Key Points
Ruxolitinib provided greater clinical benefit than BAT in steroid-refractory GVHD, regardless of concomitant treatment with azoles.
The starting dose of ruxolitinib 10 mg twice daily was well tolerated in most patients who received concomitant azoles.
Visual Abstract
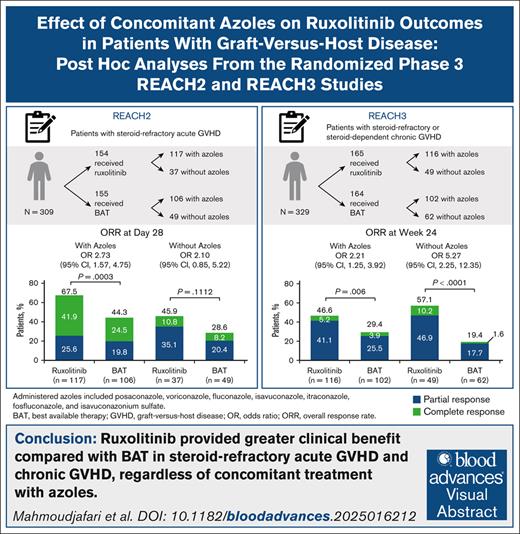
Azole antifungal agents, commonly used for preventing invasive fungal infections in graft-versus-host disease (GVHD), are known to affect ruxolitinib metabolism. Post hoc analyses of the REACH2/REACH3 phase 3 trials examined the impact of these clinically relevant drug interactions on ruxolitinib treatment outcomes in patients with steroid-refractory acute GVHD (aGVHD; REACH2) and steroid-refractory/steroid-dependent chronic GVHD (cGVHD; REACH3). In REACH2, overall response rate (ORR) at day 28 was significantly higher with ruxolitinib vs best available therapy (BAT; 67.5% vs 44.3%; P = .0003) among patients who received concomitant azoles; among those who did not, day 28 ORR was 45.9% vs 28.6%, respectively. In REACH3, ORR at week 24 was significantly higher with ruxolitinib vs BAT in patients who did (46.6% vs 29.4%; P = .006) or did not receive (57.1% vs 19.4%; P < .0001) concomitant azoles. Concomitant azoles neither increased the rate of cytopenias in patients treated with ruxolitinib in REACH2/REACH3, nor affected the median dose of ruxolitinib up to day 28 in REACH2 (azoles/no azoles, 20.0 mg/d [range, 9.0-21.0]/20.0 mg/d [range, 8.4-20.0]) or week 24 in REACH3 (azoles/no azoles, 19.4 mg/d [range, 4.8-20.5]/19.9 mg/d [range, 5.5-20.0]). Patients receiving concomitant azoles were more likely to have ruxolitinib dose modifications in REACH2/REACH3, highlighting the importance of dose optimization in these patients. Overall, concomitant azole treatment was generally well tolerated and did not affect treatment outcomes with appropriate ruxolitinib dose optimization. Consistent with primary REACH2/REACH3 results, ruxolitinib provided greater clinical benefit than BAT in patients with steroid-refractory aGVHD and steroid-refractory/steroid-dependent cGVHD, irrespective of concomitant azole treatment. These trials were registered at www.ClinicalTrials.gov as #NCT02913261 (REACH2) and #NCT03112603 (REACH3).
Introduction
Graft-versus-host disease (GVHD) is a serious and potentially fatal complication of allogeneic hematopoietic stem cell transplantation (HSCT) that develops when alloreactive donor T cells mount an immunologic attack against recipient organs or tissues.1,2 Despite prophylactic immunosuppressive therapies, GVHD develops in ∼50% of allogeneic HSCT recipients,3 leading to severe morbidity, reduced quality of life, and increased mortality.4-6 Acute GVHD (aGVHD) manifests primarily in the skin, gastrointestinal tract, and liver, and it accounts for up to one-third of deaths within a year of HSCT.3,6-8 Chronic GVHD (cGVHD) manifests with multiorgan pathology, including the skin, mouth, eye, gastrointestinal tract, liver, genitalia, lungs, and musculoskeletal system,3,9,10 and is a leading cause of nonrelapse mortality (22%-37% at 5 years).11,12
Systemic corticosteroids are the recommended first-line treatment for aGVHD and cGVHD,13 but ∼35% to 60% of patients become either steroid refractory (SR) or steroid dependent (SD; patients with cGVHD become SD because of an inability to taper corticosteroids below 0.25 mg/kg per day in 2 attempts over 8 weeks), requiring second-line therapies.9,13-15 Since 2017, the US Food and Drug Administration has approved 4 therapies (ibrutinib, belumosudil, axatilimab, and ruxolitinib) for GVHD after failure of at least 1 line of systemic therapy.16,17 Ibrutinib, a selective Bruton tyrosine kinase inhibitor; belumosudil, a selective Rho-associated coiled-coil kinase 2 inhibitor; and axatilimab, a colony-stimulating factor 1 receptor–blocking antibody, are approved for treatment of cGVHD only. Ruxolitinib, a selective Janus kinase 1/2 (JAK1/JAK2) inhibitor, is approved for the treatment of both aGVHD and cGVHD in adult and pediatric patients aged ≥12 years16,18 and is the only category 1 systemic option recommended by the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology.13
The efficacy and safety of ruxolitinib in patients with SR-aGVHD are supported by results from the pivotal randomized, open-label, phase 3 REACH2 study.19 In REACH2, the overall response rate (ORR) was significantly higher at day 28 for ruxolitinib compared with investigator-selected best available therapy (BAT; 62% vs 39%; P < .001) in patients with grade 2 to 4 SR-aGVHD.19 Ruxolitinib was also associated with improvement in failure-free survival (5.0 vs 1.0 month; hazard ratio, 0.46; 95% confidence interval [CI], 0.35-0.60) and numeric improvement in overall survival (11.1 vs 6.5 months; hazard ratio, 0.83; 95% CI, 0.60-1.15) compared with BAT. Furthermore, the efficacy and safety of ruxolitinib in patients with SR- or SD-cGVHD are supported by findings from the pivotal randomized, open-label, phase 3 REACH3 study.20 In REACH3, the ORR at week 24 was significantly higher for ruxolitinib vs BAT (50% vs 26%; P < .001) in patients with moderate to severe SR- or SD-cGVHD.20 Patients receiving ruxolitinib also had significantly longer failure-free survival (>18.6 vs 5.7 months; P < .001) and a significantly higher modified Lee Symptom Scale response rate at week 24 (24.2% vs 11.0%; P < .001) than those receiving BAT.
The safety profile in both REACH2 and REACH3 was consistent with the known safety profile of ruxolitinib.21-26 In REACH2, cytopenias, including thrombocytopenia (33%) and anemia (30%), and cytomegalovirus infection (26%) were the most common adverse events (AEs) up to day 28.19 The corresponding rates with BAT were 18%, 28%, and 21%, respectively. Similarly, in REACH3, cytopenias were the most common AEs (anemia, 29.1%; thrombocytopenia, 21.2%; and neutropenia, 10.9%) up to week 24.20 The corresponding rates with BAT were 12.7%, 14.6%, and 5.1%. Other common AEs with ruxolitinib vs BAT included hypertension (15.8% vs 12.7%, respectively), pyrexia (15.8% vs 9.5%), alanine aminotransferase increased (15.2% vs 4.4%), creatinine increased (13.9% vs 4.4%), and pneumonia (10.9% vs 12.7%).20 Overall, infections occurred in 61.2% of patients treated with ruxolitinib vs 54.7% given BAT (viral, 42.8% vs 33.3%; bacterial, 29.6% vs 32.0%; fungal, 8.6% vs 4.0%) in REACH2,19 and 63.6% vs 56.3% (viral, 33.9% vs 29.1%; bacterial, 27.9% vs 25.9%; fungal, 11.5% vs 5.7%), respectively, in REACH3.
Because of an increased risk of invasive fungal infections in patients with GVHD, antifungal prophylaxis with azoles is recommended for 16 weeks or until corticosteroid dose is <10 mg daily prednisolone equivalent.27 However, azoles are inhibitors of cytochrome P450 (CYP) 3A4, and in some cases CYP2C9 as well.28 Because ruxolitinib is metabolized by CYP3A4 and to a lesser extent by CYP2C9, there is a potential risk for drug-drug interactions.18 Pharmacokinetic (PK) and PK modeling studies have shown that concomitant administration of azoles can double the plasma concentration of ruxolitinib.29-31 Therefore, when ruxolitinib and azoles are concomitantly administered, it is recommended to monitor patients and modify the ruxolitinib dose as needed.18 The post hoc analyses of the REACH2 and REACH3 trials presented here evaluated the efficacy and safety of ruxolitinib in patients with SR-aGVHD or SR- or SD-cGVHD who did or did not receive concomitant azoles.
Methods
Study design and patients
The study design and eligibility criteria for REACH2 (ClinicalTrials.gov identifier: NCT02913261) and REACH3 (ClinicalTrials.gov identifier: NCT03112603) have been reported previously.19,20 REACH2 was a randomized, open-label phase 3 trial that compared the efficacy and safety of ruxolitinib with investigator-selected BAT in patients aged ≥12 years who had grade 2 to 4 SR-aGVHD that involved the use of systemic immunosuppressive therapy and excluded those who received ≥1 previous treatment for SR-aGVHD or JAK inhibitor therapy for any indication after initiation of allogeneic HSCT conditioning. REACH3 was a randomized, open-label phase 3 trial that compared the efficacy and safety of ruxolitinib with investigator-selected BAT after allogeneic HSCT in patients aged ≥12 years who had moderate or severe SR- or SD-cGVHD (here collectively referred to as patients with SR-cGVHD) and excluded those who previously received ≥2 systemic therapies for cGVHD in addition to corticosteroids with or without calcineurin inhibitors. In both studies, patients were randomly assigned 1:1 to receive ruxolitinib 10 mg twice daily (dose modifications allowed per clinician discretion for safety/efficacy per protocol) or investigator-selected BAT. All patients continued to receive corticosteroids with/without calcineurin inhibitors and were allowed to receive infection prophylaxis according to local institutional guidelines. The REACH trials were approved by the institutional review board at each participating institution and were performed in accordance with the Declaration of Helsinki, the International Council for Harmonisation guideline for good clinical practice, and applicable local regulations. Patients or their legal guardians (eg, for patients aged <18 years) provided written informed consent or assent.
These post hoc analyses examined efficacy and safety outcomes in the ruxolitinib and BAT treatment groups from the REACH2 and REACH3 trials according to receipt of concomitant azoles (ie, ≥1 dose of azoles used during the study since randomization as captured on case report forms). All patients from the original studies were included in the analyses, whether or not they received concomitant azoles. The full analysis set included patients randomized to REACH2/REACH3 study treatment; the safety analysis set included patients who received ≥1 dose of study treatment. Administered azoles included posaconazole, voriconazole, fluconazole, fosfluconazole, itraconazole, isavuconazonium sulfate, and isavuconazole; patients may have received >1 azole during the follow-up period.
Outcomes and assessments
Patient demographics (eg, age, sex, and race) and disease characteristics at baseline were reported by treatment group and concomitant azole use, defined as any concomitant use of azoles for which the start date is before or after initiation of ruxolitinib. Ruxolitinib treatment patterns (dose modification, discontinuation, and dose intensity) were examined according to concomitant azole administration. In REACH2, efficacy outcomes evaluated were ORR at day 28 (defined as the percentage of patients who had complete or partial responses [CR/PR]), best overall response (BOR; defined as the percentage of patients with CR or PR at any time up to day 28), and duration of response (DOR; defined as time from first response until aGVHD progression or the addition of new systemic therapy for aGVHD). Competing events for DOR included death without progression of aGVHD or onset of cGVHD. The efficacy outcomes evaluated in REACH3 were ORR at week 24, BOR up to week 24, and DOR (defined as the time from first documented CR or PR among patients with a response at week 24 until cGVHD progression, death, or change/addition of systemic cGVHD therapies). No competing events were defined for DOR, and it was calculated only in patients who achieved a BOR of CR or PR. Safety data in both REACH2 and REACH3 included the frequency of cytopenias, with clinically relevant cytopenias defined as any platelet count of <30 × 109/L, hemoglobin of <8 g/dL, white blood cell count of <5 × 109/L, or absolute neutrophil count <1 × 109/L. These criteria were chosen based on their clinical relevance for the population of patients with GVHD being managed in inpatient and GVHD clinics. These definitions identified potential thresholds for supportive care interventions, such as platelet or red blood cell transfusions and growth factor support, as well as treatment decision-making; the same definitions were used in previously published REACH2/3 analyses.32
Statistical analyses
Efficacy analyses were performed in the full analysis set (all randomized patients), and safety analyses were performed in the safety analysis set (all patients who received ≥1 dose of study drug). Demographics and baseline patient characteristics, ruxolitinib treatment patterns, and safety data were summarized using descriptive statistics. Statistical tests were informative and hypothesis generating, with no adjustment for multiplicity testing. ORR and BOR in patients who did or did not receive azoles were compared between ruxolitinib and BAT treatment groups. Odds ratios and 95% CIs were calculated using a Cochran-Mantel-Haenszel χ2 test stratified by SR-aGVHD grade (ie, 2 vs 3 vs 4; REACH2) or severity of SR-cGVHD (REACH3). For REACH2, DOR was plotted by cumulative incidence curve, and median and quartiles were estimated using the Kaplan-Meier method. For REACH3, DOR was analyzed using the Kaplan-Meier method.
Results
Baseline characteristics
Patients with SR-aGVHD treated in the REACH2 study
All 309 randomized patients from the REACH2 study were included in the analysis. Of 154 patients who received ruxolitinib, 117 (76.0%) received a concomitant azole and 37 (24.0%) did not; of 155 patients who were given BAT, 106 (68.4%) received a concomitant azole and 49 (31.6%) did not. Patient demographics and clinical characteristics were generally similar between groups (Table 1).
Demographics and baseline clinical characteristics of patients with SR-aGVHD (REACH2) by azoles subgroup (full analysis set)
| . | Ruxolitinib (n = 154) . | BAT (n = 155) . | ||
|---|---|---|---|---|
| With azoles (n = 117) . | Without azoles (n = 37) . | With azoles (n = 106) . | Without azoles (n = 49) . | |
| Age, median (range), y | 51.0 (13.0-71.0) | 58.0 (12.0-73.0) | 54.0 (13.0-71.0) | 58.0 (16.0-70.0) |
| Male, n (%) | 67 (57.3) | 25 (67.6) | 62 (58.5) | 29 (59.2) |
| Time from transplant to aGVHD grade ≥2 diagnosis, median (range), d | 36.0 (3-380) | 29.0 (7-247) | 34.5 (6-412) | 33.0 (10-190) |
| aGVHD grade,∗n (%) | ||||
| 2 | 56 (47.9) | 12 (32.4) | 62 (58.5) | 12 (24.5) |
| 3 | 51 (43.6) | 17 (45.9) | 36 (34.0) | 26 (53.1) |
| 4 | 10 (8.5) | 8 (21.6) | 8 (7.5) | 11 (22.4) |
| aGVHD organ involvement at randomization, n (%) | ||||
| Skin | 75 (64.1) | 18 (48.6) | 56 (52.8) | 18 (36.7) |
| Liver | 26 (22.2) | 10 (27.0) | 14 (13.2) | 12 (24.5) |
| Upper GI | 19 (16.2) | 9 (24.3) | 22 (20.8) | 15 (30.6) |
| Lower GI | 71 (60.7) | 25 (67.6) | 76 (71.7) | 39 (79.6) |
| Missing | 4 (3.4) | 0 | 1 (0.9) | 0 |
| Steroid dose at randomization,† median (range), mg/d | 120.0 (16.0-1000.0) | 129.0 (50.0-200.0) | 122.5 (16.0-680.0) | 120.0 (20.0-200.0) |
| Azole received as previous medication,‡n (%) | 99 (64.3) | 92 (59.4) | ||
| Posaconazole | 62 (40.3) | 49 (31.6) | ||
| Fluconazole | 21 (13.6) | 25 (16.1) | ||
| Voriconazole | 18 (11.7) | 19 (12.3) | ||
| Itraconazole | 5 (3.2) | 7 (4.5) | ||
| Isavuconazole | 3 (1.9) | 1 (0.6) | ||
| Isavuconazonium sulfate | 0 | 1 (0.6) | ||
| . | Ruxolitinib (n = 154) . | BAT (n = 155) . | ||
|---|---|---|---|---|
| With azoles (n = 117) . | Without azoles (n = 37) . | With azoles (n = 106) . | Without azoles (n = 49) . | |
| Age, median (range), y | 51.0 (13.0-71.0) | 58.0 (12.0-73.0) | 54.0 (13.0-71.0) | 58.0 (16.0-70.0) |
| Male, n (%) | 67 (57.3) | 25 (67.6) | 62 (58.5) | 29 (59.2) |
| Time from transplant to aGVHD grade ≥2 diagnosis, median (range), d | 36.0 (3-380) | 29.0 (7-247) | 34.5 (6-412) | 33.0 (10-190) |
| aGVHD grade,∗n (%) | ||||
| 2 | 56 (47.9) | 12 (32.4) | 62 (58.5) | 12 (24.5) |
| 3 | 51 (43.6) | 17 (45.9) | 36 (34.0) | 26 (53.1) |
| 4 | 10 (8.5) | 8 (21.6) | 8 (7.5) | 11 (22.4) |
| aGVHD organ involvement at randomization, n (%) | ||||
| Skin | 75 (64.1) | 18 (48.6) | 56 (52.8) | 18 (36.7) |
| Liver | 26 (22.2) | 10 (27.0) | 14 (13.2) | 12 (24.5) |
| Upper GI | 19 (16.2) | 9 (24.3) | 22 (20.8) | 15 (30.6) |
| Lower GI | 71 (60.7) | 25 (67.6) | 76 (71.7) | 39 (79.6) |
| Missing | 4 (3.4) | 0 | 1 (0.9) | 0 |
| Steroid dose at randomization,† median (range), mg/d | 120.0 (16.0-1000.0) | 129.0 (50.0-200.0) | 122.5 (16.0-680.0) | 120.0 (20.0-200.0) |
| Azole received as previous medication,‡n (%) | 99 (64.3) | 92 (59.4) | ||
| Posaconazole | 62 (40.3) | 49 (31.6) | ||
| Fluconazole | 21 (13.6) | 25 (16.1) | ||
| Voriconazole | 18 (11.7) | 19 (12.3) | ||
| Itraconazole | 5 (3.2) | 7 (4.5) | ||
| Isavuconazole | 3 (1.9) | 1 (0.6) | ||
| Isavuconazonium sulfate | 0 | 1 (0.6) | ||
GI, gastrointestinal.
At diagnosis of aGVHD grade ≥2.
Data regarding steroid dose at randomization were missing in 4 and 1 patients treated with ruxolitinib with and without azoles, respectively, and 2 and 2 patients treated with BAT with and without azoles, respectively.
Previous medication defined as any nonstudy medication started before first dose of ruxolitinib or BAT in the randomized phase, regardless of concomitant azole use.
Patients with SR-cGVHD treated in the REACH3 study
All 329 randomized patients from the REACH3 study were included in the analysis. Of 165 patients who received ruxolitinib in REACH3, 116 (70.3%) received a concomitant azole and 49 (29.7%) did not; of 164 patients who were given BAT, 102 (62.2%) received a concomitant azole and 62 (37.8%) did not. Patient demographics and clinical characteristics were generally well balanced between groups (Table 2).
Demographics and baseline clinical characteristics with SR-cGVHD (REACH3) by azoles subgroup (full analysis set)
| . | Ruxolitinib (n = 165) . | BAT (n = 164) . | ||
|---|---|---|---|---|
| With azoles (n = 116) . | Without azoles (n = 49) . | With azoles (n = 102) . | Without azoles (n = 62) . | |
| Age, median (range), y | 49.0 (15.0-73.0) | 48.0 (13.0-71.0) | 48.5 (13.0-76.0) | 51.0 (12.0-72.0) |
| Male, n (%) | 77 (66.4) | 32 (65.3) | 61 (59.8) | 31 (50.0) |
| Previous aGVHD, n (%) | 58 (50.0) | 34 (69.4) | 57 (55.9) | 31 (50.0) |
| Grade 1 | 17 (14.7) | 8 (16.3) | 19 (18.6) | 11 (17.7) |
| Grade 2 | 34 (29.3) | 19 (38.8) | 26 (25.5) | 17 (27.4) |
| Grade 3 | 7 (6.0) | 7 (14.3) | 10 (9.8) | 2 (3.2) |
| Grade 4 | 0 | 0 | 2 (2.0) | 1 (1.6) |
| SR-aGVHD | 11 (9.5) | 7 (14.3) | 6 (5.9) | 11 (17.7) |
| SR-cGVHD severity at study entrance, n (%) | ||||
| Mild | 0 | 0 | 0 | 1 (1.6) |
| Moderate | 42 (36.2) | 26 (53.1) | 46 (45.1) | 27 (43.5) |
| Severe | 74 (63.8) | 23 (46.9) | 56 (54.9) | 34 (54.8) |
| Time from cGVHD diagnosis to randomization, median (range), d | 185.5 (7-998) | 120.0 (8-2017) | 122.0 (10-1154) | 186.0 (14-1947) |
| Previous cGVHD therapy, n (%) | ||||
| Steroid only | 42 (36.2) | 28 (57.1) | 47 (46.1) | 34 (54.8) |
| Steroid + CNI | 56 (48.3) | 12 (24.5) | 48 (47.1) | 21 (33.9) |
| Steroid + CNI + other systemic therapy | 7 (6.0) | 3 (6.1) | 2 (2.0) | 2 (3.2) |
| Steroid + other systemic therapy | 10 (8.6) | 4 (8.2) | 4 (3.9) | 5 (8.1) |
| Missing | 1 (0.9) | 2 (4.1) | 1 (1.0) | 0 |
| Azole received as previous medication,∗n (%) | 97 (58.8) | 86 (52.4) | ||
| Posaconazole | 43 (26.1) | 48 (29.3) | ||
| Fluconazole | 26 (15.8) | 30 (18.3) | ||
| Voriconazole | 25 (15.2) | 9 (5.5) | ||
| Itraconazole | 4 (2.4) | 4 (2.4) | ||
| Isavuconazole | 2 (1.2) | 1 (0.6) | ||
| Fosfluconazole | 1 (0.6) | 0 | ||
| Isavuconazonium sulfate | 1 (0.6) | 1 (0.6) | ||
| . | Ruxolitinib (n = 165) . | BAT (n = 164) . | ||
|---|---|---|---|---|
| With azoles (n = 116) . | Without azoles (n = 49) . | With azoles (n = 102) . | Without azoles (n = 62) . | |
| Age, median (range), y | 49.0 (15.0-73.0) | 48.0 (13.0-71.0) | 48.5 (13.0-76.0) | 51.0 (12.0-72.0) |
| Male, n (%) | 77 (66.4) | 32 (65.3) | 61 (59.8) | 31 (50.0) |
| Previous aGVHD, n (%) | 58 (50.0) | 34 (69.4) | 57 (55.9) | 31 (50.0) |
| Grade 1 | 17 (14.7) | 8 (16.3) | 19 (18.6) | 11 (17.7) |
| Grade 2 | 34 (29.3) | 19 (38.8) | 26 (25.5) | 17 (27.4) |
| Grade 3 | 7 (6.0) | 7 (14.3) | 10 (9.8) | 2 (3.2) |
| Grade 4 | 0 | 0 | 2 (2.0) | 1 (1.6) |
| SR-aGVHD | 11 (9.5) | 7 (14.3) | 6 (5.9) | 11 (17.7) |
| SR-cGVHD severity at study entrance, n (%) | ||||
| Mild | 0 | 0 | 0 | 1 (1.6) |
| Moderate | 42 (36.2) | 26 (53.1) | 46 (45.1) | 27 (43.5) |
| Severe | 74 (63.8) | 23 (46.9) | 56 (54.9) | 34 (54.8) |
| Time from cGVHD diagnosis to randomization, median (range), d | 185.5 (7-998) | 120.0 (8-2017) | 122.0 (10-1154) | 186.0 (14-1947) |
| Previous cGVHD therapy, n (%) | ||||
| Steroid only | 42 (36.2) | 28 (57.1) | 47 (46.1) | 34 (54.8) |
| Steroid + CNI | 56 (48.3) | 12 (24.5) | 48 (47.1) | 21 (33.9) |
| Steroid + CNI + other systemic therapy | 7 (6.0) | 3 (6.1) | 2 (2.0) | 2 (3.2) |
| Steroid + other systemic therapy | 10 (8.6) | 4 (8.2) | 4 (3.9) | 5 (8.1) |
| Missing | 1 (0.9) | 2 (4.1) | 1 (1.0) | 0 |
| Azole received as previous medication,∗n (%) | 97 (58.8) | 86 (52.4) | ||
| Posaconazole | 43 (26.1) | 48 (29.3) | ||
| Fluconazole | 26 (15.8) | 30 (18.3) | ||
| Voriconazole | 25 (15.2) | 9 (5.5) | ||
| Itraconazole | 4 (2.4) | 4 (2.4) | ||
| Isavuconazole | 2 (1.2) | 1 (0.6) | ||
| Fosfluconazole | 1 (0.6) | 0 | ||
| Isavuconazonium sulfate | 1 (0.6) | 1 (0.6) | ||
CNI, calcineurin inhibitor.
Previous medication defined as any nonstudy medication started before first dose of ruxolitinib or BAT in the randomized phase, regardless of concomitant azole use.
Use of concomitant azoles
A total of 302 patients in REACH2 and 323 patients in REACH3 received ≥1 dose of study drug and were included in the safety analysis. More than two-thirds of patients in REACH2 and REACH3 received concomitant azoles (Table 3).
Use of concomitant azoles (safety analysis set)
| . | Patients with SR-aGVHD [REACH2] (n=302) . | Patients with SR-cGVHD [REACH3] (n=323) . | ||
|---|---|---|---|---|
| Ruxolitinib (n = 152) . | BAT (n = 150) . | Ruxolitinib (n = 165) . | BAT (n = 158) . | |
| Triazole derivatives, n (%) | 117 (77.0) | 106 (70.7) | 116 (70.3) | 102 (64.6) |
| Posaconazole | 79 (52.0) | 69 (46.0) | 48 (29.1) | 54 (34.2) |
| Voriconazole | 38 (25.0) | 22 (14.7) | 38 (23.0) | 20 (12.7) |
| Fluconazole | 18 (11.8) | 17 (11.3) | 40 (24.2) | 39 (24.7) |
| Isavuconazole | 7 (4.6) | 6 (4.0) | 4 (2.4) | 4 (2.5) |
| Itraconazole | 7 (4.6) | 7 (4.7) | 5 (3.0) | 2 (1.3) |
| Isavuconazonium sulfate | 1 (0.7) | 2 (1.3) | 2 (1.2) | 3 (1.9) |
| Fosfluconazole | 2 (1.3) | 0 | 1 (0.6) | 0 |
| . | Patients with SR-aGVHD [REACH2] (n=302) . | Patients with SR-cGVHD [REACH3] (n=323) . | ||
|---|---|---|---|---|
| Ruxolitinib (n = 152) . | BAT (n = 150) . | Ruxolitinib (n = 165) . | BAT (n = 158) . | |
| Triazole derivatives, n (%) | 117 (77.0) | 106 (70.7) | 116 (70.3) | 102 (64.6) |
| Posaconazole | 79 (52.0) | 69 (46.0) | 48 (29.1) | 54 (34.2) |
| Voriconazole | 38 (25.0) | 22 (14.7) | 38 (23.0) | 20 (12.7) |
| Fluconazole | 18 (11.8) | 17 (11.3) | 40 (24.2) | 39 (24.7) |
| Isavuconazole | 7 (4.6) | 6 (4.0) | 4 (2.4) | 4 (2.5) |
| Itraconazole | 7 (4.6) | 7 (4.7) | 5 (3.0) | 2 (1.3) |
| Isavuconazonium sulfate | 1 (0.7) | 2 (1.3) | 2 (1.2) | 3 (1.9) |
| Fosfluconazole | 2 (1.3) | 0 | 1 (0.6) | 0 |
aGVHD, acute graft-versus-host disease; BAT, best available therapy; cGVHD, chronic graft-versus-host disease; SR, steroid refractory.
Effect of concomitant azoles on efficacy
Effect on patients with SR-aGVHD treated in the REACH2 study
In patients receiving concomitant azoles, ORR at day 28 was significantly higher among those treated with ruxolitinib than those given BAT (67.5% [95% CI, 58.2-75.9] vs 44.3% [95% CI, 34.7-54.3]; P = .0003; Figure 1A). In patients who did not receive azoles, ORR was 45.9% (95% CI, 29.5-63.1) among those treated with ruxolitinib and 28.6% (95% CI, 16.2-43.3; P = .1112 vs ruxolitinib) among those given BAT.
ORR and BOR by azole subgroup in patients with GVHD. Patients with SR-aGVHD (A) and patients with SR-cGVHD (B) treated with ruxolitinib (RUX) or BAT, with or without azoles. Panel A shows ORR (CR + PR) and BOR at day 28 (aGVHD [full analysis set]). Panel B shows ORR (CR + PR) and BOR at week 24 (cGVHD [full analysis set]). OR, odds ratio.
ORR and BOR by azole subgroup in patients with GVHD. Patients with SR-aGVHD (A) and patients with SR-cGVHD (B) treated with ruxolitinib (RUX) or BAT, with or without azoles. Panel A shows ORR (CR + PR) and BOR at day 28 (aGVHD [full analysis set]). Panel B shows ORR (CR + PR) and BOR at week 24 (cGVHD [full analysis set]). OR, odds ratio.
Similarly, BOR up to day 28 was significantly higher among patients treated with ruxolitinib vs BAT who received azoles (87.2% [95% CI, 79.7-92.6] vs 66.0% [95% CI, 56.2-75.0], respectively; P = .0001). Among those who did not receive azoles, BOR was 64.9% (95% CI, 47.5-79.8) for patients treated with ruxolitinib and 49.0% (95% CI, 34.4-63.7; P = .1394; Figure 1A) for patients treated with BAT. Irrespective of ruxolitinib or BAT treatment, there was a trend toward higher ORR and BOR in patients who received azoles than in those who did not.
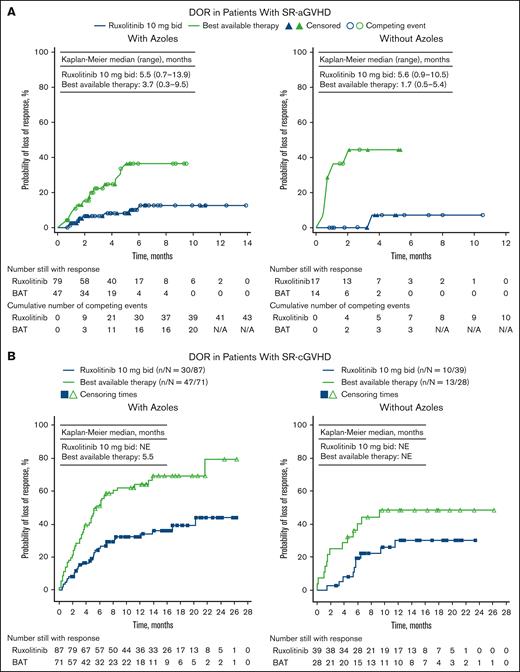
Median DOR was 5.5 months (range, 0.7-13.9) with ruxolitinib and 3.7 months (range, 0.3-9.5) with BAT in patients who received azoles. Without azoles, median DOR was 5.6 months (range, 0.9-10.5) with ruxolitinib and 1.7 months (range, 0.5-5.4) with BAT (Figure 2A).
DOR by azole subgroup in patients with GVHD. Patients with SR-aGVHD (A) and patients with SR-cGVHD (B) treated with ruxolitinib (RUX) or BAT, with or without azoles. Data reported for full analysis set. bid, twice daily; N/A, not available; NE, not evaluable.
DOR by azole subgroup in patients with GVHD. Patients with SR-aGVHD (A) and patients with SR-cGVHD (B) treated with ruxolitinib (RUX) or BAT, with or without azoles. Data reported for full analysis set. bid, twice daily; N/A, not available; NE, not evaluable.
Effect on patients with SR-cGVHD treated in the REACH3 study
ORR at week 24 was significantly higher in patients treated with ruxolitinib than in those given BAT among those who received concomitant azoles (46.6% [95% CI, 37.2-56.0] vs 29.4% [95% CI, 20.8-39.3]; P = .006) or did not receive azoles (57.1% [95% CI, 42.2-71.2] vs 19.4% [95% CI, 10.4-31.4]; P < .0001; Figure 1B).
BOR up to week 24 was similar between treatments, at 75.0% (95% CI, 66.1-82.6) in patients treated with ruxolitinib and azoles and 69.6% (95% CI, 59.7-78.3) in patients who received BAT and azoles (P = .2775 vs ruxolitinib). BOR was significantly higher in patients treated with ruxolitinib than in patients treated with BAT, among those who did not receive concomitant azoles (79.6% [95% CI, 65.7-89.8] vs 45.2% [95% CI, 32.5-58.3], respectively; P = .0003; Figure 1B).
DOR was prolonged in patients treated with ruxolitinib vs BAT, both in patients who received and did not receive concomitant azoles (Figure 2B). Median DOR was 5.5 months in patients given BAT and concomitant azoles and was not evaluable in the other subgroups.
Safety
The mean dose of ruxolitinib 10 mg twice daily was generally well tolerated when administered with concomitant azoles in patients with SR-aGVHD and SR-cGVHD (Figure 3).
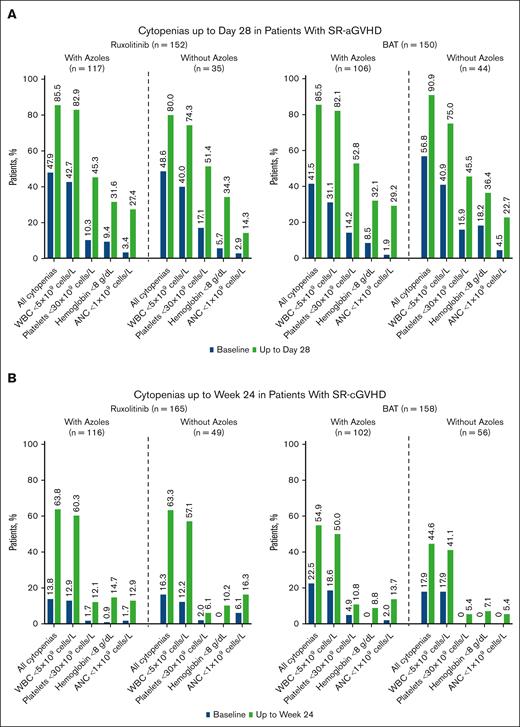
Incidence of cytopenias by azole subgroup in patients with GVHD. Patients with SR-aGVHD (A) and patients with SR-cGVHD (B) treated with ruxolitinib (RUX) or BAT, with or without azoles. Data reported for safety analysis set. ANC, absolute neutrophil count; WBC, white blood cell.
Incidence of cytopenias by azole subgroup in patients with GVHD. Patients with SR-aGVHD (A) and patients with SR-cGVHD (B) treated with ruxolitinib (RUX) or BAT, with or without azoles. Data reported for safety analysis set. ANC, absolute neutrophil count; WBC, white blood cell.
Impact on clinically relevant cytopenias
Minimal differences were observed in the development of cytopenias at day 28 in patients with SR-aGVHD who received ruxolitinib (85.5% vs 80.0%) or BAT (85.8% vs 90.9%) with azoles vs without azoles. No differences were observed in the development of cytopenias at week 24 for patients with SR-cGVHD who received ruxolitinib with vs without azoles (63.8% vs 63.3%). Among patients who were given BAT with vs without azoles, cytopenia rates were 54.9% and 44.6%, respectively.
Effect of concomitant azoles on ruxolitinib treatment patterns
Despite more dose modifications in ruxolitinib-treated patients who received concomitant azoles, the median ruxolitinib dose was similar in patients who received vs did not receive concomitant azoles up to day 28 in REACH2 (20.0 mg/d [range, 9.0-21.0] vs 20.0 mg/d [range, 8.4-20.0]) and up to week 24 in REACH3 (19.4 mg/d [range, 4.8-20.5] vs 19.9 mg/d [range, 5.5-20.0]; Table 4). Rates of ruxolitinib discontinuation were similar across both subgroups in REACH2 (108/117 [92.3%] vs 33/35 [94.3%]) and REACH3 (62/116 [53.4%] vs 23/49 [46.9%]). In REACH2, the primary reasons for ruxolitinib discontinuation among patients receiving concomitant azoles were treatment completion (n = 29 [24.8%]), AEs (n = 24 [20.5%]), lack of efficacy (n = 19 [16.2%]), and death (n = 15 [12.8%]), and among those not receiving azoles were lack of efficacy (n = 11 [31.4%]), death (n = 6 [17.1%]), AEs (n = 5 [14.3%]), and treatment completion (n = 4 [1.4%]). In REACH3, the primary reasons for ruxolitinib discontinuation among patients receiving concomitant azoles were AEs (n = 25 [21.6%]) and lack of efficacy (n = 13 [11.2%]), and among those not receiving azoles were lack of efficacy (n = 9 [18.4%]) and AEs (n = 5 [10.2%]).
Effect of concomitant azoles on ruxolitinib treatment patterns (safety analysis set)
| . | Patients with SR-aGVHD (REACH2; n = 152) . | Patients with SR-cGVHD (REACH3; n = 165) . | ||
|---|---|---|---|---|
| With azoles (n = 117) . | Without azoles (n = 35) . | With azoles (n = 116) . | Without azoles (n = 49) . | |
| Ruxolitinib dose intensity up to day 28 (REACH2) or week 24 (REACH3), mg/d | ||||
| Mean (standard deviation) | 18.1 (3.0) | 18.6 (2.7) | 17.0 (4.0) | 18.5 (2.9) |
| Median (range) | 20.0 (9.0-21.0) | 20.0 (8.4-20.0) | 19.4 (4.8-20.5) | 19.9 (5.5-20.0) |
| Ruxolitinib dose modifications, n (%) | 97 (82.9) | 21 (60.0) | 83 (71.6) | 31 (63.3) |
| Ruxolitinib discontinuations, n (%) | 108 (92.3) | 33 (94.3) | 62 (53.4) | 23 (46.9) |
| Reasons for ruxolitinib discontinuation, n (%) | ||||
| Completed study treatment | 29 (24.8) | 4 (11.4) | 1 (0.9) | 0 |
| AE | 24 (20.5) | 5 (14.3) | 25 (21.6) | 5 (10.2) |
| Lack of efficacy | 19 (16.2) | 11 (31.4) | 13 (11.2) | 9 (18.4) |
| Death | 15 (12.8) | 6 (17.1) | 5 (4.3) | 0 |
| Other∗ | 21 (17.9) | 7 (20.0) | 18 (15.5) | 9 (18.4) |
| . | Patients with SR-aGVHD (REACH2; n = 152) . | Patients with SR-cGVHD (REACH3; n = 165) . | ||
|---|---|---|---|---|
| With azoles (n = 117) . | Without azoles (n = 35) . | With azoles (n = 116) . | Without azoles (n = 49) . | |
| Ruxolitinib dose intensity up to day 28 (REACH2) or week 24 (REACH3), mg/d | ||||
| Mean (standard deviation) | 18.1 (3.0) | 18.6 (2.7) | 17.0 (4.0) | 18.5 (2.9) |
| Median (range) | 20.0 (9.0-21.0) | 20.0 (8.4-20.0) | 19.4 (4.8-20.5) | 19.9 (5.5-20.0) |
| Ruxolitinib dose modifications, n (%) | 97 (82.9) | 21 (60.0) | 83 (71.6) | 31 (63.3) |
| Ruxolitinib discontinuations, n (%) | 108 (92.3) | 33 (94.3) | 62 (53.4) | 23 (46.9) |
| Reasons for ruxolitinib discontinuation, n (%) | ||||
| Completed study treatment | 29 (24.8) | 4 (11.4) | 1 (0.9) | 0 |
| AE | 24 (20.5) | 5 (14.3) | 25 (21.6) | 5 (10.2) |
| Lack of efficacy | 19 (16.2) | 11 (31.4) | 13 (11.2) | 9 (18.4) |
| Death | 15 (12.8) | 6 (17.1) | 5 (4.3) | 0 |
| Other∗ | 21 (17.9) | 7 (20.0) | 18 (15.5) | 9 (18.4) |
Includes failure to meet protocol continuation, disease relapse, physician decision, graft loss, patient decision, and protocol deviation.
Discussion
Post hoc analyses of the REACH2 and REACH3 trials demonstrated that concomitant treatment with azoles did not affect the safety and efficacy of ruxolitinib in patients with SR-aGVHD and SR-cGVHD. Consistent with the overall results of the REACH2 and REACH3 trials,19,20 at day 28 and week 24, respectively, ORR was significantly higher for patients treated with ruxolitinib vs BAT for those who received concomitant azoles. In REACH2, patients receiving an azole had a better response than those who did not, irrespective of whether they were treated with ruxolitinib or BAT.
Concomitant azoles did not increase the rate of cytopenias in patients treated with ruxolitinib and did not affect the median dose of ruxolitinib; however, patients receiving concomitant azoles were more likely to require ruxolitinib dose optimization. These findings may be explained by more rigorous monitoring of ruxolitinib dosing in patients receiving concomitant azoles and dose optimization based on blood cell count fluctuations. A starting dose of ruxolitinib 10 mg twice daily appears to be well tolerated in most patients with SR-aGVHD and SR-cGVHD receiving concomitant azoles, but, nonetheless, our results highlight the need to monitor patients closely and dose optimize in the setting of blood cell count fluctuations.
Antifungal prophylaxis with azoles is recommended and commonly used in patients with GVHD because of an increased risk of infection, attributed both to the disease itself and its treatment with corticosteroids and other immunosuppressive therapies.5,9 In addition, therapeutic drug monitoring of azoles is being increasingly used to optimize antifungal therapy.33 Azoles are inhibitors of the CYP450 system, the major enzymatic pathway involved in ruxolitinib metabolism.29,34 PK and PK modeling studies of the coadministration of ruxolitinib with azoles, specifically fluconazole, posaconazole, and voriconazole, showed an approximately twofold increase in ruxolitinib plasma concentrations.29-31 The impact of azole interaction on ruxolitinib area under the plasma concentration-time curve and maximum plasma concentration is highest with fluconazole, likely because of its properties of dual CYP3A4 and CYP2C9 inhibition, requiring avoidance of doses >200 mg/d and empiric dose reduction for doses ≤200 mg/d as recommended in the ruxolitinib prescribing information.18 In contrast, voriconazole and posaconazole are primarily CYP3A4 inhibitors with a reduced impact on ruxolitinib area under the plasma concentration-time curve and maximum plasma concentration and therefore do not require empiric dose reduction as per the ruxolitinib prescribing information.18,29,30,35 Fluconazole, posaconazole, and voriconazole were included in the concomitant azoles administered with ruxolitinib in REACH2 and REACH3. Although analysis of individual azoles was outside the scope of this study, the overall results of this analysis suggest that current ruxolitinib dosing information is sufficient to maintain safety and efficacy of ruxolitinib when administered concomitantly with azoles.
Limitations of this study include the lack of blinding in the REACH2 and REACH3 studies; the relatively small number of patients who did not receive azoles, which possibly contributed to loss of significance in efficacy outcomes for patients with SR-aGVHD not treated with concomitant azoles; and the hypothesis-generating nature of post hoc analyses. Specific limitations of these post hoc analyses include a lack of standardization for exposure to azoles, and differences in disease and in drug exposure for SR-aGVHD vs SR-cGVHD. REACH2 and REACH3 were not designed to evaluate the use of azoles, which might contribute to potential selection bias for specific azoles in SR-aGVHD vs SR-cGVHD and complications regarding different clinical characteristics in the context of SR-aGVHD or SR-cGVHD (eg, differing organ involvement or comorbidities). Additionally, the original REACH2/3 PK analyses were not designed to support development of conclusions regarding concomitant azole administration on ruxolitinib PK exposure; therefore, a dedicated drug interaction study would be necessary to assess the relationship of ruxolitinib exposure to outcomes.
In conclusion, these post hoc analyses of the REACH2 and REACH3 trials showed that ruxolitinib provided greater clinical benefit compared with BAT in patients with SR-aGVHD or SR-cGVHD, regardless of concomitant treatment with azoles. The objectives of these analyses were to provide clinical guidance in patients with GVHD treated with ruxolitinib and concomitant azoles. In addition, development of cytopenias was similar between patients who were treated with azoles and those who were not. However, dose optimization of ruxolitinib in patients being treated with ruxolitinib and concomitant azoles is crucial to maintaining ruxolitinib dose intensity over time. The starting dose of ruxolitinib 10 mg twice daily appeared to be well tolerated in most patients who received concomitant azoles; however, the ruxolitinib prescribing information should be followed for empiric dose adjustments.
Acknowledgments
Writing assistance, including drafting the first version of the manuscript, was provided by Mitali Choudhury, an employee of ICON (Blue Bell, PA), and was funded by Incyte Corporation (Wilmington, DE).
Authorship
Contribution: Z.M., E.K., Z.X., V.B., and R.Z. designed the post hoc analysis; E.K., Z.X., and V.B. analyzed the data; and all authors interpreted the data, provided critical revision of the manuscript, and approved the content for publication.
Conflict-of-interest disclosure: Z.M. has served on advisory boards for Bristol Myers Squibb, Incyte Corporation, Jazz, Kite, and Sanofi. E.K., Z.X., V.B., and J.G. are employees and stockholders of Incyte Corporation. F.L. reports honoraria from Amgen, Gilead, Miltenyi, and Novartis. R.Z. reports honoraria from Incyte Corporation, Mallinckrodt, Novartis, and Sanofi.
Correspondence: Zahra Mahmoudjafari, Division of Hematologic Malignancies & Cellular Therapeutics, The University of Kansas Cancer Center, 2330 Shawnee Mission Parkway, Suite 1107, Westwood, KS 66205; email: zmahmoudjafari@kumc.edu.
References
Author notes
Incyte (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized data sets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized data sets from any interventional study (except phase 1 studies) for which the product and indication have been approved on, or after, 1 January 2020 in at least 1 major market (eg, United States, European Union, or Japan). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960

![ORR and BOR by azole subgroup in patients with GVHD. Patients with SR-aGVHD (A) and patients with SR-cGVHD (B) treated with ruxolitinib (RUX) or BAT, with or without azoles. Panel A shows ORR (CR + PR) and BOR at day 28 (aGVHD [full analysis set]). Panel B shows ORR (CR + PR) and BOR at week 24 (cGVHD [full analysis set]). OR, odds ratio.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/16/10.1182_bloodadvances.2025016212/2/m_blooda_adv-2025-016212-gr1.jpeg?Expires=1769083646&Signature=wFzPUR-5XAgjqsiR3FjB1JvQ0YXPDAdYSy094yrZulDzvy-Sh6ndn0YEwylNhvHu~wL871D6IRTkG4vKqFScvCOIcZ478A2v1NBwBeoMT2cwQLk0i5bSdQwlehPae2TfiUaKrFfT7qtuia0deA216IhzaOvD1QxMMSp0UQVo8S8ERJiOae~ZdWoMm4J1glwfFN66pNGFffB4fLaTwKwyCA79eZkOGbq5Wg69TEAuUhCo9kxbPZk-EoDIVOAcSQKzPqQ3DrPlCN5x9z54gadX3sQ4JhUF2F~41eeKHZdN1SqRlcUMyY~jecdz5SCuOZLYssolhGJRBs6nGId2jl1EAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)