Persistent detection of ADAMTS13 inhibitors may delay recovery of ADAMTS13 activity.
A delay in administering rituximab may potentially lead to further delays in the ADAMTS13 recovery.
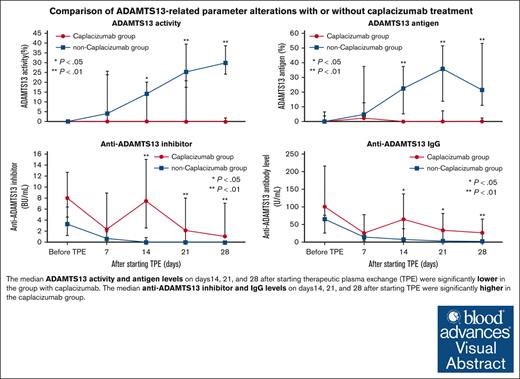
Visual Abstract
For patients with immune-mediated thrombotic thrombocytopenic purpura (iTTP), caplacizumab, a nanobody against von Willebrand factor A1 domain, has become crucial. Delayed normalization of ADAMTS13 activity during caplacizumab therapy has been identified. In a retrospective analysis, we compared platelet count, ADAMTS13 activity, its inhibitor, and anti-ADAMTS13 immunoglobulin G (IgG) levels in acute iTTP cases treated with caplacizumab (n = 14) or without it (n = 16). The median time from initial therapeutic plasma exchange (TPE) to the first rituximab administration was 12 days in the caplacizumab group (n = 11) and 10 days in the group without caplacizumab (n = 13). We evaluated ADAMTS13-related parameters at onset and once a week until day 28 after the first TPE. The number of days until the platelet counts reached ≥150 × 109/L was significantly shorter in the caplacizumab group than in the non-caplacizumab group. The median ADAMTS13 activity levels on days 14, 21, and 28 were significantly lower in the caplacizumab group. The median titers of the ADAMTS13 inhibitor and anti-ADAMTS13 IgG on the same days were significantly higher in the caplacizumab group. Furthermore, the median number of days from the first TPE until finally achieving an ADAMTS13 activity of ≥10% was significantly longer in the caplacizumab group than in the non-caplacizumab group (42 vs 23 days, P = .014). We observed delayed ADAMTS13 activity recovery and continued inhibitor and anti-ADAMTS13 IgG detection in patients with acute iTTP on caplacizumab, possibly because of the decreased number of TPEs and delayed frontline rituximab.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a fatal disease when not treated immediately, characterized by the formation of platelet thrombi in the microvasculature throughout the body, leading to ischemic organ damage.1 The onset of TTP is attributed to a significant decrease in the activity of a disintegrin and metalloprotease with thrombospondin type 1 motif, member 13 (ADAMTS13), an enzyme that cleaves von Willebrand factor (VWF).2,3 Approximately 95% of all TTP cases are acquired and attributed to anti-ADAMTS13 autoantibodies and are often referred to as immune-mediated TTP (iTTP). Therapeutic plasma exchange (TPE) using fresh frozen plasma (FFP) as a replacement fluid has been established as the standard treatment for iTTP.4 Additionally, corticosteroids5 and rituximab6 are widely used to suppress the production of anti-ADAMTS13 autoantibodies. In Japan, owing to the paucity of data on the efficacy and safety of rituximab in patients with acute iTTP, upfront rituximab administration in the acute phase, that is, the period until clinical remission is achieved, has not yet been approved by the current Japanese national health insurance system. Therefore, the Japanese national guidelines suggest that add-on rituximab in the acute phase of iTTP should be considered on a case-by-case basis.7
Mortality in the acute phase remains an issue even with these drugs, prompting the development of new treatments aimed at suppressing thrombosis. Among treatment candidates, caplacizumab, an anti-VWF antibody, has attracted significant attention. Caplacizumab is a humanized nanobody consisting of a single variable region of the heavy chain that binds to the A1 domain of VWF and inhibits the adhesion between VWF and platelets, thereby preventing thrombus formation.8 In addition to the phase 2 TITAN trial9 and phase 3 HERCULES trial,10 the Japanese domestic phase 2/3 trial demonstrated a reduction in the time to platelet count normalization, an improvement in organ damage markers, and a reduction in the recurrence rate in Japanese patients with caplacizumab-treated acute iTTP.11 Based on these results, caplacizumab was approved for use in Japan in September 2022.
Recently, Prasannan et al reported a delay in the recovery of ADAMTS13 activity with caplacizumab administration in a subset of patients compared with in a non-caplacizumab cohort.12 The authors indicated a potential link between an increase in anti-ADAMTS13 immunoglobulin G (IgG) levels from the onset to the time of stopping caplacizumab and the delayed normalization of ADAMTS13 activity. However, there have been no evaluations of the temporal changes in anti-ADAMTS13 IgG levels or ADAMTS13 functional inhibitors. Nonetheless, this delay in ADAMTS13 activity recovery has not been observed in other real-world studies conducted in Germany,13 France,14 and Spain.15
In this study, we retrospectively compared the changes in ADAMTS13 activity and the titers of ADAMTS13 inhibitors and anti-ADAMTS13 IgG among patients with caplacizumab-treated acute iTTP with those of historical controls in Japan.
Methods
Patients
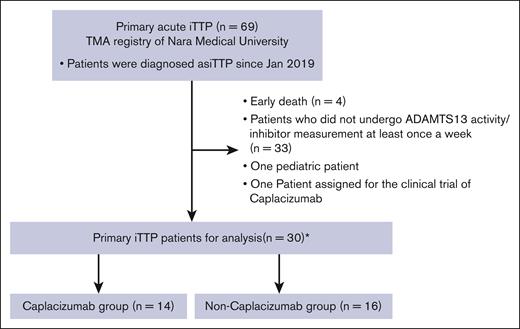
Our laboratory serves as a reference center for thrombotic microangiopathy in Japan and manages the thrombotic microangiopathy registry.16 Since January 2019, we have identified 69 patients with primary acute iTTP across Japan diagnosed with hemolytic anemia and thrombocytopenia with a severely decreased ADAMTS13 activity (<10%) and the presence of ADAMTS13 inhibitor (≥0.5 Bethesda units [BU]/mL or more).7 Patients who underwent weekly screening to determine the activities of ADAMTS13 and its inhibitors during the acute phase were included in this study. Patients with secondary iTTP treated with certain medications, vaccinations, or various underlying conditions, such as autoimmune diseases, malignancy, and pregnancy, were excluded from the analysis. One patient who was administered caplacizumab as an investigational drug before approval and 1 pediatric patient were excluded from the analysis. In patients diagnosed with acute iTTP and an ADAMTS13 inhibitor titer of <0.5 BU/mL, the study included those whose titer rose above 0.5 BU/mL during treatment. Consequently, the analysis encompassed 30 individuals: 14 received caplacizumab (caplacizumab group), whereas 16 did not (non-caplacizumab group; Figure 1).
Incorporation of patients treated with caplacizumab and patients not receiving caplacizumab treatment. We identified 69 patients with iTTP from the thrombotic microangiopathy (TMA) registry. Among those who satisfied the exclusion criteria, 30 acute episodes in 29 patients were included in this study. Among these, 14 received caplacizumab treatment (designated as the caplacizumab group), whereas the remaining 16 did not receive caplacizumab treatment (referred to as the non-caplacizumab group).
Incorporation of patients treated with caplacizumab and patients not receiving caplacizumab treatment. We identified 69 patients with iTTP from the thrombotic microangiopathy (TMA) registry. Among those who satisfied the exclusion criteria, 30 acute episodes in 29 patients were included in this study. Among these, 14 received caplacizumab treatment (designated as the caplacizumab group), whereas the remaining 16 did not receive caplacizumab treatment (referred to as the non-caplacizumab group).
Measurements
A sensitive chromogenic enzyme-linked immunosorbent assay (ELISA; Kainos Laboratories, Tokyo, Japan) was used to measure ADAMTS13 activity.17 A plasma mixing assay was used to measure the ADAMTS13 inhibitor titers. A titer of >0.5 BU/mL was considered to be significant. Plasma anti-ADAMTS13 IgG levels were analyzed using TECHNOZYM ADAMTS13 INH ELISA according to the manufacturer’s instructions (Technoclone, Vienna, Austria). The reference range for this test was classified as negative for values of <12 U/mL, borderline for values between 12 and 15 U/mL, and positive for values of >15 U/mL. ADAMTS13 antigen levels were measured by our in-house ELISA.18 Briefly, microtiter plates were precoated with 5 μg/mL of anti-ADAMTS13 murine monoclonal antibodies (A10) in phosphate-buffered saline, overnight. Patient plasma was then diluted by 20-fold with the assay buffer and incubated on the plate at 37°C for 1 hour. Horseradish peroxidase–conjugated C7 monoclonal antibodies (1.1 mg/mL) were subsequently used at a 2000-fold dilution to detect the bound ADAMTS13. The coloring reaction was developed for 10 minutes by o-phenylenediamine dihydrochloride and quenched with 4M H2SO4 for 10 minutes. Optical density values were measured at 492 nm. To investigate the modulator of B-cell activation, we measured the B-cell activating factor (BAFF) levels in the patients, which were quantified by ELISA according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). The average levels reported in healthy controls were 850 pg/mL (range, 584-1186 pg/mL) in serum, 790 pg/mL (range, 501-1078 pg/mL) in EDTA plasma, and 765 pg/mL (range, 528-1073 pg/mL) in heparin plasma, as per the manufacturer’s instructions. These data in both groups were compared on the day of onset (before any iTTP treatment), and day 7, day 14, day 21, and day 28 from first TPE (with an error margin of ±2 days for each).
We calculated the number of days from the start of TPE until the platelet count reached ≥150 × 109/L. Currently, the Japanese TTP guidelines recommend TPE until 2 days after the platelet count exceeds 150 × 109/L.7 The duration required for ADAMTS13 activity levels to continuously exceed 10% and 20% was also determined using the start and end of TPE as reference points. The difference in the duration until the recovery of ADAMTS13 activity, based on the presence or absence of rituximab, was also evaluated. In addition, we analyzed the correlations between the initial ADAMTS13 inhibitor levels/anti-ADAMTS13 IgG levels and the duration until ADAMTS13 activity recovered to ≥10% in the caplacizumab and non-caplacizumab groups.
Statistical analysis
Categorical variables were expressed as frequencies and percentages, and continuous variables were expressed as medians and interquartile ranges (IQRs). Fisher exact test was used to compare categorical variables, and the Mann-Whitney U test was used to compare continuous variables between the 2 groups. The correlation between the initial ADAMTS13 inhibitor/anti-ADAMTS13 IgG levels and the duration until the ADAMTS13 recovered to ≥10% was represented using Spearman correlation coefficient. P values of < .05 were judged as statistically significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).19
Ethics statement
This study was approved by the ethics committee of Nara Medical University and was conducted in accordance with the principles of the Declaration of Helsinki. Patients were considered to have provided informed consent if they opted out of the study on a specific website. Those who opted out of the study were excluded.
Results
Clinical characteristics and laboratory parameters
Overall, 14 patients treated with caplacizumab, and 16 patients not treated with caplacizumab were analyzed in this study. We retrospectively compared the differences in clinical characteristics, laboratory parameters, and treatments between patients in both groups (Table 1). The demographic data for each patient are presented in supplemental Tables 1-3. Patients were followed-up for a median of 55 days (IQR, 49.3-72.8) in the caplacizumab group, and 44 days (IQR, 35.3-61.3) in the non-caplacizumab group. Age at onset and sex proportion were not statistically significant between the 2 groups. Of 14 patients in the caplacizumab group, and of 16 patients in the non-caplacizumab group, 13 and 15, respectively, were enrolled during their initial iTTP episode.
Demographic data of the caplacizumab group and non-caplacizumab group
| . | Caplacizumab group (n = 14) . | Non-caplacizumab group (n = 16) . | P value . |
|---|---|---|---|
| Baseline patient characteristics | |||
| Follow-up period, d | 55.0 (49.3-72.8) | 44 (35.3-61.3) | .197 |
| Age, y | 71 (62.8-80.0) | 66.5 (52.0-84.3) | .983 |
| Female sex, n/n total (%) | 6/14 (42.9%) | 9/16 (56.3%) | .715 |
| Initial episode, n/n total (%) | 13/14 (92.9%) | 15/16 (93.8%) | 1 |
| Hemoglobin, initial, g/dL | 8.1 (7.5-9.1) | 7.9 (6.7-10.0) | .901 |
| Platelet, initial, ×109/L | 11 (8-12) | 11 (7-13) | .754 |
| Lactate dehydrogenase, initial, IU/L | 1086 (931-1282) | 1038 (857-1272) | .854 |
| Serum creatinine, initial, mg/dL | 1.01 (0.68-1.51) | 1.15 (0.82-1.39) | .739 |
| Total bilirubin, initial, mg/dL | 3.9 (2.6-4.8) | 3.1 (2.5-4.2) | .394 |
| Neuropsychiatric symptoms, n/n total (%) | 11/14 (78.6%) | 9/16 (56.3%) | .26 |
| ADAMTS13 activity of <0.5%, initial, n/n total (%) | 14/14 (100%) | 16/16 (100%) | - |
| ADAMTS13 inhibitor, initial, BU/mL | 8.0 (4.6-12.7) | 3.3 (1.2-6.4) | .058 |
| Anti ADAMTS13 IgG antibody level, initial, U/mL | 100.7 (59.6-215.9) | 65.1 (24.6-76.0) | .212 |
| Plasma BAFF level, initial, pg/mL | 861.1 (685.3-1228.4) | 824.2 (704.9-1075) | .697 |
| Treatment | |||
| TPE, n/n total (%) | 14/14 (100%) | 16/16 (100%) | - |
| Number of TPE treatment, n | 8 (7-8.8) | 15 (8-20) | .025 |
| Date of final TPE, d | 8 (7-16.5) | 22 (9-27.3) | .049 |
| Glucocorticoids, n/n total (%) | 14/14 (100%) | 16/16 (100%) | - |
| Rituximab, n/n total (%) | 11/14 (78.6%) | 13/16 (81.4%) | 1 |
| Time from first TPE to first rituximab dose, d | 12 (4.5-15.5) | 10 (7-13) | .954 |
| Duration of caplacizumab treatment, d | 49 (39.5-59.5) | - | |
| Patient outcomes | |||
| Time from first TPE to final ADAMTS13 activity of ≥10%, d | 42 (31.0-55.0) | 23 (14.5-33.3) | .014 |
| Time from first TPE to final ADAMTS13 activity of ≥20%, d | 52 (33.0-55.0) | 25 (20.5-43.5) | .134 |
| Time after final TPE to final ADAMTS13 activity of ≥10%, d | 35 (11.3-41.3) | 2 (2.0-3.0) | <.001 |
| Time after final TPE to final ADAMTS13 activity of ≥20%, d | 38.5 (17.5-48.5) | 11.0 (3.0-18.8) | .008 |
| Time to achieve initial platelet counts of ≥100 × 109/L, d | 5 (4.0-5.0) | 8.5 (5.8-15) | <.001 |
| Time to achieve initial platelet counts of ≥150 × 109/L, d | 6 (5.0-7.0) | 14.5 (7-22.8) | .002 |
| . | Caplacizumab group (n = 14) . | Non-caplacizumab group (n = 16) . | P value . |
|---|---|---|---|
| Baseline patient characteristics | |||
| Follow-up period, d | 55.0 (49.3-72.8) | 44 (35.3-61.3) | .197 |
| Age, y | 71 (62.8-80.0) | 66.5 (52.0-84.3) | .983 |
| Female sex, n/n total (%) | 6/14 (42.9%) | 9/16 (56.3%) | .715 |
| Initial episode, n/n total (%) | 13/14 (92.9%) | 15/16 (93.8%) | 1 |
| Hemoglobin, initial, g/dL | 8.1 (7.5-9.1) | 7.9 (6.7-10.0) | .901 |
| Platelet, initial, ×109/L | 11 (8-12) | 11 (7-13) | .754 |
| Lactate dehydrogenase, initial, IU/L | 1086 (931-1282) | 1038 (857-1272) | .854 |
| Serum creatinine, initial, mg/dL | 1.01 (0.68-1.51) | 1.15 (0.82-1.39) | .739 |
| Total bilirubin, initial, mg/dL | 3.9 (2.6-4.8) | 3.1 (2.5-4.2) | .394 |
| Neuropsychiatric symptoms, n/n total (%) | 11/14 (78.6%) | 9/16 (56.3%) | .26 |
| ADAMTS13 activity of <0.5%, initial, n/n total (%) | 14/14 (100%) | 16/16 (100%) | - |
| ADAMTS13 inhibitor, initial, BU/mL | 8.0 (4.6-12.7) | 3.3 (1.2-6.4) | .058 |
| Anti ADAMTS13 IgG antibody level, initial, U/mL | 100.7 (59.6-215.9) | 65.1 (24.6-76.0) | .212 |
| Plasma BAFF level, initial, pg/mL | 861.1 (685.3-1228.4) | 824.2 (704.9-1075) | .697 |
| Treatment | |||
| TPE, n/n total (%) | 14/14 (100%) | 16/16 (100%) | - |
| Number of TPE treatment, n | 8 (7-8.8) | 15 (8-20) | .025 |
| Date of final TPE, d | 8 (7-16.5) | 22 (9-27.3) | .049 |
| Glucocorticoids, n/n total (%) | 14/14 (100%) | 16/16 (100%) | - |
| Rituximab, n/n total (%) | 11/14 (78.6%) | 13/16 (81.4%) | 1 |
| Time from first TPE to first rituximab dose, d | 12 (4.5-15.5) | 10 (7-13) | .954 |
| Duration of caplacizumab treatment, d | 49 (39.5-59.5) | - | |
| Patient outcomes | |||
| Time from first TPE to final ADAMTS13 activity of ≥10%, d | 42 (31.0-55.0) | 23 (14.5-33.3) | .014 |
| Time from first TPE to final ADAMTS13 activity of ≥20%, d | 52 (33.0-55.0) | 25 (20.5-43.5) | .134 |
| Time after final TPE to final ADAMTS13 activity of ≥10%, d | 35 (11.3-41.3) | 2 (2.0-3.0) | <.001 |
| Time after final TPE to final ADAMTS13 activity of ≥20%, d | 38.5 (17.5-48.5) | 11.0 (3.0-18.8) | .008 |
| Time to achieve initial platelet counts of ≥100 × 109/L, d | 5 (4.0-5.0) | 8.5 (5.8-15) | <.001 |
| Time to achieve initial platelet counts of ≥150 × 109/L, d | 6 (5.0-7.0) | 14.5 (7-22.8) | .002 |
Continuous data are presented as medians (first to third quartiles), whereas categorical data are presented as percentages.
Laboratory data and the incidence of neurological symptoms at presentation were not significantly different between the 2 groups. ADAMTS13 activity in all enrolled patients was <0.5%, and the median initial ADAMTS13 antigen levels were below the detection limit (<1.5%) in both groups (P = .077). Median initial ADAMTS13 inhibitor levels measured by Bethesda assay were 8.0 BU/mL (IQR, 4.6-12.7) in the caplacizumab group and 3.3 BU/mL (IQR, 1.2-6.4) in the non-caplacizumab group (P = .058). The median initial levels of anti-ADAMTS13 IgG antibodies were 100.7 U/mL (IQR, 59.6-215.8) in the caplacizumab group and 65.1 U/mL (IQR, 24.6-76) in the non-caplacizumab group (P = .212).
Comparison of treatment methods in patients treated with or without caplacizumab
TPE using FFP was performed in all enrolled patients. The median number of TPE days in the caplacizumab group was significantly lower than that in the non-caplacizumab group (8 days [IQR, 7-8.8] and 15 days [IQR, 8-20], respectively; P = .025). We observed a significant decrease in the median number of days required for TPE completion, with the median date of final TPE being 8 days (IQR, 7-16.5) in the caplacizumab group, compared with 22 days (IQR, 9-27.3) in the non-caplacizumab group (P = .049). Corticosteroids were administered to all patients. Furthermore, the proportion of patients receiving rituximab treatment was 78.6% in the caplacizumab group and 81.4% in the non-caplacizumab group, showing no significant difference (P = 1.0). Additionally, the median time from the initial TPE to the first rituximab administration was 12 days (IQR, 4.5-15.5) in the caplacizumab group and 10 days (IQR, 7-13) in the non-caplacizumab group (P = .954). Specifically, for patients treated with caplacizumab, the median duration of caplacizumab treatment was 49 days (IQR, 39.5-59.5; Table 1). Most cases started caplacizumab either on the same day or the second day after the first plasma exchange.
Platelets and ADAMTS13 response in patients with and without caplacizumab
Earlier platelet recovery was observed in patients treated with caplacizumab. The median duration until the platelet counts initially recovered to >150 × 109/L was 6 days (IQR, 5-7) in the caplacizumab group and 14.5 days (IQR, 7-22.8) in the non-caplacizumab group (P = .002; Table 1). In the weekly analysis from disease onset, the median platelet counts of the non-caplacizumab group on day 14 were significantly lower than those of the caplacizumab group (207 × 109/L [IQR, 170 × 109/L to 302 × 109/L] in the caplacizumab group vs 110 × 109/L [IQR, 46 × 109/L to 167 × 109/L] in the non-caplacizumab group; P = .024; Figure 2E). No differences in platelet counts were observed at other time points. As for the duration to achieve ADAMTS13 activity of ≥10% after the first TPE, the caplacizumab group took a median of 42 days (IQR, 31-55), which was significantly longer than that of the non-caplacizumab group (median, 23 days; IQR, 14.5-33.3; P = .021). The caplacizumab group required a median of 35 days (IQR, 11.3-41.3) to attain ADAMTS13 activity of ≥10% after the final TPE, whereas the non-caplacizumab group reached this level at a median of 2 days (IQR, 2-3; P < .001; Table 1). In the caplacizumab group, there was no difference in the median duration to achieve ADAMTS13 activity of ≥10% after the first TPE, based on the presence or absence of rituximab administration (43 days with rituximab [n = 11; IQR, 37.5-61.5] vs 19 days without [n = 3; IQR, 14.5-32.5]; P = .184). Similarly, in the non-caplacizumab group, there was no significant difference (23 days with rituximab [n = 13; IQR, 15-25], vs 34 days without rituximab [n = 3; IQR, 23.5-38.5]; P = .346).
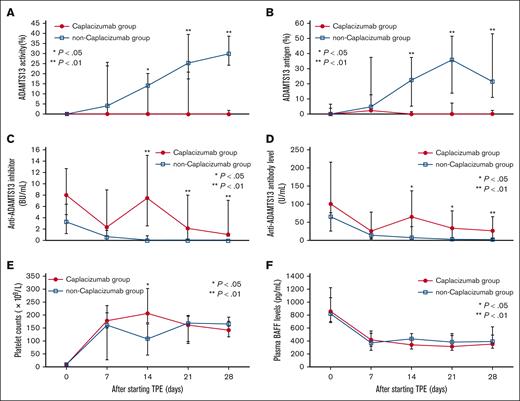
Temporal changes of platelet counts, ADAMTS13-related markers, and BAFF in acute iTTP cases. The levels of (A) ADAMTS13 activity, (B) ADAMTS13 antigen, (C) anti-ADAMTS13 inhibitor, (D) anti-ADAMTS13 IgG, (E) platelet counts, and (F) plasma BAFF were compared between the day of onset and days 7, 14, 21, and 28 after initiating TPE. The error bars depict the first and third quartiles. ∗P < .05 and ∗∗P < .01.
Temporal changes of platelet counts, ADAMTS13-related markers, and BAFF in acute iTTP cases. The levels of (A) ADAMTS13 activity, (B) ADAMTS13 antigen, (C) anti-ADAMTS13 inhibitor, (D) anti-ADAMTS13 IgG, (E) platelet counts, and (F) plasma BAFF were compared between the day of onset and days 7, 14, 21, and 28 after initiating TPE. The error bars depict the first and third quartiles. ∗P < .05 and ∗∗P < .01.
Temporal changes in ADAMTS13-related markers
The median ADAMTS13 activity level was significantly lower in the caplacizumab group than in the non-caplacizumab group on days 14 (0% [IQR, 0-0] vs 14.2% [IQR, 0-20.2], P = .027), 21 (0% [IQR, 0-20.8] vs 25.5% [IQR, 17.5-39.6], P = .002), and 28 (0% [IQR, 0-1.8] vs 29.9% [IQR, 24.4-38.7], P < .001; Figure 2A). Similarly, the median ADAMTS13 antigen level was significantly lower in the caplacizumab group on days 14 (<1.5% [IQR, <1.5] vs 22.6% [IQR, 4.9-37.4], P < .001), 21 (<1.5% [IQR, <1.5-7.2] vs 37.1% [IQR, 13.6-51.5], P < .001), and 28 (<1.5% [IQR, <1.5-2.2] vs 25.6% [IQR, 11-53], P < .001; Figure 2B). The median ADAMTS13 inhibitor level was significantly higher in the caplacizumab group on the same days: day 14 (7.5 BU/mL [IQR, 2.6-15] vs <0.5 BU/mL [IQR, <0.5], P = .001), day 21 (2.2 BU/mL [IQR, <0.5-8] vs <0.5 BU/mL [IQR, <0.5], P = .001), and day 28 (1.1 BU/mL [IQR, 0.7-7.1] vs <0.5 BU/mL [IQR, <0.5], P < .001; Figure 2C). The median level of anti-ADAMTS13 IgG antibody was also significantly higher in the caplacizumab group than in the non-caplacizumab group at identical time points on days 14 (65.4 U/mL [IQR, 39.2-136.9] vs 7.6 U/mL [IQR, 3.7-37.9], P = .013), 21 (33.9 U/mL [IQR, 9.9-81.5] vs 3.7 U/mL [IQR, 2-7.4], P = .015), and 28 (26.9 U/mL [IQR, 5.9-65.6] vs 2.5 U/mL [IQR, 1.6-4.2], P = .009; Figure 2D).
Temporal changes in plasma BAFF levels
The median initial plasma BAFF level in the caplacizumab group was 861.1 pg/mL (IQR, 685.3-1228.4), compared with 824.2 pg/mL (IQR, 704.9-1075) in the non-caplacizumab group. This difference was not statistically significant (P = .946; Figure 2F). Furthermore, these values align closely with the average levels reported in healthy controls, as per the manufacturer’s instructions, which are 850 pg/mL (range, 584-1186 pg/mL) in serum, 790 pg/mL (range, 501-1078 pg/mL) in EDTA plasma, and 765 pg/mL (range, 528-1073 pg/mL) in heparin plasma.
Moreover, weekly assessments of plasma BAFF levels, from 7 to 28 days after initiation of TPE, revealed no statistically significant differences at any time point between the 2 groups.
Correlations between the ADAMTS13 activity recovery and the levels of initial ADAMTS13 inhibitor and anti-ADAMTS13 IgG
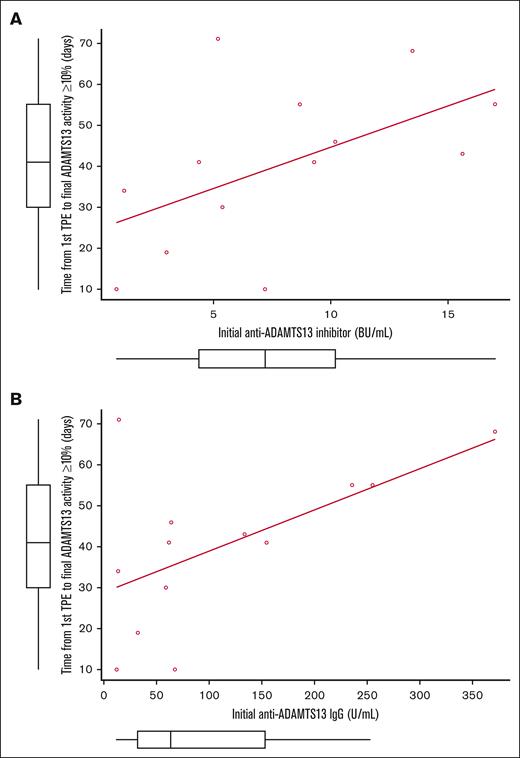
Subsequently, our attention shifted to examining the initial levels of ADAMTS13 inhibitor/anti-ADAMTS13 IgG and the duration until ADAMTS13 activity restoration. Importantly, a correlation coefficient of 0.668 (P = .009) was observed between the initial ADAMTS13 inhibitor titer and the time from the commencement of TPE to the eventual elevation of ADAMTS13 activity to ≥10% in the caplacizumab group. Similarly, the correlation coefficient for the initial anti-ADAMTS13 IgG levels and the aforementioned period was 0.615 (P = .019). Notably, in the caplacizumab group, 1 patient presented with an initial ADAMTS13 inhibitor level of 185.3 BU/mL and an IgG level of 1647.2U/mL. This patient, during and after the administration of caplacizumab, experienced recurrent relapses requiring additional TPE until ADAMTS13 activity levels reached stability. Excluding this patient, the remaining 13 patients treated with caplacizumab showed a correlation of 0.585 (P = .036) for ADAMTS13 inhibitor (Figure 3A) and 0.519 (P = .069) for anti-ADAMTS13 IgG (Figure 3B), in relation to the time required for ADAMTS13 activity to recover to ≥10%. In the non-caplacizumab group, no correlation was observed between the titers of the ADAMTS13 inhibitor and anti-ADAMTS13 IgG and the comparable time taken for the recovery of ADAMTS13 activity.
Correlations between the ADAMTS13 activity recovery and the levels of initial ADAMTS13 inhibitor and anti-ADAMTS13 IgG. Data shown for n = 13. The correlations between the period from the start of TPE until the final recovery of ADAMTS13 activity to ≥10% and the initial titers of (A) ADAMTS13 inhibitor and (B) anti-ADAMTS13 IgG in the caplacizumab group. In this calculation, we excluded 1 patient who presented with an initial ADAMTS13 inhibitor level of 185.3 BU/mL and an IgG level of 1647.2 U/mL.
Correlations between the ADAMTS13 activity recovery and the levels of initial ADAMTS13 inhibitor and anti-ADAMTS13 IgG. Data shown for n = 13. The correlations between the period from the start of TPE until the final recovery of ADAMTS13 activity to ≥10% and the initial titers of (A) ADAMTS13 inhibitor and (B) anti-ADAMTS13 IgG in the caplacizumab group. In this calculation, we excluded 1 patient who presented with an initial ADAMTS13 inhibitor level of 185.3 BU/mL and an IgG level of 1647.2 U/mL.
Subsequently, the time required for the ADAMTS13 activity to restore to ≥10% was analyzed in relation to whether the initial ADAMTS13 inhibitor levels were above or below the median value. In the caplacizumab group, the median duration was 55 days (IQR, 44.5-61.5) for the high-titer group (ADAMTS13 inhibitor of ≥8.0 BU/mL) and 30 days (IQR, 14.5-37.5) for the low-titer group (ADAMTS13 inhibitor of <8.0 BU/mL; P = .025). The same evaluation was applied to initial anti-ADAMTS13 IgG levels. The median duration was 55 days (IQR, 46-64.8) for the high-IgG group (IgG ≥ 100.7 U/mL) and 32 days (IQR, 16.8-42.3) for the low-IgG group (IgG < 100.7 U/mL) in the caplacizumab group (P = .038). Similarly, in the non-caplacizumab group, the time taken for ADAMTS13 activity to reach 10% was compared based on whether the titers of the ADAMTS13 inhibitor and anti-ADAMTS13 IgG were higher or lower than their respective median values. However, no significant differences were observed between groups.
Discussion
In our study, similar to the TITAN and HERCULES studies, introducing caplacizumab in addition to existing therapies for acute iTTP resulted in a rapid improvement in platelet counts and a reduction in the number of TPE procedures. Consistent with the findings of Prasannan et al, we observed a delay in the recovery of ADAMTS13 activity in the caplacizumab group. We conducted consecutive measurements of ADAMTS13-related parameters during the acute phase. Our findings showed delayed recovery of ADAMTS13 activity in caplacizumab-treated acute iTTP cases and a deceleration in the reduction of ADAMTS13 inhibitor and anti-ADAMTS13 IgG levels. Moreover, in the caplacizumab group, correlations were observed between the initial ADAMTS13 inhibitor/anti-ADAMTS13 IgG levels and the period of ADAMTS13 activity recovery, revealing aspects that were previously masked by prolonged TPE in patients not treated with caplacizumab. These findings suggest that higher titers of anti-ADAMTS13 antibodies delay the recovery of ADAMTS13 activity.
One reason for the delayed recovery of ADAMTS13 activity in patients with iTTP treated with caplacizumab could be the decreased number of TPE. With the introduction of caplacizumab, the platelet count recovered more rapidly, which reduced the need for TPE. Consequently, this led to fewer opportunities for ADAMTS13 replenishment and ADAMTS13 inhibitor removal, which were expected with TPE. Moreover, BAFF did not increase, and there was no difference in its levels between the 2 groups, making B-cell activation by caplacizumab unlikely. Considering the established half-life of IgG, which ∼21 days, the reduced number of TPE in the caplacizumab cohort may have led to suboptimal clearance of the initial IgG found at presentation. This may have contributed to the differences in ADAMTS13 activity and inhibitor/IgG levels between the 2 groups during the acute phase.
Furthermore, a correlation was observed between initial ADAMTS13 inhibitor/anti-ADAMTS13 IgG levels and the duration of ADAMTS13 activity recovery in patients treated with caplacizumab. Kremer Hovinga et al reported that an ADAMTS13 inhibitor titer of ≥2 at initial iTTP presentation was associated with lower survival than in those with lower ADAMTS13 titer.20 Another study conducted by Alwan et al demonstrated that a high anti-ADAMTS13 antibody level adversely affected outcomes in patients with iTTP, with greater mortality.21 However, these studies were conducted in the precaplacizumab era. Moreover, to our knowledge, there are no reports detailing the degree of increase in ADAMTS13 inhibitor or anti-ADAMTS13 antibody levels, or on the duration until ADAMTS13 recovery. In patients undergoing caplacizumab treatment, the diminished necessity for TPE has facilitated a more precise representation of iTTP’s disease course through ADAMTS13-related parameters, in contrast to the precaplacizumab era.
Another potential factor contributing to the delayed recovery of ADAMTS13 activity in the caplacizumab group could be attributed to the postponed administration of rituximab in our cohort. In their comparative study, Coppo et al analyzed the outcomes of conventional and triplet therapies, which include TPE, initial immunosuppression with corticosteroids and rituximab, along with caplacizumab.14 Notably, they observed a more rapid restoration of ADAMTS13 activity in the group receiving caplacizumab. However, in Japan, the use of rituximab is limited to relapsed or refractory iTTP. In this study, there was a time lag of ∼12 days for the initial administration of rituximab from the start of TPE. In a phase 2 trial of rituximab in the acute phase of acquired TTP,22 recovery of ADAMTS13 activity and a decrease in anti-ADAMTS13 IgG levels were observed ∼8 days after rituximab administration, and platelet counts started to recover after ∼12 days. In our study, we observed a re-elevation in the anti-ADAMTS13 inhibitor and anti-ADAMTS13 IgG on the 14th day after the initiation of TPE in the caplacizumab group. This phenomenon has been reported as inhibitor boosting even in the precaplacizumab era.23 However, with the advent of caplacizumab and the consequent reduction in the frequency of TPE, this phenomenon has become more apparent. Because patients with caplacizumab-treated iTTP rely solely on the effects of immunosuppressants for inhibitor boosting, intensifying immunosuppressive therapy, namely, upfront rituximab, is necessary to reduce ADAMTS13-related inhibitors more quickly.
This study presents some limitations. Notably, the most significant limitation is its nature as a small-scale, retrospective registry study, focused exclusively on Japanese patients with iTTP. The clinical data were collected from various institutions based on transfer forms, constraining the breadth of information available. Furthermore, because of the rarity of the disease and the retrospective design of the study, accruing cases that allowed for frequent monitoring of ADAMTS13-related markers during the acute phase proved challenging. Therefore, future research necessitates the accumulation of prospective case studies. Moreover, considering the homogeneity of the ethnic groups involved, caution should be exercised when generalizing these results to other ethnic populations.
Another possible limitation is the impact of using FFP for TPE to analyze ADAMTS13-related parameters. The median end date of the TPE period was 8 days for the caplacizumab group and 22 days for the non-caplacizumab group, suggesting that exogenous ADAMTS13 replenished through TPE may affect the actual measurement values during the TPE period. In the non-caplacizumab group, the time between the last TPE and ADAMTS13 of >10% was 2 days; however, the activity was maintained at >10% thereafter. Considering a half-life of ADAMTS13 of a few days, the impact of exogenous ADAMTS13 after completing TPE in this setting is considered to be limited.
In this study, although there was no significant difference, a tendency toward higher ADAMTS13 inhibitor titers was observed in the caplacizumab group, which may have influenced the delayed recovery of ADAMTS13 activity in this group. One possible reason for this result could be the small sample size. Additionally, all 4 cases of early mortality, which were excluded from this study, belonged to the non-caplacizumab group. The exclusion of severe cases from the analysis may have also had an impact.
In conclusion, we have demonstrated the delayed recovery of ADAMTS13 activity in patients with iTTP who were treated with caplacizumab. We have indicated that this delay in recovery may be attributable to the persistently detected presence of ADAMTS13 inhibitors or anti-ADAMTS13 IgG antibodies. These findings strongly suggest the importance of closely monitoring ADAMTS13-related parameters in patients with iTTP who are undergoing caplacizumab treatment. Furthermore, it underscores the significance of promptly initiating rituximab therapy at the onset of the disease.
Acknowledgments
The authors extend their gratitude to the primary care teams who supplied the specimens for analysis.
This study was financially supported by research grants from the Ministry of Health, Labour and Welfare of Japan (20FC1024 to M.M.).
Authorship
Contribution: K. Saito designed the research, conducted laboratory testing, analyzed data, and wrote the manuscript; K. Sakai designed the research and reviewed the manuscript; M.K. reviewed the manuscript; A.H. and H. Azumi conducted laboratory testing, and reviewed the manuscript; S.O., H. Amagase, H.K., Y.O., and H.Y. provided data on patients with iTTP; and M.M. reviewed the study design and manuscript.
Conflict-of-interest disclosure: K. Sakai received the research funding from Takeda. M.M. provided consultancy services for Takeda, Alexion Pharma, and Sanofi; received speaker fees for Takeda, Alexion Pharma, Asahi Kasei Pharma, and Sanofi; and received research funding from Alexion Pharma, Chugai Pharmaceutical, Asahi Kasei Pharma, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Masanori Matsumoto, Department of Blood Transfusion Medicine and Hematology, Nara Medical University, 840 Shijo-Cho, Kashihara, Nara 634-8522, Japan; email: mmatsumo@naramed-u.ac.jp.
References
Author notes
Original data are available on request from the corresponding author, Masanori Matsumoto (mmatsumo@naramed-u.ac.jp).
The full-text version of this article contains a data supplement.