TO THE EDITOR:
von Willebrand disease (VWD) is an inherited bleeding disorder caused by the deficiency or dysfunction of von Willebrand factor (VWF).1 Given the increased risk of bleeding, patients with VWD are advised to avoid aspirin, P2Y12 inhibitors, or anticoagulants. Patients with VWD may develop thrombotic complications that require treatment with these agents.2-4 For example, 1% to 2% of the population has atrial fibrillation (AF) that may be treated with aspirin or anticoagulation depending on CHA2DS2-VASC risk stratification.5 Furthermore, ∼805 000 people each year develop an acute coronary syndrome (ACS), defined as non-ST segment myocardial infarction, ST elevation myocardial infarction, or unstable angina.6 Treatment includes aspirin, P2Y12 inhibitors, dual antiplatelet therapy (DAPT), and/or anticoagulation after percutaneous coronary intervention (PCI).
Few data exist to guide the management of ACS and AF in patients with VWD. Present recommendations, based on low certainty of evidence, suggest giving necessary antiplatelet therapy or anticoagulants to patients with VWD with assessment of bleeding risk throughout the course and potentially providing factor prophylaxis, which is also reflected in hemophilia guidelines.1,7,8 This study aimed to assess anticoagulant and antiplatelet prescription patterns, bleeding risk, and other outcomes in patients with VWD who develop ACS and/or AF.
This study is a retrospective analysis of coded data from institutional electronic medical records approved by the Mass General Brigham Institutional Review Board. Patients were identified from 1980 to 2020 who had a diagnosis of VWD or any abnormal VWF panel along with a diagnosis of non-ST segment myocardial infarction, ST elevation myocardial infarction, unstable angina, AF, or atrial flutter. Six hundred twenty-three patients met the initial search criteria. After a manual review to confirm diagnoses and sufficient data for analysis by 2 independent physicians with 10% overlap to ensure concordance, a total of 117 patients were included. Primary end point was the rate of major bleeding while on an antiplatelet agent for ACS and anticoagulation for AF. The International Society for Thrombosis and Haemostasis definition for major bleeding was used, which is defined as a fatal bleeding and/or symptomatic bleeding in a critical area or organ such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome, and/or bleeding causing a fall in hemoglobin levels by ≥2 g/dL or leading to a transfusion of ≥2 units of red blood cells.9,10 Secondary end points included time to an event while on anticoagulation or antiplatelet therapy, prevalence of anticoagulation or antiplatelet use, and complications of AF and ACS such as stroke or death within 1 year of an event. Descriptive data are reported in counts and percentages for categorical variables and medians and interquartile ranges for continuous variables. Time-to-event end points were estimated by means of the Kaplan-Meier method. Analyses were performed in SAS 9.4 (SAS Institute, Cary, NC).
Demographic information, ever prescription of anticoagulant or antiplatelet therapy, and length of time on these therapies are shown in Table 1. In patients with AF, 89.9% (80/89) had CHA2DS2-VASC of ≥2 at the time of diagnosis. Of those with CHA2DS2-VASC of ≥2, 57.5% (46/80) were ever prescribed anticoagulation. Among those undergoing PCI for ACS, 85% (17/20) were initiated on DAPT. A total of 75% (15/20) were prescribed DAPT for at least 28 days and 30% (6/20) for at least 1 year.
Demographic data and prescribing patterns among patients with VWD and AF alone, ACS alone, and both AF and ACS
| . | AF alone (n = 62) . | ACS alone (n = 28) . | AF and ACS (n = 27) . |
|---|---|---|---|
| Demographic data | |||
| Age, median (IQR), y | 64.1 (52.0-73.6) | 59.5 (55.5-68.1) | 66.9 (59.6-74.3) |
| Sex, female, n (%) | 45 (72.6%) | 14 (50.0%) | 12 (44.4%) |
| Deceased, n (%) | 13 (21.0%) | 10 (35.7%) | 10 (37.0%) |
| Diabetes, n (%) | 12 (19.4%) | 11 (39.3%) | 13 (48.1%) |
| HTN, n (%) | 53 (85.5%) | 27 (96.4%) | 27 (100.0%) |
| CHF, n (%) | 26 (41.9%) | 13 (46.4%) | 23 (85.2%) |
| Smoking, n (%) | 16 (25.8%) | 8 (28.6%) | 10 (37.0%) |
| CHA2DS2-VASC score, median (IQR) | 3 (2-4) | — | 5 (4-6) |
| PCI, n (%) | — | 10 (35.7%) | 10 (37.0%) |
| CABG, n (%) | — | 3 (10.7%) | 10 (37.0%) |
| Ever prescribed,n (%) | |||
| Aspirin | 17 (27.4%) | 21 (75.0%) | 20 (74.1%) |
| P2Y12 inhibitor | 1 (1.6%) | 12 (42.9%) | 11 (40.7%) |
| DAPT | — | 11 (39.3%) | 10 (37.0%) |
| AC | 38 (61.3%) | 1 (3.6%) | 12 (44.4%) |
| No AC or AP | 18 (29.0%) | 6 (21.4%) | 3 (11.1%) |
| Duration of use, median (IQR), y | |||
| Aspirin | 8.6 (0.8-11.7) | 6.2 (3.4-17.0) | 13.0 (8.7-15.7) |
| DAPT | — | 0.1 (0.1-0.1) | 1.2 (0.1-9.0) |
| AC | 2.7 (1.5-8.6) | — | 8.4 (2.7-13.2) |
| . | AF alone (n = 62) . | ACS alone (n = 28) . | AF and ACS (n = 27) . |
|---|---|---|---|
| Demographic data | |||
| Age, median (IQR), y | 64.1 (52.0-73.6) | 59.5 (55.5-68.1) | 66.9 (59.6-74.3) |
| Sex, female, n (%) | 45 (72.6%) | 14 (50.0%) | 12 (44.4%) |
| Deceased, n (%) | 13 (21.0%) | 10 (35.7%) | 10 (37.0%) |
| Diabetes, n (%) | 12 (19.4%) | 11 (39.3%) | 13 (48.1%) |
| HTN, n (%) | 53 (85.5%) | 27 (96.4%) | 27 (100.0%) |
| CHF, n (%) | 26 (41.9%) | 13 (46.4%) | 23 (85.2%) |
| Smoking, n (%) | 16 (25.8%) | 8 (28.6%) | 10 (37.0%) |
| CHA2DS2-VASC score, median (IQR) | 3 (2-4) | — | 5 (4-6) |
| PCI, n (%) | — | 10 (35.7%) | 10 (37.0%) |
| CABG, n (%) | — | 3 (10.7%) | 10 (37.0%) |
| Ever prescribed,n (%) | |||
| Aspirin | 17 (27.4%) | 21 (75.0%) | 20 (74.1%) |
| P2Y12 inhibitor | 1 (1.6%) | 12 (42.9%) | 11 (40.7%) |
| DAPT | — | 11 (39.3%) | 10 (37.0%) |
| AC | 38 (61.3%) | 1 (3.6%) | 12 (44.4%) |
| No AC or AP | 18 (29.0%) | 6 (21.4%) | 3 (11.1%) |
| Duration of use, median (IQR), y | |||
| Aspirin | 8.6 (0.8-11.7) | 6.2 (3.4-17.0) | 13.0 (8.7-15.7) |
| DAPT | — | 0.1 (0.1-0.1) | 1.2 (0.1-9.0) |
| AC | 2.7 (1.5-8.6) | — | 8.4 (2.7-13.2) |
Each column represents a cohort that is assessed separately (AF alone, ACS alone, and both AF and ACS). The percentages in the table indicate the proportion of each cohort who had a given condition or medication use. Thus, the percentages do not add to 100% across a given row.
AC, anticoagulation; AP, antiplatelet agent; CABG, coronary artery bypass grafting; CHF, congestive heart failure; HTN, hypertension; IQR, interquartile range.
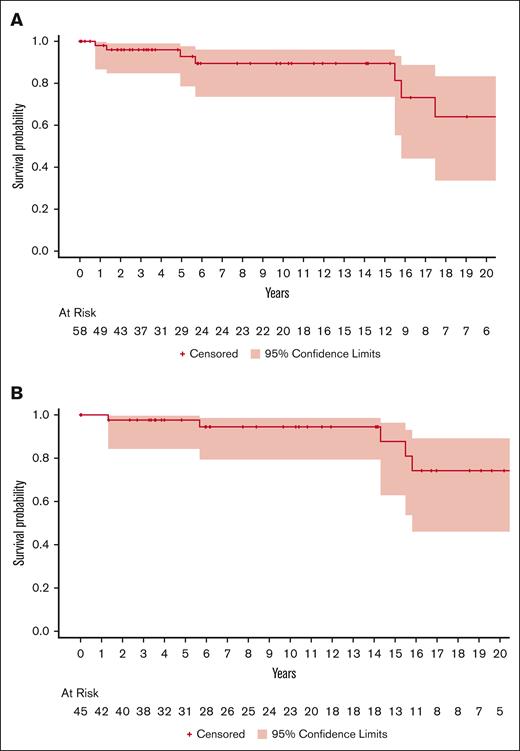
Time to first bleed while prescribed anticoagulation for AF and antiplatelet agents for ACS is shown in Figure 1. Among patients with AF alone, the rate of major bleeding among patients prescribed antiplatelet agents was 1.51 events per 100 person-years (2 events per 132.38 years) and 8.99 events per 100 person-years (21 events per 233.59 years) among those prescribed anticoagulation. Among patients with ACS alone (n = 28), there were 5.9 events per 100 person-years (12 events per 201.77 years) among those prescribed aspirin monotherapy and 0 events in those receiving DAPT (6.31 years). Among patients with AF and ACS, there were 17.82 events per 100 person-years (41 events per 230 years) among those prescribed aspirin alone, 7.5 events per 100 person-years (3 events per 39.95 years) in patients prescribed DAPT, and 16.7 events per 100 person-years (21 events per 125.6 years) in patients prescribed anticoagulation. There were 38.2 events per 100 person-years (56 events per 146.6 years) in those who were on both anticoagulation and antiplatelet therapy simultaneously.
Time to first International Society for Thrombosis and Haemostasis major bleed event. (A) Time to first bleed event among those with a diagnosis of AF who were prescribed any anticoagulant. (B) Time to first bleed event among those with a diagnosis of ACS who were prescribed an antiplatelet agent. Censoring occurred for death or cessation of treatment.
Time to first International Society for Thrombosis and Haemostasis major bleed event. (A) Time to first bleed event among those with a diagnosis of AF who were prescribed any anticoagulant. (B) Time to first bleed event among those with a diagnosis of ACS who were prescribed an antiplatelet agent. Censoring occurred for death or cessation of treatment.
Thromboembolic stroke occurred in 15.7% (14/89) of patients with AF, and 78.6% (11/14) of those strokes occurred in patients who were not prescribed anticoagulation in the preceding 90 days. One stroke occurred within 90 days of starting anticoagulation. There was 1 fatal stroke (1/14 [7.1%]). Among those with a thromboembolic stroke, median CHA2DS2-VASC was 3 (range, 1-5) among those prescribed anticoagulation (n = 3) and 3 (range, 0-6) among those without an anticoagulation prescription. There were 2 deaths (2/55 [3.6%]) within 1 year of ACS that both occurred 3 days after the ACS event.
This carefully selected cohort of patients with both VWD and either ACS or AF elucidates the prescribing patterns of anticoagulation and antiplatelet agents and the risk of bleeding or stroke. Among patients with AF, only 57.5% of patients with CHA2DS2-VASC score of ≥2 were ever prescribed the standard-of-care anticoagulation. We found that 27.4% of patients were prescribed aspirin for AF alone, which is not currently a guideline recommended therapy. We speculate this approach was an attempt to reduce stroke risk while simultaneously minimizing bleeding risk. We did find significantly lower rates of bleeding with antiplatelet use than anticoagulation use in patients with AF alone, but we have insufficient data to support that aspirin monotherapy sufficiently reduces stroke risk in this population. Importantly, most thromboembolic strokes occurred in patients who were not prescribed recommended anticoagulation.
Among any patients with ACS, ∼3 in 4 were prescribed guideline recommended DAPT after PCI for at least 28 days. There were no bleeding events reported among those with ACS alone who received DAPT, suggesting that it is safe to prescribe a limited course of this recommended treatment. There is also a low rate of bleeding on aspirin monotherapy, and most events occurred after 10 years of use. In contrast to patients with VWD and AF or ACS alone, there are much higher rates of bleeding with all medication types among those with VWD, ACS, and AF, possibly reflecting a more medically frail population prone to bleeding complications than those with ACS or AF alone. Furthermore, concomitant anticoagulation and antiplatelet use had much higher rates of bleeding than anticoagulation use alone. The best approach for patients with VWD, AF, and ACS is unknown at present.
The retrospective nature of the data with lack of granularity in the coded data about VWD severity and subtype is a limitation to this study. Dates associated with any diagnosis or medication before electronic medical record launch in 2015 were often inaccurate and required manual verification, and incomplete older charts limited analysis. Data on severity of VWD were often missing or incomplete and thus not included in the analysis. In addition, direct-acting oral anticoagulants and P2Y12 inhibitors were introduced after our cohort's first AF diagnosis in 1980; therefore, the availability of medications to prescribe changed over time.
In summary, patients with VWD and AF or ACS are inconsistently prescribed recommended anticoagulation or antiplatelet agents, likely due to concerns about bleeding risk, with the highest bleeding rates found in patients with VWD with both ACS and AF. Shared decision-making is advised. These data suggest short-course DAPT is relatively safe in those with VWD, but long-term aspirin should be approached with caution. Bleeding risk on anticoagulation for patients with VWD and AF is higher than that observed in the general population, but the risk of stroke in this population is high without anticoagulant use as well. Shared decision-making around stroke and bleeding risk is advised in considering anticoagulation for patients with VWD and AF, but standard anticoagulation should be strongly considered for those with CHA2DS2-VASC score of ≥2 and average bleeding risk for VWD.
Contribution: L.E.M., D.A., and N.T.C. designed the research; L.E.M., D.A., D.M.K., and P.M. performed the research; L.E.M. and S.F. analyzed the data; L.E.M. and N.T.C. wrote the manuscript; and D.A., D.M.K., and P.M. edited the manuscript.
Conflict-of-interest disclosure: N.T.C. reports advisory board fees from Takeda, Genentech, and Medzown; consultancy fees from Takeda; travel support and honorarium from Octapharma; and holds equity in Doximity and Medzown. The remaining authors declare no competing financial interests.
Correspondence: Lauren E. Merz, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: lauren_merz@dfci.harvard.edu.
References
Author notes
Data sets are available on request from the corresponding author, Lauren E. Merz (lauren_merz@dfci.harvard.edu).