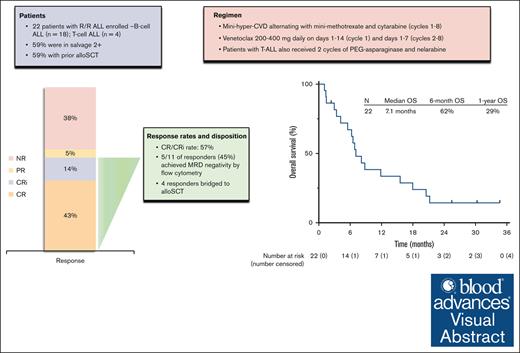
Low-intensity chemotherapy plus venetoclax was safe and active in heavily pretreated relapsed/refractory ALL.
The CR/CRi rate of 57% compares favorably with historical expectations with intensive chemotherapy in this population.
Visual Abstract
Preclinical studies suggest that Bcl-2 inhibition with venetoclax has antileukemic activity in acute lymphoblastic leukemia (ALL) and may synergize with conventional chemotherapy. We designed a phase 1/2 clinical trial to evaluate the safety and efficacy of low-intensity chemotherapy in combination with venetoclax in adults with relapsed or refractory ALL. Patients received the mini-hyper-CVD regimen (dose-attenuated hyperfractionated cyclophosphamide, vincristine, and dexamethasone alternating with methotrexate and cytarabine) in combination with venetoclax (200 mg or 400 mg daily) on days 1 to 14 in cycle 1 and on days 1 to 7 in consolidation cycles. Twenty-two patients were treated. The median number of prior therapies was 2 (range, 1-6). Thirteen patients (59%) had undergone prior allogeneic stem cell transplant (allo-SCT), and 7 of 18 patients (39%) with B-cell ALL had previously received both inotuzumab ozogamicin and blinatumomab. The recommended phase 2 dose of venetoclax in the combination regimen was 400 mg daily. The composite complete remission (CR) and CR with incomplete hematologic recovery (CRi) rate was 57% (CR, 43%; CRi, 14%), and 45% of responders achieved measurable residual disease negativity by multiparameter flow cytometry. Four patients proceeded to allo-SCT. The median duration of response was 6.3 months. The median overall survival was 7.1 months, and the 1-year overall survival rate was 29%. The most common grade ≥3 nonhematologic adverse events were infection in 17 patients (77%) and febrile neutropenia in 4 patients (18%). Overall, the combination of mini-hyper-CVD plus venetoclax was active in heavily pretreated relapsed/refractory ALL. Further development of venetoclax-based combinations in ALL is warranted. This trial is registered at www.clinicaltrials.gov as #NCT03808610.
Introduction
In the past decade, there have been several new advances in the treatment of relapsed/refractory acute lymphoblastic leukemia (ALL), including the approval of blinatumomab, inotuzumab ozogamicin (INO), and CD19 chimeric antigen receptor (CAR) T cells.1-4 Although blinatumomab and INO improve response rates and survival compared with conventional chemotherapy, the median overall survival (OS) when used as monotherapy is only 7 to 8 months. Better outcomes have been observed when both agents are combined; the combination of these agents with the mini-hyper-CVD regimen (dose-attenuated hyperfractionated cyclophosphamide, vincristine, and dexamethasone alternating with methotrexate and cytarabine) resulted in a median OS of 17 months and 3-year OS rate of 40%.5,6 However, the outcomes are dismal for patients after failure of INO and blinatumomab, with a median OS of only 3.8 months.7 Although there are several salvage options available for B-cell ALL, there are relatively fewer effective therapies for patients with relapsed/refractory T-cell ALL. Nelarabine is active in this setting as monotherapy, but the durations of remissions are short.8 Unlike in B-cell ALL, there are also no commercially available CAR T-cell therapies for patients with T-cell ALL.
B-cell leukemia/lymphoma-2 (Bcl-2) is an antiapoptotic protein overexpressed in many hematologic malignancies, including ALL.9 Venetoclax is a potent oral small molecular inhibitor of Bcl-2 that leads to activation of the intrinsic apoptotic pathway, ultimately resulting in cell death.10 Preclinically, venetoclax has activity against B-lymphoblasts and T-lymphoblasts both in vitro and in xenograft models derived from patients.11-14 Retrospective analyses in children and adults have also suggested efficacy of chemotherapy plus venetoclax combinations in heavily pretreated relapsed/refractory ALL.15-17 In 1 retrospective analysis of 13 patients with relapsed/refractory T-cell ALL who had received a median of 2 prior lines of therapy, venetoclax-based salvage therapy resulted in marrow remission (ie, bone marrow blasts < 5%) in 6 of 10 evaluable patients (60%), which translated to a median OS of 7.7 months.15 Based on the preclinical and clinical activity of venetoclax in ALL, we developed a phase 1/2 clinical trial to evaluate the combination of mini-hyper-CVD in combination with venetoclax in patients with relapsed/refractory ALL.
Methods
Study design and participants
This was a single-center, phase 1/2 study to assess the efficacy and safety of the combination of the mini-hyper-CVD regimen (dose-attenuated hyperfractionated cyclophosphamide, vincristine, and dexamethasone, alternating with methotrexate and cytarabine) in combination with venetoclax in patients with relapsed or refractory ALL. This study was conducted at a single academic center (The University of Texas MD Anderson Cancer Center) and approved by the Institutional Review Board of the university. All patients provided informed consent according to institutional guidelines, and the study was conducted according to the Declaration of Helsinki.
Patients aged ≥18 years who had relapsed/refractory ALL after any number of prior therapies were eligible. Additional eligibility criteria included: Eastern Cooperative Oncology Group performance status 0 to 3; total bilirubin ≤2× upper limit of normal; alanine aminotransferase and aspartate aminotransferase (AST) ≤3× upper limit of normal; and creatinine clearance ≥30 mL per minute. Key exclusion criteria were: Philadelphia chromosome-positive ALL or Burkitt leukemia, prior treatment with venetoclax, history of significant bleeding disorder unrelated to cancer, left ventricular ejection fraction <40%, and use of moderate or strong cytochrome P450 3A4 (CYP3A4) inducers or strong CYP3A4 inducers within 7 days.
Treatment regimen
Patients received the mini-hyper-CVD regimen at doses that have been previous reported.18 Briefly, patients received mini-hyper-CVD on cycles 1, 3, 5, and 7, as well as mini-methotrexate and cytarabine on cycles 2, 4, 6, and 8. In cycle 1, patients received venetoclax orally with ramp-up to a maximum dose of 200 mg to 400 mg (determined by the dose level in phase 1) on days 1 to 14. For cycles 2 to 8, patients received venetoclax orally at the assigned dose on days 1 to 7. The venetoclax dose was decreased by 50% with moderate CYP3A4 inhibitors or p-glycoprotein inhibitors and by 75% with strong CYP3A4 inhibitors (except for posaconazole, in which the dose was reduced by ∼83%, eg, from 400 mg to 70 mg). Patients with CD20 expression ≥20% by flow cytometry also received rituximab 375 mg/m2 IV, with 2 doses administered per cycle, up to 8 total doses. Patients were recommended to receive intrathecal prophylaxis with alternating doses of cytarabine 100 mg and methotrexate 12 mg, with 2 doses administered per cycle, up to 8 total doses. Pegfilgrastim 6 mg subcutaneously was given on ∼day 5 of each mini-hyper-CVD or mini-methotrexate and cytarabine cycle after the completion of chemotherapy. Cycles were intended to be given every 21 to 28 days, depending on peripheral blood count recovery. Patients with T-cell ALL also received additional cycles of nelarabine and PEGylated asparaginase (PEG-asparaginase), given in cycles 4N and 5N (intercalated after cycle 4 and cycle 5, respectively, of the mini-hyper-CVD regimen). Nelarabine was given at a dose of 650 mg/m2 IV daily on days 1 to 5, and PEG-asparaginase was given on day 5 at a dose of 1500 IU/m2 (capped at 3750 IU) in patients aged <60 years and at a dose of 1000 IU/m2 (capped at 2000 IU) in patients aged ≥60 years.
Responding patients who did not proceed to allogeneic stem cell transplantation (allo-SCT) could receive a maintenance regimen of vincristine 2 mg IV on day 1, prednisone 50 mg orally on days 1 to 5, and venetoclax orally (at the assigned dose) on days 1 to 14, with cycles intended to be given every 28 days. Patients with T-cell ALL could receive 2 additional cytarabine of nelarabine and PEG-asparaginase during cycles 6 and 7 of maintenance. Maintenance was given for up to 24 cycles.
Outcomes
The primary objective of the phase 1 portion of the study was to define the maximum tolerated dose of venetoclax and dose-limiting toxicities of the combination regimen. The primary objective of the phase 2 portion of the study was to determine the combined rate of complete remission (CR) and CR with incomplete hematologic recovery (CRi). Secondary objectives include determination of the measurable residual disease (MRD) negativity rate by multiparameter flow cytometry, duration of response, relapse-free survival (RFS), OS, and the safety of the regimen.
CR was defined as <5% blasts in the bone marrow with an absolute neutrophil count of ≥1 × 109/L and a platelet count of ≥100 × 109/L, along with complete resolution of all sites of extramedullary disease. CRi was defined as the same as CR, but without complete peripheral blood count recovery. MRD assessment with a sensitivity of 10-4 was performed on fresh bone marrow aspiration samples using multiparameter flow cytometry as described previously.19 RFS was calculated from the time of response until relapse or death from any cause, censored if alive at last follow-up. OS was calculated from the time of treatment initiation until death from any cause, censored if alive at last follow-up. Safety was assessed with the Common Terminology Criteria for Adverse Events version 4.03.
Statistical analysis
The phase 1 portion of the study was conducted using a standard “3 + 3” design and evaluated venetoclax at a dose of 200 mg (dose level 0) and 400 mg (dose level +1). This was followed by a phase 2 portion of the study using the recommended phase 2 dose of venetoclax. Interim monitoring rules for efficacy and toxicity were used throughout the phase 2 portion. The study was continuously monitored for efficacy and treatment-related toxicities using a Bayesian design.20,21 The regimen was considered promising if the CR/CRi rate was ≥50% and the grade 4 to 5 drug-related toxicity rate was <20%.
Patient characteristics were summarized using the median (range) for continuous variables and frequencies (percentages) for categorical variables. Remission duration, RFS, and OS were calculated with Kaplan-Meier estimates, and survival estimates were compared with the log-rank test. The data cutoff for this analysis was 1 March 2023. The data analyses were done using GraphPad Prism 9. This study was registered at www.clinicaltrials.gov as #NCT03808610.
Results
Patient characteristics
Between June 2019 and February 2021, a total of 22 patients with relapsed/refractory ALL were treated (Table 1). The median age was 46 years (range, 20-70). Eighteen patients (82%) had B-cell ALL, and 4 patients (18%) had T-cell ALL. One patient with T-cell ALL had an early T-cell precursor immunophenotype. Thirteen patients (59%) were in salvage 2 or beyond, and 13 patients (59%) had undergone prior allo-SCT. Among patients with B-cell ALL, 89% had received prior blinatumomab, 39% had received prior INO (all of whom had also received prior blinatumomab), and 17% had received prior CAR T-cell therapy. Overall, 6 of 17 tested patients (35%) had a detectable TP53 mutation. Three patients had findings consistent with Philadelphia chromosome-like ALL (2 with CRLF2 overexpression by flow cytometry and 1 with NUP214-ABL1 rearrangement).
Baseline characteristics of the study population
| Characteristic . | n (%) or median [range] . |
|---|---|
| Age, y | 46 [20-70] |
| ≥60 y | 4 (18) |
| ALL subtype | |
| T-cell ALL | 4 (18) |
| B-cell ALL | 18 (82) |
| Ph-like | 3/18 (33) |
| Isolated extramedullary disease | 4 (18) |
| Baseline blood parameters | |
| White blood cells (×109/L) | 3.6 [0.1-19.5] |
| Hemoglobin (g/dL) | 9.4 [7.8-12.4] |
| Platelets (×109/L) | 32 [9-257] |
| Bone marrow blasts (%) | 60 [0-87] |
| Cytogenetics | 7/22 (32) |
| Diploid | 3/22 (14) |
| Complex | 3/22 (14) |
| Triploid t(4;11) | 1/22 (4) |
| Miscellaneous | 3/22 (14) |
| Insufficient metaphases/not done | 5/22 (23) |
| TP53 mutation | 5/22 (23) |
| Median prior lines of therapy | 2 [1-6] |
| B-cell ALL | 3 [1-6] |
| Prior INO | 7/18 (39) |
| Prior blinatumomab | 16/18 (89) |
| Prior INO and blinatumomab | 7/18 (39) |
| Prior CD19 CAR T cells | 3/18 (17) |
| Prior allo-SCT | 12/18 (67) |
| T-ALL | 1 [1-3] |
| Prior nelarabine | 1/4 (25) |
| Prior allo-SCT | 1/4 (25) |
| Characteristic . | n (%) or median [range] . |
|---|---|
| Age, y | 46 [20-70] |
| ≥60 y | 4 (18) |
| ALL subtype | |
| T-cell ALL | 4 (18) |
| B-cell ALL | 18 (82) |
| Ph-like | 3/18 (33) |
| Isolated extramedullary disease | 4 (18) |
| Baseline blood parameters | |
| White blood cells (×109/L) | 3.6 [0.1-19.5] |
| Hemoglobin (g/dL) | 9.4 [7.8-12.4] |
| Platelets (×109/L) | 32 [9-257] |
| Bone marrow blasts (%) | 60 [0-87] |
| Cytogenetics | 7/22 (32) |
| Diploid | 3/22 (14) |
| Complex | 3/22 (14) |
| Triploid t(4;11) | 1/22 (4) |
| Miscellaneous | 3/22 (14) |
| Insufficient metaphases/not done | 5/22 (23) |
| TP53 mutation | 5/22 (23) |
| Median prior lines of therapy | 2 [1-6] |
| B-cell ALL | 3 [1-6] |
| Prior INO | 7/18 (39) |
| Prior blinatumomab | 16/18 (89) |
| Prior INO and blinatumomab | 7/18 (39) |
| Prior CD19 CAR T cells | 3/18 (17) |
| Prior allo-SCT | 12/18 (67) |
| T-ALL | 1 [1-3] |
| Prior nelarabine | 1/4 (25) |
| Prior allo-SCT | 1/4 (25) |
Ph-like, Philadelphia like.
Phase 1 results
Nine patients (7 patients with B-cell ALL and 2 patients with T-cell ALL) were enrolled into the phase 1 portion of the study (3 patients at dose level 1 with venetoclax 200 mg and 6 patients at dose level 2 with venetoclax 400 mg). No dose-limiting toxicities were observed at either of the venetoclax doses that were evaluated. Two of the 3 patients in the 200 mg cohort and 3 of the 6 patients in the 400 mg cohort achieved CR. Given the safety and efficacy in the 400 mg cohort, venetoclax at a dose of 400 mg daily was chosen as the recommended phase 2 dose for further study.
Response rates and treatment intensity
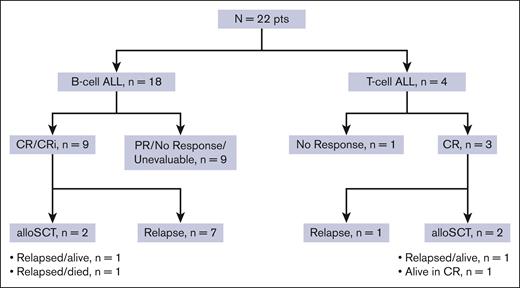
Twenty-one patients were evaluable for response; 1 patient was unevaluable due to receiving radiation for extramedullary disease during cycle 1. Among the 21 evaluable patients, 9 patients (43%) achieved CR, and 3 patients (14%) achieved CRi, resulting in composite CR/CRi rate of 57% (Figure 1). One additional patient with extramedullary-only disease achieved a partial response. CR/CRi rates were similar across subgroups analyzed. CR/CRi was achieved in 9 of 17 (53%) with B-cell ALL and 3 of 4 (75%) with T-cell ALL; in 5 of 9 (56%) in salvage 1 and 7 of 12 (58%) in salvage 2 and beyond; in 7 of 12 (58%) with prior allo-SCT; and in 4 of 7 (57%) who had previously received both blinatumomab and INO. Among the 12 patients who achieved CR/CRi as best response, 9 patients (75%) achieved best response after cycle 1 and 3 patients (25%) after cycle 2. Among the 3 patients who achieved best response after cycle 2, a total of 2 had achieved partial response with cycle 1. Five of 11 responding patients with bone marrow involvement at the time of enrollment (45%) achieved MRD negativity by multiparameter flow cytometry.
The median number of cycles of the mini-hyper-CVD plus venetoclax regimen received was 2 (range, 1-4 cycles). Eight patients (36%) received 1 cycle, 7 patients (32%) received 2 cycles, 4 patients (18%) received 3 cycles, and 3 patients (14%) received 4 cycles. No patients proceeded to maintenance therapy. Among 4 patients with T-cell ALL, 3 patients received at least 1 cycle of nelarabine plus PEG-asparaginase.
Disposition and survival outcomes
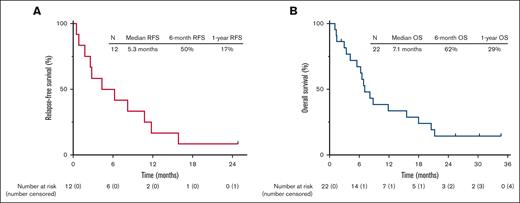
The median follow-up is 26 months (range, 1.1-35). Four patients (18% overall and 33% of the patients achieving CR/CRi) proceeded to allo-SCT (Figure 1). Among the 12 responding patients, 11 patients subsequently relapsed, with a median duration of response of 6.3 months (range, 0.5-30). Among the 4 patients who underwent allo-SCT, 3 relapsed. As of the last follow-up, 18 patients (82%) have died. One patient is still alive and in continuous remission at the last follow-up with a remission duration of 30 months. This patient had T-cell ALL, was treated in first salvage, achieved MRD negativity by flow cytometry after 2 cycles of protocol therapy, and underwent allo-SCT after 4 cycles of therapy. The median RFS was 5.3 months, and the 1-year RFS rate was 17% (Figure 2A). The median OS was 7.1 months, and the 1-year OS rate was 29% (Figure 2B). For the 12 responders, the median OS was 8.5 months, and the 1-year OS was 42% (supplemental Figure 1). RFS and OS were similar in patients in salvage 1 vs salvage 2 or beyond (supplemental Figure 2). For patients with exposure to prior INO and blinatumomab (n = 7), the median OS was 7.1 months, and 1-year OS was 29% (supplemental Figure 3).
Safety
All patients who received at least 1 dose of study therapy were eligible for safety analysis. Nonhematologic adverse events across both cohorts are shown in Table 2. The most common grade ≥3 nonhematologic adverse events were infection in 17 patients (77%) and febrile neutropenia in 4 patients (18%). The types of infection were pneumonia in 8 patients (3 bacterial, 3 fungal, and 2 unspecified), line-associated bacteremia, upper respiratory infection, skin/soft tissue infection, and disseminated fungal infection in 2 patients each, and possible infectious hepatitis in 1 patient. All patients were receiving antibacterial, antifungal, and antiviral prophylaxis at the time of the infection. There were 2 on-study deaths (both due to disseminated fungal infection). The 30-day and 60-day mortality rates were 0% and 14%, respectively. Among the responding patients, the median time in cycle 1 to absolute neutrophil counts >0.5 × 109/L and >1 × 109/L were 14 and 18 days, respectively, and to platelet counts >50 × 109/L and >100 × 109/L were 14 and 26 days, respectively. Five patients (23%) had at least 1 dose reduction or interruption of venetoclax (all due to myelosuppression). One patient discontinued therapy due to treatment-related toxicity; this patient achieved CR as best response but came off study due to prolonged neutropenia and thrombocytopenia after cycle 3.
Treatment emergent nonhematologic adverse events occurring in at least 10% of patients and all grade 3 or higher events
| Adverse events . | Grade 1-2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| Abdominal distension | 3 (14) | 0 | 0 | 0 |
| Abdominal pain | 6 (27) | 0 | 0 | 0 |
| ALT increased | 2 (9) | 2 (9) | 0 | 0 |
| Anorexia | 8 (36) | 0 | 0 | 0 |
| Anxiety | 5 (23) | 0 | 0 | 0 |
| Arthralgia | 6 (27) | 0 | 0 | 0 |
| Bone pain | 2 (9) | 1 (5) | 0 | 0 |
| Bruising | 5 (23) | 0 | 0 | 0 |
| Chills | 4 (18) | 1 (5) | 0 | 0 |
| Constipation | 8 (36) | 2 (9) | 0 | 0 |
| Cough | 3 (14) | 1 (5) | 0 | 0 |
| Depression | 3 (14) | 0 | 0 | 0 |
| Diarrhea | 3 (14) | 2 (9) | 0 | 0 |
| Dizziness | 5 (23) | 0 | 0 | 0 |
| Dysphagia | 3 (14) | 0 | 0 | 0 |
| Epistaxis | 2 (9) | 1 (5) | 0 | 0 |
| Fatigue | 2 (9) | 2 (9) | 0 | 0 |
| Febrile neutropenia | 0 | 4 (18) | 0 | 0 |
| Hyperbilirubinemia | 3 (14) | 1 (5) | 1 (5) | 0 |
| Hyperkalemia | 2 (9) | 1 (5) | 0 | 0 |
| Hyponatremia | 0 | 1 (5) | 0 | 0 |
| Infection | 2 (9) | 13 (59) | 2 (9) | 2 (9) |
| Insomnia | 4 (18) | 0 | 0 | 0 |
| Mucositis oral | 1 (5) | 2 (9) | 0 | 0 |
| Myalgia/muscle weakness | 5 (23) | 1 (5) | 0 | 0 |
| Sepsis | 0 | 1 (5) | 0 | 0 |
| Thromboembolic event | 0 | 1 (5) | 0 | 0 |
| Tumor lysis syndrome | 0 | 1 (5) | 0 | 0 |
| Adverse events . | Grade 1-2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| Abdominal distension | 3 (14) | 0 | 0 | 0 |
| Abdominal pain | 6 (27) | 0 | 0 | 0 |
| ALT increased | 2 (9) | 2 (9) | 0 | 0 |
| Anorexia | 8 (36) | 0 | 0 | 0 |
| Anxiety | 5 (23) | 0 | 0 | 0 |
| Arthralgia | 6 (27) | 0 | 0 | 0 |
| Bone pain | 2 (9) | 1 (5) | 0 | 0 |
| Bruising | 5 (23) | 0 | 0 | 0 |
| Chills | 4 (18) | 1 (5) | 0 | 0 |
| Constipation | 8 (36) | 2 (9) | 0 | 0 |
| Cough | 3 (14) | 1 (5) | 0 | 0 |
| Depression | 3 (14) | 0 | 0 | 0 |
| Diarrhea | 3 (14) | 2 (9) | 0 | 0 |
| Dizziness | 5 (23) | 0 | 0 | 0 |
| Dysphagia | 3 (14) | 0 | 0 | 0 |
| Epistaxis | 2 (9) | 1 (5) | 0 | 0 |
| Fatigue | 2 (9) | 2 (9) | 0 | 0 |
| Febrile neutropenia | 0 | 4 (18) | 0 | 0 |
| Hyperbilirubinemia | 3 (14) | 1 (5) | 1 (5) | 0 |
| Hyperkalemia | 2 (9) | 1 (5) | 0 | 0 |
| Hyponatremia | 0 | 1 (5) | 0 | 0 |
| Infection | 2 (9) | 13 (59) | 2 (9) | 2 (9) |
| Insomnia | 4 (18) | 0 | 0 | 0 |
| Mucositis oral | 1 (5) | 2 (9) | 0 | 0 |
| Myalgia/muscle weakness | 5 (23) | 1 (5) | 0 | 0 |
| Sepsis | 0 | 1 (5) | 0 | 0 |
| Thromboembolic event | 0 | 1 (5) | 0 | 0 |
| Tumor lysis syndrome | 0 | 1 (5) | 0 | 0 |
Discussion
In this study of mini-hyper-CVD chemotherapy combined with venetoclax in patients with relapsed/refractory ALL, a CR/CRi rate of 57% and median OS of 7.1 months were achieved. Encouraging response rates were observed even in patients with poor-risk features, including those in second or later salvage, those who had undergone prior allo-SCT, and those with prior exposure to INO and blinatumomab. These findings compare favorably with historical expectations of conventional chemotherapy in this population, suggesting a possible benefit of venetoclax in this setting.
Although outcomes have improved for patients with relapsed/refractory B-cell ALL due to the development of INO, blinatumomab, and CD19 CAR T cells, novel therapies are still needed, and outcomes are particularly poor for those who have already been exposed to these agents.1-4 In 1 report, the CR/CRi rate to salvage therapy in patients who had previously received INO and blinatumomab was only 30%, and the median OS after INO and blinatumomab failure was 3.8 months overall and 4.7 months in patients who received subsequent ALL-directed therapy. For patients with relapsed/refractory T-cell ALL, there are no approved immune-based strategies or CAR T cells, and thus, effective salvage strategies are particularly limited. In this setting, nelarabine monotherapy achieves a CR/CRi of 31% and a median OS of <5 months.8 The response rates and survival outcomes observed in this study appear superior to these historical expectations. Notably, in patients with prior INO and blinatumomab exposure, the mini-hyper-CVD plus venetoclax regimen yielded response rates and survival outcomes that mirrored that of the general trial population, suggesting that this regimen may offer a valuable treatment option even after failure of the available commercial therapies.
In the absence of a randomized study, it is challenging to determine the potential benefit of venetoclax to the chemotherapy backbone; however, comparisons with conventional chemotherapy (without venetoclax) in this setting are informative. In the randomized INO-VATE and TOWER studies, which lead the approvals of INO and blinatumomab, respectively, for relapsed/refractory B-cell ALL, the chemotherapy control arm resulted in CR/CRi rates of 25% to 29% and median OS of 4.0 to 6.7 months.1,2 Acknowledging the limits of such cross-trial comparisons, the results obtained with the mini-hyper-CVD plus venetoclax regimen compare favorably with those achieved with conventional chemotherapy alone and suggest a possible benefit to the addition of venetoclax to a standard chemotherapy backbone.
Our results are consistent with several retrospective studies suggesting promising clinical activity with venetoclax-based combinations in ALL.15-17 Venetoclax has also been explored in a few other small prospective clinical trials. In a study of mini-hyper-CVD plus venetoclax, the combination was evaluated in older adults with newly diagnosed ALL and in those with relapsed/refractory disease.22 In an interim analysis, the CR/CRi rates in these 2 cohorts were 9 of 10 (90%) and 3 of 8 (38%), respectively. In another study, venetoclax was combined with navitoclax, a Bcl-2/Bcl-xL inhibitor, and asparaginase in children and adults with relapsed/refractory ALL.23 In this phase 1 study in a relatively young population (median age, 29 years), the CR/CRi rate was 60%, and the median OS was 7.8 months, results that are similar to those observed in our study. Importantly, this study also found that most of the evaluated cases of relapsed/refractory ALL, including both B-cell and T-cell ALL, were dependent on Bcl-2 and/or Bcl-xL, a finding that supports the development of a dual strategy with venetoclax and navitoclax. Based on the encouraging data observed from our study, as well as the translational and clinical data supporting the antileukemic activity of navitoclax in ALL, we have now amended this study to evaluate the combination of mini-hyper-CVD, venetoclax, and navitoclax in both relapsed/refractory ALL and in older adults with ALL who are not suitable for intensive chemotherapy.
In this study, no new safety signals were observed. The regimen was myelosuppressive, as has been consistently observed in studies of the combination of chemotherapy and venetoclax in acute myeloid leukemia,24-27 and infections were common, including grade ≥3 infections in 77% of patients. Therefore, despite the “low-intensity” chemotherapy backbone, it is imperative that patients’ laboratory parameters are monitored carefully when receiving this regimen, and dose reductions of venetoclax are to be performed in patients exhibiting excessive myelosuppression (as was required in 23% of patients in the present study). However, despite the risk of myelosuppression, it is notable that the CR rate was 43%, and most patients who responded to the regimen experienced full hematologic recovery.
In conclusion, the combination of mini-hyper-CVD with venetoclax in patients with relapsed/refractory ALL resulted in encouraging response rates and survival outcomes in this heavily pretreated population. These data support the further development of venetoclax-based combination strategies in ALL. An expansion of this study incorporating the Bcl-xL inhibitor navitoclax will attempt to further improve on these outcomes.
Acknowledgments
This research is supported in part by The University of Texas MD Anderson Cancer Center Leukemia SPORE CA100632 and the National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA016672.
Authorship
Contribution: N.J.S. and E.J. designed the study, enrolled patients, collected and analyzed the data, and wrote the manuscript; H.K., F.R., N.J., F.-G.H., N.P., M.O., W.G.W., G.M-B., G.B., R.A., M.Z., and M.K. enrolled patients; J.S., L.N., and R.G. collected and analyzed the data and performed statistical analyses; L.H. and X.H. performed statistical analyses; Z.L., Y.-C.H., J.J.Y., and L.X. performed genomic and pathological analyses; and all authors reviewed and edited the manuscript and approved of the final version.
Conflict-of-interest disclosure: E.J. has received research funding and honoraria from AbbVie. M.K. has received research funding from AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Nicholas J. Short, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; email: nshort@mdanderson.org; and Elias Jabbour, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; email: ejabbour@mdanderson.org.
References
Author notes
N.J.S. and E.J. contributed equally to this manuscript.
The data sets used and/or analyzed during this study are available upon reasonable request from the corresponding authors, Nicholas J. Short (nshort@mdanderson.org) and Elias Jabbour (ejabbour@mdanderson.org).
The full-text version of this article contains a data supplement.