TO THE EDITOR:
Acute myeloid leukemia (AML) is an aggressive, heterogeneous disease with genomic subtypes that are increasingly treated differently. There is a growing interest in going beyond mutations and cytogenetics to stratify AML. Recent work has combined proteomics,1 signaling,2,3 or immunophenotypes4 with integrated genomic-transcriptomic measurements to improve AML risk classifications.5 Although invaluable as resources, such approaches can neither extend retroactively to existing repositories nor prospectively to new AML cases lacking these data types. We sought to develop a more extensible approach involving sphingolipids (Figure 1A), a family of bioactive molecules implicated in AML pathogenesis and therapeutic resistance6,7 that differentially regulate cell proliferation,8 differentiation,9 autophagy,10 apoptosis,11 and immune cell activation.12 Sphingolipid abundances in AML vary heterogeneously and ratiometrically,13 prompting us to ask whether systematic sphingolipidomic profiling could meaningfully stratify patients with AML and common AML cell lines.
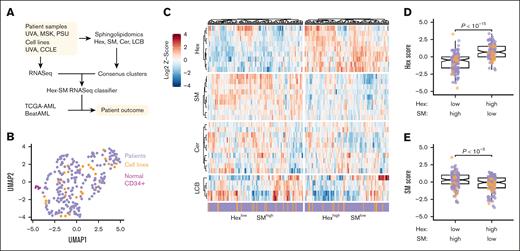
AML cell lines and patients with AML separate into 2 sphingolipidomic clusters that differ in their abundance of hexosylceramide and sphingomyelin. (A) Strategy to identify sphingolipidomic subtypes in AML. Sphingolipidomics of hexosylceramide (Hex), sphingomyelin (SM), ceramide (Cer), and LCB (comprised of sphingosine and its derivatives) was performed on primary AML samples and AML cell lines by liquid chromatography mass spectrometry, and the normalized data were consensus clustered to identify a stable number of sphingolipid clusters. Cluster-specific gene signatures were extracted to train a Hex-SM classifier that infers sphingolipidomic subtype from RNA sequencing (RNASeq). (B) Sphingolipidomic heterogeneity is similar in AML cell lines and patient samples but distinct from normal CD34+ bone marrow. Normalized sphingolipidomics for normal bone marrow samples (magenta, n = 6), primary AML samples (purple, n = 213), and AML cell lines (orange, n = 30) were displayed by Uniform Manifold Approximation and Projection (UMAP). (C) Row-standardized lipid abundances organized by the sphingolipid family: Hex, SM, Cer, and LCB. The HexlowSMhigh and HexhighSMlow consensus clusters are separately clustered and annotated as cell lines (orange) and patient samples (purple). (D-E) Normalized Z-scores of lipid species within the Hex (D) and SM (E) families were summed and differences between consensus clusters were assessed by the Mann-Whitney test with continuity correction. Colors indicate the sample type: AML cell lines (orange, n = 30) and primary samples (purple, n = 213).
AML cell lines and patients with AML separate into 2 sphingolipidomic clusters that differ in their abundance of hexosylceramide and sphingomyelin. (A) Strategy to identify sphingolipidomic subtypes in AML. Sphingolipidomics of hexosylceramide (Hex), sphingomyelin (SM), ceramide (Cer), and LCB (comprised of sphingosine and its derivatives) was performed on primary AML samples and AML cell lines by liquid chromatography mass spectrometry, and the normalized data were consensus clustered to identify a stable number of sphingolipid clusters. Cluster-specific gene signatures were extracted to train a Hex-SM classifier that infers sphingolipidomic subtype from RNA sequencing (RNASeq). (B) Sphingolipidomic heterogeneity is similar in AML cell lines and patient samples but distinct from normal CD34+ bone marrow. Normalized sphingolipidomics for normal bone marrow samples (magenta, n = 6), primary AML samples (purple, n = 213), and AML cell lines (orange, n = 30) were displayed by Uniform Manifold Approximation and Projection (UMAP). (C) Row-standardized lipid abundances organized by the sphingolipid family: Hex, SM, Cer, and LCB. The HexlowSMhigh and HexhighSMlow consensus clusters are separately clustered and annotated as cell lines (orange) and patient samples (purple). (D-E) Normalized Z-scores of lipid species within the Hex (D) and SM (E) families were summed and differences between consensus clusters were assessed by the Mann-Whitney test with continuity correction. Colors indicate the sample type: AML cell lines (orange, n = 30) and primary samples (purple, n = 213).
We quantified 33 sphingolipid metabolites by mass spectrometry in 213 primary AML samples, 30 human AML cell lines, and 6 normal CD34+ bone marrow samples after carefully controlling for cell purity and viability. Normalized sphingolipid profiles in AML cell lines and primary AML cases were highly dispersed (while remaining intermixed) and separable from normal samples (Figure 1B), motivating a pan-AML stratification. Consensus clustering of the normalized lipidomics data identified 2 sphingolipidomic clusters that were statistically robust (supplemental Figure 1A-C). The 2 clusters were equally populated with cell lines and primary samples, and neither was differentially enriched for common AML mutations (supplemental Figure 1D; supplemental Tables 1-3). In contrast, the clusters were divergent in their abundance of hexosylceramide (Hex) and sphingomyelin (SM) species (Figure 1C). Cluster 1 exhibited proportionally less Hex and more SM (HexlowSMhigh), whereas cluster 2 exhibited more Hex and less SM (HexhighSMlow; Figure 1D-E). Additionally, the HexhighSMlow cluster was elevated in long-chain, C14 to C20 carbon chains relative to the HexlowSMhigh cluster (supplemental Figure 1E). There were no detectable differences in ceramide (Cer), other long-chain or sphingoid bases (LCB, comprising sphingosine and its derivatives), or very long chain, C22 to C26 carbon sphingolipids (supplemental Figure 1F-H). The analyses supported 2 sphingolipidomic subtypes with biochemical states that were uncoupled from AML derivation or mutation status.
We next examined whether the subtypes differed in clinical outcomes. Complete data were available for 70 HexlowSMhigh and 72 HexhighSMlow cases sampled at diagnosis before intensive induction chemotherapy (supplemental Tables 2-3). Based on European LeukemiaNet (ELN) 2022 criteria,5 patients with HexlowSMhigh had twice the failure rate of HexhighSMlow (supplemental Figure 2A). The HexlowSMhigh subtype also trended toward shorter event-free survival (EFS) and overall survival (OS), although the difference was not statistically significant (Figure 2A-B). To eliminate confounding caused by events that occurred before the first round of induction chemotherapy was complete, we excluded patients with EFS <20 days and observed significant differences in EFS and OS (supplemental Figure 2B-C). These results were robust to EFS threshold (supplemental Figure 2D) and suggested prognostic value.
The HexlowSMhigh and HexhighSMlow AML subtypes differ in gene expression and survival outcome. (A-B) Kaplan-Meier plots of (EFS; npurple = 69, ngreen = 72) (A) and (OS; npurple = 70, ngreen = 72) (B) for patients with AML grouped into HexlowSMhigh (purple) and HexhighSMlow (green) subtypes. The study cohort comprised patients from 3 institutions (University of Virginia [UVA], Memorial Sloan Kettering [MSK], and Pennsylvania State University [PSU]). Patients who received intensive induction chemotherapy treatment were included in the analyses. Within each plot are the corresponding risk tables for the 2 groups. (C) Volcano plot of differentially expressed genes between the HexlowSMhigh and HexhighSMlow subtypes. Purple genes are upregulated in the HexlowSMhigh cluster whereas green genes are upregulated in the HexhighSMlow cluster (−log10Padj >1). (D) The HexlowSMhigh subtype is enriched for the LSC program. Gene set enrichment analysis score plot for an LSC signature of 104 genes.14 The y-axis is the running enrichment score along the ranked gene list. The enrichment score is the maximum deviation from 0 encountered along the list and represents the degree to which a gene set is overrepresented at the top or the bottom of the ranked gene list. The normalized enrichment score (NES) is the enrichment score normalized for variation in gene set sizes. The adjusted P-value (Padj) for the NES is shown. (E-F) The deconvolved HexlowSMhigh subtype is enriched for LSPCs and depleted in differentiated myeloid cell types. The CIBERSORTx log2-transformed absolute abundance score of total LSPCs (sum of quiescent, primed, and cycling LSPCs) (E) and total myeloid-lineage cells (sum of promono-like, mono-like, and monocyte) (F) is shown for the HexlowSMhigh (n = 217) and HexhighSMlow (n = 199) subtypes colored by study. Full deconvolution of leukemic cell types including the different LSPC and myeloid cell types is shown in supplemental Figure 3I and J. Differences between the 2 sphingolipid subtypes were assessed by a 2-sided Wilcoxon rank-sum test. (G-H) Frequency of CD34+CD38− cells between HexlowSMhigh and HexhighSMlow subtypes. Log10-transformed proportions of CD34+CD38− cells (G) and CD34+CD38−CD96+ cells (H) among 100 000 viable cells are shown for the HexlowSMhigh (n = 11) and HexhighSMlow (n = 12) subtypes. Differences between the 2 sphingolipid subtypes were assessed by a 1-sided Wilcoxon rank-sum test. (I) An RNASeq-based classifier accurately distinguishes sphingolipidomic subtypes. Receiver operating characteristics curve for a 284-gene support vector machine classifier applied to test data that includes both primary AML samples and cell lines with paired RNASeq and sphingolipidomic data. The area under the curve (AUC), its 95% confidence interval in brackets, and the 1-sided binomial test P-value (Pbinom) of the classifier are shown. (J-L) Kaplan-Meier plots for patients with AML inferred to be HexlowSMhigh (purple) or HexhighSMlow (green) in BeatAML (npurple = 102, ngreen = 72) (J), TCGA-AML (npurple = 67, ngreen = 84) (K), or the molecularly–defined intermediate-risk group combined for both BeatAML and TCGA-AML (npurple = 80, ngreen = 58) (L). Only patients with AML who received standard intensive induction chemotherapy were included in the analyses for both datasets. Log-rank P-values, Hazard ratio and 95% confidence interval in brackets are shown. The bottom of each plot shows risk tables for the 2 subtypes.
The HexlowSMhigh and HexhighSMlow AML subtypes differ in gene expression and survival outcome. (A-B) Kaplan-Meier plots of (EFS; npurple = 69, ngreen = 72) (A) and (OS; npurple = 70, ngreen = 72) (B) for patients with AML grouped into HexlowSMhigh (purple) and HexhighSMlow (green) subtypes. The study cohort comprised patients from 3 institutions (University of Virginia [UVA], Memorial Sloan Kettering [MSK], and Pennsylvania State University [PSU]). Patients who received intensive induction chemotherapy treatment were included in the analyses. Within each plot are the corresponding risk tables for the 2 groups. (C) Volcano plot of differentially expressed genes between the HexlowSMhigh and HexhighSMlow subtypes. Purple genes are upregulated in the HexlowSMhigh cluster whereas green genes are upregulated in the HexhighSMlow cluster (−log10Padj >1). (D) The HexlowSMhigh subtype is enriched for the LSC program. Gene set enrichment analysis score plot for an LSC signature of 104 genes.14 The y-axis is the running enrichment score along the ranked gene list. The enrichment score is the maximum deviation from 0 encountered along the list and represents the degree to which a gene set is overrepresented at the top or the bottom of the ranked gene list. The normalized enrichment score (NES) is the enrichment score normalized for variation in gene set sizes. The adjusted P-value (Padj) for the NES is shown. (E-F) The deconvolved HexlowSMhigh subtype is enriched for LSPCs and depleted in differentiated myeloid cell types. The CIBERSORTx log2-transformed absolute abundance score of total LSPCs (sum of quiescent, primed, and cycling LSPCs) (E) and total myeloid-lineage cells (sum of promono-like, mono-like, and monocyte) (F) is shown for the HexlowSMhigh (n = 217) and HexhighSMlow (n = 199) subtypes colored by study. Full deconvolution of leukemic cell types including the different LSPC and myeloid cell types is shown in supplemental Figure 3I and J. Differences between the 2 sphingolipid subtypes were assessed by a 2-sided Wilcoxon rank-sum test. (G-H) Frequency of CD34+CD38− cells between HexlowSMhigh and HexhighSMlow subtypes. Log10-transformed proportions of CD34+CD38− cells (G) and CD34+CD38−CD96+ cells (H) among 100 000 viable cells are shown for the HexlowSMhigh (n = 11) and HexhighSMlow (n = 12) subtypes. Differences between the 2 sphingolipid subtypes were assessed by a 1-sided Wilcoxon rank-sum test. (I) An RNASeq-based classifier accurately distinguishes sphingolipidomic subtypes. Receiver operating characteristics curve for a 284-gene support vector machine classifier applied to test data that includes both primary AML samples and cell lines with paired RNASeq and sphingolipidomic data. The area under the curve (AUC), its 95% confidence interval in brackets, and the 1-sided binomial test P-value (Pbinom) of the classifier are shown. (J-L) Kaplan-Meier plots for patients with AML inferred to be HexlowSMhigh (purple) or HexhighSMlow (green) in BeatAML (npurple = 102, ngreen = 72) (J), TCGA-AML (npurple = 67, ngreen = 84) (K), or the molecularly–defined intermediate-risk group combined for both BeatAML and TCGA-AML (npurple = 80, ngreen = 58) (L). Only patients with AML who received standard intensive induction chemotherapy were included in the analyses for both datasets. Log-rank P-values, Hazard ratio and 95% confidence interval in brackets are shown. The bottom of each plot shows risk tables for the 2 subtypes.
To associate broader transcriptional differences with the subtypes, we collected RNA-sequencing (RNASeq) data for 33 primary AML samples and 30 AML cell lines with sphingolipidomic profiles. We appended transcriptomes from additional AML cell lines available through the Cancer Cell Line Encyclopedia,15 which were batch-corrected and merged with 2 clinical RNASeq repositories for AML: TCGA-AML16 and BeatAML17 (supplemental Figure 3A-B; supplemental Tables 4-6). For the cell lines and primary samples with sphingolipidomics, we identified 734 increased transcripts in HexlowSMhigh and 1125 increased transcripts in HexhighSMlow (Figure 2C; supplemental Table 7), including 6 enzymes with complex roles in sphingolipid metabolism (UGCG, FUT4, NAGA, SMPD3, GANC, and B3GALT1 are discussed in supplemental Table 8). The clinically favorable HexhighSMlow subtype was enriched for hallmark gene sets related to immune activation (supplemental Figure 3C-D). In contrast, the clinically unfavorable HexlowSMhigh subtype was enriched for leukemic stem cell (LSC) signatures14,18-20 (Figure 2D and supplemental Figure 3E-H). We unmixed bulk transcriptomes computationally by CIBERSORTx21 using customized signatures for AML differentiation states22,23 and repopulating activities.23 HexlowSMhigh was highly enriched for leukemia stem-progenitor cell (LSPC) states with enriched repopulating activity, whereas HexhighSMlow was enriched for differentiated myeloid lineages (Figure 2E-F, supplemental Figure 3I-J). Next, we identified sphingolipidomics-profiled AML cases that had not been sequenced to be analyzed using flow cytometry. Statistical power was limited because comparatively few samples had enough remaining viable material for recovery from thaw and staining (supplemental Figure 3K), but we identified multiple HexlowSMhigh (n = 11) and HexhighSMlow (n = 12) samples to evaluate general trends. Standard flow paradigms for LSCs involved surprisingly few genes from the LSC gene signatures themselves, but CD34 was increased (log2 fold-change = 1.56, P = .02). Mean CD34 immunoreactivity was unchanged between sphingolipid subtypes (supplemental Figure 3L) and consistently lower rather than higher in CD34+ subsets of the HexlowSMhigh subtype (supplemental Figure 3M), arguing against overall changes in gene expression. In contrast, upon gating with standard LSC markers, we noted suggestive and specific increases in CD34+CD38−CD96+/− cells for the HexlowSMhigh subtype (Figure 2G-H and supplemental Figure 3N). Although more cases are needed, the available samples favored increased proportions of LSC-like cells in the HexlowSMhigh subtype. We concluded that the 2 sphingolipid subtypes were more coupled to transcriptomic states and differentiation status than AML driver mutations (supplemental Figure 1D), suggesting different disease mechanisms.
For inferring sphingolipidomic subtypes from transcriptional states alone, we developed a support vector-machine classifier of Hex-SM status using the 284 most variable and differential genes between subtypes (supplemental Table 9). When trained on 60% of the samples with paired transcriptomics and sphingolipidomics (including both primary cases and AML cell lines), the classifier showed excellent predictive performance on the remaining 40% of samples (Figure 2I). We then used the classifier with the batch-corrected transcriptomic data from TCGA-AML and BeatAML to infer sphingolipidomic subtypes. For both repositories, the classifier predicted a balanced proportion of HexlowSMhigh and HexhighSMlow cases, supporting that neither subtype is rare (supplemental Tables 4-5). Consistent with our independent cohort (supplemental Figure 2B-C), patients inferred to be HexlowSMhigh had significantly worse outcomes than those predicted to be HexhighSMlow (Figure 2J-K). Importantly, sphingolipid subtype remained an independent predictor of outcomes in a multivariable Cox regression analysis that included age, sex, white blood count, mutation statuses of NPM1 and FLT3, and study as covariates (supplemental Table 10). The HexhighSMlow subtype was enriched for patients in the molecularly–defined favorable or good group, the HexlowSMhigh subtype was enriched for the adverse or poor group, and the intermediate-risk group was not detectably skewed by subtype (supplemental Figure 4A-B). Thus, we stratified cases by risk group and examined whether the sphingolipid subtypes differed in their clinical outcomes. OS of HexlowSMhigh was similar to that of HexhighSMlow in the favorable or good risk group, slightly worse in the adverse or poor risk group, and significantly worse in the intermediate-risk group (Figure 2L and supplemental Figure 4C-D). This extension to public AML data sets strengthens the conclusion that HexlowSMhigh is a high-risk subtype with poor outcomes, especially for patients whose molecular risk classification is intermediate.
By examining AML from the perspective of sphingolipid metabolism, our work uncovers a stratification that eluded prior gene-based classifications.14,17,24 Sphingolipidomic subtypes are embedded in a fraction of the transcriptome that is not prominent when clustering is performed in an unsupervised manner. Indeed, the sphingolipid profiles of AML cell lines are much more concordant with primary samples (Figure 1C), unlike when their whole transcriptomes are coclustered (supplemental Figure 5A-C). The LSC programs in the high-risk HexlowSMhigh subtype are consistent with the importance of sphingolipid homeostasis in LSC maintenance.25 Our sphingolipid-guided transcript classifier could be useful for identifying patients who are least likely to benefit from intensive induction chemotherapy or stem-cell transplant and thus most eligible for experimental therapeutics. Stratification of the intermediate-risk subgroup provides an additional tool to suggest therapy in this highly heterogeneous population. Many patients with intermediate risk are offered transplants in first remission; thus, prognostic biomarkers are of great interest. Future studies should confirm these findings and investigate the pharmacologic vulnerabilities of the 2 subtypes, which presumably arise from complex differences in sphingolipid metabolism (supplemental Table 8). Given the promise of targeting sphingolipid biology in AML7,25 we envision that sphingolipidomic subtyping could contribute to tailored treatment selections for patients with AML who otherwise lack targetable alternatives.
Acknowledgments: The authors thank Samuel Haddox for help with RNA preparation for RNA sequencing (RNASeq), Emily Sullins for processing patient samples and cell lines, Galina Diakova for help with RNASeq data from the Oncology Research Information Exchange Network, UVA Biorepository and Tissue Research Facility (Research Resource Identifiers [RRID]: SCR_022971), Memorial Sloan Kettering Tissue Bank for assistance with sample processing, and the Flow Cytometry Core facility at the Penn State University College of Medicine (RRID: SCR_021134). The authors appreciate comments from Cameron Griffiths, Russell Hawes, and Kenley Ellis on the manuscript. This work was financially supported by the National Institutes of Health (NIH) under the National Cancer Institute (NCI) award number P01 CA171983 (to T.P.L. Jr, M.K., and M.C.), NIH/NCI Cancer Center support grant P30 CA044579 (to T.P.L. Jr), NCI R35 CA197594 (to R.L.L.), NIH/NCI Cancer Center support grant P30 CA008748 (to R.L.L.), NIH/NCI F31 CA271809 and UVA Robert R. Wagner Fellowship (to J.U.), National Science Foundation Graduate Research Fellowship NSF grant #1842490 (to W.A.F.), NCI K08 CA215317 and Edward P. Evans Foundation (to A.D.V.), NCI R03 CA252825 (to B.M.B.).
Contribution: B.B.P., S.-F.T., T.E.F., U.G., M.C., F.E.G.-B., M.K., D.C., D.J.F., K.A.J., and T.P.L. designed the research; B.B.P., S.-F.T., T.E.F., W.D., I.L., U.G., A.S., A.D.V., and D.C. performed the research; T.E.F. and F.E.G.-B. contributed in providing vital new reagents or analytical tools; S.-F.T., T.E.F., J.U., U.G., W.D., I.L., S.P., A.S., A.D.V., F.E.G.-B., D.C., and D.J.F. collected data; B.B.P., S.-F.T., T.E.F., J.U., U.G., J.J.P.S., W.A.F., B.M.B., M.S.T., M.C., F.E.G.-B., R.L.L., D.C., D.J.F., K.A.J., and T.P.L. analyzed and interpreted data; B.B.P., F.E.G.-B., and K.A.J. performed statistical analysis; B.B.P., S.-F.T., T.E.F., J.U., F.E.G.-B., D.J.F., K.A.J., and T.P.L. wrote the manuscript; all authors read and approved the manuscript.
Conflict-of-interest disclosure: A.D.V. is a scientific adviser to Arima Genomics. B.M.B. is the owner and founder of Tahosa Bio, LLC (Rapid City, SD). M.S.T. has received research funding from AbbVie, Orsenix, BioSight, Glycomimetics, Rafael Pharmaceuticals, and Amgen; is on the advisory boards for AbbVie, Daiichi-Sankyo, Orsenix, KAHR Medical, Oncolyze, Jazz Pharma, Roche, BioSight, Novartis, Innate Pharma, Kura, Syros Pharmaceuticals, Ipsen Biopharmaceuticals, Cellularity; has received royalties from UpToDate (for writing); is chair for the data and safety monitoring board for HOVON 156; is chair of the adjudication committee for Foghorn Therapeutics; has received honoraria from Northwell Health, Japan Society of Hematology, MetroHealth Cleveland, Ohio State University, American Society of Hematology; and is on the board for the American Society of Hematology. R.L.L. is on the supervisory board of Qiagen; is a scientific adviser to Imago, Mission Bio, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics, and Isoplexis; receives research support from Ajax and Zentalis; has consulted for Incyte, Janssen, AstraZeneca, and Novartis; and has received honoraria from AstraZeneca, Roche, Lilly, and Amgen for invited lectures and from Gilead for grant reviews. D.J.F. has received research funding, honoraria, and/or stock options from AstraZeneca, Dren Bio, Recludix Pharma, and Kymera Therapeutics. K.A.J. serves on the scientific advisory board of BridgeBio. T.P.L. has received scientific advisory board membership, consultancy fees, honoraria, and/or stock options from Keystone Nano, Flagship Labs 86, Dren Bio, Recludix Pharma, Kymera Therapeutics, and Prime Genomics. The remaining authors declare no competing financial interests.
Mark Kester died on 20 July 2022.
Correspondence: Thomas P. Loughran, Jr., Department of Medicine, Division of Hematology & Oncology, The University of Virginia School of Medicine, Charlottesville, VA, 22908; email: TL7CS@virginia.edu; and Kevin A. Janes, Department of Biomedical Engineering, Biochemistry & Molecular Genetics, The University of Virginia, Charlottesville, VA, 22908; email: kjanes@virginia.edu.
References
Author notes
RNA-sequencing data are available at the Gene Expression Omnibus (GSE229032; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE229032). Normalized lipidomics data are provided in supplemental Table 12. Some data on primary AML samples analyzed in this study (supplemental Table 11, Column D) are subject to restrictions set by the ORIEN network, and access is controlled by M2Gen and the ORIEN consortium. Requests to access these data sets should be directed to https://www.oriencancer.org/request-an-account.
The full-text version of this article contains a data supplement.

![The HexlowSMhigh and HexhighSMlow AML subtypes differ in gene expression and survival outcome. (A-B) Kaplan-Meier plots of (EFS; npurple = 69, ngreen = 72) (A) and (OS; npurple = 70, ngreen = 72) (B) for patients with AML grouped into HexlowSMhigh (purple) and HexhighSMlow (green) subtypes. The study cohort comprised patients from 3 institutions (University of Virginia [UVA], Memorial Sloan Kettering [MSK], and Pennsylvania State University [PSU]). Patients who received intensive induction chemotherapy treatment were included in the analyses. Within each plot are the corresponding risk tables for the 2 groups. (C) Volcano plot of differentially expressed genes between the HexlowSMhigh and HexhighSMlow subtypes. Purple genes are upregulated in the HexlowSMhigh cluster whereas green genes are upregulated in the HexhighSMlow cluster (−log10Padj >1). (D) The HexlowSMhigh subtype is enriched for the LSC program. Gene set enrichment analysis score plot for an LSC signature of 104 genes.14 The y-axis is the running enrichment score along the ranked gene list. The enrichment score is the maximum deviation from 0 encountered along the list and represents the degree to which a gene set is overrepresented at the top or the bottom of the ranked gene list. The normalized enrichment score (NES) is the enrichment score normalized for variation in gene set sizes. The adjusted P-value (Padj) for the NES is shown. (E-F) The deconvolved HexlowSMhigh subtype is enriched for LSPCs and depleted in differentiated myeloid cell types. The CIBERSORTx log2-transformed absolute abundance score of total LSPCs (sum of quiescent, primed, and cycling LSPCs) (E) and total myeloid-lineage cells (sum of promono-like, mono-like, and monocyte) (F) is shown for the HexlowSMhigh (n = 217) and HexhighSMlow (n = 199) subtypes colored by study. Full deconvolution of leukemic cell types including the different LSPC and myeloid cell types is shown in supplemental Figure 3I and J. Differences between the 2 sphingolipid subtypes were assessed by a 2-sided Wilcoxon rank-sum test. (G-H) Frequency of CD34+CD38− cells between HexlowSMhigh and HexhighSMlow subtypes. Log10-transformed proportions of CD34+CD38− cells (G) and CD34+CD38−CD96+ cells (H) among 100 000 viable cells are shown for the HexlowSMhigh (n = 11) and HexhighSMlow (n = 12) subtypes. Differences between the 2 sphingolipid subtypes were assessed by a 1-sided Wilcoxon rank-sum test. (I) An RNASeq-based classifier accurately distinguishes sphingolipidomic subtypes. Receiver operating characteristics curve for a 284-gene support vector machine classifier applied to test data that includes both primary AML samples and cell lines with paired RNASeq and sphingolipidomic data. The area under the curve (AUC), its 95% confidence interval in brackets, and the 1-sided binomial test P-value (Pbinom) of the classifier are shown. (J-L) Kaplan-Meier plots for patients with AML inferred to be HexlowSMhigh (purple) or HexhighSMlow (green) in BeatAML (npurple = 102, ngreen = 72) (J), TCGA-AML (npurple = 67, ngreen = 84) (K), or the molecularly–defined intermediate-risk group combined for both BeatAML and TCGA-AML (npurple = 80, ngreen = 58) (L). Only patients with AML who received standard intensive induction chemotherapy were included in the analyses for both datasets. Log-rank P-values, Hazard ratio and 95% confidence interval in brackets are shown. The bottom of each plot shows risk tables for the 2 subtypes.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/5/10.1182_bloodadvances.2023010535/2/m_blooda_adv-2023-010535-gr2.jpeg?Expires=1769083653&Signature=puNXa-BgMph0zqG~mOXp3~eznPCwPxOrZ52CsK-BNywh12dSx3O-Csf80htNQq5LHV0E0Czp9TKbtfiTLoiJeBnacDvOip7G82lUNsWOwPu9pWvRLrzov4Yycl0blfy3HEKKX~JxWtNelJHgvt7h36CgqRypZ6~086qgBxrooURijbGtOFKVMQTuJJxG8EPQnrzctLnVzfvDzl72GiKCvx1QOBhvdAsYu~pUCrz-gkCm4e5~-HOv4QLjn-YVWK4GPvEx5e1V3HUFe2XLd22EKE5KzzRZKckRkIDIcJ~b5WCyzdqNHZ5cuRs9m2nxtmmIFLLmydF5VLH6UBBTAbWWaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)