TO THE EDITOR:
Waldenström’s macroglobulinemia (WM) is characterized by recurring MYD88 (95%-97%) and CXCR4 (40%) mutations.1 Mutated MYD88 (MYD88Mut) triggers hemtopoietic cell kinase transcription, which enables Bruton tyrosine kinase (BTK) activation.2,3 BTK triggers nuclear factor kappa-light-chain enhancer of activated B-cells prosurvival signaling, as well as extracellular signal regulated kinase (ERK) 1/2 activation leading to inflammatory cytokine release supporting autocrine- and paracrine-mediated growth and survival signaling.3,4 More than 40 frameshift and nonsense variants have been reported in CXCR4Mut patients.1,5CXCR4Mut are subclonal to MYD88Mut and show heterogeneous clonality in patients with WM.5 WM cells expressing CXCR4Mut show enhanced BTK, protein kinase B, and ERK1/2 signaling in response to the CXCR4 ligand CXCL12.6,7 These findings supported the development of BTK-inhibitors, and regulatory approval for ibrutinib and zanubrutinib for treating WM.
Differences in treatment outcomes have been reported in patients with WM receiving ibrutinib and zanubrutinib by CXCR4Mut status.8-12CXCR4Mut patients with WM can show a longer time to major response, fewer major and very good partial response responses, and/or shorter progression-free survival (PFS) in response to BTK inhibitors.8-13 The effect is particularly noteworthy in patients with WM treated with ibrutinib who carry nonsense CXCR4 variants.14 Clonality of CXCR4Mut can also contribute to inferior responses and shorter PFS in patients with WM receiving ibrutinib.15 Detecting CXCR4Mut in patients with WM can be problematic, particularly by next-generation sequencing.15CXCR4Mut were missed in two-thirds of patients with WM using next-generation sequencing, particularly in those with lower bone marrow (BM) disease involvement and clonality.16 Although presence of CXCR4Mut can predict inferior outcomes with BTK inhibitors, many wild-type CXCR4 (CXCR4WT) patients can also have inferior outcomes. Conversely, some patients with CXCR4Mut disease can have robust responses to BTK inhibitors. Thus, we sought to identify more robust biomarker(s) to better predict BTK inhibitor response activity using a multiomic approach. Such biomarkers may help position BTK inhibitors relative to other available therapeutics such as bendamustine or proteasome-inhibitor–based regimens. For these studies, we focused on ibrutinib, using BM samples obtained in a prospective clinical trial with long-term follow-up in symptomatic, treatment-naïve patients with WM.
We used CD19-selected BM samples obtained at baseline from a prospective clinical trial of ibrutinib monotherapy (NCT02604511). Thirty symptomatic, treatment-naïve patients were enrolled whose MYD88 and CXCR4 mutation status and treatment outcomes are published.9 Samples were available for whole exome sequencing (WES), RNA sequencing (RNA-seq), and assay for transposase-accessible chromatin using sequencing (ATAC) sequencing (ATAC-seq) for 23, 27, and 20 patients, respectively. Methods for WES, RNA-seq, and ATAC-seq appear in the supplemental Material. We used a previous validated model for PFS prediction in patients with WM treated with ibrutinib.17 ElasticNet regression using the glmnet package was used for feature selection with the response variable defined as responders (partial response [PR], or better) vs nonresponders with predictors from the relevant combined features of the WES, RNA-seq and ATAC-seq analyses.18 To determine the most robust predictors, this analysis was bootstrapped 500 times, and only features identified in >95% of the bootstraps were considered further. Raw counts were used to run the default DESeq2 pipeline to obtain differentially expressed (DE) ATAC regions and transcripts between response groups using a l2fc > |1| and adjusted P value (adj P) of < .1. Adjusted P values for false discovery rate (Benjamini-Hochberg)-corrected P values determined by DESeq2 are reported.19 Survival and survminer R modules were used to perform Kaplan-Meier time to response (TTR) and PFS analyses. The study was approved by our Institutional Review Board, and written consent was obtained from all patients for their sample use.
The baseline characteristics for the 27 patients appear in supplemental Table 1. All 27 were MYD88Mut; 13 were CXCR4Mut of whom 12 had nonsense variants, and 1 a frameshift mutation. Their median follow-up was 50.1 months. At best response, the overall (minor response or better), major (partial response or better), and very good partial response rates were 100%, 87%, and 30%, respectively. The median time to major response was significantly longer in CXCR4Mut vs CXCR4WT patients (7.3 vs 1.8 months; P = .01). The median PFS was not reached. At 4 years, the PFS rate was 76% for all study patients and was shorter for CXCR4Mut vs CXCR4WT patients (59% vs 92%; P = .06). Among the 27 patients with WM included in this study, PFS showed a trend for being longer among those who attained a major vs less than a major response at 6 months (median not reached vs 64.5 months; P = .10).
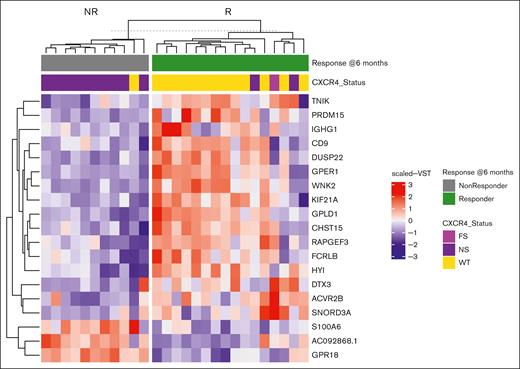
We next compared multiomic findings between the 2 response groups to identify factors influencing ibrutinib response. By WES, the only somatic mutation that showed significant association with major response attainment at 6 months was CXCR4. CXCR4Mut patients showed fewer major responses at 6 months (16.7% vs 93%; P < .0002) and shorter PFS (36.4 months vs not reached; P = .046) vs CXCR4WT patients. While the presence of CXCR4Mut associated with nonresponse status at 6 months and shorter PFS, 3 of 13 (23%) CXCR4Mut patients were major responders at 6 months. By RNA-seq, 64 DE genes were identified that included WNK2 (adj P = .00005), DUSP22 (adj P = .0008), and GPER1 (adj P = .0008) as the top hits expressed in CXCR4WT and CXCR4Mut patients who attained a major response at 6 months (supplemental Table 2). Other top DE genes identified by RNA-seq in major responders included OSBPL3, PRDM15, GPLD1, GPR18, NEB, and DDR1 (supplemental Table 2). ATAC-seq revealed 1 differentially open genomic region at chr12, which mapped to the 5′ region of the KIF21A locus. This region was found to be more open or accessible in the 16 patients with WM who attained a major response at 6 months. KIF21A was also a significant gene in the DE RNA-seq data as being upregulated for those who attained a major response at 6 months (supplemental Tables 2 and 3).
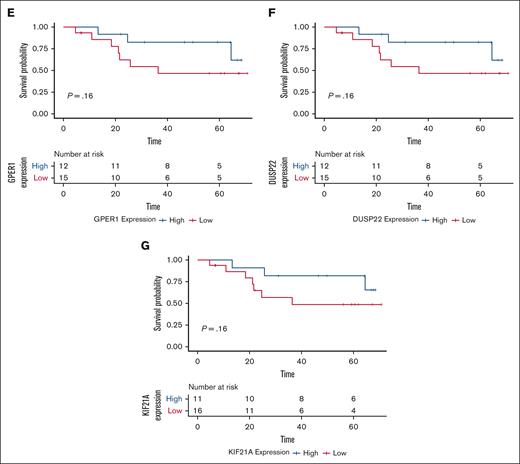
Elasticnet analysis, using the significant hits from WES, RNA, and ATAC-seq analysis, identified a set of 19 RNA transcripts that best distinguished major and nonmajor 6-month responders. Notably, many of these are known regulators of ERK1/2 signaling including WNK2, DUSP22, GPR18, GPER1, and PRDM15 (Figure 1).20-24 The suppression of ERK1/2 signaling has emerged as an important signaling pathway for ibrutinib response, as well as acquired resistance related to its role in inflammatory cytokine release and microenvironmental support.4,6 A TTR analysis revealed that the attainment of a major response at 6 months was most strongly associated with baseline expression of DUSP22, GPER1, CHST15, GPR18, ACVR2B, KIF21A, and WNK2 (supplemental Table 4). To identify which of these candidates might also influence long-term response, a PFS analysis was performed. At baseline, only GPR18 (P = .013) significantly impacted PFS, with low GPR18 transcript levels predicting longer PFS. A trend for longer PFS was also notable in those with high vs low levels of WNK2 expression (P = .097). Differential gene expression of CHST15, KIF21A, PRDM15, DUSP22, and KIF21A also showed an impact on PFS but were not statistically significant. The small sample size of our study may have contributed to these findings (Figure 2).
Top gene candidates from ElasticNet analysis distinguishing response groups. ElasticNet heat map for baseline expressed genes in MYD88-mutated patients with WM who received ibrutinib monotherapy on a clinical trial, and who attained a major response (green) or no major response (gray) at 6 months. The heat map shows the scaled VST gene expression levels selected by ElasticNet, which best predicts differences observed in response between the 2 groups. Each patient’s CXCR4 mutation status is shown above the heat map. FS, frameshift mutation; NS, nonsense mutation; VST, variance stabilizing transformation; WT, wild type.
Top gene candidates from ElasticNet analysis distinguishing response groups. ElasticNet heat map for baseline expressed genes in MYD88-mutated patients with WM who received ibrutinib monotherapy on a clinical trial, and who attained a major response (green) or no major response (gray) at 6 months. The heat map shows the scaled VST gene expression levels selected by ElasticNet, which best predicts differences observed in response between the 2 groups. Each patient’s CXCR4 mutation status is shown above the heat map. FS, frameshift mutation; NS, nonsense mutation; VST, variance stabilizing transformation; WT, wild type.
Multiomic identified biomarkers and progression-free survival in patients with WM receiving ibrutinib. Kaplan-Meier curves for PFS and risk tables for (A) GPR18, (B) WNK2, (C) CHST15, (D) PRDM15, (E) GPER1, (F) DUSP22, and (G) KIF21A. Tables show progression for high and low expressors of each candidate. Risk tables show progression data for each group.
Multiomic identified biomarkers and progression-free survival in patients with WM receiving ibrutinib. Kaplan-Meier curves for PFS and risk tables for (A) GPR18, (B) WNK2, (C) CHST15, (D) PRDM15, (E) GPER1, (F) DUSP22, and (G) KIF21A. Tables show progression for high and low expressors of each candidate. Risk tables show progression data for each group.
In summary, by use of comprehensive multiomics, we identified putative biomarkers for clinical investigation to predict ibrutinib major responders at 6 months, TTR, and PFS outcomes in MYD88Mut patients with WM. Low baseline expression of GPR18 as well as high levels of WNK2 showed the strongest associations with major response attainment at 6 months, shorter TTR, and longer PFS in this study. In addition, differential gene expression of CHST15, KIF21A, PRDM15, DUSP22, S100A6, and KIF21A also associated with major response attainment at 6 months and TTR but showed more marginal association with predicting PFS outcomes to ibrutinib. Many of these biomarkers including GPR18 and WNK2 are known modulators of ERK1/2 signaling.20-24 Further validation of these findings in a larger cohort will be required, as well as their potential use as predictors with other covalent and noncovalent BTK inhibitors. The applied use of such validated biomarkers may better BTK inhibitors over other available treatment options in WM. The findings may also be relevant for other B-cell malignancies in which BTK inhibitors are used. Our studies thereby provide a framework for the clinical investigation of novel, multiomic-identified genes as predictive biomarkers for BTK inhibitors in WM.
Acknowledgments: Clinical and genomic studies were supported by Pharmacyclics.
The authors gratefully acknowledge the generous support of Peter Bing, International Waldenström’s Macroglobulinemia (WM) Foundation, Leukemia and Lymphoma Society (grant: 6673-24), National Institutes of Health Specialized Programs of Research Excellence in Multiple Myeloma (grant: 2P50CA100707-16A1), the Orszag and Kitchen Fund for WM Research, the Kerry Robertson Fund for WM Research, the Siegel Family Fund for WM, the WM Foundation of Canada, the WMR Fund, and the patients with WM who provided samples for these studies.
Contribution: S.P.T., K.R., and Z.R.H. designed the study; J.J.C., S.R.S., A.R.B., C.A.F., and S.P.T. provided patient care; K.M. and C.J.P. provided study administrative support; J.N.G. coordinated clinical trial and sample collection; X.L., A.K., S.L., N.T., and M.L.G. performed sample processing and genomic and translational studies; K.R. and Z.R.H. performed the genomic analyses; K.R., Z.R.H., S.P.T., and J.J.C. analyzed the data; K.R. and S.P.T. drafted the initial manuscript; K.A.K. and J.L.W. performed statistical analyses; and all authors critically reviewed the manuscript and approved the final version for submission.
Conflict-of-interest disclosure: J.J.C. received research funds from AbbVie, AstraZeneca, BeiGene, Cellectar, LOXO, Pharmacyclics, TG Therapeutics, and honoraria from AbbVie, Beigene, Cellectar, Kite, LOXO, Janssen, Pharmacyclics, and Roche Pharmaceuticals. S.R.S. received research funding and/or consulting fees from BeiGene, Cellectar Biosciences, and ADC Therapeutics. A.R.B. received research funds and/or honoraria from Adaptive, BeiGene, CSL Behring, Genzyme, Karyopharm, Pharmacyclics, and Sanofi. S.P.T. received research funds and/or consulting fees from AbbVie/Pharmacyclics, Janssen Pharmaceuticals, BeiGene, Bristol Myers Squibb, and Eli Lilly. The remaining authors declare no competing financial interests.
Correspondence: Steven P. Treon, Bing Center for Waldenstrom's Macroglobulinemia, Dana Farber Cancer Institute, SM630, 450 Brookline Ave, Boston, MA 02115; email: steven_treon@dfci.harvard.edu.
References
Author notes
Deidentified data from this study will be available to investigators through email to the corresponding author, Steven P. Treon (steven_treon@dfci.harvard.edu).
The full-text version of this article contains a data supplement.