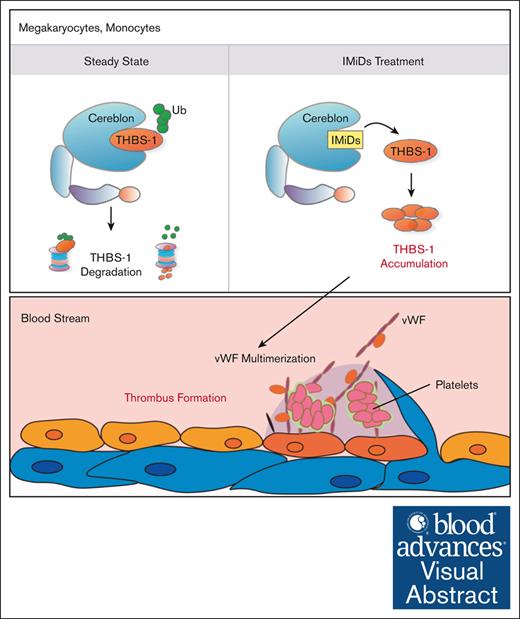
IMiDs inhibit the degradation of thrombospondin-1 in human megakaryocytes, resulting in the aberrant accumulation of thrombospondin-1.
The increment of thrombospondin-1, which promotes the multimerization of von Willebrand factor could be associated with IMiDs-induced venous thromboembolisms.
Visual Abstract
Immunomodulatory drugs (IMiDs) are key drugs for treating multiple myeloma and myelodysplastic syndrome with chromosome 5q deletion. IMiDs exert their pleiotropic effects through the interaction between cell-specific substrates and cereblon, a substrate receptor of the E3 ubiquitin ligase complex. Thus, identification of cell-specific substrates is important for understanding the effects of IMiDs. IMiDs increase the risk of thromboembolism, which sometimes results in fatal clinical outcomes. In this study, we sought to clarify the molecular mechanisms underlying IMiDs-induced thrombosis. We investigated cereblon substrates in human megakaryocytes using liquid chromatography–mass spectrometry and found that thrombospondin-1 (THBS-1), which is an inhibitor of a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13, functions as an endogenous substrate in human megakaryocytes. IMiDs inhibited the proteasomal degradation of THBS-1 by impairing the recruitment of cereblon to THBS-1, leading to aberrant accumulation of THBS-1. We observed a significant increase in THBS-1 in peripheral blood mononuclear cells as well as larger von Willebrand factor multimers in the plasma of patients with myeloma, who were treated with IMiDs. These results collectively suggest that THBS-1 represents an endogenous substrate of cereblon. This pairing is disrupted by IMiDs, and the aberrant accumulation of THBS-1 plays an important role in the pathogenesis of IMiDs-induced thromboembolism.
Introduction
Immunomodulatory drugs (IMiDs) such as lenalidomide and pomalidomide are key drugs for treating multiple myeloma (MM)1 and myelodysplastic syndromes with chromosome 5q deletion.2 These compounds exert their pharmacological effects by binding to cereblon, a substrate receptor of CRL4-cereblon-E3 ubiquitin ligase, and modulate the ubiquitination of target substrates.3 Lenalidomide-bound cereblon acquires the ability to bind and target proteasomal degradation of neosubstrates such as IKZF1/3 and CK1α, which underlie the clinical effects of IMiDs in MM and myelodysplastic syndromes with del (5q), respectively,4-8 while losing the ability to bind endogenous substrates such as MEIS2.9 Because the pleiotropic effects of IMiDs depend on the various functions of each cereblon substrate, identification of cell-specific novel substrates is a critical step in understanding the effects of IMiDs.
We have recently identified aromatase as a novel neosubstrate of cereblon responsible for IMiDs-induced thrombocytopenia.10 In addition to thrombocytopenia, venous thromboembolism (VTE) represents another major adverse effect of IMiDs. Previous studies have reported that VTEs occur in 17% to 26% of patients with MM treated with IMiDs plus dexamethasone. Importantly, higher incidences of VTE were observed in patients treated with IMiDs in addition to dexamethasone than with dexamethasone alone.11,12 Because severe VTE can be associated with fatal clinical outcomes in patients,13 high incidence of IMiDs-induced VTE hampers the continuous treatment of MM in clinical practice. Thus, elucidation of the mechanisms underlying IMiDs-induced VTE is important for establishing safe and effective treatment strategies against MM. However, the molecular mechanisms specific for IMiDs-induced VTEs remains elusive. Furthermore, unlike common VTEs, platelets play a peculiar role in IMiDs-induced VTEs; venous and arterial thrombosis also increase with IMiDs treatment,14,15 and clinical studies suggest the efficacy of antiplatelet therapy in the prevention of IMiDs-induced VTEs.16,17 Based on these observations, we hypothesized that platelets or megakaryocyte-specific substrates of cereblon could be involved in the pathogenesis of IMiDs-induced VTEs.
In this study, we investigated the substrates of cereblon in human megakaryocytes using liquid chromatography–mass spectrometry (LC-MS). We found an α-granular protein thrombospondin-1 (THBS-1), which induces VTEs by promoting the multimerization of von Willebrand factor (vWF),18-22 as a novel endogenous substrate of cereblon. IMiDs inhibited the proteasomal degradation of THBS-1 by impairing the recruitment of cereblon to THBS-1, leading to aberrant accumulation of THBS-1 in human megakaryocytes. We also found that dexamethasone facilitated the expression of THBS-1 by activating its transcription activity, providing the rationale for the synergistic increase in VTE risk in patients with MM treated with IMiDs and dexamethasone. In patients with MM treated with IMiDs, we confirmed increased levels of THBS-1 and enhanced vWF multimerization in peripheral blood mononuclear cells (PBMNCs) and plasma, respectively. These results collectively suggest that THBS-1 represents an endogenous substrate of cereblon, and the aberrant accumulation of THBS-1 plays an important role in the pathogenesis of IMiDs-induced VTEs.
Materials and methods
Reagents
Lenalidomide, pomalidomide, thalidomide, cycloheximide, histamine (Merck & Co, Rahway, NJ), MG132 (Abcam, Cambridge, United Kingdom), and dexamethasone (Abcam) were dissolved in dimethyl sulfoxide (Tokyo Chemical Industry Co, Ltd, Tokyo, Japan) at concentrations ranging from 1 to 100 mM and stored at −20°C for up to 6 months.
Clinical samples
Peripheral blood samples of adult patients with MM, diagnosed according to the criteria set by the World Health Organization, were included in this study. In all patients, the bone marrow sample was aspirated from the posterior superior iliac spine, and peripheral blood was drawn from the peripheral vein. Cord blood cells from umbilical cord and placenta obtained after full-term deliveries were provided by the Kyushu Block Red Cross Blood Center, Japan Red Cross Society, and Ishida Ladies Clinic (Fukuoka, Japan). Informed consent was obtained from all patients and ethical guidelines set forth by the Declaration of Helsinki of 1975 (revised in 1983) were followed. The institutional review board of Kyushu University Hospital approved all the studies on human study participants.
Cell lines and primary cells
Human megakaryocytic leukemia cell line MEG01,23 human monocytic cell line THP-1, transfectable human embryonic kidney cells 293T, and human umbilical vein endothelial cells were obtained from the American Type Culture Collection (Manassas, VA). PBMNCs from patients with MM and from healthy donors were isolated from whole blood using Lymphoprep (Abbott Diagnostics Technologies AS, Oslo, Norway) according to the manufacturer’s protocol. MEG01, THP-1 cells, and primary PBMNCs were grown in RPMI-1640 (Wako, Richmond, VA), and 293T cells were grown in Dulbecco modified Eagle medium (Wako, Richmond, VA) supplemented with 10% heat-inactivated fetal bovine serum (Merck & Co) and penicillin plus streptomycin (Thermo Fischer Scientific, Waltham, MA). Human umbilical vein endothelial cells were grown in EGM BulletKit (Lonza Pharma & Biotech, Basel, Switzerland). Cells were grown at 37°C in a humidified incubator with 5% carbon dioxide.
In vitro culture of megakaryocytes derived from CD34+ hematopoietic stem and progenitor cells (HSPCs)
CD34+ HSPCs were isolated using the Indirect CD34 MicroBead Kit (Miltenyi Biotec, Germany) and grown in serum-free medium, as previously reported.24 Briefly, to generate mature megakaryocytes, cells were cultured in Iscove modified Dulbecco medium supplemented with 20% BIT serum substitute (bovine serum albumin, insulin, and transferrin; STEMCELL Technologies, Vancouver, Canada), 40 μg/mL low-density lipoprotein, 50 μM 2-mercaptoethanol, and antibiotics in the presence of a cytokine cocktail including stem cell factor (1 ng/mL), thrombopoietin (30 ng/mL), interleukin-6 (7.5 ng/mL), and interleukin-9 (13.5 ng/mL). All cytokines were purchased from R&D Systems (Minneapolis, MN). Ten days after the initiation of culture, dead cells were removed using the Dead Cell Removal Kit (Miltenyi Biotec) and live megakaryocytes were stored at −80°C until used in experiments.
Flow cytometric analysis
For the analysis of megakaryocytes derived from CD34+ HSPCs, cells were stained and analyzed using the fluorescence-activated cell sorter Aria II (BD Biosciences, NJ), as previously reported.25 Briefly, cells were stained with allophycocyanin-conjugated anti-CD41 (HIP8) antibody (BioLegend, CA) and phycoerythrin-conjugated anti-CD42b (HIP1) antibody (BioLegend). Dead cells were excluded using propidium iodide staining. Appropriate isotype-matched, irrelevant control monoclonal antibodies were used to determine the background staining.
LC-MS analysis
Co-immunoprecipitated protein samples were subjected to LC-MS analysis. In brief, proteins were digested by the addition of trypsin and desalted before LC-MS analysis. Peptide mixtures were analyzed on an EASY nLC-1000 nanoflow LC system (Thermo Fischer Scientific) coupled to an LTQ Orbitrap Fusion Lumos mass spectrometer (serial number FSN20221; Thermo Fischer Scientific). Data were processed using MaxQuant26 with the human UniProt sequence database for peptide-spectrum matching. Detailed experimental procedures are described in supplemental Materials and Methods.
Transient overexpression of cereblon, virus production, and generation of THBS-1– or cereblon-depleted cells by CRISPR–CRISPR-associated protein 9 (CRISPR-Cas9) genome editing
For transient overexpression, 293T cells were transfected with FLAG-tagged human cereblon. The cells were collected 48 hours after transfection.
To generate THBS-1– or cereblon-depleted cells, MEG01 and THP-1 cells were transfected with a lentiviral vector encoding Streptococcus pyogenes Cas9 and the blasticidin resistance gene. After blasticidin selection, cells were transfected with a lentiviral vector encoding crimson fluorescent protein and single-guide RNA against THBS-1 or cereblon. THBS-1– or cereblon-depleted cells were established by sorting crimson-positive cells by Aria II (BD Biosciences). Detailed experimental procedures are described in supplemental Materials and Methods.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was reverse transcribed to complementary DNA using SuperScript II reverse transcriptase (Thermo Fischer Scientific). The messenger RNA (mRNA) levels were measured by qRT-PCR in triplicate using THUNDERBIRD Probe One-step qRT-PCR Kit (TOYOBO, Osaka, Japan) in an Mx3000P Real-Time QPCR System (Agilent Technologies, CA). The probe sets used were THBS-1 and 18S Eukaryotic ribosomal RNA Endogenous Control (Thermo Fischer Scientific). The relative expression of each gene was calculated by the ΔΔCT method, which uses changes in the cycling threshold. The mRNA levels of 18S ribosomal RNA were used for normalization. Primer sequences are available upon request.
Western blotting analysis
Cells were lysed in RIPA lysis buffer (Nacalai Tesque, Kyoto, Japan) for 30 minutes on ice. The lysates were denatured in equal volumes of 2× sodium dodecyl sulfate (SDS) sample buffer (Bio-Rad, CA) containing 5% (volume per volume) 2-mercaptoethanol (Sigma-Aldrich, MO) and heated for 5 minutes at 95°C. Next, they were fractionated by SDS–polyacrylamide gel electrophoresis on precast gels (Wako, Richmond, VA) and transferred onto nitrocellulose membranes (Wako). Before incubation with primary antibodies, the blots were blocked in 5% dry nonfat milk in Tris (hydroxymethyl) aminomethane–buffered saline with 0.1% Tween for 60 minutes. For protein detection, primary antibodies detecting THBS-1, IKZF1, Meis2, damage-specific DNA binding protein 1 (DDB1), glutathione S-transferases, His (Abcam), cereblon (Merck), and β-actin (Cell Signaling Technology, MA) were used. Secondary antibodies were horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse heavy chain– (BioLegend) and light chain–specific antibodies (Jackson ImmunoResearch, PA). The blots were visualized using ECL Prime Western Blot Detection Reagent (GE Healthcare, IL).
Immunoprecipitation
To assess the binding of THBS-1 to cereblon, a lysate mixture of MEG01 cells and 293T cells, transiently transfected with FLAG-tagged cereblon, was incubated, and subjected to immunoprecipitation with an antibody against FLAG. The direct binding of THBS-1 to cereblon was analyzed by coimmunoprecipitation using purified recombinant THBS-1 and cereblon. Each experiment was performed using magnetic beads and, after washing, bound proteins were eluted with elution buffer and analyzed by immunoblotting. Detailed experimental procedures are described in supplemental Materials and Methods.
Enzyme-linked immunosorbent assay (ELISA)
Culture medium of PBMNCs from healthy donors was subjected to ELISA analysis, following the manufacturer’s instructions (Quantikine ELISA Human Thrombospondin-1 Immunoassay, R&D systems, MN). Briefly, THBS-1 was captured by a monoclonal antibody specific for human THBS-1 coated on a microplate, followed by detection with an HRP-linked polyclonal antibody for THBS-1 after washing out unbound substances. The amount of THBS-1 was analyzed by measuring the absorbance of the developed color by adding the HRP substrate using a microplate reader (Thermo Fischer Scientific).
Platelet-poor plasma (PPP) collection
To detect vWF multimers in PPP, 10 mL of peripheral blood was drawn, using sodium citrate, from patients with MM and healthy donors. The plasma layer was collected after centrifugation at 760g for 15 minutes, followed by another round of centrifugation at 10 000g for 10 minutes. PPP was collected from the upper layer of the supernatant and stored at −80°C until analysis.
SDS–agarose gel electrophoresis of plasma vWF multimers
SDS–agarose gel electrophoresis of plasma vWF was performed by modifying previously described methods.27 In brief, PPP samples from patients with MM and from healthy donors were denatured, subjected to electrophoresis using SDS-agarose gel, and transferred onto a nitrocellulose membrane. The blots were immunoblotted using antibodies against vWF. Detailed experimental procedures are described in supplemental Materials and Methods.
Optical density (OD) analysis of vWF multimers
The OD level of each vWF multimers observed in the SDS-agarose gel electrophoresis of plasma vWF multimers was evaluated by ImageJ 1.53t. To quantify the amount of each multimer, the area of each peak observed in OD analysis was measured. To exclude the background signals, the peak area above the OD level reflecting the background adjacent to each band was measured. The vWF multimers were classified into small (band 1-5), intermediate (band 6-10), and large (band ≥11) multimers according to their molecular weight.
Statistical analysis
All statistical analyses were performed with JMP Pro version 15 (SAS Institute, NC) designed to add statistical functions frequently used in biostatistics. Significance was assessed using a 2-tailed Student t test or Wilcoxon 2-sample exact test. Statistical significance was set at P value < .05. Data are presented as mean ± standard error of the mean.
Results
Lenalidomide alters the protein interaction between THBS-1 and cereblon in human megakaryocytes
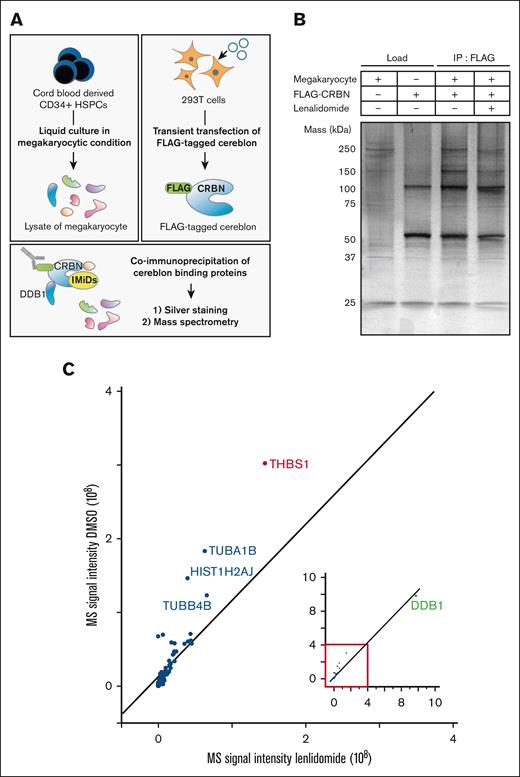
We sought to identify human megakaryocyte–specific proteins that interact with cereblon. Cereblon is a substrate receptor within the CRL4-cereblon-E3 ligase complex. DDB1 interacts with cereblon and serves as a core component of the CRL4-cereblon-E3 ligase complex. The interaction between cereblon and DDB1 is vital for the selective degradation of various proteins.3 The experimental design is shown in Figure 1A. Cord blood–derived CD34+ HSPCs differentiated into CD41+CD42b+ mature human megakaryocytic cells after 10 days of liquid culture (supplemental Figure 1), after which proteins were extracted. Cellular extracts from human megakaryocytes and those from HEK293T cells expressing FLAG-tagged cereblon were incubated with or without lenalidomide. Proteins interacting with cereblon were immunoprecipitated using an anti-FLAG antibody, and the immunoprecipitants were subsequently subjected to silver staining after SDS–polyacrylamide gel electrophoresis and LC-MS analysis. First, in the silver staining, besides proteins estimated to be cereblon (51 kDa), the 120-, 150-, and 250-kDa proteins were coimmunoprecipitated. The 120-kDa protein was assumed to be DDB1 (127 kDa) that directly binds to cereblon,3 because it was the most abundant protein coimmunoprecipitated with cereblon. The coimmunoprecipitated 150- and 250-kDa proteins were abundant in megakaryocyte lysate. We observed a reduction in the immunoprecipitated yield of these proteins in the presence of lenalidomide, suggesting that lenalidomide reduced the binding capacity of these proteins to cereblon (Figure 1B). Next, LC-MS analysis of cell lysates obtained from HSPC-derived human megakaryocytes identified the proteins abundantly expressed in human megakaryocytes (supplemental Figure 2). The whole data of the proteins detected in LC-MS analysis is listed in supplemental Table 1. Among these proteins, THBS-1 (150 kDa), tubulin α1b (TUBA1B, 50 kDa), histone H2A type1-J (HIST1H2AJ, 14 kDa), and tubulin β4B (TUBB4B, 50 kDa) exhibited reduced signal intensity when coimmunoprecipitated with cereblon in the presence of lenalidomide (Figure 1C). Although we could not assume the identity of the 250-kDa protein observed in the silver staining, the 150 kDa protein was assumed to be THBS-1, considering its molecular weight. Moreover, previous studies have reported the association between THBS-1 and thrombus formation.18-22 We, therefore, hypothesized that THBS-1 could bind to cereblon, and that IMiDs interfere with their binding.
Lenalidomide-induced changes in megakaryocyte proteins coimmunoprecipitated with cereblon. (A) Experimental design for identifying human megakaryocyte–specific proteins interacting with cereblon. Lysate mixture of human megakaryocytes differentiated from CD34+ HSPCs and 293T cells transfected with FLAG-tagged cereblon were incubated with and without 10 μM lenalidomide and subjected to immunoprecipitation with antibody against FLAG. Immunoprecipitates were analyzed by sliver staining and MS. (B) Whole-cell lysates (lanes 1-2) and FLAG-specific immunoprecipitates (lanes 3-4) were subjected to silver staining after SDS–polyacrylamide gel electrophoresis. The proteins were denatured in a nonreduced condition. (C) MS signal intensities of FLAG-specific immunoprecipitates in the presence of dimethyl sulfoxide (DMSO) or 10 μM lenalidomide. Each dot shows the yield of the top 25 proteins abundant in megakaryocyte lysate (supplemental Figure 2) and DDB1 (green dot).
Lenalidomide-induced changes in megakaryocyte proteins coimmunoprecipitated with cereblon. (A) Experimental design for identifying human megakaryocyte–specific proteins interacting with cereblon. Lysate mixture of human megakaryocytes differentiated from CD34+ HSPCs and 293T cells transfected with FLAG-tagged cereblon were incubated with and without 10 μM lenalidomide and subjected to immunoprecipitation with antibody against FLAG. Immunoprecipitates were analyzed by sliver staining and MS. (B) Whole-cell lysates (lanes 1-2) and FLAG-specific immunoprecipitates (lanes 3-4) were subjected to silver staining after SDS–polyacrylamide gel electrophoresis. The proteins were denatured in a nonreduced condition. (C) MS signal intensities of FLAG-specific immunoprecipitates in the presence of dimethyl sulfoxide (DMSO) or 10 μM lenalidomide. Each dot shows the yield of the top 25 proteins abundant in megakaryocyte lysate (supplemental Figure 2) and DDB1 (green dot).
IMiDs induce the accumulation of THBS-1 in megakaryocytic cells in a proteasome-dependent manner
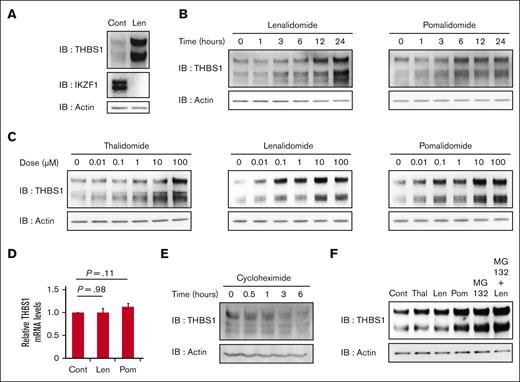
THBS-1 promotes thrombus formation and increases the risk of VTE onsets.18,20-22 Therefore, using the MEG01 cell line,23 we tested whether IMiDs could affect the protein level of THBS-1. We confirmed the expression of THBS1 and cereblon in MEG01 cells (supplemental Figure 3). Treatment with 10 μM lenalidomide increased THBS-1 levels in MEG01 cells in vitro, whereas IKZF1 decreased, consistent with previous reports4,5 (Figure 2A). In addition to lenalidomide, pomalidomide and thalidomide also induced the accumulation of THBS-1 in a time- and dose-dependent manner (Figures 2B and 2C, respectively). Although the protein levels of THBS-1 increased with IMiDs treatment, the mRNA level of THBS1, a gene encoding THBS-1, was not affected by lenalidomide or pomalidomide treatment in MEG01 cells (Figure 2D). Furthermore, the protein synthesis inhibitor cycloheximide decreased THBS-1 expression (Figure 2E), whereas the proteasome inhibitor MG132 increased THBS-1 in MEG01 cells (Figure 2F), indicating that the accumulation of THBS-1 was mediated by posttranscriptional regulation through the ubiquitin-proteasome pathway. Based on these results, we infer that IMiDs could induce THBS-1 accumulation by inhibiting its binding to cereblon and subsequent proteasomal degradation.
IMiDs upregulate intracellular THBS-1 expression in a proteasome-dependent manner. (A) Immunoblot analysis for THBS-1 and IKZF1 protein levels in MEG01 cells treated for 24 hours with DMSO or 10 μM lenalidomide. (B) Time course analysis of THBS-1 protein expression in MEG01 cells treated with 10 μM lenalidomide and pomalidomide for the indicated times. (C) Immunoblot analysis for THBS-1 in MEG01 cells treated with thalidomide, lenalidomide, and pomalidomide at indicated concentrations for 24 hours. (D) Relative mRNA expression levels of THBS1 in MEG01 cells. Cells were treated with DMSO, 10 μM lenalidomide, or pomalidomide for 24 hours. Data are from 9 independent experiments and are expressed as means ± standard error of the mean (SEM). P values were determined by 2-tailed Student t test. (E) Immunoblot analysis for THBS-1 in MEG01 cells treated with 10 μg/mL cycloheximide for the indicated times. (F) Immunoblot analysis for THBS-1 in MEG01 cells treated with 10 μM IMiDs and/or 1 μM MG132 for 24 hours. Results are representative of 2 (panel A) and 3 (panels B, C, E, and F) independent experiments. Cont, control; IB, immunoblotting; Len, lenalidomide; Pom, pomalidomide; Thal, thalidomide.
IMiDs upregulate intracellular THBS-1 expression in a proteasome-dependent manner. (A) Immunoblot analysis for THBS-1 and IKZF1 protein levels in MEG01 cells treated for 24 hours with DMSO or 10 μM lenalidomide. (B) Time course analysis of THBS-1 protein expression in MEG01 cells treated with 10 μM lenalidomide and pomalidomide for the indicated times. (C) Immunoblot analysis for THBS-1 in MEG01 cells treated with thalidomide, lenalidomide, and pomalidomide at indicated concentrations for 24 hours. (D) Relative mRNA expression levels of THBS1 in MEG01 cells. Cells were treated with DMSO, 10 μM lenalidomide, or pomalidomide for 24 hours. Data are from 9 independent experiments and are expressed as means ± standard error of the mean (SEM). P values were determined by 2-tailed Student t test. (E) Immunoblot analysis for THBS-1 in MEG01 cells treated with 10 μg/mL cycloheximide for the indicated times. (F) Immunoblot analysis for THBS-1 in MEG01 cells treated with 10 μM IMiDs and/or 1 μM MG132 for 24 hours. Results are representative of 2 (panel A) and 3 (panels B, C, E, and F) independent experiments. Cont, control; IB, immunoblotting; Len, lenalidomide; Pom, pomalidomide; Thal, thalidomide.
THBS-1 is an endogenous substrate of cereblon, and IMiDs inhibit its proteasomal degradation in megakaryocytic cells
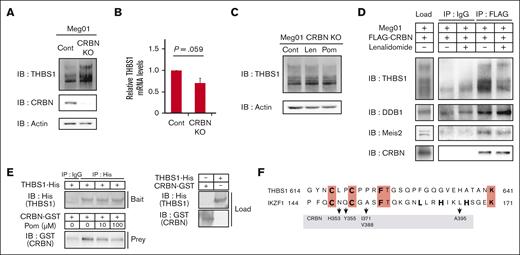
To test this hypothesis, we analyzed the effects of cereblon depletion in MEG01 cells by CRISPR-Cas9 genome editing. THBS-1 accumulated in the cereblon-knockout MEG01 cells (Figure 3A), whereas there was no increase in its transcription level (Figure 3B). Moreover, IMiDs-induced THBS-1 accumulation was not observed in cereblon-knockout MEG01 cells (Figure 3C), suggesting that THBS-1 is endogenously regulated in a cereblon-dependent manner. Upon examining the effect of IMiDs on the interaction between THBS-1 and the cereblon ubiquitin ligase complex, and conducting immunoprecipitation assay using an anti-FLAG antibody, we observed coimmunoprecipitation of THBS-1 with FLAG-tagged cereblon, which was attenuated in the presence of lenalidomide (Figure 3D). THBS-1 exhibited the same alteration as Meis2, an endogenous substrate of cereblon.9 Specifically, both lost their binding capacity to cereblon in the presence of IMiDs. Next, we tested whether THBS-1 could directly bind to cereblon using purified recombinant His-tagged cereblon and glutathione S-transferases–tagged THBS-1. As shown in Figure 3E, THBS-1 and cereblon were coimmunoprecipitated, and their interaction was attenuated by pomalidomide in a dose-dependent manner. Thus, IMiDs-dependent binding inhibition of purified recombinant cereblon and THBS-1 strongly suggested their direct interaction potential. Interestingly, THBS-1 harbors a similar sequence to the cereblon binding site of IKZF1/3 in C617-T625 residues (Figure 3F). The sequence includes residues that compose a zinc-finger structure of IKZF1/3, representing the protein backbone required for IKZF1/3 binding to cereblon.28,29 This suggests the possibility that THBS-1 could interact with cereblon via the zinc-finger structure commonly observed in cereblon substrates. These results further suggest that THBS-1 is an endogenous substrate of cereblon, and that IMiDs induce THBS-1 accumulation by attenuating its proteasomal degradation.
IMiDs interfere with the direct binding of THBS-1 and cereblon. (A and B) Protein expression of THBS-1 (A) and relative mRNA expression levels of THBS-1 (B) in cereblon-depleted MEG01 cells. In panel B, data are from 3 independent experiments and expressed as means ± SEM. P values were determined by 2-tailed Student t test. (C) Immunoblot analysis for THBS-1 in cereblon-depleted MEG01 cells treated with DMSO, 10 μM lenalidomide, or 10 μM pomalidomide for 24 hours. (D) Lysate mixture of MEG01 cells and 293T cells transfected with FLAG-tagged cereblon were incubated with or without 10 μM lenalidomide and subjected to immunoprecipitation with antibody against FLAG. (E) Purified recombinant His-tagged THBS-1–loaded beads were incubated with glutathione S-transferases–tagged cereblon in the presence of pomalidomide at indicated concentration. Mixture was immunoprecipitated with antibody against His. Results are representative of 3 independent experiments (panels A, C, D, and E). (F) Homologous sequences in THBS-1 and cereblon binding site of IKZF1 are aligned. The identical residues are highlighted in red. Residues of IKZF1 composing backbone of zinc-finger structure are shown in bold characters. Arrows represent indicated interacting residues between IKZF1 and cereblon in previous report. Sequence similarity with each protein was analyzed using the protein-protein BLAST program on The National Center for Biotechnology Information. CRBN, cereblon; Cont, control; GST, glutathione S-transferases; IB, immunoblotting; IP, immunoprecipitation; Len, lenalidomide; Pom, pomalidomide; Thal, thalidomide.
IMiDs interfere with the direct binding of THBS-1 and cereblon. (A and B) Protein expression of THBS-1 (A) and relative mRNA expression levels of THBS-1 (B) in cereblon-depleted MEG01 cells. In panel B, data are from 3 independent experiments and expressed as means ± SEM. P values were determined by 2-tailed Student t test. (C) Immunoblot analysis for THBS-1 in cereblon-depleted MEG01 cells treated with DMSO, 10 μM lenalidomide, or 10 μM pomalidomide for 24 hours. (D) Lysate mixture of MEG01 cells and 293T cells transfected with FLAG-tagged cereblon were incubated with or without 10 μM lenalidomide and subjected to immunoprecipitation with antibody against FLAG. (E) Purified recombinant His-tagged THBS-1–loaded beads were incubated with glutathione S-transferases–tagged cereblon in the presence of pomalidomide at indicated concentration. Mixture was immunoprecipitated with antibody against His. Results are representative of 3 independent experiments (panels A, C, D, and E). (F) Homologous sequences in THBS-1 and cereblon binding site of IKZF1 are aligned. The identical residues are highlighted in red. Residues of IKZF1 composing backbone of zinc-finger structure are shown in bold characters. Arrows represent indicated interacting residues between IKZF1 and cereblon in previous report. Sequence similarity with each protein was analyzed using the protein-protein BLAST program on The National Center for Biotechnology Information. CRBN, cereblon; Cont, control; GST, glutathione S-transferases; IB, immunoblotting; IP, immunoprecipitation; Len, lenalidomide; Pom, pomalidomide; Thal, thalidomide.
Dexamethasone and IMiDs synergistically increase THBS-1
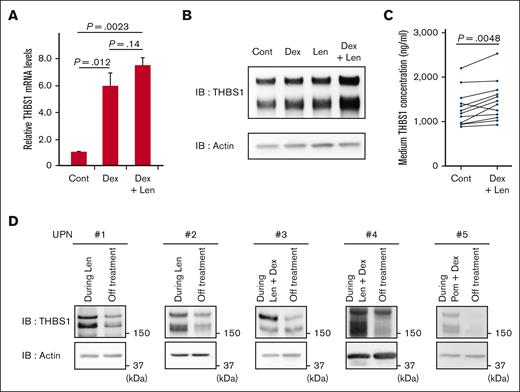
In clinical practice, it is observed that the risk of IMiDs-induced VTEs increases greatly with the combination therapy of dexamethasone and IMiDs.1,11,12,30 Therefore, we examined whether there is any effect of dexamethasone on THBS-1. Although dexamethasone showed a modest increment in mRNA levels in MEG01 cells (supplemental Figure 4), dexamethasone significantly increased the mRNA level of THBS1 in the THP-1 cells, which harbored biological features of monocytes and macrophages, consistent with previous reports.31,32 Because THBS-1 is reportedly abundant not only in platelets and megakaryocytes but also in monocytes and macrophages,33,34 we attempted to evaluate the effect of IMiDs and dexamethasone on THBS-1 using THP-1 cells. The upregulation of the mRNA level of THBS-1 was maintained when lenalidomide was added with dexamethasone simultaneously (Figure 4A). Moreover, a remarkable increase in THBS-1 was observed when THP-1 cells were treated with lenalidomide in combination with dexamethasone, compared with a single treatment with either dexamethasone or lenalidomide (Figure 4B), suggesting the synergistic augmentation of THBS-1 by the combination treatment with IMiDs and dexamethasone.
Lenalidomide and dexamethasone synergistically increase intracellular THBS-1 level. (A) Relative mRNA expression levels of THBS1 in THP1 cells. Cells were treated with DMSO, 1 μM dexamethasone with or without 10 μM lenalidomide for 24 hours. Data are from 3 independent experiments and expressed as means ± SEM. P values were determined by 2-tailed Student t test. (B) Immunoblot analysis for THBS-1 in THP1 cells treated for 24 hours with each drug. (C) THBS-1 concentration in the culture medium of PBMNCs. PBMNCs from healthy donor were cultured in RPMI-1640 for 24 hours with or without 1 μM dexamethasone and 10 μM lenalidomide, and THBS-1 concentration of medium was evaluated by ELISA assay. (D) Serial changes in THBS-1 protein level of PBMNCs from case 1 to 5 listed in supplemental Table 1. Cont, control; Dex, dexamethasone; IB, immunoblotting; Len, lenalidomide; Pom, pomalidomide; UPN, unique patient number.
Lenalidomide and dexamethasone synergistically increase intracellular THBS-1 level. (A) Relative mRNA expression levels of THBS1 in THP1 cells. Cells were treated with DMSO, 1 μM dexamethasone with or without 10 μM lenalidomide for 24 hours. Data are from 3 independent experiments and expressed as means ± SEM. P values were determined by 2-tailed Student t test. (B) Immunoblot analysis for THBS-1 in THP1 cells treated for 24 hours with each drug. (C) THBS-1 concentration in the culture medium of PBMNCs. PBMNCs from healthy donor were cultured in RPMI-1640 for 24 hours with or without 1 μM dexamethasone and 10 μM lenalidomide, and THBS-1 concentration of medium was evaluated by ELISA assay. (D) Serial changes in THBS-1 protein level of PBMNCs from case 1 to 5 listed in supplemental Table 1. Cont, control; Dex, dexamethasone; IB, immunoblotting; Len, lenalidomide; Pom, pomalidomide; UPN, unique patient number.
Several types of blood cells, such as megakaryocytes, platelets,35,36 and monocytes/macrophages33,34 express THBS-1. THBS-1 is secreted into the plasma or extracellular matrix from such cells, triggering its pleiotropic effects, including thrombus formation.18,37 Therefore, we tested whether combination therapy with IMiDs and dexamethasone could increase the extracellular level of THBS-1. As shown in Figure 4C, THBS-1 levels in the culture medium of PBMNCs from healthy donors increased with lenalidomide plus dexamethasone treatment. In clinical samples (supplemental Table 2), THBS-1 increased in PBMNCs from patients with MM undergoing IMiDs single therapy or combination therapy using IMiDs and dexamethasone, whereas THBS-1 levels decreased after the cessation of the treatment in all 5 cases examined (Figure 4D). Patient characteristics are summarized in supplemental Table 1. These results collectively suggest that dexamethasone and IMiDs synergistically increase THBS-1 levels in patients with MM during IMiDs-based treatment.
Multimerization of vWF is promoted in patients with MM during treatment including lenalidomide and dexamethasone
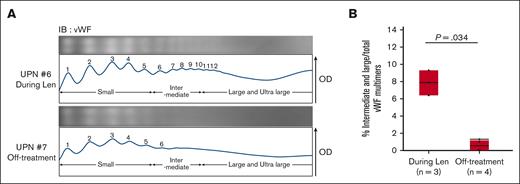
Previous studies have shown that THBS-1 inhibits the cleavage activity of a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13 (ADAMTS13) against vWF multimers and enhances the development of larger vWF multimers,19,38,39 which has an enhanced ability to promote platelet adhesion and aggregation. Therefore, we collected plasma samples from patients with MM and POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, skin changes) syndrome (n = 7) and evaluated the size of vWF multimers by electrophoresis, using SDS-agarose gel followed by immunoblotting (supplemental Figure 5), as previously reported.27,40 The vWF multimers were classified into small, intermediate, and large multimers according to their molecular weight. The proportion of intermediate and large multimers, which possess platelet adhesion and aggregation capabilities,41 was higher in the plasma samples from patients treated with lenalidomide and dexamethasone than in those not receiving these treatments (Figure 5A-B). The patient information is listed in supplemental Table 2. Moreover, we compared the activity level of ADAMTS13 between patients undergoing combination therapy of IMiDs and dexamethasone with those without combination treatment. As shown in supplemental Figure 6 and supplemental Table 3, patients receiving the combination therapy exhibited lower levels of ADAMTS13 activity than those without combination treatment. There was no change in vWF antigen level between the 2 groups. All patients were negative for ADAMTS13 inhibitor (supplemental Table 3). Previous studies have suggested that a higher level of plasma THBS-1 increases the risk of VTEs20,22 via inhibition of ADAMTS13. Collectively, our results indicate that IMiDs-induced THBS-1 accumulation can promote multimerization of vWF, resulting in an increased risk of VTE in patients with MM treated with IMiDs and dexamethasone.
Lenalidomide and dexamethasone therapy induces multimerization of vWF. (A) Representative electrophoresis (above) and densitometric analysis (below) of vWF multimers in patients undergoing treatment including lenalidomide (UPN #6) and off-treatment (UPN #7). The OD pattern was analyzed using ImageJ 1.53t. Peaks 1 through 5 represent the small multimers, and peaks 6 through 10 represent the intermediate multimers. The large and ultra-large multimers have >10 peaks. (B) Comparison of the proportion of intermediate and large multimers to total vWF multimers between patients during treatment including lenalidomide (n = 3) and off-treatment (n = 4). Each area of vWF multimer peaks in the OD analysis was measured by ImageJ 1.53t. Statistical analysis was performed by Wilcoxon 2-sample exact test. Len, lenalidomide; UPN, unique patient number.
Lenalidomide and dexamethasone therapy induces multimerization of vWF. (A) Representative electrophoresis (above) and densitometric analysis (below) of vWF multimers in patients undergoing treatment including lenalidomide (UPN #6) and off-treatment (UPN #7). The OD pattern was analyzed using ImageJ 1.53t. Peaks 1 through 5 represent the small multimers, and peaks 6 through 10 represent the intermediate multimers. The large and ultra-large multimers have >10 peaks. (B) Comparison of the proportion of intermediate and large multimers to total vWF multimers between patients during treatment including lenalidomide (n = 3) and off-treatment (n = 4). Each area of vWF multimer peaks in the OD analysis was measured by ImageJ 1.53t. Statistical analysis was performed by Wilcoxon 2-sample exact test. Len, lenalidomide; UPN, unique patient number.
Discussion
In this study, we identified THBS-1 as a novel endogenous substrate of cereblon. IMiDs alter the capacity of THBS-1 to bind to cereblon and induce THBS-1 accumulation by suppressing its proteasomal degradation in humans. Furthermore, dexamethasone, another key drug for MM treatment, synergistically potentiates THBS-1 accumulation via transcriptional enhancement of THBS-1 expression. The increase in THBS-1 levels observed in patients with MM treated with IMiDs and dexamethasone could promote the development of larger vWF multimers, which possess an enhanced ability to promote platelet adhesion and aggregation.
Previous studies have reported that vWF multimers play an important role in developing thrombosis by their enhanced ability to recruit platelets. Because vWF multimerization is regulated by a shear flow–dependent conformation change of vWF,42-44 such a pathological mechanism is important in arterial rather than venous thrombosis formation. Recent studies, however, have suggested the pathogenic importance of vWF in VTE. Some clinical studies have implied the association between vWF and VTE because a higher level of vWF is observed in the patients with VTE.45-47 In addition, the underlying mechanisms are studied in mouse models. Studies have demonstrated that platelet recruitment by vWF is important in developing venous thrombosis because venous thrombosis is inhibited by blocking the A1 domain of vWF, which is essential for promoting platelet aggregation.48,49 Although, vWF is reported to carry factor VIII, which activates the coagulation system,41 infusion of factor VIII in vWF-depleted mice did not promote venous thrombosis.49 From these previous studies, platelet aggregation by vWF is considered to play an important role in VTE, therefore, an increase in larger vWF multimers possessing an enhanced ability to recruit platelets,41 could promote VTE in addition to arterial thrombosis.
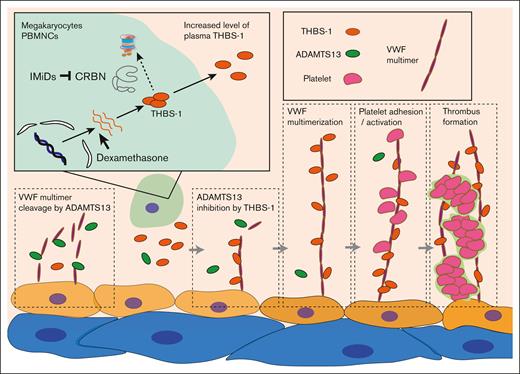
Although none of the patients analyzed in this study developed VTEs, this might be because of the recommended prophylactic use of antithrombotic drugs in clinical practice. Therefore, we were unable to validate the association between IMiDs-induced THBS-1 increment and the occurrence of VTEs. However, consistent with previous findings,20,22 our results propose a novel molecular mechanism underlying the increased risk of VTE in patients with MM treated with IMiDs and dexamethasone. The proposed model is shown in Figure 6.
Proposed molecular mechanism of IMiDs-induced VTEs. THBS-1 is an endogenous substrate of cereblon. IMiDs interfere with the binding of cereblon and THBS-1, leading to the accumulation of intracellular THBS-1. Secreted THBS-1 in plasma inhibits the cleavage activity of ADAMTS13, resulting in the multimerization of vWF. Platelet attachment to large-sized vWF multimer induces platelet activation and promotes thrombus formation during IMiDs treatment. CRBN, cereblon.
Proposed molecular mechanism of IMiDs-induced VTEs. THBS-1 is an endogenous substrate of cereblon. IMiDs interfere with the binding of cereblon and THBS-1, leading to the accumulation of intracellular THBS-1. Secreted THBS-1 in plasma inhibits the cleavage activity of ADAMTS13, resulting in the multimerization of vWF. Platelet attachment to large-sized vWF multimer induces platelet activation and promotes thrombus formation during IMiDs treatment. CRBN, cereblon.
In clinical practice, prophylaxis of VTE is recommended in patients with MM treated with IMiDs-containing regimens.50 Unlike general VTEs, aspirin is as effective as warfarin,16 implying the possibility that platelets can play additional roles in the pathogenesis of IMiDs-induced VTEs. Although there is a lack of understanding of the basic mechanisms, in clinical practice, administration of aspirin is recommended for the prevention of IMiDs-induced VTEs in patients who are not at high risk.50 Our study proposes a peculiar mechanism of IMiDs-induced VTEs whereby increment of larger vWF multimers is promoted by IMiDs-induced THBS-1 accumulation in humans. Furthermore, recent studies have demonstrated that THBS-1 contributes to platelet activation via CD36-dependent phosphodiesterase 3A activation under flow condition,51,52 suggesting its important role in flow-associated “arterial” thrombosis. Thus, our study provides a possible rationale for why IMiDs increase the incidence of venous and arterial thrombosis.14,15
Another consideration from this study is that accumulation of THBS-1 might not only be associated with VTE onset but also contribute to the antiangiogenic effects of IMiDs. It has been reported that bone marrow angiogenesis contributes to disease progression of MM and monoclonal gammopathy of undetermined significance.53 Previous reports have shown that interactions between myeloma, stromal, and endovascular cells are mediated by vascular endothelial growth factor, interleukin-6, and basic fibroblast growth factor, each of which play a role in constructing the microenvironment.54-56 In vitro and in vivo experiments have shown that thalidomide and its derivatives possess the ability to inhibit angiogenic activities.57-59 However, the IMiDs-specific molecular mechanism underlying the antiangiogenic effect remains elusive. THBS-1 has been reported to negatively control angiogenesis by inhibiting vascular endothelial growth factor signaling via CD4760 and CD36,61 and inhibiting nitric oxide–stimulated response in endothelial cells.62 Moreover, other reports suggest that THBS-1 promotes apoptosis-dependent inhibition of neovascularization in an animal model.63 These studies have shown the potent antiangiogenic effect of THBS-1, and, therefore, THBS-1 might be an endogenous substrate of cereblon, exerting anti-MM effects by inhibiting angiogenesis required for the MM-specific tumor microenvironment.
In summary, THBS-1 represents an endogenous substrate of cereblon responsible for IMiDs-induced thrombus formation, and this study provides a novel molecular mechanism for IMiDs-induced VTE in humans. Further studies are warranted to clarify the pleotropic functions of THBS-1 in thrombosis formation, as well as its antiangiogenic effect.
Acknowledgments
This study was supported, in part, by a grant-in-aid for scientific research (21407314; K.A.), grant-in-aid for scientific research (21385681; T.M.) and (22494899; Y. Kikushige), from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was also supported, in part, by a grant-in-aid from the Shinnihon Foundation of Advanced Medical Treatment Research (Y. Kikushige).
Authorship
Contribution: K.H., Y. Kikushige, T.I., H.H., and K.A. designed the research; K.H., Y. Kikushige, Y. Kunisaki, T.T., T.M., T.S., A.C., M.F., D.I., S.Y., G.K., and K.K. performed research and collected data; K.H., Y. Kikushige, and K.A. analyzed and interpreted data; and K.H, Y. Kikushige, and K.A. wrote the manuscript.
Conflict-of-interest disclosure: A.C. is an employee of Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Yoshikane Kikushige, Department of Medicine and Biosystemic Sciences, Kyushu University Graduate School of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; email: kikushige.yoshikane.726@m.kyushu-u.ac.jp.
References
Author notes
Data are available on request from the corresponding author, Yoshikane Kikushige (kikushige.yoshikane.726@m.kyushu-u.ac.jp).
The full-text version of this article contains a data supplement.