ratios at baseline (Figure 2D). However, although exercise induced significant decreases in mean corpuscular volume, we did not observe a significant mean corpuscular volume difference between the 2 models (Figure 2E). These shared changes are, in part, recapitulated by SEM analyses, which demonstrated an increased percentage of reversibly altered morphologies (echinocytes, as opposed to unaltered discocytes) after exercise to exhaustion in both groups (Figure 2F and details elaborated in supplemental Figure 4), with no significant differences between hG6PDND and hG6PDMed− RBCs (Figure 2G). Similar to morphology, significant postexercise increases in circulating protein markers of hemolysis (eg, hemoglobin-α chain and hemoglobin-β1 chain) were not observed in the plasma of either group in response to exercise. Unexpectedly, however, circulating bilirubin levels increased significantly only in hG6PDND mice, suggesting a minor yet appreciable hemolytic effect of exercise on exhaustion in these models (Figure 2H; supplemental Table 1). No strain-specific differences in free heme or biliverdin were observed, suggesting that the differences in bilirubin are not attributable to constraints in NADPH-dependent reactions in this pathway (eg, those catalyzed by heme oxygenase 1, or biliverdin reductase A or B). These results are consistent with the observed lack of hematological differences before and after exercise between the canonical and the deficient strains (supplemental Table 1). RBC counts were found to be lower at baseline (6.924 × 106/μL vs 7.68 × 106/μL, P = .043), despite higher mean cell volumes (51.66 vs 50.3 fL; P = .02) in the hG6PDMed− group, with no other observed difference in complete blood counts. Despite minor (15.7 vs 15.275 pg; P < .001) increases in reticulocyte hemoglobin in the hG6PDMed− group, no differences were observed in reticulocyte counts or any other hemoglobin parameters in mature RBCs.
G6PDMed− mice have improved cardiac function
In the absence of negligible differential exercise-induced effects on RBC counts, metabolism, morphology, and hemolysis between the 2 groups, we hypothesized that alternative factors could contribute to explaining the improved CS in G6PDMed− mice. To further explore the physiological underpinnings of exercise performance, hemodynamics measurements were performed on both hG6PDND and hG6PDMed− mice after exercise to exhaustion. Mouse weights, cardiac output, stroke volume, heart rate, and preload were all significantly increased in G6PDMed− mice, whereas the afterload decreased (Figure 3A). No changes in heart weights were observed. Cardiac muscle sarcomere length was measured in both the hG6PDND and hG6PDMed− heart sections using TEM (Figure 3B), which revealed significantly longer sarcomere lengths in hG6PDMed−. Additionally, TEM analyses showed an increase in the count and morphology of mitochondria present in cardiac muscle (Figure 3C-D). In addition, mass spectrometry–based proteomics characterization of left ventricle (LV) tissue revealed differential proteome composition between the 2 genotypes (Figure 3E; labeled heat maps in supplemental Figure 5A). Gene Ontology (GO) analysis of the differentially expressed proteins in the hearts of hG6PDMed− compared with hG6PDND mice highlighted an increased response to muscle activity, protein kinase A signaling, regulation with decreased in actin filamin distribution, calcium import, and long-chain FA import (Figure 3F). Statistical analyses of individual proteins within these GO terms highlighted higher levels of key contractile, and regulatory proteins indicate enhancements in muscle strength and contractile capacity (Figure 3G). Additionally, there was a notable rise in oxidoreductase proteins, suggesting heightened metabolic activity. Conversely, a decrease in proteins responsible for muscle organization was noted, potentially signaling alterations in muscle structure (Figure 3G). In line with the observed increase in mitochondrial content, a significant increase in mitochondrial transport proteins and complexes are also seen in the hG6PDMed− genotype (Figure 3H; details elaborated in supplemental Figure 5B). Consistent with the compositional and structural sarcomere changes described above, proteomics analyses tailored to optimize the extraction and quantification of extracellular matrix components in the heart proteome highlighted a lower degree of soluble collagen deposition and higher level of total extracellular matrix glycoproteins in hG6PDMed− mice (Figure 3I).
Cardiac physiology and protein expression in heart muscle. (A) Hemodynamics measurements. (B) Sarcomere length differences as determined through TEM analysis. (C) Mitochondrial quantification of TEM images. (D) TEM sections at 1 μm (mf, myofibrils; mi, mitochondria). (E) Genotype-specific protein expression was observed in the LV, as measured by t test. Protein abundances were grouped as being significantly increased (red box) or significantly decreased (blue box) in hG6PDMed− mice. (F) Each group was entered for GO enrichment analysis, per LV. Red bars indicate significantly increased and blue bars indicate significantly decreased biological processes in hG6PDMed− mice. (G) Proteomics identification of heart muscle–specific proteins. (H) Significant mitochondrial proteins identified in the heart muscle. (I) Extracellular matrix of LV composition differences identified via proteomics (significance ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001).
Cardiac physiology and protein expression in heart muscle. (A) Hemodynamics measurements. (B) Sarcomere length differences as determined through TEM analysis. (C) Mitochondrial quantification of TEM images. (D) TEM sections at 1 μm (mf, myofibrils; mi, mitochondria). (E) Genotype-specific protein expression was observed in the LV, as measured by t test. Protein abundances were grouped as being significantly increased (red box) or significantly decreased (blue box) in hG6PDMed− mice. (F) Each group was entered for GO enrichment analysis, per LV. Red bars indicate significantly increased and blue bars indicate significantly decreased biological processes in hG6PDMed− mice. (G) Proteomics identification of heart muscle–specific proteins. (H) Significant mitochondrial proteins identified in the heart muscle. (I) Extracellular matrix of LV composition differences identified via proteomics (significance ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001).
Skeletal muscle proteomic profiles reveal higher levels of mitochondrial proteins in G6PDMed− mice
Considering the functional and compositional changes observed in the heart and associated cardiac muscles, we expanded the analyses to skeletal muscles (Figure 4A). Proteomic analyses of white and red muscles (gastrocnemius and soleus) revealed distinct separations based on tissue type, with partial differentiation observed by genotype (principal component analyses in Figure 4B). Upon investigating genotype-level disparities between the gastrocnemius and soleus muscles, a distinct proteomic contrast is evident between hG6PDND and hG6PDMed− (Figure 4C; labeled heat maps in supplemental Figure 5C). GO analysis of the top upregulated proteins in hG6PDMed− mice highlighted enrichment in mitochondrial protein translation, protein folding, mitochondrial biogenesis, and cellular energy respiration (Figure 4D). Upregulation of mitochondrial pathways was even more evident when focusing exclusively on mitochondrial proteins of both white and red muscles (Figure 4E-F).
Proteomics analysis of muscle revealed both tissue- and genotype-specific differences. (A) Muscle tissues (soleus and gastrocnemius) were isolated from hG6PDND and hG6PDMed− mice for proteomics analysis. (B) Principal component analysis (PCA) showed tissue-type specific differences. (C) Significant genotype-specific differences were observed for each tissue type, as measured by t test. Protein abundances were clustered as being significantly increased (red box) or significantly decreased (blue box) in hG6PDMed− mice. (D) Network analysis (OmicsNet, GO) of significant proteins in hG6PDMed− muscle tissues illustrating enriched biological pathways. Protein nodes were colored according to their associated enriched biological pathway. Significantly increased mitochondrial proteins in the (E) gastrocnemius and (F) soleus.
Proteomics analysis of muscle revealed both tissue- and genotype-specific differences. (A) Muscle tissues (soleus and gastrocnemius) were isolated from hG6PDND and hG6PDMed− mice for proteomics analysis. (B) Principal component analysis (PCA) showed tissue-type specific differences. (C) Significant genotype-specific differences were observed for each tissue type, as measured by t test. Protein abundances were clustered as being significantly increased (red box) or significantly decreased (blue box) in hG6PDMed− mice. (D) Network analysis (OmicsNet, GO) of significant proteins in hG6PDMed− muscle tissues illustrating enriched biological pathways. Protein nodes were colored according to their associated enriched biological pathway. Significantly increased mitochondrial proteins in the (E) gastrocnemius and (F) soleus.
Metabolomics of heart and muscle
Both cardiac muscles and skeletal muscles of G6PDMed− mice are characterized by increased levels of mitochondrial proteins and higher mitochondrial counts. We thus performed metabolomics analyses of skeletal muscles (gastrocnemius and soleus) and the heart (LV and right ventricle; Figure 5A). Although tissue was the strongest driver of the principal component analyses clustering pattern (Figure 5B), genotype had a more marked effect on the metabolic profiles of the soleus and the LV (Figure 5C; detailed metabolite names for each map are provided in supplemental Figure 6A). Pathway analyses revealed skeletal muscle tissue-specific differences between genotypes: nucleotide and tricarboxylic acid cycle metabolism in the soleus (supplemental Figure 6B) amino acid metabolism, and glutathione metabolism in the gastrocnemius (supplemental Figure 6C). In the heart, disparities were observed between the genotype-associated metabolic differences in the right ventricle and LV (Figure 5D; details elaborated in supplemental Figure 6D), which related predominantly to differences in carbohydrate metabolism, especially the PPP and glycolysis (supplemental Figure 6E-F). G6PDMed− hearts were characterized by significantly lower levels of high-energy phosphate compounds adenosine triphosphate and diphosphate at the end of exercise to exhaustion (Figure 5E); these changes were accompanied by corresponding increases in the levels of polyamines, particularly spermidine and spermine in the heart (Figure 5E). Within the skeletal muscle tissues, a decrease in acylcarnitine pools was noted in both the gastrocnemius and the soleus (Figure 5F), suggestive of differential FA oxidation in mitochondria.
Metabolomics analysis of muscle and heart revealed both tissue- and genotype-specific differences. (A) Muscle and heart tissues were collected from hG6PDND and hG6PDMed− mice. The gastrocnemius and soleus excised from isolated muscle (as well as the LV and right ventricle), separated from the isolated heart were subjected to metabolomics analysis. (B) PCA showed differences between tissue types. Comparison between hG6PDND and hG6PDMed− genotypes showed significant differences in both (C) muscle and (D) heart tissues, as measured by t test. Genotype-specific differences were observed for (E) energy-related metabolites and polyamines in the heart tissues, as well as (F) acylcarnitines (AC) detected in muscle tissues. Genotype-specific comparisons were made using the t test (significance ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001).
Metabolomics analysis of muscle and heart revealed both tissue- and genotype-specific differences. (A) Muscle and heart tissues were collected from hG6PDND and hG6PDMed− mice. The gastrocnemius and soleus excised from isolated muscle (as well as the LV and right ventricle), separated from the isolated heart were subjected to metabolomics analysis. (B) PCA showed differences between tissue types. Comparison between hG6PDND and hG6PDMed− genotypes showed significant differences in both (C) muscle and (D) heart tissues, as measured by t test. Genotype-specific differences were observed for (E) energy-related metabolites and polyamines in the heart tissues, as well as (F) acylcarnitines (AC) detected in muscle tissues. Genotype-specific comparisons were made using the t test (significance ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001).
Protein ubiquitination is higher in G6PDMed− mice
Given the apparent increase in mitochondrial proteins and metabolism in G6PDMed− mice, we investigated whether increased protein turnover may be triggered as a compensatory mechanism to cope with the diminished capacity to fuel antioxidant systems via the NADPH-generating PPP, especially in oxidant stress–sensitive organelles such as mitochondria. This phenomenon would also contribute to explaining the overall stability of total G6PD protein levels in transcriptionally competent tissues (cardiac and skeletal muscles but not RBCs) through degradation of damaged proteins and de novo synthesis of damaged enzymes. To this end, we identified a significant increase in protein ubiquitination (KGG)46 (Figure 6A) in G6PDMed− heart and muscle. The top 100 LV proteins with abundant KGG (Figure 6B) included proteins involved in mitochondrial cellular respiration, FA oxidation, and carboxylic acid processes (full list provided in supplemental Tables 2 and 3). Ubiquitinated proteins in the soleus (Figure 6D) were similar, with higher modifications to proteins involved in cellular respiration, FA oxidation, and carboxylic acid processes (Figure 6G; supplemental Tables 2 and 3). The gastrocnemius, however, (Figure 6C) included components of muscle twitch stimulation, signaling, and glycolytic processes (Figure 6F; supplemental Tables 2 and 3). Overall, these results support the hypothesis that G6PDMed− primes a prooxidant milieu, triggering elevated protein turnover and compensatory upregulation of de novo expression of mitochondrial proteins and muscle fibers that are more susceptible to stress and degradation via ubiquitination.
Protein ubiquitination profiles are tissue and genotype dependent. (A) Summary of protein ubiquitination of the formation of K-GG. (B-D) Top 100 proteins identified through gly-gly searches as measured by t test for the (B) LV, (C) the gastrocnemius, and the (D) soleus. Significant proteins in hG6PDMed− tissues (E) LV, (F) gastrocnemius, and (G) soleus were entered into OmicsNet for network analysis and GO analysis of enriched biological pathways. Protein nodes were colored according to their associated enriched biological pathway.
Protein ubiquitination profiles are tissue and genotype dependent. (A) Summary of protein ubiquitination of the formation of K-GG. (B-D) Top 100 proteins identified through gly-gly searches as measured by t test for the (B) LV, (C) the gastrocnemius, and the (D) soleus. Significant proteins in hG6PDMed− tissues (E) LV, (F) gastrocnemius, and (G) soleus were entered into OmicsNet for network analysis and GO analysis of enriched biological pathways. Protein nodes were colored according to their associated enriched biological pathway.
Discussion
Without prospective clinical evidence and established safety, patients with G6PDd are currently recommended to refrain from strenuous exercise based on an assumed risk for hemolytic complications. However, these recommendations are not supported by the scarce, and often anecdotal, literature. In light of inconsistencies in the literature on human G6PDd, here we leveraged a murine model to interrogate exercise performance.7,28,47 After confirming that their mature RBCs have <10% residual G6PD activity, we subjected these mice to multiple bouts of exercise to exhaustion. The CS model, a robust approach for evaluating the maximum sustainable running speed before fatigue sets in, holds promise for delineating physiological limitations and cardiovascular health. Thus, addressing the complexities of G6PDd and its association with oxidative stress, intrinsic (eg, RBCs) and extrinsic (cardiopulmonary function) factors during exercise necessitates a comprehensive understanding of underlying mechanisms. When testing G6PDMed− mice for CS, we did not observe a significant elevation in hemolysis or RBC morphological alterations, however, unexpectedly, we observed an improvement in exercise performance (∼8% improvement). Similar complete blood counts before or after exercise (except for borderline significant decreases in RBC counts at baseline) did not justify the observed functional CS phenotype.
Physiologically, elevated glycolytic fluxes were accompanied by higher 2,3-BPG levels. While 2,3-BPG can be scavenged for adenosine triphosphate generation in response to exercise-associated decreases in intracellular pH,48 these results are suggestive of maintained oxygen off-loading capacity of deoxyhemoglobin to meet the increased oxygen demand of exercising tissues. While these changes were significant, yet relatively minor in terms of fold-changes to controls, extremely significant increases in cardiac function were noted in the deficient mice. Such changes were explained by increased sarcomere length and composition at the proteome level, also accompanied by increased mitochondrial counts and total mitochondrial proteins. Altogether, these changes resulted in increased cardiac output, stroke volume and heart rate, with decreased afterload.
Increased cardiac function, mitochondrial content of cardiac muscles, and relative maintenance of RBC 2,3-bisphosphoglyceric acid (2,3-BPG) were not the only physiological adaptations observed in the better performing G6PDMed− mice. Elevation in mitochondrial proteins and altered acylcarnitine metabolism were observed in striated muscles, both white (gastrocnemius) and red muscle types (soleus), suggestive of differential FA oxidation profiles in the deficient mice. Continued reliance on FA oxidation during prolonged endurance exercise stress is a hallmark of improved performance, whereby glucose oxidation and mitochondria-dependent lactate clearance capacity represent not only a metabolic bottleneck constraining performance in all athletes, from recreational to elite,20,49 but also represent a metabolic hallmark of exercise intolerance in cardiometabolic conditions from diabetes50 to pulmonary hypertension,51,52 from aging53 to postacute sequelae, to COVID-19.54
It is worth noting that the S188F G6PD mutation results in an unstable protein whose oligomerization is impaired, and activity declined in cells (such as enucleated RBCs) that cannot compensate with de novo protein synthesis. In nucleated cells, de novo protein synthesis and redundant NADPH synthesis pathways3 through mitochondrial routes including folate metabolism, isocitrate dehydrogenase, malic enzyme 1, dihydroorotate dehydrogenase, and nicotinamide nucleotide transhydrogenase, may kick in to compensate for the genetic abnormality targeting the PPP, which is consistent with previous reports appreciating the independent regulation of NADPH fluxes in the cytosol vs mitochondria.55 Depression of NADPH-consuming pathways such as de novo FA synthesis and oxidation of storage lipids and free FAs may thus occur in the context of G6PDd, although further studies with stable isotope–labeled tracers may be necessary to corroborate the steady state evidence reported herein.
Cardiac functional and biochemical analyses allude to hG6PDMed− mice having better cardiac function. Here, we show that cardiac output (calculated by stroke volume divided by heart rate) is increased despite increases in both stroke volume and heart rate. In support, an increase in preload with a decrease in afterload was determined throughout hemodynamics analyses. Omics profiles additionally show increases in contractile proteins such as Tnnc1 and Nrap and decrease in Pin1, a signature that has been shown to lead to cardiac fibrosis and collagen deposition.56 GO analysis of proteomics data highlights an increase in protein kinase A signaling in hG6PDMed− mice, which helps increase cardiac muscle, contractility aiding in cardiac homeostasis.57 Indeed, hG6PDMed− mice have longer sarcomere lengths, which contain more mitochondria. These results collectively suggest that hG6PDMed− mice have higher mitophagy.
Higher protein turnover as a function of G6PD status, as gleaned by elevated protein ubiquitination, is consistent with an increased basal level of stress even under homeostatic conditions in G6PD mice. Moreover, G6PDd might confer increased risk of age-associated comorbidities including cardiovascular,58 metabolic, and immune-related diseases.42 Indeed, 1 main limitation of this study is that only young male mice were tested, whereas literature suggests that G6PD expression and activity declines with age, and that G6PD-overexpressing mice have longer lifespans and delayed onset of frailty,12,43 suggesting a potential negative association between G6PDd and healthspan and life span. From a mechanistic standpoint, we did not observe a G6PD-dependent exacerbation of molecular signatures associated with the activation of common oxidant stress-activated transcriptional reprogramming previously linked to adaptations to exercise, such as nuclear factor erythroid 2–related factor 259 or mutated in ataxia telangenctasica, a redox sensitive transcriptional regulator that directly promotes G6PD expression while also regulating mitochondrial metabolism.60 Elevated circulating succinate-to-fumarate ratios in the deficient mice are consistent with a potential signaling role of circulating succinate, recently reported to promote neuromuscular adaptations to exercise via signaling through its cognate receptor succinate receptor 1.45 In this view, the linkage between circulating succinate levels (a marker of CS and exercise tolerance in our previous studies in mice and humans39,42,43) and polymorphisms in the region coding for G6PD associated with deficient variants (eg, S188F rs5030868 Mediterranean and V68M rs1050828 for the African variant) in >13 000 blood donor volunteers further strengthens the linkage between G6PD status on mitochondrial homeostasis, and the translational relevance of our murine findings in humans. The observed increases in succinate levels and succinate-to-fumarate ratios in G6PD-deficient mice upon CS test is likely a consequence of the more strenuous exercise and concomitant mitochondrial activity, as result of increased counts and changes in morphologies. Although speculative at this stage, a potential additional explanation to this phenomenon is consistent with an exacerbation of oxidant stress to the redox-sensitive cysteine in the active site of succinate dehydrogenase (SDH), which metabolizes succinate to fumarate. In other hematologically relevant contexts of oxidant stress to mitochondria,61 glutathionylation of SDH preserves mitochondrial capacity but also slows down metabolic fluxes in mitochondria. Given the role of G6PD-derived NADPH chemistry, one could speculate that altered NADPH/glutathione homeostasis would impact this process of mitochondrial salvage from oxidant stress via glutathionylation of relevant enzymes (eg, SDH). Redox regulation of SDH cysteines has been reported in the context of aging,62 adding to our interpretation of the role of G6PD (deficiency) both in exercise tolerance, mitochondrial function and, potentially, aging.
In conclusion, here we report that G6PD-deficient mice do not hemolyze after being challenged by exercise to exhaustion. Functionally, this outcome is substantiated by cardiac adaptations, resulting in increased output and stroke volume and decreased afterload. Molecularly, we report that G6PD-deficient mice are characterized by increased mitochondrial count/protein levels in both cardiac and skeletal muscles, along with elevated protein turnover via ubiquitination and an increase in polyamines (spermidine and spermine), regulators of mitophagy.63 Altogether, these data are suggestive of a model in which G6PD-deficient mice are subject to molecular adaptations that enhance performance, while pushing the system to its limits, akin to a “star that shines twice as bright that burns half of its time.”
Acknowledgments
The authors acknowledge support from the Recipient Epidemiology and Donor Evaluation study (REDS) RBC Omics and REDS-IV-P CTLS programs, sponsored by the National Heart, Lung, and Blood Institute (NHLBI) contract 75N2019D00033, and from the NHLBI REDS-III RBC Omics project, which was supported by NHLBI contracts HHSN2682011-00001I, -00002I, -00003I, -00004I, -00005I, -00006I, -00007I, -00008I, and -00009I. Pathway graphs were prepared on BioRender.com.
This study was supported by funds by the NHLBI (R01HL161004 [D.C.I., P.W.B., and A.D.] and R01HL146442, R01HL149714, and R01HL148151 [A.D.]).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.C.Z. developed the G6PD animal models and A.M.H. and K.H.D.-C. characterized and shared the animals for further testing; F.I.C., C.L., J.H., and D.C.I. performed critical speed and hemodynamics tests; F.I.C., M.D., J.A.R., D.S., and K.C.H. performed metabolomics, inductively coupled plasma-mass spectrometry, and proteomics analyses; C.L., M.D., I.S.L., E.P.W., A. Darehshouri, E.J.E., K.H.D.-C., X.D., P.W.B., G.P.P., K.C.H., J.C.Z., D.C.I., T.N., and A. D’Alessandro performed method or model development, quality control, and omics data analysis; E.P.W. and A. Darehshouri performed SEM and TEM analyses; D.C.I., P.W.B., P.J.N., M.P.B., G.P.P., and A. Darehshouri designed, supervised, or interpreted the human studies; E.J.E. and G.P.P. performed mQTL analyses; F.I.C., E.J.E., G.P.P., and A. D’Alessandro performed statistical analyses; F.I.C. prepared figure panels; and F.I.C. and A. D’Alessandro wrote and revised the manuscript which was critically reviewed and approved by all authors.
Conflict-of-interest disclosure: A. D'Alessandro, K.C.H., and T.N. are founders of Omix Technologies Inc and Altis Biosciences LLC. A. D'Alessandro is a scientific advisory board member for Hemanext Inc and Macopharma Inc. The remaining authors declare no competing financial interests.
Correspondence: Angelo D’Alessandro, Department of Biochemistry and Molecular Genetics, University of Colorado Anschutz Medical Campus, 12801 E 17th Ave, Aurora, CO 80045; email: angelo.dalessandro@cuanschutz.edu.
References
Author notes
All raw data and elaborations are submitted in supplemental Tables 1-3.
The full-text version of this article contains a data supplement.


![Metabolic alterations detected in the blood before and after exercise. (A) RBC and (B) plasma heat map with hierarchical clustering of the top 25 metabolites significantly different (analysis of variance [ANOVA]) between hG6PDND and hG6PDMed− taken from the before/after exercise fold change. Autoscaled metabolite abundances were compared before and after exercise between hG6PDND and hG6PDMed− genotypes. (C) Glycolysis, PPP, and long-chain FA intermediates before and after CS tests. Metabolite name is color coded according to fraction with plasma (blue) and RBC (red) and genotype is indicated by hG6PDND (blue) and hG6PDMed− (orange). (D) Succinate accumulation after exercise in the plasma and RBCs. (E) Mean corpuscular volumes (MCV) as a function of exercise in RBCs. (F) SEM images of RBC morphology before and after run to exhaustion. (G) Echinocyte-to-discocyte ratios before and after exercise expressed as relative percentages. (H) Before to after changes in markers of hemolysis in plasma (significance ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/2/10.1182_bloodadvances.2024013968/3/m_blooda_adv-2024-013968-gr2.jpeg?Expires=1763482243&Signature=wU~Jll-hLMxwcq85ENAfAZDrkUNSnzbCoyN07nEy9nMOswK8K7JRqXkbPZM8Jfqo-m7TntJRJfw2E3cTrzibrOI5s8gE5g-wxjvgoPpy6h11MoOFyiMVxARTjlyYGgQwhQ2wSXkYa06J4AMgJU-lPmpGKB~pK9Iubdpp69m1Lt7ebwr3BceHp5bJqF5FpPLwjntYQTU2DswpCwgBbweSIeTiCR13CZkqsWS1fJGVmp41JHPXV4cknTZm-JjaNlZrZkjl9nb4KGOgRUCp8OC1PnHczwX0s3Up8wlSx9Dti5FUtcIQLSWGLe0sFDee3DKm9LPj0Rh5TaGo~EdaGPrf6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)