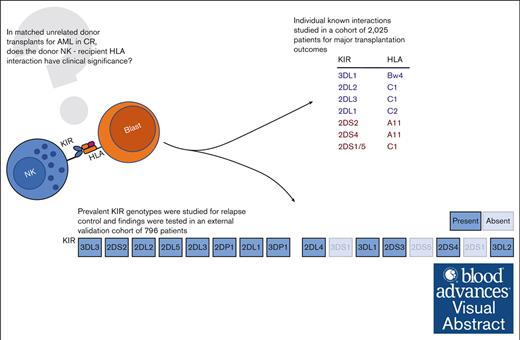
Individual donor-KIR and recipient-HLA interactions were not associated with outcomes of MUD HCT for AML.
Three prevalent donor KIR genotypes were differentially associated with relapse risk in 1 large cohort but not in a validation group.
Visual Abstract
In acute myeloid leukemia (AML), donor natural killer cell killer immunoglobulin–like receptors (KIR) and recipient HLA interactions may contribute to the graft-versus-leukemia effect of allogeneic hematopoietic cell transplantation (HCT). Analyses of individual KIR/HLA interactions, however, have yielded conflicting findings, and their importance in the HLA-matched unrelated donor (MUD) setting remains controversial. We systematically studied outcomes of individual donor-KIR/recipient-HLA interactions for HCT outcomes and empirically evaluated prevalent KIR genotypes for clinical benefit. Adult patients with AML (n = 2025) who received HCT with MUD grafts in complete remission reported to the Center for International Blood and Marrow Transplantation were evaluated. Only the donor-2DL2+/recipient-HLA-C1+ pair was associated with reduced relapse (hazard ratio [HR], 0.79; 95% confidence interval [CI], 0.67-0.93; P = .006) compared with donor-2DL2–/recipient-HLA-C1+ pair. However, no association was found when comparing HLA-C groups among KIR-2DL2+–graft recipients. We identified 9 prevalent donor KIR genotypes in our cohort and screened them for association with relapse risk. Genotype 5 (G5) in all recipients and G3 in Bw4+ recipients were associated with decreased relapse risk (HR, 0.52; 95% CI, 0.35-0.78; P = .002; and HR, 0.32; 95% CI, 0.14-0.72; P = .006; respectively) and G2 (HR 1.63, 95% CI, 1.15-2.29; P = .005) with increased relapse risk in C1-homozygous recipients, compared with other patients with the same ligand. However, we could not validate these findings in an external data set of 796 AML transplants from the German transplantation registry. Neither a systematic evaluation of known HLA-KIR interactions nor an empiric assessment of prevalent KIR genotypes demonstrated clinically actionable associations; therefore, these data do not support these KIR-driven strategies for MUD selection in AML.
Introduction
Allogeneic hematopoietic stem cell transplantation (HCT) has been described as “Janus-faced,” with the desirable graft-versus-leukemia (GVL) effect inseparable from the morbidity and mortality of its alter ego, graft-versus-host disease (GVHD).1 Separating GVL from GVHD, thus permitting disease control with less toxicity, has been a core mission of transplantation science over the past half-century. Among patients with acute myeloid leukemia (AML), previous studies held out the promise of natural killer (NK) cell–driven GVL without GVHD by using the presence of individual killer immunoglobulin-like receptor (KIR) genes to optimize donor selection.2-4 Activating and inhibiting signals from the interactions of donor NK cell KIRs and target cell-surface antigens (including classical and nonclassical HLA) guide NK cell cytotoxicity. Previous analyses have suggested that choosing donors based on KIR genotyping to accentuate this effect may increase leukemia cell death and have a clinically relevant impact on HCT outcome.5 However, initial, promising results linking individual donor KIR–recipient HLA interactions or groups of donor KIR with relapse and mortality after HCT have been inconsistently replicated.6,7 Recent work has proposed that these interactions are best treated as summative rather than independent,8,9 although how NK cells integrate multiple KIR signals remains incompletely understood.8,10
In this analysis, we studied, to our knowledge, the largest extant cohort of patients with AML in complete remission (CR) who underwent HCT with available KIR genotyping to systematically characterize outcomes associated with individual, known HLA-KIR interactions. We then took an empiric and pragmatic approach to identify clinically relevant KIR sets, which may act synergistically by evaluating the differential risk of relapse among patients treated with the most prevalent KIR genotypes in the cohort. We framed our analysis from the recipient (patient) perspective, interrogating the role of donor KIRs in the setting of known recipient HLA. Although several other analyses have looked from the perspective of the donor KIRs, we believe that the opposite outlook approximates the clinical question. The recipient HLA is fixed, whereas the donor can be chosen; thus, we asked whether the selection of an HLA-matched unrelated donor (MUD) by consideration of their KIR repertoire can optimize patient outcomes.
Methods
To define the clinical significance of donor KIR–recipient HLA interactions in HCT for AML, we analyzed a retrospective cohort of adult patients whose transplantation data had been reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). This cohort has been described previously by Weisdorf et al.11 The CIBMTR includes reporting from >450 transplantation centers internationally, using standardized processes and forms to collect data on patient and donor demographics, transplantation strategies, and outcomes. KIR genotyping was performed by the National Marrow Donor Program through retrospective typing at the presence/absence level.12 Patients and donors provided consent for data collection and research use with approval by participating institutions and the National Marrow Donor Program institutional review board.
Patients and donors
We included patients aged ≥18 years with AML in CR who had donor KIR genotyping available through the registry and who underwent their first HCT between 2010 and 2016 from unrelated donors matched for HLA alleles A, B, C, and DRB1. An independent validation cohort from the German Registry for Stem Cell Transplantation (DRST) comprised patients with similar inclusion and exclusion criteria but who underwent transplantation between 2005 and 2017, with donor KIR genotyping performed as previously described.6
End points
Overall survival (OS) was defined as the interval between graft infusion and death or last reported follow-up. Nonrelapse mortality (NRM) and relapse were defined as the time until death from any cause, with relapse as competing event, and time until relapse, with NRM as competing event, respectively. Leukemia-free survival (LFS) was defined as the time until either relapse or NRM and was censored at last follow-up. Acute GVHD severity was defined using the CIBMTR scale.13 Chronic GVHD incidence was defined by the incidence of chronic GVHD of any severity.
Statistical analysis
First, known donor-KIR–recipient-HLA ligand pairs were studied for each of the traditional transplantation outcomes: OS, NRM, relapse, LFS, severe acute GVHD (grade ≥3), and chronic GVHD. We included receptor-ligand pairs previously cataloged by Cooley et al.5 Adjustment covariates included for each model were chosen using backward selection from traditional transplantation variables including recipient age, Karnofsky performance status, disease status (first vs subsequent CR), conditioning intensity (myeloablative vs reduced intensity), recipient cytomegalovirus serostatus, receipt of in vivo T-cell depletion (either antithymocyte globulin [ATG] or alemtuzumab), donor age, female-to-male sex mismatch, time from diagnosis to transplantation, and transplantation year. The Cox proportional hazards model was used to determine the association of donor-KIR–recipient-HLA pairs with each outcome. A z score was calculated by dividing the regression coefficient for the main effect in each model by its standard error, and polar coordinate plots were constructed to graphically depict multiple outcomes simultaneously. Kaplan-Meier curves were constructed for each studied pair and outcome.
Next, an unbiased approach based on evaluation of prevalent donor KIR genotypes was adopted to uncover combinations of KIRs or KIR-HLA correlated with clinically meaningful alteration in relapse incidence. KIR genotypes (ie, the sum total of KIR alleles in an individual donor graft) were ranked based on their prevalence in the cohort, and those identified in >50 cases were studied. The most prevalent, corresponding to haplotype AA, served as baseline for a screening Cox regression model including each of the prevalent genotypes as well as the regression covariates previously selected. The centromeric-B/B genotype was similarly evaluated.14 Genotypes were compared across all recipients as well as in HLA-B– and HLA-C–defined subsets (ie, Bw4-homozygous, Bw4/Bw6, etc). KIR genotypes and genotype-HLA pairs significantly associated with differential relapse in this screening were selected and studied using Cox regression and Kaplan-Meier plots in comparison to all other patients. External validity was then assessed on an independent DRST cohort.
The proportional hazard assumption was tested for each model by graphical assessment of Schoenfeld residuals, and models were stratified by variables violating the assumption.15 Interactions between receptor-ligand pairs and conditioning intensity and in vivo T-cell depletion were studied. In the first phase, a P value < .0083 was considered significant, corresponding to a Bonferroni correction for 6 tests, the number of outcomes studied. In the second phase (KIR genotypes), analyses were considered hypothesis generating and a threshold of P < .05 was considered significant. Multiple testing in the second phase was further constrained by validation of each finding on an independent cohort. In both phases, interactions were deemed significant at a corrected P value < .025.
Results
Patient and donor characteristics
A total of 2025 patient-donor pairs were included. Patients had a median age at transplantation of 57 years, and the majority (n = 1587; 78%) received transplantation in first CR (Table 1). Overall, 1189 (59%) received myeloablative conditioning and 1791 (88%) received tacrolimus-based GVHD prophylaxis. A minority of patients (n = 802; 40%) received in vivo T-cell depletion. Grafts were matched for the DQB1 locus (ie, 10/10 HLA) in 95% of cases (n = 1918), and the median donor age was 28 years (interquartile range [IQR], 23-35). KIR haplotype AA grafts were used for 649 cases (32%). The median survival was 52 months (95% confidence interval [CI], 44-58), and the median follow-up was 48 months (IQR, 35-64).
Population characteristics
| Characteristic . | N (%)/median [IQR] . | P∗ . | |
|---|---|---|---|
| CIBMTR cohort . | DRST cohort . | ||
| Number of patients | 2025 | 796 | |
| Patient age (y) | 57 (44-65) | 56 (47-64) | .26 |
| Disease status | .40 | ||
| CR 1 | 1587 (78) | 636 (80) | |
| CR ≥2 | 438 (22) | 160 (20) | |
| Cytogenetics (SWOG) | <.001 | ||
| Good | 121 (6) | 5 (1) | |
| Intermediate | 504 (25) | 712 (89) | |
| Poor | 144 (7) | 79 (10) | |
| Unknown | 1256 (62) | ||
| HCT-CI score | —† | ||
| 0 | 506 (25) | 215 (27) | |
| 1-2 | 687 (34) | — | |
| ≥3 | 832 (41) | — | |
| >0‡ | — | 581 (73) | |
| Conditioning | <.001 | ||
| RIC/nonmyeloablative | 836 (41) | 589 (74) | |
| MAC | 1189 (59) | 207 (26) | |
| GVHD prophylaxis | <.001 | ||
| Tac based | 1791 (88) | 55 (7) | |
| CSA based | 234 (12) | 712 (93) | |
| Other | — | 29 (4) | |
| Donor age (y) | 28 (23-35) | 31 (25-39) | <.001 |
| Recipient CMV serostatus§ | <.001 | ||
| Seronegative | 655 (33) | 328 (41) | |
| Seropositive | 1358 (67) | 463 (58) | |
| Graft source | <.001 | ||
| Peripheral blood | 1738 (86) | 768 (96) | |
| Bone marrow | 287 (14) | 28 (4) | |
| In vivo T-cell depletion | <.001 | ||
| ATG or campath | 802 (40) | 628 (79) | |
| Neither | 1221 (60) | 168 (21) | |
| F donor to M recipient | 230 (11) | 62 (8) | |
| Year | <.001 | ||
| 2005-2009 | 0 (0) | 60 (8) | |
| 2010-2013 | 1129 (56) | 161 (20) | |
| 2014-2017 | 896 (44) | 575 (72) | |
| DQ | .005 | ||
| Matched | 1918 (95) | 774 (97) | |
| Mismatched | 107 (5) | 22 (3) | |
| KIR haplotype | .94 | ||
| AA | 649 (32) | 257 (32) | |
| Bx | 1376 (68) | 539 (68) | |
| Characteristic . | N (%)/median [IQR] . | P∗ . | |
|---|---|---|---|
| CIBMTR cohort . | DRST cohort . | ||
| Number of patients | 2025 | 796 | |
| Patient age (y) | 57 (44-65) | 56 (47-64) | .26 |
| Disease status | .40 | ||
| CR 1 | 1587 (78) | 636 (80) | |
| CR ≥2 | 438 (22) | 160 (20) | |
| Cytogenetics (SWOG) | <.001 | ||
| Good | 121 (6) | 5 (1) | |
| Intermediate | 504 (25) | 712 (89) | |
| Poor | 144 (7) | 79 (10) | |
| Unknown | 1256 (62) | ||
| HCT-CI score | —† | ||
| 0 | 506 (25) | 215 (27) | |
| 1-2 | 687 (34) | — | |
| ≥3 | 832 (41) | — | |
| >0‡ | — | 581 (73) | |
| Conditioning | <.001 | ||
| RIC/nonmyeloablative | 836 (41) | 589 (74) | |
| MAC | 1189 (59) | 207 (26) | |
| GVHD prophylaxis | <.001 | ||
| Tac based | 1791 (88) | 55 (7) | |
| CSA based | 234 (12) | 712 (93) | |
| Other | — | 29 (4) | |
| Donor age (y) | 28 (23-35) | 31 (25-39) | <.001 |
| Recipient CMV serostatus§ | <.001 | ||
| Seronegative | 655 (33) | 328 (41) | |
| Seropositive | 1358 (67) | 463 (58) | |
| Graft source | <.001 | ||
| Peripheral blood | 1738 (86) | 768 (96) | |
| Bone marrow | 287 (14) | 28 (4) | |
| In vivo T-cell depletion | <.001 | ||
| ATG or campath | 802 (40) | 628 (79) | |
| Neither | 1221 (60) | 168 (21) | |
| F donor to M recipient | 230 (11) | 62 (8) | |
| Year | <.001 | ||
| 2005-2009 | 0 (0) | 60 (8) | |
| 2010-2013 | 1129 (56) | 161 (20) | |
| 2014-2017 | 896 (44) | 575 (72) | |
| DQ | .005 | ||
| Matched | 1918 (95) | 774 (97) | |
| Mismatched | 107 (5) | 22 (3) | |
| KIR haplotype | .94 | ||
| AA | 649 (32) | 257 (32) | |
| Bx | 1376 (68) | 539 (68) | |
CMV, cytomegalovirus; CR1, first complete remission; CR ≥2, second or later CR; CsA, cyclosporine A; DQ, HLA-DQB1 match; F, female; HCT-CI, HCT-specific comorbidity index; M, male; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning; SWOG, Southwestern Oncology Group; Tac, tacrolimus.
P value from the χ2 statistic for categorical variables and the Wilcoxon test for continuous variables.
Not calculated because of absent information in 1 cohort.
Patients in the DRST cohort were grouped based on absence vs presence of HCT-CI comorbidities.
In the DRST cohort, recipient CMV serostatus was missing for 5 patients (<1%).
Individual interactions: donor KIR–recipient HLA outcomes
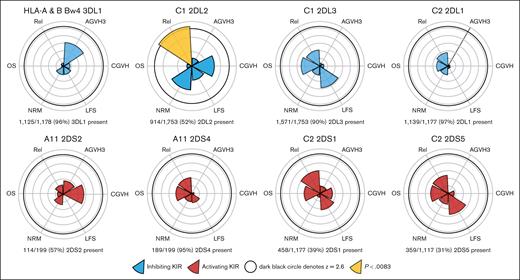
We screened for potential interactions between donor KIRs, their respective recipient HLA ligand, and any of the 6 traditional transplantation outcomes; results were depicted in terms of z scores, with z = 2.6 corresponding closely to a statistically significant result (P < .0083; Figure 1; supplemental Table 1). Among the unique activating KIR-HLA interactions studied (2DS2/A11, 2DS4/A11, 2DS1/C2, and 2DS5/C2), none were associated with any of the studied outcomes. Similarly, among the inhibitory KIR interactions 3DL1/Bw4, 2DL3/C1, and 2DL1/C2, no interactions were observed. Patients with ≥1 C1 alleles receiving 2DL2+ grafts had decreased relapse incidence (hazard ratio [HR], 0.79; [95% CI, 0.67-0.93]; P = .006; n = 914 of 1753 [52%]). However, when considering all patients who received KIR-2DL2+ grafts (the KIR perspective), there was no difference in relapse between recipients with and those without HLA-C1 (HR, 0.91; 95% CI, 0.64-1.23; P = .593), arguing against the decrease in relapse depending on an interaction between KIR-2DL2 and HLA-C1. KIR-2DS4 was present in nearly all recipients with the A11 allele (n = 189 of 199 [95%]), limiting the analysis of this interaction. To account for the possibility that activating KIRs potentially tolerize to constitutive ligand exposure, we performed a supplemental analysis studying the same donor KIRs in the context of heterozygous-only recipient HLA.16 No significant differences were seen for any studied outcomes (supplemental Table 2)
Individual donor-KIR–patient-HLA interactions are generally not associated with differential outcomes. Although a difference in relapse is seen in the HLA-C1/KIR-2DL2 pairing, this does not extend to an OS or LFS difference and may reflect these donor KIR belonging exclusively to the Bx haplotype. AGVH3, grade ≥3 acute GVHD; CGVH, chronic GVHD; Rel, relapse.
Individual donor-KIR–patient-HLA interactions are generally not associated with differential outcomes. Although a difference in relapse is seen in the HLA-C1/KIR-2DL2 pairing, this does not extend to an OS or LFS difference and may reflect these donor KIR belonging exclusively to the Bx haplotype. AGVH3, grade ≥3 acute GVHD; CGVH, chronic GVHD; Rel, relapse.
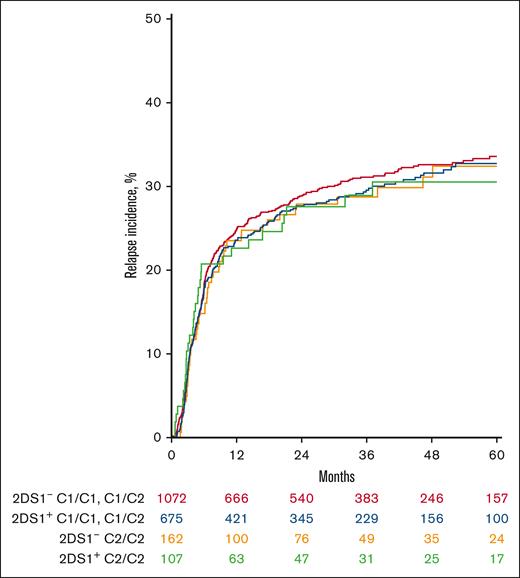
Although the interaction between C2 and 2DS1 in this analysis was nonsignificant for all outcomes, previous work has proposed that it bears meaningful clinical impact on relapse.3 We, therefore, studied the same interaction from the reverse perspective, asking, in the setting of a 2DS1+ donor graft, whether there is a meaningful difference in the risk of relapse based on the number of recipient C2 antigens? In a multivariable Cox model comparing with a baseline of C2-homozygous recipients, no significant difference in hazard of relapse was observed with heterozygous vs C1-homozygous recipients (supplemental Table 3; Figure 2A). These results were consistent when interactions with in vivo T-cell depletion or conditioning intensity were also included in the model.
KIR 2DS1 interaction with HLA-C2 does not show any association with relapse. Cumulative incidence curve depicting relapse in the presence or absence of KIR 2DS1 and its ligand HLA-C2 does not show any difference in outcome.
KIR 2DS1 interaction with HLA-C2 does not show any association with relapse. Cumulative incidence curve depicting relapse in the presence or absence of KIR 2DS1 and its ligand HLA-C2 does not show any difference in outcome.
We further investigated the decreased risk of relapse seen in recipients with C1 receiving 2DL2+ grafts. Interaction analyses suggested that improved relapse control may be abrogated by myeloablative conditioning, although this result was nonsignificant when controlling for multiple testing (HR interaction, 1.32; 95% CI, 1.02, 1.72; P = .036; supplemental Table 4). KIR-2DL2 was present in 77% of haplotype Bx grafts, such that the effect of C1-2DL2 interactions observed plausibly reflects a haplotype Bx-dependent association with relapse control previously described by Weisdorf in an overlapping data set.11 No interactions with in vivo T-cell depletion or conditioning intensity were identified in other inhibitory or activating KIR-HLA pairs.
Combinatorial interactions: donor KIR genotype–recipient HLA outcomes
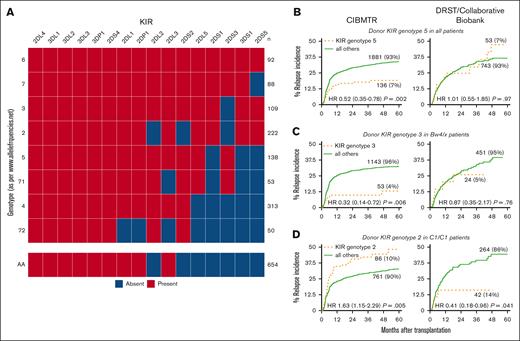
Applying a pragmatic approach to identify potential KIR gene combinations that might affect clinical outcomes, we selected the most common donor Bx KIR genotypes in this cohort. Genotypes were labeled per the Allele Frequency Net Database nomenclature.17 Nine Bx KIR genotypes with a prevalence of >50 donors in the cohort were screened for differential relapse when compared with the AA genotype, in separate models for (1) the entire cohort and (2) patients bearing or lacking specific B or C antigens. Three genotypes or genotype–antigen pairs were found to have a significant association with relapse and was selected for further study. Genotype 5 (G5; n = 136 of 2025; all KIRs except 2DS1, 2DS5, and 3DS1) was associated with a hazard of 0.52 (95% CI, 0.35-0.78; P = .002; Figure 3A) for relapse when compared with all other recipients, irrespective of HLA antigen. In recipients with at least 1 HLA-Bw4 antigen, G3 (n = 53 of 1196; all KIRs except 2DS3) was associated with a hazard of 0.32 (95% CI, 0.14-0.72; P = .006; Figure 3B) for relapse. In the opposite direction, among patients homozygous for the C1 antigen, G2 (n = 86 of 847; all KIRs present except 2DL2, 2DS2, and 2DS3) was associated with increased risk of relapse (HR, 1.63; 95% CI, 1.15-2.29; P = .005; Figure 3C). No significant interaction with either conditioning intensity or in vivo T-cell depletion was observed. Similarly, the presence of the centromeric-B/B KIR genotype was not associated with differential relapse risk in the entire cohort or HLA subgroups (P > .05).
Three candidate KIR genotypes are associated with altered relapse incidence in the CIBMTR cohort but not the DRST cohort. (A) Heat map of the KIRs included in each of the candidate genotypes, with haplotype AA as a reference. Of these, 3 were selected through a screening for association with relapse compared with patients receiving AA grafts. (B) G5 in all patients and (C) G3 in patients with HLA-Bw4/Bx were associated with significant decrease in relapse incidence, and (D) G2 was associated with increased relapse incidence among patients homozygous for HLA-C1; however, these results could not be replicated in an independent external cohort.
Three candidate KIR genotypes are associated with altered relapse incidence in the CIBMTR cohort but not the DRST cohort. (A) Heat map of the KIRs included in each of the candidate genotypes, with haplotype AA as a reference. Of these, 3 were selected through a screening for association with relapse compared with patients receiving AA grafts. (B) G5 in all patients and (C) G3 in patients with HLA-Bw4/Bx were associated with significant decrease in relapse incidence, and (D) G2 was associated with increased relapse incidence among patients homozygous for HLA-C1; however, these results could not be replicated in an independent external cohort.
Independent analysis: validation cohort
We sought to replicate the relapse effect seen in the patients of the CIBMTR cohort in an independent validation cohort comprising 796 patients treated in Germany between 2005 and 2017 (DRST). The median age within the DRST group was 59 years (IQR, 49-65) with similar proportions of first vs subsequent CR, cytomegalovirus seropositivity, donor age, and female-to-male sex mismatch vs the CIBMTR cohort (Table 2). However, there were several important differences; patients in the DRST cohort were more likely to receive reduced-intensity conditioning (74% vs 41%), in vivo T-cell depletion (79% vs 37%), and cyclosporine rather than tacrolimus-based GVHD prophylaxis (93% vs 12%).
Multivariable regression for relapse in 3 candidate KIR genotypes
| Model . | G5 . | G3/HLA-Bw4 . | G2/HLA-C1C1 . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| CIBMTR | G5, n = 136 vs all others, n = 1881 | G3, n = 53 vs other Bw4, n = 1143 | G2, n = 86 vs other C1C1 n = 761 | |||
| Base model∗ | 0.52 (0.35, 0.78) | .002 | 0.32 (0.14, 0.72) | .006 | 1.63 (1.15, 2.29) | .005 |
| Int∗ T-cell depletion† | 0.55 (0.34, 0.92) | .021 | 0.50 (0.21, 1.21) | .13 | 1.35 (0.81, 2.27) | .30 |
| Int∗ cond intensity | 0.43 (0.23, 0.78) | .005 | 0.17 (0.04, 0.68) | .013 | 1.93 (1.17, 3.18) | .010 |
| DRST | G5, n = 73 vs all others, n = 743 | G3, n = 24 vs other Bw4, n = 451 | G2, n = 42 vs other C1C1, n = 264 | |||
| 1.01 (0.55, 1.85) | .97 | 0.87 (0.35, 2.17) | .76 | 0.41 (0.18, 0.96) | .041 | |
| Model . | G5 . | G3/HLA-Bw4 . | G2/HLA-C1C1 . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| CIBMTR | G5, n = 136 vs all others, n = 1881 | G3, n = 53 vs other Bw4, n = 1143 | G2, n = 86 vs other C1C1 n = 761 | |||
| Base model∗ | 0.52 (0.35, 0.78) | .002 | 0.32 (0.14, 0.72) | .006 | 1.63 (1.15, 2.29) | .005 |
| Int∗ T-cell depletion† | 0.55 (0.34, 0.92) | .021 | 0.50 (0.21, 1.21) | .13 | 1.35 (0.81, 2.27) | .30 |
| Int∗ cond intensity | 0.43 (0.23, 0.78) | .005 | 0.17 (0.04, 0.68) | .013 | 1.93 (1.17, 3.18) | .010 |
| DRST | G5, n = 73 vs all others, n = 743 | G3, n = 24 vs other Bw4, n = 451 | G2, n = 42 vs other C1C1, n = 264 | |||
| 1.01 (0.55, 1.85) | .97 | 0.87 (0.35, 2.17) | .76 | 0.41 (0.18, 0.96) | .041 | |
Cond, conditioning; int, interaction between donor KIR and recipient-HLA.
The base model for relapse includes the genotype and age, as selected by backward variable selection (see “Methods”).
Interactions with T-cell depletion and conditioning intensity were nonsignificant in all analyses.
None of the associations in relapse risk noted in the patients in the CIBMTR cohort were reproducible in the DRST cohort. The HR for relapse in recipients of G5-present grafts was 1.01 compared with all others (95% CI, 0.55-1.86; P = .97); similarly, G3 in Bw4/X recipients had a hazard ratio of 0.87 (95% CI, 0.35-2.17; P = .76). G2 in C1-homozygous recipients was associated with a decrease in relapse, the opposite of what was found in the CIBMTR cohort (HR, 0.41; 95% CI, 0.18, 0.96; P = .041).
Discussion
Optimizing donor selection in HCT remains a central goal to improve its curative potential. The integration of donor-KIR–recipient-HLA interactions into the donor selection algorithm has been evaluated in numerous retrospective and prospective studies over the past 2 decades with variable results.3,4,7,11-14,18-22 We, therefore, sought to systematically address a vital clinical question: is there clinical benefit in selecting between HLA-MUDs based on the presence or absence of individual KIRs or based on frequently available KIR genotype combinations? Using a large cohort of patients with AML in CR treated with 8/8 HLA–matched unrelated grafts with known KIR genotypes, we evaluated the hazard for standard transplantation outcomes associated with individual donor KIRs encountering recipient HLA ligand. We aimed to rigorously consider each known biological interaction in a large cohort of patients treated for a single indication, AML in CR. Following previous models that showed benefit to the Bx haplotype, we explored relapse incidence among patients receiving grafts with the KIR genotypes most prevalent among the studied donors. Although we identified 3 genotypes linked to marked alteration in relapse rates in our initial cohort, we were unable to validate these findings in an independent group of patients, albeit 1 with differences in transplantation strategy. Our findings suggest that there is no unambiguous benefit to the selection of donors by either individual receptor-ligand pairing or by common KIR genotypes using current KIR-HLA–typing approaches.
The interaction between individual KIRs and HLA ligands in the HCT recipient can be considered from the perspective of either the graft or the host. We framed the question from a clinical perspective: in context of recipient HLA, what is the effect of a potential donor’s KIRs? In our analysis, none of the KIR-HLA interactions were associated with OS or LFS. The receptor-ligand mismatch has been implicated in the incidence of GVHD18,23,24 and early posttransplantation infection,25-28 leading determinants of morbidity and NRM. Effect size and even direction (increasing or minimizing GVHD) vary across studies.7 We did not identify any individual KIR/ligand pair linked to NRM or acute or chronic GVHD. In an analysis of a previous CIBMTR cohort that also included <8/8 HLA–MUDs, Venstrom et al identified KIR-3DS1 as potentially linked to increased grade 2 to 4 acute GVHD, although not grade 3 to 4, disease.24 KIR-3DS1 does not have a definitively identified class 1 HLA ligand29; thus, we did not include it in this receptor-ligand pair analysis.
We identified 1 pair, recipient HLA-C1 and donor KIR-2DL2, which was associated with improved relapse control despite no improvement in survival. Importantly, KIR-2DL2 is exclusively present in the Bx haplotype. Our findings are, therefore, consistent with those of Weisdorf et al in an overlapping data set, in which they showed improved relapse control of HLA-C1 recipients with Bx grafts.11 The unique contribution of 2DL2 to the Bx haplotype is challenging to parse in this case, although we note its presence in centromeric-B haplotypes associated with decreased relapse.30,31 Moreover, 2DL2 is present in the 2 protective genotypes (G3 and G5) that we identified and absent in the 1 adverse genotype (G2). The pairing of 2DL2/C1 merits mechanistic investigation into its role in an integrated effect of KIRs in combination; however, the absence of an independent association with OS or LFS argues against its consideration in donor selection. An alternative approach to identifying clinically beneficial KIR genotypes, described by Krieger et al and validated on an overlapping data set, likewise suggests the importance of 2DL2; 2DL2 is constitutively present in donors whose KIR genotypes have the maximal inhibitory content by this method.8,9
Previous studies demonstrated varying support for the hypothesis that other individual KIR-HLA pair models may prognosticate relapse risk.7 Most prominently, Venstrom et al described the interaction of donor KIR2DS1 and recipient homozygous HLA-C2 leading to higher relapse rates than in cases with HLA-C1 present, suggesting a KIR-mediated tolerance of the recipient host when presented with the appropriate ligand. However, in a recent study of a large cohort of patients with myeloid malignancies, Schetelig et al found no difference in relapse between C2-homozygous and C1/x recipients in the presence of the KIR-2DS1+ grafts.6 Our results similarly could not replicate this effect when studied from either the recipient or donor perspective. Additional studies have sought to further refine our understanding of the KIR-HLA interaction by using more nuanced groupings of donor KIRs, for instance looking at NK cell 3DL1 surface density.19 The DRST has performed allele-level sequencing of donor KIRs, opening the door to KIR allele–HLA allele–level interaction analyses.32 Both KIR3DL1 expression and KIR allelic typing were unavailable in this analysis, which is an important limitation of our study. Although these approaches promise the possibility of identifying powerful mechanisms of relapse control, they further narrow the subset of patients for whom KIR-driven strategies would be possible, potentially limiting their broad clinical applicability.
KIR-mediated NK cell killing requires the integration of activating and inhibiting signals from multiple receptors.33,34 Reductionist approaches based on individual receptor-ligand pairs may “miss the forest for the trees.” A number of models have been proposed to describe the summative effect of multiple KIRs.21 The model by Krieger et al, which ranks KIR genotypes according to the inhibitory KIR content or number of recipient ligand–donor receptor pairs showed that recipients of the most inhibitory-rich genotypes had improved OS and decreased relapse.8 These findings were validated on a data set overlapping with this analysis.9 Other studies demonstrated that haplotype B, containing multiple activating KIRs, is associated with improved relapse control.4,11 Subsets of the B haplotype enriched for centromeric KIRs (especially KIR-2DS2, KIR-2DL2, and KIR-2DL3) have been associated with longer OS and disease-free survival,14,30 although, here too, the findings have been inconsistently replicated.21 Our analysis took an empirical rather than biologically driven approach. We identified candidate KIR groupings based on the most frequently observed KIR genotypes in our data set and considered these in conjunction with as well as independent of recipient HLAs. Recipients of grafts with 3 prevalent genotypes, when compared with recipients from all other donors, showed dramatically altered relapse trajectories. G5 across all patients and G3 alongside recipient Bw4 appeared to be beneficial, and G2 in HLA-C1+ recipients was linked to adverse outcomes. Of note, both G5 and G3 contain the centromeric B01/Bx pattern elsewhere associated with improved relapse control, whereas G2 contains the centromeric A region linked to greater treatment failure.31 G5 and G3 also contain the maximal inhibitory KIR content following the Krieger et al model, which they associated with longer OS and decreased relapse incidence.8,9 Previous work correlating NK cell responsiveness with the number of different inhibitory receptors on individual murine cells may account for this finding.35 Further investigation with high-resolution genotyping to identify component diplotypes may clarify the contributions of individual centromeric and telomeric regions.31 However, no corresponding effect on disease control was seen when the analysis was repeated on an independent data set. There are certain key distinctions between the 2 cohorts, including conditioning intensity, use of ATG, and the range of years during which patients underwent treatment. Although ATG and conditioning intensity were not found to have significant interactions in our model, previous work points to their importance in NK cell activity after HCT.9,11 The selection of calcineurin inhibitors differ markedly between the 2 cohorts, raising the possibility that cyclosporine and tacrolimus may differently modulate NK cell cytotoxicity differently. A comparison of the effect of tacrolimus and cyclosporine A on NK cell activity merits future study. The contribution of population-level ethnic diversity through minor histocompatibility antigens cannot be accounted for in this study but may also contribute differently to GVL in these 2 groups.36 The German cohort was considerably smaller than the CIBMTR cohort, limiting the overall power to detect KIR-HLA interactions. Still, the possibility of false discovery cannot be ignored, especially given the retrospective nature of these data. Further validation for other large cohorts with similar transplantation strategies as well as for patients who received transplantation during active leukemia is warranted.
Several limitations must be considered when interpreting these results. First, assembling subgroups of donor KIR and recipient HLA pairs leads inescapably to small patient numbers. True but subtle effects may be missed; conversely, effect sizes are also liable to overestimation. Although we find no individual KIR-HLA pairs clearly linked to outcome, the absence of evidence is not evidence of absence, and a P value > .05 is definitionally not dispositive for lack of any effect. That said, we would anticipate that a true difference in effect sizes large enough to be clinically relevant has a reasonable likelihood of being detected in a large sample. Alternative statistical techniques such as Bayesian approaches could assign a direct probability that an interaction has no association with an outcome. Our analysis is based on a retrospective and, necessarily, a varied cohort of patients, with transplantation practices varying over time as well as by center and treating-physician practices. To the extent possible, this has been mitigated by adjusting for likely confounders. Notably, our results are in line with the largest prospective study undertaken, in which donor selection by KIR2DS1/C1 pairing or the presence of centromeric-B/B donors were not associated with benefit (although they found that KIR3DL1 inhibition, not assessed in this analysis, did stratify outcomes).7 The absence of cytogenetic data for a large portion of the patients in the CIBMTR cohort led to that variable’s absence in our model. In 1 recent study including most patients with AML who received transplantation in European Society for Blood and Marrow Transplantation centers from 2014 to 2016, >70% of patients with AML with reported cytogenetics had intermediate cytogenetic risk.37 The overwhelming proportion of intermediate risk in reference cohorts suggests that the inclusion of cytogenetics may not decisively alter results; however, it clearly merits further evaluation. Moreover, the study includes only patients who received transplantation in CR, likely biasing selection toward a molecularly favorable risk profile. Although our analysis was restricted to 8/8 HLA–matched donors and few had mismatches at HLA-DBQ1, HLA-DBP1 matching is not known for these patients. No patients in this analysis received posttransplant cyclophosphamide, now rapidly emerging as a standard strategy in the MUD setting.38 Evaluation of the donor-KIR–recipient-HLA interaction in the posttransplant cyclophosphamide MUD setting is needed. Finally, the differences between the CIBMTR and DRST cohorts are both a limitation and a strength, increasing the barrier to replicability of our initial findings but also suggesting broad generalizability had we replicated any effect.
In summary, in a systematic analysis of a large cohort of patients with AML undergoing transplantation in first CR from MUDs, we were unable to identify a widely applicable strategy for donor selection using either individual KIR-HLA pairs or donor KIR genotypes. The evaluation of discrete pairs did not reveal clinically actionable interactions, although whether KIR-2DL2 exerts a relapse-protective influence beyond its membership in the Bx haplotype warrants further exploration. The absence of a positive finding may suggest that the biologically present interaction between graft NK cells and host leukemia is unable to overcome other, stronger factors in the setting of MUD transplantation. In line with our theoretical understanding of the summative nature of NK cell activation and inhibition, combinatorial approaches integrating population-level findings on HLA and KIR diversity remain the most compelling for future study. Allele- and expression intensity–level analyses hold biological promise but risk identifying subgroups too narrow for broad clinical benefit. Perhaps most importantly, the lack of any correspondence between results in the CIBMTR and DRST cohorts is a stark reminder of the critical nature of external validation, echoing the difficulty of replication seen across KIR-driven analyses. Based on this analysis, we find no evidence of clinical benefit from individual KIR-HLA pairings or by common KIR genotypes using current approaches, and do not recommend incorporating KIR typing into current donor selection algorithms.
Acknowledgment
J.A.F. received support from a Hematology Opportunities for the Next Generation of Research Scientists award from the American Society of Hematology.
Authorship
Contribution: J.A.F., R.S., E.K., S.R.S., A.A.T., R.R., and J.K. were responsible for study concept and design; J.A.F., R.S., E.K., S.R.S., A.A.T., K.-J.M., J.S., R.R., and J.K. were responsible for the acquisition, analysis, and interpretation of data; J.A.F. was responsible for statistical analyses, with analyses of DRST data performed by H.B. and overall statistical oversight by T.W.; J.A.F., R.S., and E.K. were responsible for initial drafting of the manuscript; J.A.F., R.S., E.K., S.R.S., T.W., H.B., K.F., N.K., M.H., M. Maiers, J.S.M., M. Mohty, A.N., D.W., K.-J.M., A.A.T., J.S., R.R., and J.K. were responsible for critical revision of the manuscript; S.R.S., M.H., M. Maiers, K.F., N.K., M. Mohty, A.N., and J.S. provided and oversaw access to registry data resources; R.R. and J.K. were responsible for study supervision and provided administrative, technical, and material support; H.B. and J.S. had full access to included DRST data; and J.A.F., R.S., E.K., S.R.S., A.A.T., R.R., and J.K. had full access to included CIBMTR data.
Conflict-of-interest disclosure: R.R. receives research funding from Crispr Therapeutics and Skyline Therapeutics; holds equity in and is cofounder of InnDura Therapeutics; and reports consultancy and serves on the scientific advisory board for Glycostem and xNK Therapeutics. J.K. reports serving on the scientific advisory board for Cugene and Mallinckrodt; consulting for Equilibrium, Gentbio, Cue biopharma, Biolojic Design, and Tr1X; and research for Amgen, Clinigen Laboratories, Bristol Myers Squibb, Miltenyi Biotec, Regeneron, and Equilibrium. The remaining authors declare no competing financial interests.
Correspondence: Rizwan Romee, Transplant and Cellular Therapies, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: rizwan_romee@dfci.harvard.edu.
References
Author notes
∗J.A.F., R.S., and E.K. are joint first authors.
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 12 December 2021.
Data are available through the requesting platforms, CIBMTR (inforequest@mcw.edu) and DRST (support@drst.de).
The full-text version of this article contains a data supplement.