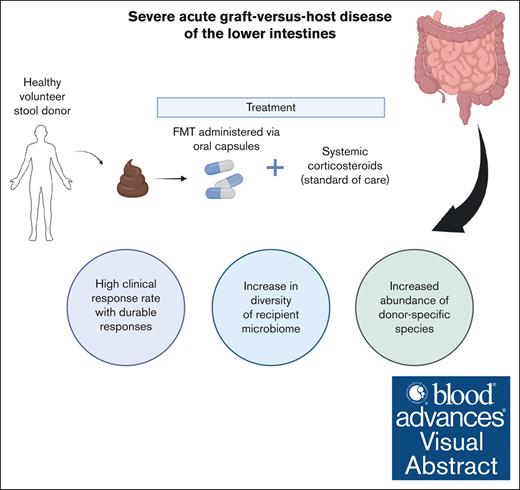
Initial treatment with third-party FMT and corticosteroids is associated with high response rates in LGI acute GVHD.
Expansion of recipient microbiome diversity and donor-specific microbial species was observed.
Visual Abstract
Disruption of the intestinal microbiome is observed with acute graft-versus-host disease (GVHD) of the lower gastrointestinal (LGI) tract, and fecal microbiota transplantation (FMT) has successfully cured steroid-refractory cases. In this open-label, single-arm, pilot study, third-party, single-donor FMT was administered in combination with systemic corticosteroids to participants with high-risk acute LGI GVHD, with a focus on treatment-naïve cases. Participants were scheduled to receive 1 induction dose (15 capsules per day for 2 consecutive days), followed by 3 weekly maintenance doses, consisting of 15 capsules per dose. The primary end point of the study was feasibility, which would be achieved if ≥80% of participants able to swallow ≥40 of the 75 scheduled capsules. Ten participants (9 treatment-naïve; 1 steroid-refractory) were enrolled and treated. The study met the primary end point, with 9 of 10 participants completing all eligible doses. Organ-specific LGI complete response rate at day 28 was 70%. Initial clinical response was observed within 1 week for all responders, and clinical responses were durable without recurrent LGI GVHD in complete responders. Exploratory analyses suggest that alpha diversity increased after FMT. Although recipient microbiome composition never achieved a high degree of donor similarity, expansion of donor-derived species and increases in tryptophan metabolites and short-chain fatty acids were observed within the first 7 days after FMT. Investigation into the use of microbiome-targeted interventions earlier in the treatment paradigm for acute LGI GVHD is warranted. This trial was registered at www.ClinicalTrials.gov as #NCT04139577.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment for patients with malignant and nonmalignant hematologic conditions. Severe acute graft-versus-host disease (GVHD), particularly of the lower gastrointestinal (LGI) tract, is a leading cause of early nonrelapse mortality (NRM) after HCT.1-3 In addition to known and emerging biological mechanisms of disease,4,5 disruption of the intestinal microbiome and microbial metabolites is now recognized as a key contributor to the development of acute GVHD.6-11
These studies established the foundation for microbiome-targeted interventions as a novel class of therapeutics for HCT recipients. Fecal microbiota transplantation (FMT), the administration of fecal matter from donors into a recipient with the intent of directly modifying the recipient’s intestinal microbiome composition, is an established intervention, which has been explored for multiple medical conditions.12-14 In recent years, FMT has been investigated with promising preliminary results in both the prevention and treatment of acute LGI GVHD.15-21 To date, the application of FMT as acute LGI GVHD therapy has been limited to treatment-refractory disease. Given the demonstrated clinical responses to FMT in refractory disease and the concern that longer duration of acute LGI GVHD may result in less responsive biology, we hypothesized that incorporating microbiome-directed interventions earlier in the treatment course may improve clinical outcomes. Thus, we conducted a pilot study to treat participants with high-risk acute LGI GVHD, with a focus on treatment-naïve cases, which allowed FMT to be administered in combination with systemic corticosteroids in the initial treatment of acute GVHD.
Methods
Study design
This was an open-label, single-arm, pilot study (ClinicalTrials.gov identifier: NCT04139577) of third-party single-donor FMT administered by oral capsules to participants with high-risk acute GVHD of the LGI tract occurring after allogeneic HCT. The study was performed at Massachusetts General Hospital. The study was approved by the institutional review board at the Dana-Farber Harvard Cancer Center, and all participants provided written informed consent. Participants were scheduled to 1 induction dose, consisting of 15 capsules per day for 2 consecutive days (days 1 and 2), followed by 3 weekly maintenance doses, consisting of 15 capsules per day (days 8, 15, and 22; all ±3 days). Participants could also complete administration of the induction dose over 3 days (10 capsules per day), if requested. Participants fasted for 2 hours before and 1 hour after capsule intake (sips of water were allowed). Participants swallowed the daily allotment of FMT capsules within 1 hour. No antibiotics or bowel preparation was provided in preparation to FMT capsules administration. FMT was administered in conjunction with systemic corticosteroids. For treatment-naïve GVHD, participants were initiated on systemic corticosteroids at a dose of ≥1 mg/kg per day of prednisone equivalent, with exact dose left to physician discretion. For steroid-refractory GVHD, baseline systemic corticosteroid dose was continued with the initiation of FMT and without any additional systemic therapy. Anti-infective prophylaxis was according to institutional standard of care, with patients receiving antiviral, antifungal, and Pneumocystis jirovecii pneumonia prophylaxis. The choice of agent was at the discretion of the treating physician. Nutritional considerations followed institutional practice, in which patients with acute LGI GVHD are in a nothing-by-mouth status at the time of endoscopy and diagnosis. Oral intake is subsequently advanced per the discretion of the treating clinician. For patients who remain with nothing-by-mouth status for more than a few days, total parenteral nutrition (TPN) is started.
Participants were assessed daily (in person or by phone) for 7 days after the first FMT dose for fever, abdominal pain, vomiting, diarrhea, constipation, bloating, and flatulence. Once FMT capsule administration has been completed, participants were seen at least monthly until 3 months after the first FMT dose, and also at 6 months after first FMT dose.
FMT capsule preparation
FMT capsules were generated as described under US Food and Drug Administration Investigational New Drug 16857 (Hohmann). FMT candidate donors were required to be healthy, nonpregnant adults aged between 18 and 50 years. Candidate donors had a normal body mass index (18.5-25 kg/m2) and did not take any medications on a regular basis. Volunteers were excluded for any significant medical history, employment as a health care worker, travel outside the United States, or use of antibiotics in the preceding 1 year. All candidates passed the American Association of Blood Banks donor questionnaire, physical examination, and general laboratory screening tests. Screening tests performed are listed in supplemental Table 1. All tests were within normal ranges or negative for all infectious screening tests except for hepatitis A/B serologies consistent with vaccination. All donations were stored without use for an additional 4 weeks after the last donation to allow retesting of donors. In compliance with US Food and Drug Administration requirements, donors were assessed for COVID-19 by clinical symptoms and temperature screening, and had nasopharyngeal swab testing by polymerase chain reaction 2 weeks before, every 2 weeks during, and 2 weeks after donation periods. If a donor test for COVID-19 was positive within this timeframe, donations were not used.
For this study, fecal suspensions from a single donor were prepared in normal saline without preservatives, using a commercial blender, and sequentially sieved to remove particulates. Final slurries were concentrated by centrifugation and resuspended in saline at one-tenth the volume of the initial sample, with 40% glycerol added as a bacterial cryoprotectant. Final fecal microbial solutions were pipetted into size-0 capsules (650 μl) that were closed and then secondarily sealed in size-00 capsules (DR Capsules; Capsugel, Greenwood, SC). Capsules were stored frozen at −80°C (−112°F). Capsules were transported to the clinic or bedside on dry ice. For each recipient, a batch of 30 capsules contained the microbial content of ∼48 g of fecal matter (mean per capsule, 1.6 g; range, 1.0-2.05). FMT capsules were generated from a single FMT donor, from 6 stool donations over a period of 1 month in 2021.
Participants
Participants were aged ≥18 years, recipients of allogeneic HCT (regardless of donor type, conditioning regimen intensity, or graft source), and were clinically suspected to have grade 2 to 4 acute GVHD, as per MAGIC (Mount Sinai Acute GVHD International Consortium) criteria.22 Participants were required to have a diagnosis of high-risk LGI acute GVHD, which included high-risk, treatment-naïve GVHD or steroid-refractory GVHD. High-risk, treatment-naïve GVHD was defined as high-risk by Refined Minnesota Criteria23 or AA3 risk by MAGIC GVHD biomarker scoring risk system,1 and receiving <3 days of therapy with systemic corticosteroids (≥1 mg/kg per day of prednisone equivalent). Steroid-refractory disease was defined as progressive GVHD after at least 3 days of systemic corticosteroids (≥ 1 mg/kg per day of prednisone equivalent), or no improvement in GVHD after at least 7 days on ≥ 1 mg/kg per day of prednisone equivalent or insufficient improvement, which warranted the addition of another agent, or flare of GVHD symptoms during taper. Participants with steroid-refractory GVHD were required to be at least 2 weeks from initiation of most recent systemic treatment (institutional standard or investigational agent). Concurrent skin or liver organ involvement of acute GVHD was allowed. Exclusion criteria included history of inflammatory bowel disease, delayed gastric emptying syndrome, active gastrointestinal infection, or the inability to swallow pills.
Outcomes
The primary end point of the study was feasibility. Secondary end points included GVHD overall response rate, time to GVHD response, duration of GVHD response, cumulative incidence of infectious events, NRM, and overall survival (OS). Complete response (CR) was defined as a score of 0 for acute GVHD grading in all evaluable organs, without administration of additional systemic therapies. Partial response (PR) was defined as improvement in GVHD stage by ≥1 point in ≥1 organs, without progression in other organs and without administration of additional systemic therapies. When evaluating organ-specific responses in the LGI tract, CR was defined as LGI score of 0, and PR was defined as improvement in LGI score by ≥1 point but not achieving a score of 0. Exploratory objectives included longitudinal assessments of fecal microbiota composition and microbial-derived metabolites and urine 3-indoxyl sulfate (3-IS) levels.
Whole metagenomic shotgun sequencing and profiling
Bacterial genomic DNA was extracted from human fecal samples using the QIAamp Fast DNA Stool Mini Kit (Qiagen), following the manufacturer's instructions with an additional bead beating lysis step. Individual libraries were then constructed from each sample using the Illumina DNA Prep kit and loaded onto the Illumina NovaSeq 6000 platform (Illumina, Inc, San Diego, CA) for sequencing. The sequencing was performed using the 2 × 150 bp paired-end read protocol, following the manufacturer's instructions. Metagenomic profiling analysis was carried out with MetaPhlAn424 using default settings.
Short-chain fatty acids and carbohydrates profiling by HRMS
To determine the relative abundance of short-chain fatty acids, carbohydrates, N-acetylglucosamine, and N-acetylgalactosamine in human feces samples, extracts were prepared and analyzed by ultra-high resolution mass spectrometry (HRMS). Detailed description of methods for analysis of short-chain fatty acids, carbohydrates, and N-acetylglucosamine, and N-acetylgalactosamine are provided in supplemental Table 2.
Tryptophan metabolite profiling by LC-HRMS
To determine the relative concentration of tryptophan metabolites in human feces samples, extracts were prepared, and analyzed by liquid chromatography coupled with liquid chromatography (LC)-HRMS. Detailed description of methods for analysis of tryptophan metabolites are provided in supplemental Table 2.
Urine 3-IS analysis
Analysis of the tryptophan metabolite 3-IS in human urine samples was performed by reverse-phase liquid chromatography electrospray ionization–tandem mass spectrometry in negative ion multiple reaction monitoring mode, as previously described.25,26 Obtained concentration values were corrected to urinary creatinine.
Statistical analyses
The primary end point of the study was feasibility, which was evaluated by the proportion of participants who swallowed ≥40 of the 75 scheduled FMT capsules. FMT was considered as feasible if this proportion was ≥80%, and not feasible if it was ≤50%. Based on this information, FMT was considered feasible if, among the 11 eligible participants, ≥8 participants were able to swallow ≥40 capsules. Using this design, the probability of concluding FMT is feasible is 11% if the true proportion of participants who can swallow ≥40 capsules is 50%, and 84% if the true proportion is 80%. OS was defined as the time from date of enrollment to death or date of last contact. Progression-free survival was defined as the time from date of enrollment to disease progression or death, whichever occurred first, with participants without an event being censored at last date of contact. Kaplan-Meier estimates of OS and progression-free survival were calculated. Cumulative incidence of infection, NRM, and disease relapse were estimated in competing risks setting.
Microbiome analyses
MetaPhlan4 data were imported into R environment using Phyloseq version 1.44.0. Shannon diversity index (SDI) was used for measuring alpha diversity. Bray dissimilarity distance was used to quantify betadiversity. Repeated measure correlation was measured using rmcorr package version 0.6.0.27 AncomBC version 1.6.2 was used to calculate differential abundance with prevalence cutoff set to 20% and structural 0 set to true.28 Influence of each species on the beta diversity was calculated by measuring the Bray-Curtis dissimilarity after transforming the counts to proportion between the sample and the same sample without a species. The output of the Bray-Curtis dissimilarity was then normalized by multiplying the distance by the SDI score as a mean to differentially quantify the shift resulting from the omission of a particular species as a factor of the community evenness of a particular sample. This will result in higher Bray-Curtis dissimilarity for samples with higher SDI scores. Finally, nonparametric mean with 1000 bootstrap was calculated for each time point using Hmisc package version 5.1-0 (https://CRAN.R-project.org/package=Hmisc) function smean.cl.boot to get the aggregated species influence for each time point.
The study was approved by the institutional review board at the Dana-Farber Harvard Cancer Center, and all participants provided written informed consent.
Results
Participant characteristics
Between June 2021 and July 2022, 10 participants with high-risk acute GVHD were enrolled. All enrolled participants were treated. The trial was closed before preplanned enrollment (n = 11) because of lack of FMT capsule inventory. Baseline demographics and disease characteristics are summarized in Table 1. The median age was 63 years (range, 51-72). Seven participants were male. Transplants were predominantly performed from matched donors (n = 9), with reduced intensity conditioning (n = 9), and tacrolimus/methotrexate-based GVHD prophylaxis (n = 9). The median time from HCT to acute GVHD diagnosis was 85 days (range, 20-207). At the time of enrollment, all participants had grade 3/4 acute GVHD and all cases were high-risk according to Refined Minnesota Criteria. Four participants had concurrent skin involvement at enrollment (1 with stage 3; and 3 with stage 1). Nine participants had high-risk treatment-naïve acute GVHD; and 1 participant had steroid-refractory acute GVHD and had already received corticosteroids, ruxolitinib, and vedolizumab. All participants were being treated in the inpatient setting at the time of LGI GVHD diagnosis and initiation of FMT treatment.
Baseline demographics and disease characteristics
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 63 (51-72) |
| Sex, n | |
| Male | 7 |
| Female | 3 |
| Race and ethnicity, n | |
| White | 8 |
| Asian | 1 |
| Hispanic | 1 |
| Diagnosis, n | |
| Acute leukemia | 5 |
| Myelodysplastic syndrome | 2 |
| Lymphoma | 2 |
| Aplastic anemia | 1 |
| Graft source, n | |
| Peripheral blood stem cells | 10 |
| Donor, n | |
| Matched unrelated | 9 |
| Haploidentical | 1 |
| Conditioning intensity, n | |
| Reduced | 9 |
| Myeloablative | 1 |
| GVHD prophylaxis, n | |
| Tacrolimus/methotrexate with/without other | 9 |
| Posttransplant cyclophosphamide based | 1 |
| Median time from HCT to acute GVHD diagnosis (range), d | 85 (20-207) |
| GVHD treatment history at enrollment | |
| Treatment-naïve | 9 |
| Treatment-refractory | 1 |
| MAGIC GVHD grading at enrollment, n | |
| Grade 3 | 9 |
| Grade 4 | 1 |
| LGI stage at enrollment, n | |
| 2 | 4 |
| 3 | 5 |
| 4 | 1 |
| Minnesota risk score at enrollment, n | |
| High | 10 |
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 63 (51-72) |
| Sex, n | |
| Male | 7 |
| Female | 3 |
| Race and ethnicity, n | |
| White | 8 |
| Asian | 1 |
| Hispanic | 1 |
| Diagnosis, n | |
| Acute leukemia | 5 |
| Myelodysplastic syndrome | 2 |
| Lymphoma | 2 |
| Aplastic anemia | 1 |
| Graft source, n | |
| Peripheral blood stem cells | 10 |
| Donor, n | |
| Matched unrelated | 9 |
| Haploidentical | 1 |
| Conditioning intensity, n | |
| Reduced | 9 |
| Myeloablative | 1 |
| GVHD prophylaxis, n | |
| Tacrolimus/methotrexate with/without other | 9 |
| Posttransplant cyclophosphamide based | 1 |
| Median time from HCT to acute GVHD diagnosis (range), d | 85 (20-207) |
| GVHD treatment history at enrollment | |
| Treatment-naïve | 9 |
| Treatment-refractory | 1 |
| MAGIC GVHD grading at enrollment, n | |
| Grade 3 | 9 |
| Grade 4 | 1 |
| LGI stage at enrollment, n | |
| 2 | 4 |
| 3 | 5 |
| 4 | 1 |
| Minnesota risk score at enrollment, n | |
| High | 10 |
Feasibility and safety
One participant completed taking 20 capsules before progression of GVHD and subsequent GVHD-related death. The other 9 participants were able to complete all eligible doses of the treatment course. Thus, the primary end point of the trial, feasibility, was met. No treatment-related significant adverse events observed. There were 2 cases of bacteremia in the first 28 days after FMT, both of which occurred in treatment nonresponders. One participant developed methicillin-resistant Staphylococcus aureus bacteremia, 1 day after the first dose of FMT. One participant developed Lactobacillus bacteremia 16 days after the first dose of FMT. Both cases were considered unrelated to FMT because neither organism was identified in donor samples.
Efficacy
Median follow-up among survivors was 311 days (range, 69-443). At day 28, the overall response rate was 70% (CR, 60%; PR, 10%). The LGI CR rate at day 28 was 70%; 1 participant with a LGI CR had acute GVHD skin rash (stage 3) at day 28, and subsequently received ruxolitinib. Clinical responses were durable, without recurrent LGI GVHD in participants achieving CR (Figure 1). At day 28, clinical responders did not require any additional GVHD therapies beyond corticosteroids and FMT. Among responders, the median duration of response was 152 days, and 7 participants had an ongoing GI response at data cut off. The 3 participants with stage 1 skin involvement at enrollment experienced complete resolution of the rash; the 1 participant with stage 3 skin involvement at enrollment (mentioned earlier) experienced initial improvement of the rash before receiving ruxolitinib for progressive skin GVHD at day 28. NRM and OS at 1 year were 21% (95% confidence interval, 2.7-52) and 79% (95% confidence interval, 38-94), respectively. There have been 2 deaths, both due to acute GVHD in nonresponders.
Clinical outcomes after FMT in the treatment of high-risk acute LGI GVHD. Swimmers plot demonstrating clinical responses and duration of response after the administration of FMT in combination with corticosteroids for acute LGI GVHD. NR, no response; SR, steroid-refractory.
Clinical outcomes after FMT in the treatment of high-risk acute LGI GVHD. Swimmers plot demonstrating clinical responses and duration of response after the administration of FMT in combination with corticosteroids for acute LGI GVHD. NR, no response; SR, steroid-refractory.
Initial clinical response was observed within 1 week for all responders. Four participants received TPN during the first 28 days after the first dose of FMT. The median duration of TPN was 15 days, and 2 participants were still receiving TPN on day 28. Three participants received systemic antibiotics beyond prophylaxis during the first 28 days after the first dose of FMT: 1 participant for 3 days (vancomycin and cefepime for bacteremia in participant before GVHD-related death), 1 participant for 22 days (vancomycin and cefepime, followed by ampicillin for bacteremia in the participant with GVHD nonresponse), and 1 participant for 3 days (levofloxacin for prostatitis before GVHD in the participant with GVHD CR). The median length of hospitalization for responders after the initiation of FMT was 9 days (range, 1-76). The median dose of systemic corticosteroids at enrollment was 1.05 mg/kg methylprednisolone equivalent daily (range, 0.40-1.75 mg/kg methylprednisolone equivalent). By day 28, the median corticosteroid dose had decreased by 67%.
Exploratory correlations between the fecal microbiome and urinary 3-IS after FMT
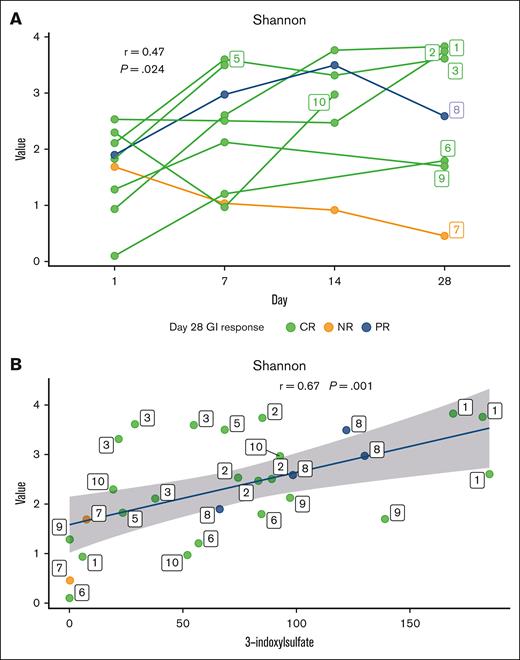
Serial samples of stool and urine collected before and after administration of FMT were analyzed to characterize changes in the microbiome after FMT. Alpha diversity was evaluated from fecal specimens with SDI and repeated measures correlation. This metric demonstrated increase in alpha diversity compared with baseline assessment over the first 28 days after FMT with R2 = 0.47 (P = .024; Figure 2A). Low urinary concentrations of 3-IS after HCT are indicative of significant and clinically relevant intestinal microbiota disruption.26 A significant increase in the median urinary 3-IS level was noted when comparing baseline with highest level over the first 28 days after FMT for clinical responders (creatinine, 21.4 vs 90.9 μmol/mmol; P = .0006). Alpha diversity metric derived from the stool demonstrated positive correlations with urinary concentrations of 3-IS with repeated measure correlation with R2 = 0.67 (P = .001; Figure 2B).
Relationship between alpha diversity and urinary 3-IS. (A) Shannon alpha diversity metric over the first 28 days from FMT for participants with CR (CR, green), participants with no response (NR, orange), and particpants with PR (PR, blue) based on the day 28 GI response. Repeated measure correlation coefficient and P value of the correlation are labeled. Solid lines represent individual participants (numbered 1-10) over days, and participant number labels indicate the last available data point for that participant. (B) Correlation between alpha diversity metric and urine 3-IS. Repeated measure correlation coefficient and P value of the correlation are labeled. Repeated measures for the patients are indicated by the label over the points.
Relationship between alpha diversity and urinary 3-IS. (A) Shannon alpha diversity metric over the first 28 days from FMT for participants with CR (CR, green), participants with no response (NR, orange), and particpants with PR (PR, blue) based on the day 28 GI response. Repeated measure correlation coefficient and P value of the correlation are labeled. Solid lines represent individual participants (numbered 1-10) over days, and participant number labels indicate the last available data point for that participant. (B) Correlation between alpha diversity metric and urine 3-IS. Repeated measure correlation coefficient and P value of the correlation are labeled. Repeated measures for the patients are indicated by the label over the points.
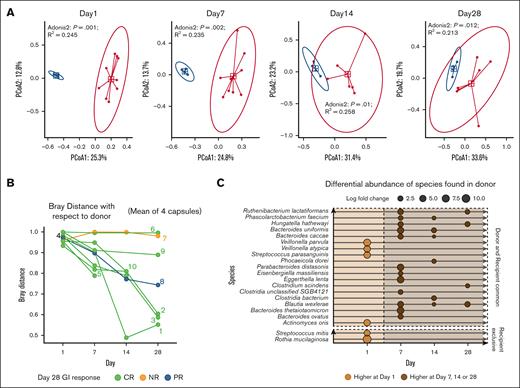
Longitudinal changes in microbial composition after FMT
Characterization of the composition of serially collected stool samples over the first 28 days after FMT demonstrated evolving microbial composition, with recipient microbial communities that became less distinct from the donor over time (R2 = 0.233 [P = .001] before FMT; R2 = 0.257 [P=.006] at day 28), indicating slight increase in the compositional variation explained by the grouping of donor and recipients (Figure 3A). However, despite this general trend, the recipient community structures at day 28 were still significantly distinct from the donor with Bray-Curtis dissimilarity distance from donor >0.85 at day 28 (Figure 3A-B). Because initial clinical responses were observed by day 7 for all responders, changes in specific species from baseline to day 7 after FMT were investigated. We identified increases in relative abundances of select species in the stool of complete responders at either day 7, day 14, or day 28 from FMT with respect to baseline (Figure 3C). Of note, 19 of 21 species were shared between donor FMT and recipients across time points, and 2 of the species were recipient-only species before FMT. Rothia mucilaginosa, 1 of the recipient-only species, had the highest decrease with log fold change of 10.70 at day 14 compared with before FMT (adjusted P = .0014), whereas Eggerthella lenta, a shared-species, had the highest increase of 6.27 log fold change at day 7 (adjusted P < .001). Blautia wexlerea was the only species that was observed to be differentially abundant on all 3 days after FMT compared with baseline with log fold change of 4.40, 1.38, and 3.09 on day 7, day 14, and day 28, respectively.
Compositional and differential changes over time. (A) Principal component ordinate analysis at baseline (day 1), day 7, day 14, and day 28 by donor (blue points) and recipient (red points). Blue square shape represents the median of the Euclidean distances for donor, and the red square shape represents median of the Euclidean distances for recipients. The red circle and blue circle showing 95% confidence interval based on the t-distribution of the Euclidean distance for donor and recipient respectively. R2 and P values derived using Adonis2 test are annotated for each day. (B) Bray-Curtis distances over the first 28 days from FMT with respect to average taxonomic composition of the 4 donor samples. Each line indicates individual participant’s beta diversity distance with respect to donor. Participant number labels indicate the last available data point for that participant. (C) Species shown to be significantly differentially abundant between baseline and day 7 or day 14 or day 28 with AncomBC analysis. Circles at day and species indicate the log fold change. Dotted line separates the days before and after FMT. Double dotted line with vertical gap separates the origins of species based on the fecal microbiome survey at baseline for recipient and donors. All species have adjusted P values <.05, minimum prevalence of 50% across all samples, and minimum absolute log fold change >1.
Compositional and differential changes over time. (A) Principal component ordinate analysis at baseline (day 1), day 7, day 14, and day 28 by donor (blue points) and recipient (red points). Blue square shape represents the median of the Euclidean distances for donor, and the red square shape represents median of the Euclidean distances for recipients. The red circle and blue circle showing 95% confidence interval based on the t-distribution of the Euclidean distance for donor and recipient respectively. R2 and P values derived using Adonis2 test are annotated for each day. (B) Bray-Curtis distances over the first 28 days from FMT with respect to average taxonomic composition of the 4 donor samples. Each line indicates individual participant’s beta diversity distance with respect to donor. Participant number labels indicate the last available data point for that participant. (C) Species shown to be significantly differentially abundant between baseline and day 7 or day 14 or day 28 with AncomBC analysis. Circles at day and species indicate the log fold change. Dotted line separates the days before and after FMT. Double dotted line with vertical gap separates the origins of species based on the fecal microbiome survey at baseline for recipient and donors. All species have adjusted P values <.05, minimum prevalence of 50% across all samples, and minimum absolute log fold change >1.
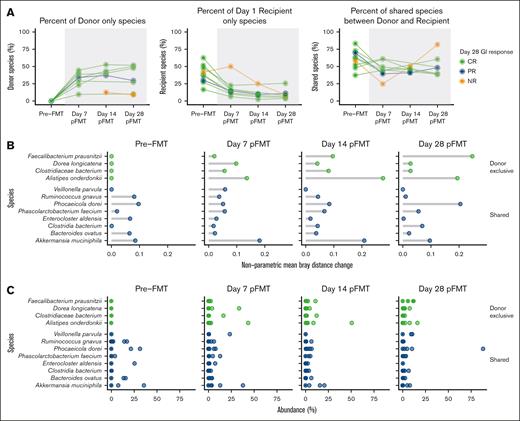
Longitudinal changes in microbial composition classified by the source
Based on the donor microbiome profile and the recipient’s microbiome profile at baseline, species were classified as donor-only, recipient-only, or donor and recipient shared species. At day 7 from FMT, the median proportion of donor-only species was 34% (range, 10-45). Donor-only species proportion increased through day 14 (median, 41% [range, 13-53]) and was similarly 30% (range, 9-52) at day 28. In contrast, recipient-only species proportions declined after FMT, from a median at baseline of 38% (range, 17-63) to 15% (range, 6-50) at day 7. The median proportion of recipient-only species further declined to 9% (range, 2-25) and 8% (range, 4-26) at day 14 and day 28, respectively. Shared species proportion saw decline at day 7 from baseline, with median proportion of 45% (range, 25-61) from 62% (range, 38-83), but were subsequently similar at day 14 (46% [range, 42-50]) and day 28 (48% [range, 39-82]), respectively (Figure 4A).
Quantifying the change in abundance of bacteria classified by source. (A) Percent of donor-only, recipient-only, and donor–recipient-shared species out of total bacterial abundance, based on donor and recipient microbiome profile at baseline. Lines are representative of individual participants, colored-coded according LGI response at day 28; CR (green), PR (blue), or NR (orange). (B) Top 5 influential species on beta diversity by nonparametric mean Bray-Curtis distance change within each sample at baseline and after FMT at day 7, day 14, and day 28. Bray-Curtis distance change was calculated by removing 1 species at a time and measuring the change in Bray-Curtis distance for each sample and adjusted with Shannon alphadiversity for that sample. Green dots show the species identified as donor exclusive, and blue dots show the shared species among donor and patients based on the first sample from donor and recipients. (C) Percent abundance of the top 5 species selected based on the nonparametric mean Bray-Curtis distance change at baseline and after FMT at day 7, day 14, and day 28.
Quantifying the change in abundance of bacteria classified by source. (A) Percent of donor-only, recipient-only, and donor–recipient-shared species out of total bacterial abundance, based on donor and recipient microbiome profile at baseline. Lines are representative of individual participants, colored-coded according LGI response at day 28; CR (green), PR (blue), or NR (orange). (B) Top 5 influential species on beta diversity by nonparametric mean Bray-Curtis distance change within each sample at baseline and after FMT at day 7, day 14, and day 28. Bray-Curtis distance change was calculated by removing 1 species at a time and measuring the change in Bray-Curtis distance for each sample and adjusted with Shannon alphadiversity for that sample. Green dots show the species identified as donor exclusive, and blue dots show the shared species among donor and patients based on the first sample from donor and recipients. (C) Percent abundance of the top 5 species selected based on the nonparametric mean Bray-Curtis distance change at baseline and after FMT at day 7, day 14, and day 28.
Quantifying the influence of a species on the Bray-Curtis distance within samples showed donor and shared species were among the top 5 species among all timepoints. Four donor-only species (Faecalibacterium praunsnitzii, Dorea longicatena, Clostridiaceae bacterium, Alistipes onderdonkii), and 8 donor–recipient-shared species (Veillonella parvula, Ruminococcus gnavus, Phocaeicola dorei, Phascolarctobacterium faecium, Enterocloster aldensis, Clostridia bacterium, Bacteroides ovatus, and Akkermansia muciniphila) showed highest mean Bray-Curtis distance change as measured with nonparametric mean Bray-Curtis distance within sample (Figure 4B). The proportion of these top influential species is also noted for comparison (Figure 4C).
Longitudinal changes in fecal metabolome after FMT
In line with the evaluation of microbial species that experienced significant changes in abundances by day 7 after FMT, stool metabolites (including carbohydrates, tryptophan metabolites, and short-chain fatty acids) were evaluated at baseline and day 7 after FMT. When evaluating for metabolites that were significantly altered in participants achieving a CR in the first week after FMT as compared with non-CR participants, we observed both increases and decreases in the stool concentration of select metabolites, respectively (Figure 5). Of note, increases in 4 indole compounds (5-HIAA, indole, indoxyl sulfate, and serotonin; P < .05) and 4 short-chain fatty acids (butyric acid, valeric acid, isobutyric acid, and isovaleric acid; P < .05) were identified (Figure 5B-C).
Significant metabolomics changes in the first week after FMT. Metabolomics changes in (A) carbohydrates, (B) tryptophan metabolites, and (C) short-chain fatty acids between baseline and day 7 after FMT administration among matched CR and non-CR participants, indicated by green and orange lines, respectively. Black bar indicates median values for the metabolite within a group. All unadjusted P values were derived using Wilcox signed rank test.
Significant metabolomics changes in the first week after FMT. Metabolomics changes in (A) carbohydrates, (B) tryptophan metabolites, and (C) short-chain fatty acids between baseline and day 7 after FMT administration among matched CR and non-CR participants, indicated by green and orange lines, respectively. Black bar indicates median values for the metabolite within a group. All unadjusted P values were derived using Wilcox signed rank test.
Discussion
This pilot study, which administered third-party FMT oral capsules in combination with corticosteroids, is, to our knowledge, the first to investigate the use of FMT with concurrent systemic corticosteroids for treatment-naïve high-risk acute LGI GVHD. The trial met its primary end point of feasibility. FMT was well tolerated, with no treatment-related adverse events, which is consistent with that observed in other GVHD studies.18-20,29,30 In the LGI tract, the target organ for the FMT intervention, the organ–specific response rate was high (CR rate, 70% at day 28) and responses were durable, without recurrent LGI GVHD in participants achieving CR. As expected, all participants displayed evidence for microbiota alternations before treatment. Exploratory analyses of stool specimens suggested that microbial richness and SDI typically increased after FMT. Although recipient microbiome composition never achieved a high degree of donor similarity, expansion of donor-derived species and increases in several indole compounds and short-chain fatty acids were observed, although the small number of samples limit the interpretation of these findings. This trial demonstrates the feasibility to successfully incorporate FMT in combination with corticosteroids into the upfront treatment of acute LGI GVHD, and the encouraging clinical outcomes should prompt further investigation of FMT and other microbiome-directed therapeutics in this setting.
Feasibility was selected as the primary end point for this study, as a cautious approach to intensifying upfront treatment for participants at high risk. Nonetheless, it is not surprising that the intervention was well tolerated. Oral capsules are often preferred to endoscopic approaches because of ease of administration, which also allowed for implementation of weekly maintenance dosing in this protocol. There have been no major issues with feasibility or adverse events reported to date in the setting of GVHD with oral capsules.31-33 One major concern with FMT in the setting of GVHD is the risk for infection from donor stool, which has occurred in HCT recipients.34 There were 2 cases of bacteremia in the current study, both considered unrelated to FMT. Bacteremia is known to occur in patients with LGI GVHD, because of the underlying compromise of the intestinal barrier and the ongoing treatment with high-dose corticosteroids and other immunosuppressive therapies. One may hypothesize that the risk for bacteremia from an enteric source may increase with time in patients with prolonged LGI involvement. Thus, the administration of FMT earlier in the GVHD disease may lower the risk for these events.
The clinical response rates were high in this small pilot. Although these clinical results need to be interpreted with caution given the small sample size, we believe that it helps establish primary proof of concept that intensifying upfront treatment may represent an important approach for patients with acute LGI GVHD. Historical rates of steroid response in acute GVHD have been ∼60%, with more severe LGI GVHD cases having lower response rates and higher mortality.23 Recent studies have investigated the combination of novel agents with corticosteroids in the upfront treatment of acute GVHD, although these studies have failed to show a clinical improvement vs corticosteroids alone.35 In this study, because all treatment-naïve participants received FMT and corticosteroids concurrently, the clinical impact attributed to FMT cannot be accurately assessed. It is reasonable to hypothesize that there are subpopulations of acute GVHD patients that may be most likely to benefit from use of FMT and these patients could be identified by GVHD–related characteristics, microbiome-related measurements, or a combination thereof. Ultimately, randomized trials will be needed to determine the clinical impact of FMT in the upfront treatment of LGI GVHD.
Improvement in dysbiosis was observed in participants after FMT, according to multiple metrics in both the stool and urine. It was also observed that intestinal microbial composition in the recipients trended away from baseline and toward the donor but without achieving a high degree of donor similarity. Nonetheless, expansion of donor-specific species was observed within 7 days of FMT. Although the microbial changes in clinical responders appear more favorable than nonresponders, the small sample size does not allow for definitive characterization of this descriptive observation. Taken together, these findings suggest that FMT contributes to the improvement in microbial diversity metrics observed in this study, while acknowledging that the impact of FMT cannot be truly delineated from other factors such as resolution of GVHD-related intestinal inflammation, the lack of broad-spectrum antibiotic use, and resumption of normal oral nutritional intake. The observation that clinical responses were observed without full engraftment of donor species could be explained by the concurrent use of systemic corticosteroids to treat GVHD. However, it also highlights areas for future research to elucidate the degree to which microbial disruption contributes to GVHD pathophysiology and what threshold of microbial change may be needed to result in a clinical response. An increase in the abundance of microbial-related metabolites was identified in the 7 days after FMT, but future endeavors will need to further investigate the interplay between microbiome composition, metabolite activity, and GVHD, as well as to the kinetics of metabolite abundance in patients recovering from GI GVHD.
A major limitation of this study is the small size and single-arm design. This limits the ability to characterize the impact of FMT on clinical responses and microbial measurements because FMT was administered concurrently with corticosteroids. Additionally, all participants received FMT from the same donor, and it is unclear whether similar results would be reproduced with different FMT donors. Finally, given the high clinical response rate, only a preliminary characterization of microbial and metabolomic changes in responders could be provided. A larger study would be needed to develop a microbial signature that could potentially stratify responders from nonresponders. However, significant logistical barriers limit the ability to conduct a larger trial with the single-donor approach used in this trial, and studies using pooled FMT products or other novel bacterial compositions represent the most promising next step.
In conclusion, the addition of third-party FMT to corticosteroids in the first-line treatment of high-risk acute LGI GVHD was feasible and safe. LGI clinical response rates were high, and responses were durable. Microbial richness increased significantly among responders. These data provide additional basis for future investigation to optimize FMT and microbiome-targeted therapeutics in the treatment of acute LGI GVHD.
Acknowledgments
This study was funded by an MGH American Cancer Society Institutional Research Grant; the Ancona Family Fund to Advance BMT Research and Treatment; was supported by a grant from the National Institutes of Health/National Cancer Institute (grant P30CA016672); and used the Microbiome and Metabolomics Facility.
Authorship
Contribution: Z.D., E.H., and Y.-B.C. designed the study; Z.D., A.E.-J., S.L.M., A.J.S.B., V.T., M.M.S., M.D., L.P., M.W., B.D., S.C., B.R.D., M.J.F., R.A.N., P.V.O., T.R.S., M.K.M., and Y.-B.C. recruited participants for the study, and collected clinical data and clinical specimens; E.H. prepared the fecal microbiota transplantation capsules; A.V.D., C.-C.C., D.W., N.J.A., and R.R.J. performed the analysis of stool and urine specimens; Z.D., A.V.D., H.T.K., C.-C.C., N.J.A., R.R.J., and Y.-B.C. analyzed the data and wrote the manuscript; and all authors approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: Z.D. receives research support from Incyte, REGiMMUNE, and Taiho Oncology; and has received consulting fees from Sanofi, Incyte, MorphoSys, Inhibrx, PharmaBiome, and ONO Pharmaceutical. R.A.N. has received equity from TimeDoc Health. M.J.F. receives research support from Incyte, Arcellx, Novartis, and Kite; and has received consulting fees from Kite, Novartis, Bristol Myers Squibb, and Iovance Biotherapeutics. T.R.S. has served on committees for bluebird bio (data monitoring committee), Syneos Health (data monitoring committee and adjudication committee), Ossium Health (scientific review committee); and has served on a scientific advisory board for Qihan Biotech. R.R.J. is an adviser and holds equity in Seres Therapeutics and Kaleido Biosciences; serves on the advisory board of MaaT Pharma, LISCure Biosciences, and Prolacta Biosciences; and consults for Da Volterra, Merck, Microbiome DX, and Karius. Y.-B.C. has received consulting fees from Takeda, Incyte, Vor Bio, Pharmacosmos, Editas, and Celularity. The remaining authors declare no competing financial interests.
Correspondence: Zachariah DeFilipp, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114; email: zdefilipp@mgh.harvard.edu.
References
Author notes
R.R.J. and Y.-B.C. contributed equally to this study.
Data are available on request from the corresponding author, Zachariah DeFilipp (zdefilipp@mgh.harvard.edu).
The full-text version of this article contains a data supplement.