Patients with hematology-oncology issues require platelet transfusion support that may potentiate pulmonary injury.
PRPCs decreased the probability of assisted mechanical ventilation during platelet transfusion.
Visual Abstract
Patients treated with antineoplastic therapy often develop thrombocytopenia requiring platelet transfusion, which has potential to exacerbate pulmonary injury. This study tested the hypothesis that amotosalen-UVA pathogen–reduced platelet components (PRPCs) do not potentiate pulmonary dysfunction compared with conventional platelet components (CPCs). A prospective, multicenter, open-label, sequential cohort study evaluated the incidence of treatment-emergent assisted mechanical ventilation initiated for pulmonary dysfunction (TEAMV-PD). The first cohort received CPC. After the CPC cohort, each site enrolled a second cohort transfused with PRPC. Other outcomes included clinically significant pulmonary adverse events (CSPAE) and the incidence of treatment-emergent acute respiratory distress syndrome (TEARDS) diagnosed by blinded expert adjudication. The incidence of TEAMV-PD in all patients (1068 PRPC and 1223 CPC) was less for PRPC (1.7 %) than CPC (3.1%) with a treatment difference of –1.5% (95% confidence interval [CI], –2.7 to –0.2). In patients requiring ≥2 PCs, the incidence of TEAMV-PD was reduced for PRPC recipients compared with CPC recipients (treatment difference, –2.4%; 95% CI, –4.2 to –0.6). CSPAE increased with increasing PC exposure but were not significantly different between the cohorts. For patients receiving ≥2 platelet transfusions, TEARDS occurred in 1.3% PRPC and 2.6% CPC recipients (P = .086). Bayesian analysis demonstrated PRPC may be superior in reducing TEAMV-PD and TEARDS for platelet transfusion recipients compared with CPC recipients, with 99.2% and 88.8% probability, respectively. In this study, PRPC compared with CPC demonstrated high probability of reduced severe pulmonary injury requiring assisted mechanical ventilation in patients with hematology disorders dependent on platelet transfusion. This trial was registered at www.ClinicalTrials.gov as #NCT02549222.
Introduction
Treatment-emergent assisted mechanical ventilation for pulmonary dysfunction (TEAMV-PD), including acute respiratory distress syndrome (ARDS), is a significant intervention that affects the long-term outcome of patients during treatment of hematology-oncology disorders.1 Immune-mediated transfusion-related acute lung injury (TRALI) is a potential cause of ARDS in patients who require platelet transfusion.2 It is postulated platelets may play a critical role in the pathogenesis of TRALI through the induction of neutrophil extracellular traps associated with inflammatory responses.3 However, ARDS and other types of pulmonary injury may arise from other mechanisms of pulmonary injury in which platelet transfusion is a potential contributing factor but not the primary causal factor.2,4,5 In addition, contamination of platelet components (PCs) with low levels of bacteria that do not cause immediate posttransfusion sepsis may contribute to subsequent pulmonary infections.6 Patients with hematology-oncology disorders have concurrent microbial infections and tissue damage during extensive antineoplastic therapy that in conjunction with platelet transfusion could potentiate pulmonary injury, resulting in ARDS.7
Pathogen reduction treatment of PCs (PRPCs) with amotosalen and UVA light was approved by the US Food and Drug Administration in 2014 to reduce the risk of transfusion-transmitted infections.8 Substantial data have been collected documenting the safety of amotosalen-UVA pathogen-reduced PCs.9-11 A previous publication reported that the incidence of assisted mechanical ventilation for all causes, including ARDS, was reduced in patients supported with PRPC compared with CPC.12 However, the effect of the intensity of platelet transfusion on the probability of all types of pulmonary injury, including ARDS, was not reported. The current report compares the impact of PRPC and CPC transfusion intensity on pulmonary injury requiring assisted mechanical ventilation and presents a Bayesian analysis to estimate the probability of assisted mechanical ventilation for severe pulmonary injury.
Methods
Design
The study was an open-label, prospective, nonrandomized, sequential, 2-cohort study with clinical standard of care conducted at 15 sites (supplemental Data; supplemental Table 1).12 Patients with hematology-oncology disorders, including hematopoietic cell transplant (HCT) recipients expected to require transfusion with at least 1 PC, were enrolled and considered the intention to treat (ITT) population. The modified ITT population (mITT) constituted all patients who received transfusion. The first cohort received CPC. After completion of the CPC cohort, each site enrolled a PRPC cohort. Patients who received CPC were not re-enrolled in the second cohort. The PRPC cohort was matched to the first cohort ±10% for 4 baseline therapy strata (chemotherapy, HCT with myeloablation, HCT without myeloablation, and HCT with reduced intensity conditioning [RIC]) within each clinical site to adjust for antineoplastic therapy impact on pulmonary injury.13 The study protocol was approved by each site’s institutional research board in compliance with local institutional regulations. On a per-site basis, written informed consent was either required or waived for data extraction with documented oral consent.
Clinical execution
The active transfusion period was 21 days with 7 days of follow-up after the last study PC. Sites were requested to enroll 50 to 100 patients in each cohort. Treating physicians, not study investigators, ordered PC and assisted mechanical ventilation per institutional standard of care. There were no study specific interventions. Clinical data were extracted from medical records into electronic case report forms with anonymity under Health Insurance Portability and Accountability Act compliance; and monitored against source data.
Outcomes
The outcome of interest was the incidence of TEAMV-PD by intubation or tight-fitting mask with positive pressure. Records of all patients receiving assisted mechanical ventilation after initiation of study platelet transfusion support (treatment emergent) were reviewed by a blinded pulmonary expert panel (PEP) for adjudication of the type of pulmonary injury, diagnosis of ARDS by the Berlin criteria,14 and assessment of causal relation to platelet transfusion. Other outcomes indicative of pulmonary injury included clinically significant pulmonary adverse events (CSPAE; Common Terminology Criteria for Adverse Events [CTCAE] grade ≥2) within 7 days of each transfusion. Blinded data submitted for PEP adjudication included: number of PC and duration of PC support, pulmonary imaging, respiratory therapy, arterial blood gas to inspired gas ratios (P/F), and clinical narratives. Adverse events (AEs) within 24 hours of each PC transfusion classified as transfusion reactions within 24 hours of each PC exposure and all serious AEs (SAEs) within 7 days of each study PC were analyzed. All other types of AEs and mortality up to 28 days were previously reported.12
Study PCs
Leukocyte-reduced whole blood or apheresis CPC were suspended in plasma or plasma with platelet additive solution (PAS). CPC were screened for bacterial contamination using current methods and gamma/X-ray irradiated as indicated. Leukocyte-reduced apheresis PRPC were suspended in plasma (Trima, Terumo) or plasma with PAS (Amicus and Intersol, Fenwal). PRPC were treated with the INTERCEPT Blood System for Platelets (Cerus, Concord, CA) in place of bacterial screening and gamma/X-ray irradiation. Both CPC and PRPC were stored for up to 5 days. Platelet dose was not measured, but blood centers complied with the US Food and Drug Administration criteria for PC dose ≥3.0 × 1011.
Statistical analysis
The ITT and mITT data sets were identical and included all patients who received transfusion with ≥1 study PC, regardless of any incorrect PC type (supplemental Data; supplemental Figure 1). Elective intubation for short-term airway protection during invasive procedures at baseline or during the study did not qualify as TEAMV-PD, based on no parenchymal pulmonary lesions within 24 hours after TEAMV.
Unless otherwise stated, clinical data were summarized descriptively at the patient level by 3 categories of primary disease therapy (chemotherapy, HCT with myeloablation, and HCT nonmyeloablative and HCT-RIC combined). For categorical measurements, summaries are presented using counts and proportions. For continuous measurements, summaries are presented using sample statistics including the mean, standard deviation, median, minimum, and maximum. All statistical analyses were performed using SAS version 9.4 (or higher).
Sample size and level of significance
The basis for the sample size of the study from which the current data are derived is described in the supplemental Data. For all outcomes, the proportions of patients with TEAMV-PD, treatment-emergent ARDS (TEARDS), CSPAE, and transfusion reactions were evaluated for statistical significance of treatment comparisons using a 2-sided .05 level. The impact of the intensity of platelet transfusion was examined for patients receiving ≥2 transfusions and then by subgroups of 1, 2 to 4, 5 to 10, and > 10 platelet transfusions. The outcomes were analyzed by both frequentist and Bayesian statistics.
A Bayesian regression model was used to compare treatment groups for TEAM-PD, TEARDS, CSPAE, and serious CSPAE. This model adjusted for baseline covariates using a normal distribution with large variance as a noninformative prior on the regression coefficients. Results were adjusted by baseline primary disease therapy, cardiac disease history, pulmonary disease history, and transfusion reaction history, when applicable. Treatment group as well as any additional covariates were included as fixed-level effects. Risk ratios and their associated credible intervals, along with the posterior probability of Bayesian superiority for the treatment group, are reported.
Sensitivity analysis
In the absence of true randomization to treatment, a propensity score method was used as a sensitivity analysis to assess the robustness of the primary analysis. The propensity score method was used to mimic random assignment to treatment by creating a comparison group of study participants matched on observable characteristics with participants in the treatment group (supplemental Data).
Analysis of outcomes
Outcomes (including the proportions with TEAMV-PD, TEARDS, CSPAE, PC exposure, and related transfusion reactions) were compared between treatment groups. Treatment differences in categorical variables were tested using the stratified Cochran-Mantel-Haenszel (CMH) test (row mean scores differ for ordinal data and general association for nonordinal data), controlling for primary disease therapy. Descriptive summary statistics are reported by type of primary disease therapy, system organ class (SOC), and preferred term (PT) as applicable. For continuous variables (eg, duration of study platelet support), P values for treatment difference were based on an analysis of variance model including treatment cohort and 3-category primary disease therapy as fixed effects. Point estimate and 95% confidence interval (CI) for the treatment difference in least-squares means are presented. The least-squares means with the associated standard errors are displayed for continuous variables by treatment group. Estimated treatment difference in proportions (PRPC vs CPC) along with corresponding 2-sided 95% CIs and P values are presented. For categorical variables, P values for treatment comparison are based on the stratified CMH test (row mean scores differ for ordinal data and general association for nonordinal data), controlling for primary disease therapy. Additionally, rank scores were used to compute the CMH statistics for ordinal categorical data. Time to onset of assisted ventilation from the date of first study PC transfusion are summarized descriptively by the Kaplan-Meier method, and the treatment difference is explored by the Cox model Wald test, adjusting for primary disease therapy.
Results
Demographics, prior medical history, primary disease, and primary therapy
The mITT analysis set included 1068 PRPC and 1223 CPC patients (supplemental Data; supplemental Figure 1). Demographics, previously reported, were similar between cohorts, except for greater mean age in the PRPC cohort (supplemental Data; supplemental Table 2).12 The PRPC cohort had a significantly higher incidence of prior pulmonary and cardiac disease, as well as prior transfusion reactions based on International Classification of Disease (IDC)-10 coding in medical records. The distributions of current hematologic diseases were statistically different between cohorts; however, both cohorts had substantial numbers of patients in each disease group. The proportions of patients by primary therapy strata were not statistically different between the cohorts; and were within the ±10% protocol criteria. PRPC recipients received more local radiation and less total body radiation.
Protocol compliance for assigned type of PC was 93.4% in the PRPC cohort and 84.7% in the CPC cohort, resulting in 6.6% of PRPC patients receiving CPC (mean exposure, 1.8 ± 1.5 CPC) and 15.3% of CPC patients receiving PRPC (mean exposure, 1.1 ± 0.3 PRPC).
Incidence of TEAMV-PD and TEARDS
Data for 87 patients with TEAMV were evaluated by the PEP resulting in 56 patients adjudicated as TEAMV-PD and 33 with ARDS.12 For all patients, the incidence of TEAMV-PD was less for PRPC compared with CPC recipients (P = .03) (Figure 1A; Table 1). PC transfusions and days of support for patients with TEAMV-PD were not statistically different between the cohorts (Table 1). More CPC patients (33.2%) than PRPC patients (27.6%) received a single PC, and TEAMV-PD was low in each cohort exposed to 1 PC (Table 1).
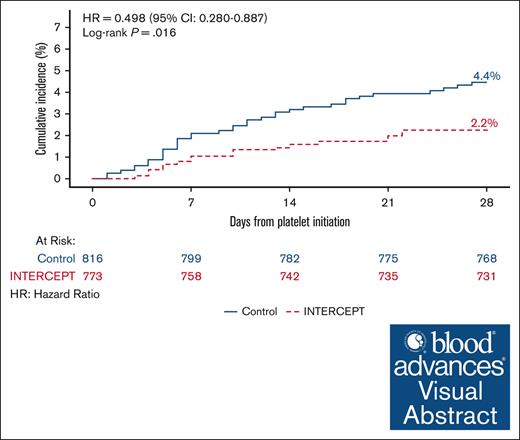
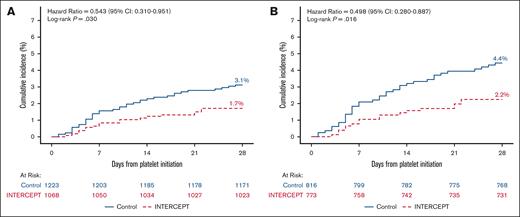
Cumulative incidence of treatment emergent assisted mechanical ventilation due to pulmonary injury for patients transfused with PRPC and CPC. (A) The cumulative incidence of TEAMV-PD in patients who received transfusion with ≥1 PC for the mITT data set (CPC, blue; PRPC-INTERCEPT), red). The hazard ratio (HR) and 95% CIs were estimated from the Cox proportional hazards regression for 1223 CPC patients and 1068 PRPC patients. The incidence of TEAMV-PD for PRPC recipients was 1.7% compared with 3.1% for CPC recipients (log rank P = .030; HR, 0.543; 95% CI, 0.310-0.951). (B) The cumulative incidence of TEAMV-PD in patients who received transfusion with ≥2 PCs for the mITT data set (CPC, blue; PRPC, red). The HR and 95% CIs were estimated from the Cox proportional hazards regression for 816 CPC patients and 773 PRPC patients. The incidence of TEAMV-PD for PRPC recipients was 2.2% compared with 4.4% for CPC recipients (log rank P = .016; HR, 0.498; 95% CI, 0.280-0.887).
Cumulative incidence of treatment emergent assisted mechanical ventilation due to pulmonary injury for patients transfused with PRPC and CPC. (A) The cumulative incidence of TEAMV-PD in patients who received transfusion with ≥1 PC for the mITT data set (CPC, blue; PRPC-INTERCEPT), red). The hazard ratio (HR) and 95% CIs were estimated from the Cox proportional hazards regression for 1223 CPC patients and 1068 PRPC patients. The incidence of TEAMV-PD for PRPC recipients was 1.7% compared with 3.1% for CPC recipients (log rank P = .030; HR, 0.543; 95% CI, 0.310-0.951). (B) The cumulative incidence of TEAMV-PD in patients who received transfusion with ≥2 PCs for the mITT data set (CPC, blue; PRPC, red). The HR and 95% CIs were estimated from the Cox proportional hazards regression for 816 CPC patients and 773 PRPC patients. The incidence of TEAMV-PD for PRPC recipients was 2.2% compared with 4.4% for CPC recipients (log rank P = .016; HR, 0.498; 95% CI, 0.280-0.887).
Incidence of TEAMV-PD, TEARDS, and platelet transfusion exposure
| mITT analysis . | |||
|---|---|---|---|
| TEAMV-PD, TEARDS, and PC exposure: all patients . | |||
| Parameter . | PRPC (n = 1068) . | CPC (n = 1223) . | PRPC vs CPC∗ . |
| Patients with TEAMV-PD†, n (%) | 18 (1.7) | 38 (3.1) | –1.5% (–2.7%, –0.2%) |
| TEARDS with TEAMV-PD‡, n (%) | 11 (1.0) | 22 (1.8) | .151 |
| PC transfused in patients with TEAMV-PD§, n ± SD) | 22.6 ± 22.1 | 13.6 ± 9.2 | .493 |
| Days of PC support in patients with TEAMV-PD‖, n ± SD | 14.8 ± 7.0 | 14.1 ± 7.2 | .632 |
| TEAMV-PD and TEARDS for patients receiving 1 PC transfusion | |||
| Parameter | PRPC (n = 295) | CPC (n = 406) | PRPC vs CPC |
| Patients with TEAMV-PD†, n (%) | 1 (0.3) | 2 (0.5) | 0.2% (–1.7%, 2.2%) |
| TEARDS with TEAMV-PD‡, n (%) | 1 (0.3) | 1 (0.2) | .793 |
| TEAMV, TEARDS, and PC exposure for patients receiving ≥2 PC transfusions | |||
| Parameter | PRPC (n = 773) | CPC (n = 816) | PRPC vs CPC∗ |
| Patients with TEAMV-PD†, n (%) | 17 (2.2) | 36 (4.4) | –2.4% (–4.2%, –0.6%) |
| TEARDS for TEAMV-PD‡, n (%) | 10 (1.3) | 21 (2.6) | .086 |
| PC transfused in patients with TEAMV-PD§, n ± SD | 23.8 ± 22.1 | 14.3 ± 9.0 | .414 |
| Days of PC support in patients with TEAMV-PD‖, n ± SD | 15.6 ± 6.3 | 14.8 ± 6.7 | .791 |
| mITT analysis . | |||
|---|---|---|---|
| TEAMV-PD, TEARDS, and PC exposure: all patients . | |||
| Parameter . | PRPC (n = 1068) . | CPC (n = 1223) . | PRPC vs CPC∗ . |
| Patients with TEAMV-PD†, n (%) | 18 (1.7) | 38 (3.1) | –1.5% (–2.7%, –0.2%) |
| TEARDS with TEAMV-PD‡, n (%) | 11 (1.0) | 22 (1.8) | .151 |
| PC transfused in patients with TEAMV-PD§, n ± SD) | 22.6 ± 22.1 | 13.6 ± 9.2 | .493 |
| Days of PC support in patients with TEAMV-PD‖, n ± SD | 14.8 ± 7.0 | 14.1 ± 7.2 | .632 |
| TEAMV-PD and TEARDS for patients receiving 1 PC transfusion | |||
| Parameter | PRPC (n = 295) | CPC (n = 406) | PRPC vs CPC |
| Patients with TEAMV-PD†, n (%) | 1 (0.3) | 2 (0.5) | 0.2% (–1.7%, 2.2%) |
| TEARDS with TEAMV-PD‡, n (%) | 1 (0.3) | 1 (0.2) | .793 |
| TEAMV, TEARDS, and PC exposure for patients receiving ≥2 PC transfusions | |||
| Parameter | PRPC (n = 773) | CPC (n = 816) | PRPC vs CPC∗ |
| Patients with TEAMV-PD†, n (%) | 17 (2.2) | 36 (4.4) | –2.4% (–4.2%, –0.6%) |
| TEARDS for TEAMV-PD‡, n (%) | 10 (1.3) | 21 (2.6) | .086 |
| PC transfused in patients with TEAMV-PD§, n ± SD | 23.8 ± 22.1 | 14.3 ± 9.0 | .414 |
| Days of PC support in patients with TEAMV-PD‖, n ± SD | 15.6 ± 6.3 | 14.8 ± 6.7 | .791 |
SD, standard deviation.
For noninferiority analysis, the treatment difference (T-C) and the 95% CI is presented. For continuous variables, P values (for treatment difference) are based on an analysis of variance model including treatment and 4-category primary disease therapy (chemotherapy, HSCT-myeloablative, HSCT- nonmyeloablative, and HSCT-RIC) as fixed effects. A point estimate and the corresponding 2-sided 95% CI for the treatment difference in LS means are also provided. For categorical variables, P values are based on a stratified CMH PRPC (general association), controlling for primary disease therapy. A P value <.05 is flagged with an “∗.”
Patients with TEAMV-PD evaluated by the blinded PEP based on review of clinical records, respiratory therapy, and all chest imaging studies in the medical record; based on review of 93 patients with protocol defined or deviant TEAMV.
TEARDS in patients with TEAMV to treat pulmonary injury assessed by the PEP was evaluated according to the Berlin criteria for ARDS.
Number of PCs transfused to patients during the active transfusion period of up to 21 days.
Days of platelet support period = (date of last study or nonstudy platelet transfusion, up to day 21 or platelet independence, whichever sooner) – (date of first study transfusion) + 1, in which platelet independence is defined as >5 days elapsed from the previous study or nonstudy platelet transfusion.
To examine TEAMV-PD in patients with more intense transfusions, an analysis was performed for patients who received ≥2 PCs. For patients with TEAMV-PD with ≥2 PC exposures, 64.7% and 69.4% of PRPC and CPC recipients, respectively, required intubation and 76.5% and 58.3% of PRPC and CPC patients, respectively, required tight-fitting mask during their clinical course. Analysis of patients with ≥2 PCs demonstrated a significantly lower incidence of TEAMV-PD in the PRPC cohort (P = .016) (Figure 1B; Table 1). The odds ratio for TEAMV-PD in PRPC patients requiring ≥2 PCs was 0.52 (95% CI, 0.29-0.94; P = .028). PC transfusions and days of transfusion were substantial for both cohorts but not statistically different between cohorts (Table 1).
Among all 56 patients with TEAMV-PD adjudicated by PEP review, 33 (11 PRPC and 22 CPC) had criteria for TEARDS (Table 1). The incidence of TEARDS assessed by the PEP for patients who received transfusion with ≥2 PCs (Table 1) was less for PRPC (1.3% vs 2.6%) but not statistically significant (P = .086; Table 1). No PRPC patient had TEARDS related to PC transfusion, and 3 CPC patients were adjudicated as TEARDS related to PC transfusion, 2 with volume overload. One CPC patient met the criteria for TRALI based on temporal relation to transfusion.
The causal factors for TEARDS were determined by the PEP (supplemental Data; supplemental Table 3). Of the 33 patients with TEARDS, 21 patients had pneumonia, and 16 had bacteremia, fungemia, sepsis, or septic shock as a causal factor. Other contributing factors in patients with TEARDS were congestive heart failure and volume overload. For the 23 patients with TEAMV-PD without TEARDS diagnostic criteria (supplemental Data; supplemental Table 4), 10 had pneumonia, and 5 had bacteremia or sepsis. The remaining patients had pulmonary edema and volume overload.
Incidence of clinically significant pulmonary AEs
Investigator assessment of treatment-emergent CSPAEs provided an integrated clinical indicator of pulmonary injury (Table 2). For patients receiving ≥2 PC, the incidences of treatment-emergent CSPAE and serious CSPAE were increased but not different between PRPC and CPC cohorts, respectively (Table 2). Mean PC transfusions for these patients were similar for PRPC and CPC cohorts (Table 2). The days of platelet support for the more intensely transfused patients were less for PRPC patients (Table 2). Patients receiving only a single PC had a low incidence of CSPAE and serious CSPAE (Table 2).
Incidence of CSPAE and PC exposure
| mITT analysis . | |||
|---|---|---|---|
| CSPAE and PC exposure: all patients . | |||
| Parameter . | PRPC, n = 1068 . | CPC, n = 1223 . | PRPC vs CPC∗ . |
| Patients with CSPAE†, n (%) | 151(14.1) | 180 (14.7) | .810 |
| Patients with serious CSPAE‡, n (%) | 67(6.3) | 85(7.0) | .705 |
| PC transfused in patients with CSPAE§, n ± SD | 9.8 ± 10.0 | 9.9 ± 7.9 | .746 |
| Days of PC support in patients with CSPAE‖, n ± SD | 11.0 ± 7.3 | 12.8 ± 7.5 | .029∗ |
| CSPAE for patients with 1 PC transfusion | |||
| Parameter | PRPC n = 295 | CPC n = 406 | PRPC vs CPC∗ |
| Patients with CSPAE†, n (%) | 9 (3.1) | 14 (3.4) | .811 |
| Patients with serious CSPAE‡, n (%) | 6 (2.0) | 7 (1.7) | .715 |
| CSPAE for patients with ≥ 2 PC transfusions | |||
| Parameter | PRPC n = 773 | CPC n = 816 | PRPC vs CPC∗ |
| Patients with CSPAE†, n (%) | 142 (18.4) | 166 (20.3) | .455 |
| Patients with serious CSPAE‡, n (%) | 61 (7.9) | 78 (9.6) | .410 |
| PC transfused in patients with CSPAE§, n ± SD | 10.4 ± 10.1 | 10.6 ± 7.8 | .677 |
| Days of PC support in patients with CSPAE‖, n ± SD | 11.6 ± 7.0 | 13.7 ± 6.9 | .011∗ |
| mITT analysis . | |||
|---|---|---|---|
| CSPAE and PC exposure: all patients . | |||
| Parameter . | PRPC, n = 1068 . | CPC, n = 1223 . | PRPC vs CPC∗ . |
| Patients with CSPAE†, n (%) | 151(14.1) | 180 (14.7) | .810 |
| Patients with serious CSPAE‡, n (%) | 67(6.3) | 85(7.0) | .705 |
| PC transfused in patients with CSPAE§, n ± SD | 9.8 ± 10.0 | 9.9 ± 7.9 | .746 |
| Days of PC support in patients with CSPAE‖, n ± SD | 11.0 ± 7.3 | 12.8 ± 7.5 | .029∗ |
| CSPAE for patients with 1 PC transfusion | |||
| Parameter | PRPC n = 295 | CPC n = 406 | PRPC vs CPC∗ |
| Patients with CSPAE†, n (%) | 9 (3.1) | 14 (3.4) | .811 |
| Patients with serious CSPAE‡, n (%) | 6 (2.0) | 7 (1.7) | .715 |
| CSPAE for patients with ≥ 2 PC transfusions | |||
| Parameter | PRPC n = 773 | CPC n = 816 | PRPC vs CPC∗ |
| Patients with CSPAE†, n (%) | 142 (18.4) | 166 (20.3) | .455 |
| Patients with serious CSPAE‡, n (%) | 61 (7.9) | 78 (9.6) | .410 |
| PC transfused in patients with CSPAE§, n ± SD | 10.4 ± 10.1 | 10.6 ± 7.8 | .677 |
| Days of PC support in patients with CSPAE‖, n ± SD | 11.6 ± 7.0 | 13.7 ± 6.9 | .011∗ |
SD, standard deviation.
P values are based on a stratified CMH PRPC (general association), controlling for 4-category primary disease therapy (chemotherapy, HSCT-myeloablative, HSCT-nonmyeloablative, and HSCT-RIC). A P value <.05 is flagged with an “∗.”
Clinically significant pulmonary adverse events (CSPAE) are AEs with CTCAE grade ≥2. CSPAEs are treatment-emergent AEs, defined as AEs with an onset on or after the start of the first study platelet transfusion. By default, AEs with missing onset date are treatment emergent. AEs with missing relationship/severity/seriousness are categorized as related/severe/serious AEs. MedDRA version 18.0 is used.
Serious CSPAE are those events that meet the criteria for serious (death, life-threatening event, inpatient hospitalization, persistent or significant disability/incapacitation, congenital anomaly/birth defect, or another significant medical event).
The number of PCs transfused during the active transfusion period of up to 21 days after enrollment.
Days of platelet support period = (date of last study or nonstudy platelet transfusion, up to day 21 or platelet independence, whichever sooner) – (date of first study transfusion) + 1, in which platelet independence is defined as >5 days elapsed from the previous study or nonstudy platelet transfusion.
The types of CSPAE were determined by SOC and PT analysis (MeDRA Version 18.0) for all patients, and the incidence of AEs by PT occurring in >1% of PRPC patients was compared between CPC and PRPC recipients (supplemental Data; supplemental Table 5). There were no significant differences by SOC. The most frequent preferred terms for these CSPAE were hypoxia, pulmonary edema, pneumonia, and pleural effusion. Except for the incidence of pulmonary edema and cough, which were less in the CPC cohort, there were no other significant differences in the frequency of CSPAE by PT. The incidences of treatment-emergent cardiac disorders were infrequent and not different between the cohorts (supplemental Data; supplemental Table 5).
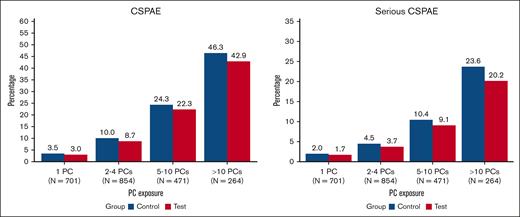
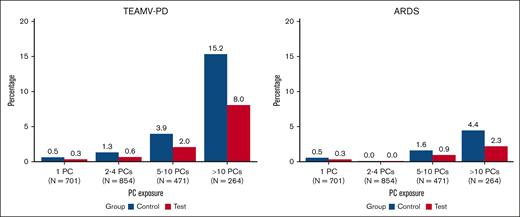
The impact of PC exposure on CSPAE, serious CSPAE, TEAMV-PD, and TEARDS was evaluated for patients who received transfusion with 1 PC, 2 to 4 PCs, 5 to 10 PCs, and >10 PCs (Figures 2 and 3). The incidence of CSPAE, serious CSPAE increased in both cohorts with the intensity of PC transfusion (Figure 2). No significant differences between PRPC and CPC were observed for recipients of 1, 2 to 4, 5 to 10, or >10 PCs, but the proportions of patients with these outcomes were consistently higher for CPC recipients. The incidence of TEAMV-PD was lower in PRPC recipients than CPC recipients transfused with 2 to 4 and 5 to 10 PCs but not statistically significant (P > .05; Figure 3). The incidence of TEAMV-PD in PRPC recipients receiving >10 PCs was less than that of CPC recipients but not significant (P = .064; Figure 3). The incidence of TEARDS was lower, but not statistically different, for PRPC recipients than that of CPC recipients who received transfusion at all levels of PC exposure (P > .05).
The impact of PC exposure on the proportions of CPC (Control) recipients and PRPC (Test) recipients with CSPAE and serious CSPAE. CPC recipients are represented in blue and PRPC in red. The proportions of patients with CSPAE and serious CSPAE increased with PC exposure but were not statistically different between CPC and PRPC cohorts.
The impact of PC exposure on the proportions of CPC (Control) recipients and PRPC (Test) recipients with CSPAE and serious CSPAE. CPC recipients are represented in blue and PRPC in red. The proportions of patients with CSPAE and serious CSPAE increased with PC exposure but were not statistically different between CPC and PRPC cohorts.
The impact of PC exposure on the proportions of CPC (Control) recipients and PRPC (Test) recipients with TEAMV-PD and TEARDS determined by blinded PEP adjudication. CPC recipients are represented in blue and PRPC in red. The incidence of TEAMV-PD increased with increasing PC exposure in both cohorts and was not statistically different between the cohorts (P > .05). The incidence of TEARDS increased with increasing PC exposure and was not statistically different between the cohorts (P > .05).
The impact of PC exposure on the proportions of CPC (Control) recipients and PRPC (Test) recipients with TEAMV-PD and TEARDS determined by blinded PEP adjudication. CPC recipients are represented in blue and PRPC in red. The incidence of TEAMV-PD increased with increasing PC exposure in both cohorts and was not statistically different between the cohorts (P > .05). The incidence of TEARDS increased with increasing PC exposure and was not statistically different between the cohorts (P > .05).
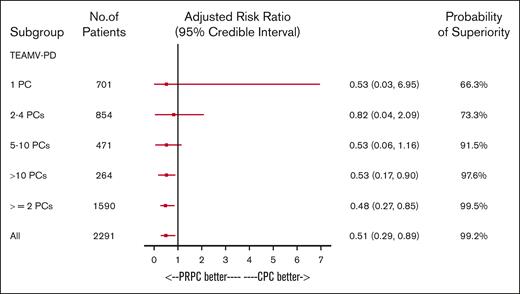
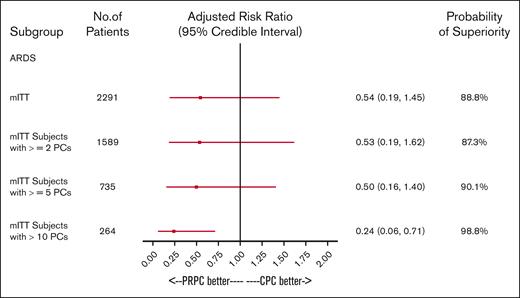
A Bayesian regression model adjusted for 3 primary therapy strata and baseline covariates (prior cardiac, pulmonary, and transfusion reaction history) was used to determine the probability that PRPC reduced the incidence of TEAMV-PD (Figure 4). For all patients who received transfusion with any PC, the probability of PRPC to reduce the incidence of TEAMV-PD was 99.2% and 99.5% for ≥2 PCs (Figure 4). PRPC demonstrated high probability of reduced TEAMV-PD (91.5%) for exposures to 5 to 10 PCs and >10 PCs (97.6%; Figure 4). PRPC exhibited moderate probability to reduce TEAMV-PD with 2 to 4 PCs (73.3%) and 1 PC (66.3%; Figure 4). Bayesian analysis for the probability of TEARDS (Figure 5) demonstrated PRPC may be superior in reducing TEARDS compared with CPC with a probability of 88.8% for the entire cohort. In patients who received transfusion with ≥5 PCs, PRPC may be superior in reducing TEARDS compared with CPC recipients with a probability of 90.1%; and for exposure to >10 PCs, 98.8%.
The impact of PC exposure on the incidence of TEAMV-PD was determined by a Bayesian regression model to compare treatment groups. The model adjusted for baseline covariates using a normal distribution with large variance as a noninformative prior on the regression coefficients. Results were adjusted by baseline primary disease therapy strata, cardiac disease history, pulmonary disease history and transfusion reaction history. Treatment group as well as any additional covariates were included as fixed-level effects. Risk ratios and their associated credible intervals, along with the posterior probability of superiority for the treatment group, are shown. The probability that PRPC were superior to CPC for lower incidence of TEAMV-PD is >90% for patients exposed from 5 to 10 and ≥10 PCs. For all patients, the probability of PRPC superiority is 99.2% in this model. For patients with limited exposures (1 PC and 2-4 PCs), PRPC is superior but with lower probability (66.3% and 73.3%, respectively).
The impact of PC exposure on the incidence of TEAMV-PD was determined by a Bayesian regression model to compare treatment groups. The model adjusted for baseline covariates using a normal distribution with large variance as a noninformative prior on the regression coefficients. Results were adjusted by baseline primary disease therapy strata, cardiac disease history, pulmonary disease history and transfusion reaction history. Treatment group as well as any additional covariates were included as fixed-level effects. Risk ratios and their associated credible intervals, along with the posterior probability of superiority for the treatment group, are shown. The probability that PRPC were superior to CPC for lower incidence of TEAMV-PD is >90% for patients exposed from 5 to 10 and ≥10 PCs. For all patients, the probability of PRPC superiority is 99.2% in this model. For patients with limited exposures (1 PC and 2-4 PCs), PRPC is superior but with lower probability (66.3% and 73.3%, respectively).
The impact of PC exposure on the incidence of TEARDS was determined by a Bayesian regression model to compare treatment groups. The model adjusted for baseline covariates using a normal distribution with large variance as a noninformative prior on the regression coefficients. Results were adjusted by baseline primary disease therapy strata, cardiac disease history, pulmonary disease history, and transfusion reaction history. Treatment group as well as any additional covariates were included as fixed-level effects. Risk ratios and their associated credible intervals, along with the posterior probability of superiority for the treatment group, are shown. For all mITT patients, the probability of PRPC superiority of a lower incidence of TEARDS is 88.8 % in this model. For patients exposed to ≥2 PCs, the probability of PRPC superiority of a lower incidence of TEARDS is 87.3 % in this model. The probability that PRPC were superior to CPC for lower incidence of TEARDS is 90.1 % for patients exposed to 5 to 10 PCs and 98.8% for patients exposed to >10 PCs. For patients with limited exposures (1 PC and 2-4 PC), the incidence of TEARDS was not informative.
The impact of PC exposure on the incidence of TEARDS was determined by a Bayesian regression model to compare treatment groups. The model adjusted for baseline covariates using a normal distribution with large variance as a noninformative prior on the regression coefficients. Results were adjusted by baseline primary disease therapy strata, cardiac disease history, pulmonary disease history, and transfusion reaction history. Treatment group as well as any additional covariates were included as fixed-level effects. Risk ratios and their associated credible intervals, along with the posterior probability of superiority for the treatment group, are shown. For all mITT patients, the probability of PRPC superiority of a lower incidence of TEARDS is 88.8 % in this model. For patients exposed to ≥2 PCs, the probability of PRPC superiority of a lower incidence of TEARDS is 87.3 % in this model. The probability that PRPC were superior to CPC for lower incidence of TEARDS is 90.1 % for patients exposed to 5 to 10 PCs and 98.8% for patients exposed to >10 PCs. For patients with limited exposures (1 PC and 2-4 PC), the incidence of TEARDS was not informative.
Incidence of transfusion-related AEs, transfusion reactions, and AEs.
The incidence of all types of transfusion AEs and related transfusion reactions reported by treating physicians were not significantly different for the mITT data set between the PRPC and CPC cohorts (Table 3). Allergic transfusion reactions were decreased in PRPC recipients (P = .009), and febrile nonhemolytic transfusion reactions were decreased in CPC recipients (P = .023). Analysis of all AEs with an incidence ≥3% by PT in either cohort showed significantly reduced differences in favor of PRPC for diarrhea, allergic transfusion reactions, and nausea (supplemental Data; supplemental Table 6). As previously reported, there was no difference in mortality between the treatment cohorts.12
Transfusion reaction AEs
| Transfusion-related AEs (all patients) . | |||
|---|---|---|---|
| Type of AE . | PRPC . | CPC . | P value . |
| Patients with any transfusion reaction AE, % | 9.6 | 10.4 | .518 |
| Patients with any related transfusion reaction, % | 8.3 | 9.7 | .246 |
| Allergic transfusion reactions, % | 3.2 | 5.6 | .009 |
| Febrile nonhemolytic transfusion reactions, % | 5.1 | 3.0 | .023 |
| Transfusion-associated cardiac overload, % | 1.1 | 1.4 | .538 |
| Transfusion-related AEs (all patients) . | |||
|---|---|---|---|
| Type of AE . | PRPC . | CPC . | P value . |
| Patients with any transfusion reaction AE, % | 9.6 | 10.4 | .518 |
| Patients with any related transfusion reaction, % | 8.3 | 9.7 | .246 |
| Allergic transfusion reactions, % | 3.2 | 5.6 | .009 |
| Febrile nonhemolytic transfusion reactions, % | 5.1 | 3.0 | .023 |
| Transfusion-associated cardiac overload, % | 1.1 | 1.4 | .538 |
Discussion
The initial report of this study analyzing all patients, without respect to platelet transfusion intensity, demonstrated a significantly reduced incidence of TEAMV-PD but not TEARDS in PRPC recipients compared with CPC recipients.12 These observations stimulated interest to examine the impact of platelet transfusion intensity on these outcomes. We observed that the cumulative incidence of TEAMV-PD was increased in patients receiving ≥2 platelet transfusions, and it was significantly reduced for PRPC recipients. However, by frequentist statistical analysis, the incidence of TEARDS was not significantly different between the PRPC and CPC cohorts. Examination of the TEARDS incidence by subgroups with different transfusion exposures demonstrated no statistically significant differences by frequentist statistics. Subsequently, we used Bayesian statistics to estimate the probability that the type of PC could affect the incidence of TEAMV-PD and TEARDS. In other studies, the Bayesian approach has demonstrated clinically useful information not always evident by the frequentist approach.15 With this approach, we observed that for recipients of ≥2 and 5 to 10 or >10 PRPCs, the probability of a lower incidence of TEAMV-PD was >90% compared with recipients of CPC. For TEARDS, the probability of a lower incidence was >90% for recipients of 5 to 10 and >10 PRPCs compared with CPC recipients. Notably, the incidence of CSPAE was comparable between the cohorts over the range of PC exposures. The similar incidence of CSPAE between the cohorts suggests that CSPAE were unlikely to be caused by the type of PC but rather driven by concurrent infections in PRPC and CPC patients with or without TEARDS. The type of PC appeared to affect the requirement for assisted mechanical ventilation.
Experimental studies suggest platelets play a role in the pathophysiology of pulmonary injury, especially with concurrent sepsis.3,16 Previously, we reported on the comparative incidence of all cause TEAMV, CSPAE, TEAMV-PD, and TEARDS in patients with hematology-oncology disorders, supported with CPC or PRPC, but did not examine the intensity of platelet transfusion.12 The study was designed to address a potential safety signal of excess pulmonary morbidity attributable to PRPC.17 In that study, the relative risk of TEAMV was less for PRPC recipients with the following baseline covariates: age <65 years, male sex, non-White race, prior chemotherapy without HCT, history of pulmonary disease, and history of cardiac disease.12
There are several hypotheses for potential reduction of TEAMV-PD with PRPC that may be relevant for intensely transfused patients. During our clinical study, the predominant method to reduce the risk of bacteria contaminated PC was culture screening, generally by aerobic culture of 8 mL of PC.18 However, not all contaminated PC can be identified6; and subsequently enhanced culture procedures with both anaerobic and aerobic cultures were implemented.6,19 Potentially, during the CPC period of this study, low levels of bacteria in PC, insufficient to cause transfusion-related sepsis but sufficient to potentiate pulmonary infections, may have affected the incidence of TEAMV-PD. In contrast to culture screening, PR inactivates bacteria to reduce low-level contamination.20 This hypothesis is consistent with the report of Aubron et al that platelet transfusion is associated with an increased risk of infection and bacteremia.21 Furthermore, residual leukocytes in donor PC may be inflammatory. Gamma/X-ray irradiation and leukocyte reduction do not completely inhibit cytokine synthesis. In contrast, leukocyte cytokine synthesis is effectively inhibited in PRPC.22 Production of residual leukocyte cytokines in CPC could have potentiated pulmonary inflammation driving TEAMV-PD.
Another possible explanation for the reduced incidence of TEAMV-PD with PRPC may be via reduction in the inflammatory effect of platelet mitochondria release during storage.23 Mitochondria from damaged platelets may initiate inflammatory responses via damage associated molecular pattern pathways with potentiation of pulmonary inflammation. Amotosalen-UVA forms DNA adducts in platelet mitochondria without impairing respiration.24,25 This could downregulate the inflammatory effects of released mitochondria from PRPC. This hypothesis may be testable.23
In this study and other studies, we have observed a significant reduction in allergic transfusion reactions in PRPC recipients, which may be partially explained by the larger proportion of PRPC suspended in platelet additive solutions with reduced donor plasma than CPC more frequently suspended in 100% donor plasma.12 However, there was no difference in the incidence of CSPAE, suggesting the difference in suspension media was not the primary cause for a reduction in TEAMV-PD.
This study has several limitations, most notably the lack of randomization and blinding of transfusing clinicians. In addition, only patients with hematology-oncology disorders were enrolled, and the results may not be generalizable to other patient populations. The patient population was heterogeneous with respect to primary disease. However, stratification by primary therapy and blinded adjudication for patients with TEAMV reduced the potential for bias in the diagnosis of TEAMV-PD and TEARDS. The subgroup analyses for the impact of PC exposure are limited by the smaller number of recipients in each group, but cumulatively, the CSPAE data suggest that PC exposure is not the primary cause of severe pulmonary injury. The outcomes could have been biased because some patients in each cohort received the contralateral type of PC due to transient platelet shortages. This occurred for 6% of PRPC patients who received CPC and 15% of CPC patients who received PRPC. Thus, both cohorts had some exposure to the other PC type; however, the amount of exposure to the contralateral PC (median exposure 1 PC) was limited. The difference in proportions of PRPC and CPC suspended in PAS with reduced plasma concentration could have affected the outcomes. The majority (78.4%) of PRPC were suspended in PAS plasma as compared with 16.5% of CPC suspended in PAS plasma. This undoubtedly contributed to the difference in allergic transfusion reactions. However, the incidence of CSPAE was not different between the cohorts, suggesting that this did not affect the differences in TEAMV-PD.
In summary, in this prospective cohort study, we observed a significant reduction in TEAMV-PD for patients supported with PRPC compared with CPC. The effect of the type of PC was enhanced in the most intensely transfused patients. Bayesian analysis demonstrated a high probability that PRPC compared with CPC reduced the incidence of TEAMV-PD and TEARDS. Adjudication of the causal factors for TEAMV-PD indicated that infectious AEs were the primary cause of pulmonary injury; but the type of PC could affect the need for TEAMV. The data suggest PRPC compared with CPC reduced the probability of assisted mechanical ventilation, especially in patients who were most heavily transfused.
Acknowledgments
The authors acknowledge the substantial contribution to the study by the pulmonary expert panel: Chairman, Ednan Bajwa, Roy Brower, Todd Rice, and Boyd Taylor Thompson. Staff at the study site blood centers and hospitals provided invaluable support to provide study platelet components. Clinical research coordinators at study sites collected and monitored clinical data.
Authorship
Contribution: L.M.C. wrote the manuscript with editorial review by R.J.B., S.B., and K.L.; S.B. and K.L. performed statistical analyses; and A.P.W., E.L.S., M.R., C.S.C., J.P., M. Fontaine, M.S., A.K.N., L.U., P.C.S., M. Fenelus, D.L., T.C., J.B., M.J., E.A.G., B.R.S., P.Y., A.J., J.J.O., G.J.S., J.D.R., E.M., R.J., S.T.A., and J.V. reviewed the manuscript.
Conflict-of-interest disclosure: P.C.S. is a consultant for Cerus, Hemanext, and CSL Behring; serves on scientific advisory boards for Octapharma and Haima; and is a cofounder and chief medical officer for Kalocyte. K.L., S.B., J.V., R.J.B., and L.M.C. are employees of Cerus Corporation, the study sponsor, and received compensation and equity options as part of their employment. A.P.W., E.L.S., M.R., C.S.C., J.P., M. Fontaine, M.S., A.K.N., L.U., M. Fenelus, D.L., T.C., J.B., M.J., E.A.G., B.R.S., P.Y., A.J., J.J.O., G.J.S., J.D.R., E.M., R.J., and S.T.A. served as study investigators and were compensated through their respective institutional research contracts with Cerus Corporation; and declare no competing financial interests.
Correspondence: Laurence M. Corash, Cerus Corporation, 1220 Concord Ave – Suite 600, Concord, CA 94520; email: lcorash@cerus.com.
References
Author notes
Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 5 years after the publication at www.cerus.com. Proposals for access should be sent to the corresponding author, Laurence Corash, (lcorash@cerus.com).
The study protocol is included as a data supplement available with the online version of this article.
The full-text version of this article contains a data supplement.