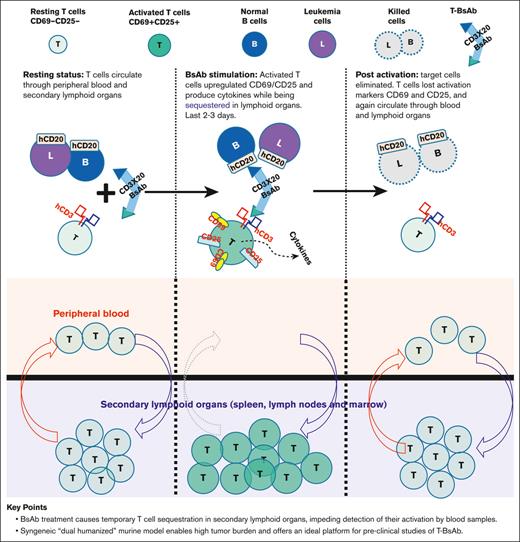
BsAb treatment causes temporary T-cell sequestration in secondary lymphoid organs, impeding detection of their activation in blood samples.
Syngeneic “dual-humanized” murine model enables high tumor burden, and offers an ideal platform for preclinical studies of T-BsAbs.
Visual Abstract
T-cell bispecific antibodies (T-BsAbs) such as blinatumomab hold great promise for cancer immunotherapy. A better understanding of the in vivo immune response induced by T-BsAbs is crucial to improving their efficacy and safety profile. However, such efforts are hindered by the limitations of current preclinical models. To address this, we developed a syngeneic murine model with humanized CD3 and target antigen (CD20). This model enables the development of disseminated leukemia with a high tumor burden, which mirrors clinical findings in human patients with relapsed/refractory acute lymphoblastic leukemia. Treatment of this model with T-BsAbs results in cytokine release syndrome, with cytokine profiles and levels reflecting observations made in human patients. This model also faithfully recapitulates the dynamics of T-cell activation seen in human patients, including the temporary disappearance of T cells from the bloodstream. During this phase, T cells are sequestered in secondary lymphoid organs and undergo activation. Clinical correlative studies that rely primarily on peripheral blood samples are likely to overlook this critical activation stage, leading to a substantial underestimation of the extent of T-cell activation. Furthermore, we demonstrate that surface expression of the T-BsAb target antigen by leukemia cells triggers a swift immune response, promoting their own rejection. Humanizing the target antigen in the recipient mice is crucial to facilitate tolerance induction and successful establishment of high tumor burden. Our findings underscore the importance of meticulously optimized syngeneic murine models for investigating T-BsAb–induced immune responses and for translational research aimed at improving efficacy and safety.
Introduction
The field of cancer therapy has experienced significant advancements through various immune-based approaches that harness both the innate and adaptive immune systems to eradicate tumor cells. T-cell bispecific antibodies (T-BsAbs) are 1 such approach. Blinatumomab, the first US Food and Drug Administration–approved T-BsAb for acute lymphoblastic leukemia, redirects T cells to kill tumor cells expressing the target antigen CD19. Numerous T-BsAbs are currently undergoing clinical trials, and some have been approved for both hematological malignancies and solid tumors, targeting antigens such as CD20, CD33, BCMA, EGFR, and EpCAM.1
In clinical trials, blinatumomab has demonstrated remarkable efficacy in patients with minimal residual disease or low-tumor-volume acute lymphoblastic leukemia (ALL).2 However, its effectiveness in high disease–burden adult ALL is modest and inferior when compared with CD19 chimeric antigen receptor (CAR) T cells.3 A similar underperformance of blinatumomab vs CAR T cells has been observed in lymphoma treatments.4-6 Common side effects of T-BsAb therapy, similar to those of CAR T cells, include cytokine release syndrome (CRS) and neurological toxicity. CRS typically occurs within hours of infusion, characterized by high fevers, hypoxemia, hypotension, vascular leak, and respiratory failure. Although the underlying mechanisms of CRS remain poorly understood, key symptoms are linked to elevated levels of various proinflammatory cytokines, including interleukin-6 (IL-6), monocyte chemoattractant protein 1 (MCP-1), tumor necrosis factor α, and interferon-γ (IFN-γ).7,8
To improve the efficacy and safety profile of T-BsAbs, it is crucial to gain a comprehensive understanding of how they stimulate T-cell immune responses in vivo, as well as the interactions between T cells and various components of the microenvironment (eg, target cells and macrophages). A significant obstacle to achieving these objectives is the lack of suitable models for in vivo studies. Immunodeficient mouse xenograft models, primate models, and correlative studies alongside human clinical trials have been used for this purpose, but all have notable limitations and fail to replicate the intricate interactions between diverse immune cell subsets and the microenvironment. Syngeneic murine models remain the primary method for investigating the T-cell immune response. Being fully immunocompetent, syngeneic models offer a native, fully matched microenvironment and secondary lymphoid organs for T-cell interactions. As the oldest and most widely used preclinical models,9,10 syngeneic models are particularly valuable for studying T-cell immunity and immunotherapy. Studies using syngeneic murine models have provided mechanistic insights into essential immunological processes, such as the roles of cytotoxic T-lymphocyte-associated protein 4 and programmed cell death protein 1 (PD-1) and have paved the way for breakthrough immunotherapy strategies.
In this study, we developed a CD3 humanized syngeneic murine model to investigate in vivo immune responses elicited by T-BsAbs designed for clinical use. We found that humanization of target antigens in this murine model is crucial for preventing target antigen–induced immune rejection, thereby enabling the development of disseminated and high tumor burden. Treatment with T-BsAbs leads to rapid and extensive T-cell activation within secondary lymphoid organs. Concurrently, these newly activated T cells temporarily vanish from the blood circulation, a phenomenon reminiscent of the transient T-cell disappearance observed in human patients receiving blinatumomab. The T-BsAbs effectively clear low-volume disease; whereas high tumor burden overwhelms the immune response and leads to significant CRS with IL-6, IFN-γ, and MCP-1 levels elevated in a pattern similar to that observed in human patients.
Material and methods
Cell lines
The E2A/PBX1 cell line was kindly provided by Terry J. Fry. It was derived from mice of C57/BL6 background and expresses CD45.2, CD19, B220, CD43, CD127, and CD93, consistent with a pre–B-ALL phenotype.11The original E2A/PBX1 cell line was transfected to express human CD19, CD20, and CD123. Stable expressing cell lines were selected by repeated fluorescence-activated cell sorting. To ensure reliable engraftment of recipient mice with predictable dynamics, each stably transfected cell line was passaged in vivo, 2 to 3 times, by engrafting the recipient mice.
Study animals
Human CD3 transgenic mice were obtained from The Jackson Laboratory (Stock no: 020456, B6.Cg-Tg [CD3E] 600Cpt/J). The mice were then crossed with wild-type C57/BL6 mice. Mice with peripheral T-cell numbers and CD4:CD8 ratios closely mimicking wild-type littermates were selected for further breeding. Mice heterozygous for the human CD3 transgene were used for the study at 12 to 16 weeks old.
The human CD20 transgenic mice on the C57/BL6 background were kindly provided by Mark Shlomchik. The mice express human CD20 on B-lineage cells and have been used to study therapeutic human CD20 antibodies such as rituximab.12 The ethical use of the mice was approved by the Ohio State University Animal Care and Use Committees.
Murine leukemia model and T-cell bispecific antibody treatment
To model human hematological malignancies, we retrovirally transfected a murine leukemia/lymphoma cell line (E2A-PBX1) with human target antigens (huCD19, huCD20, and huCD123). E2A-PBX1 is a pre–B-lymphoblastic leukemia cell line that can successfully engraft recipient mice and reliably cause disseminated disease. To induce disseminated leukemia, we engrafted recipient mice with 1 × 106 viable E2A/PBX cells expressing huCD19, huCD20, or huCD123. The recipient mice developed an aggressive, disseminated leukemia involving the bone marrow and spleen, which recapitulated human ALL. High tumor burden developed in approximately a week, and the engrafted mice began to meet the early removal criteria by ∼2 weeks after engraftment. For in vivo BsAb treatment, we intravenously injected T-BsAbs to each recipient mouse. CD3xCD20 (Xmab13676) and CD3xCD123 (XMab 14045) were kindly provided by Xencor and were given at 25 μg per mouse (approximated 1mg/kg for a mouse weighing 20-25 g). For huCD3 × CD19 BsAbs, blinatumomab was given for 2 consecutive doses at 2 μg per mouse, 12 hours apart. We measured serum cytokine levels using BD Cytometric Bead Array (Mouse Inflammation Kit, #552364).
Flow cytometry
For intracellular cytokine staining, we mixed 2 × 105 mouse T cells with E2A-PBX1 cells (expressing T-BsAb target) at a ratio of 1:3 and cocultured the cells for 6 hours with brefeldin A added for the last 3 hours. Cells were then harvested and stained with surface markers, followed by LIVE/DEAD Fixable Near-IR stain (ThermoFisher Scientific), with brefeldin A added to all staining/washing buffers. We fixed/permeabilized the cells using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer’s protocol, and then stained the cells with cytokine antibodies.
Cytotoxicity assay
Effector cells were generated by stimulating human CD3 transgenic T cells in vitro with ConA (ThermoFisher, 00-4978-03) for 2 days, and then resting the cells for 7 days. The effector cells were then labeled with CellTrace Violet (ThermoFisher Scientific, C34571). Target cells expressing human CD20 were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; C34554, ThermoFisher) and aliquoted to V-bottom 96-well plates at a density of 2 × 104 cells per well. Effector cells were added at a density of 4 × 104 cells per well to achieve an effector-to-target ratio of 2:1, and total volume were adjusted to 200 μL. The plates were centrifuged at 200g for 3 minutes and cultured at 37°C with 5% CO2. CD20 or CD123 BsAbs were added to a final concentration of 50 ng/mL. After 5 hours, cells were harvested and stained with annexin-V/propidium iodide to evaluate the viability/killing of target cells. CountBright Absolute Counting Beads (ThermoFisher Scientific) were added to estimate the absolute number of cells by flow cytometry. Target and effector cells were distinguished by prelabeled live cell dye (CFSE for target cells and CellTrace Violet for effector cells).
Results
CD3 humanized mice display comparable T-cell counts and subpopulations, resembling functionality and behavior of typical wild-type T cells
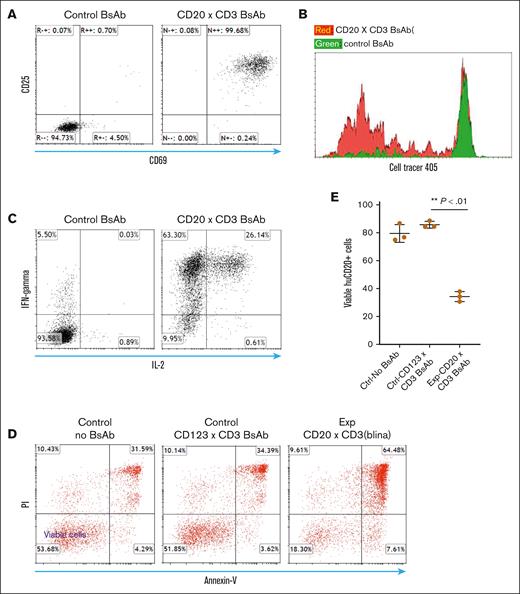
Although several human CD3 transgenic mice models are available, many of them are limited because of a skewed CD4/CD8 ratio and reduced peripheral T-cell numbers.13 We used CD3 transgenic mice generated by the Terhorst group,14,15 and crossbred them with C57/bl6 mice. We then selected those mice with good peripheral T-cell numbers and CD4/CD8 ratio that closely resemble that of wild-type littermates (supplemental Figure 1). We assessed the functionality of these human CD3e transgenic (huCD3e-Tg) T cells. We cocultured huCD3e-Tg T cells with syngeneic leukemia cells ectopically expressing target antigens (huCD20) in the presence of CD20 (CD3xCD20) bispecific antibody. As controls, we set up the same coculture system with an irrelevant T-BsAb (ie, CD3xCD123 BsAb). Our results showed that the CD20 BsAb stimulates the T cells to express activation markers such as CD25 and CD69 (Figure 1A). The CD20 T-BsAbs also induced T-cell proliferation, as assessed by CFSE dilution assay (Figure 1B). Additionally, we evaluated cytokine production by first differentiating naïve huCD3e-Tg T cells into effector cells in vitro, allowing them to rest for 1 week, and then restimulating these resting effector T cells with T-BsAb in the presence of CD20+ target cells. We found that the T-BsAbs also stimulated the production of classical T-cell cytokines such as IFN-γ in effector T cells (Figure 1C). Moreover, in the in vitro cytotoxicity assay, the resting effector T cells (huCD3e-Tg) displayed strong specific cytotoxicity against target cells in the presence of relevant T-BsAbs at a low effector-to-target ratio of 2:1 (Figure 1D-E). Thus, our study systematically demonstrated that appropriate T-BsAbs could redirect the functionalities of huCD3-Tg murine T cells in a clinically relevant setting.
Human CD3 transgenic (huCD3-Tg) T cells demonstrated appropriate functionality when stimulated with T-BsAbs and target cells expressing the corresponding human antigen. (A) Upregulation of activation markers on huCD3-Tg T cells after coculture with target cells in the presence of appropriate T-BsAbs. CD8 T cells from huCD3 transgenic mice were cocultured with huCD20-expressing E2A-PBX1 leukemia cells (B6 syngeneic) along with human CD3 X CD20 T-BsAb or CD3xCD123 T-BsAb (control) for 24 hours. Expression levels of CD69 and CD25 were measured by Flow cytometry. (B) huCD3-Tg T cells proliferate after coculture with target cells in the presence of appropriate T-BsAbs. T cells from huCD3 transgenic mice were labeled with CellTrace Violet and cocultured with huCD20-expressing E2A-PBX1 leukemia cells along with human CD3 X CD20 T-BsAb or CD3xCD123 T-BsAb (control) for 4 days. Cell proliferation was measured by the dilution of CellTrace dye. (C) Production of cytokines by effector huCD3-Tg T cells after being stimulated with appropriate T-BsAb and target cells. huCD3-Tg T cells were differentiated in vitro by stimulation with concanavalin A and then rested for 7 days. The resting effector T cells were then restimulated with CD3xCD20 T-BsAb or control T-BsAb in the presence of huCD20-expressing E2A-PBX1 cells for 5 hours. Intracellular cytokine staining was used to measure the production of cytokines. (D-E) Effector huCD3-Tg T cells demonstrated cytotoxicity against target antigen–expressing cells in the presence of appropriate T-BsAb. Effector T cells, prepared in the same way as in panel C and prelabeled with CellTrace Violet, were cocultured with CFSE-labeled E2A-PBX1-huCD20 cells. The effector-to-target ratio was adjusted to 2:1, and the mixed cells were incubated in triplicate in a 96-well plate with either CD20 T-BsAb or CD123 T-BsAb (as a control). After a 5-hour incubation, cells were stained with annexin V and propidium iodide (PI). The survival of E2A-PBX1-huCD20 target cells was then assessed. (D) Shows representative fluorescence-activated cell sorting (FACS) plots. (E) Displays a scatter plot with mean and standard deviation of calculated cytotoxicity. Data shown are representative of 3 independent experiments.
Human CD3 transgenic (huCD3-Tg) T cells demonstrated appropriate functionality when stimulated with T-BsAbs and target cells expressing the corresponding human antigen. (A) Upregulation of activation markers on huCD3-Tg T cells after coculture with target cells in the presence of appropriate T-BsAbs. CD8 T cells from huCD3 transgenic mice were cocultured with huCD20-expressing E2A-PBX1 leukemia cells (B6 syngeneic) along with human CD3 X CD20 T-BsAb or CD3xCD123 T-BsAb (control) for 24 hours. Expression levels of CD69 and CD25 were measured by Flow cytometry. (B) huCD3-Tg T cells proliferate after coculture with target cells in the presence of appropriate T-BsAbs. T cells from huCD3 transgenic mice were labeled with CellTrace Violet and cocultured with huCD20-expressing E2A-PBX1 leukemia cells along with human CD3 X CD20 T-BsAb or CD3xCD123 T-BsAb (control) for 4 days. Cell proliferation was measured by the dilution of CellTrace dye. (C) Production of cytokines by effector huCD3-Tg T cells after being stimulated with appropriate T-BsAb and target cells. huCD3-Tg T cells were differentiated in vitro by stimulation with concanavalin A and then rested for 7 days. The resting effector T cells were then restimulated with CD3xCD20 T-BsAb or control T-BsAb in the presence of huCD20-expressing E2A-PBX1 cells for 5 hours. Intracellular cytokine staining was used to measure the production of cytokines. (D-E) Effector huCD3-Tg T cells demonstrated cytotoxicity against target antigen–expressing cells in the presence of appropriate T-BsAb. Effector T cells, prepared in the same way as in panel C and prelabeled with CellTrace Violet, were cocultured with CFSE-labeled E2A-PBX1-huCD20 cells. The effector-to-target ratio was adjusted to 2:1, and the mixed cells were incubated in triplicate in a 96-well plate with either CD20 T-BsAb or CD123 T-BsAb (as a control). After a 5-hour incubation, cells were stained with annexin V and propidium iodide (PI). The survival of E2A-PBX1-huCD20 target cells was then assessed. (D) Shows representative fluorescence-activated cell sorting (FACS) plots. (E) Displays a scatter plot with mean and standard deviation of calculated cytotoxicity. Data shown are representative of 3 independent experiments.
Murine model of disseminated hematological malignancy
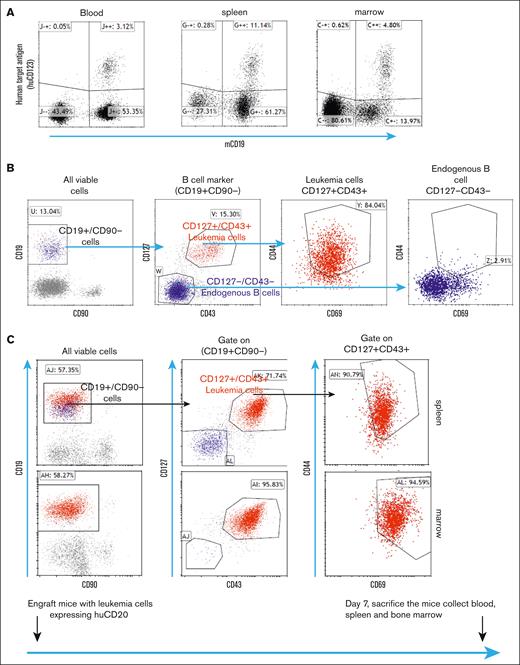
As depicted in Figure 2A, we engrafted human antigen–expressing E2A-PBX1 leukemia cells into recipient mice. One week after engraftment, these leukemia cells could be detected in the blood, bone marrow, and spleen resembling human disseminated leukemia.16 E2A-PBX1 cells express CD127 and CD43, which distinguish them from mature B cells.16,17However, these markers can also be expressed by normal pro–B cells in the bone marrow. To further differentiate leukemia cells from normal B-lineage cells, we found that the human antigen–expressing E2A cell lines also had increased levels of CD69 and CD44 (Figure 2B). Using these markers, we were able to distinguish leukemia cells from normal cell populations in the spleen and bone marrow, as well as circulating leukemia cells in the blood (Figure 2C).
After engraftment into recipient mice, the E2A-PBX1 cell line expressing human T-BsAb targets leads to disseminated leukemia that involves the bone marrow and spleen with circulating leukemia cells. (A) After engraftment of E2A-PBX1 cells expressing huCD123 into recipient mice, huCD123+/mCD19+ leukemia cells can be detected in the blood, spleen, and bone marrow 7 days later. (B) Leukemia cells expressing human antigen can be distinguished from normal B-cell lineage subsets by a combination of surface markers (CD127, CD43, CD69, and CD44). (C) Detection of CD127+/CD43+/CD69+/CD44hi leukemia cells in the bone marrow and spleen.
After engraftment into recipient mice, the E2A-PBX1 cell line expressing human T-BsAb targets leads to disseminated leukemia that involves the bone marrow and spleen with circulating leukemia cells. (A) After engraftment of E2A-PBX1 cells expressing huCD123 into recipient mice, huCD123+/mCD19+ leukemia cells can be detected in the blood, spleen, and bone marrow 7 days later. (B) Leukemia cells expressing human antigen can be distinguished from normal B-cell lineage subsets by a combination of surface markers (CD127, CD43, CD69, and CD44). (C) Detection of CD127+/CD43+/CD69+/CD44hi leukemia cells in the bone marrow and spleen.
T-BsAb therapy eliminates leukemia cells expressing human target antigen in engrafted huCD3 transgenic mice
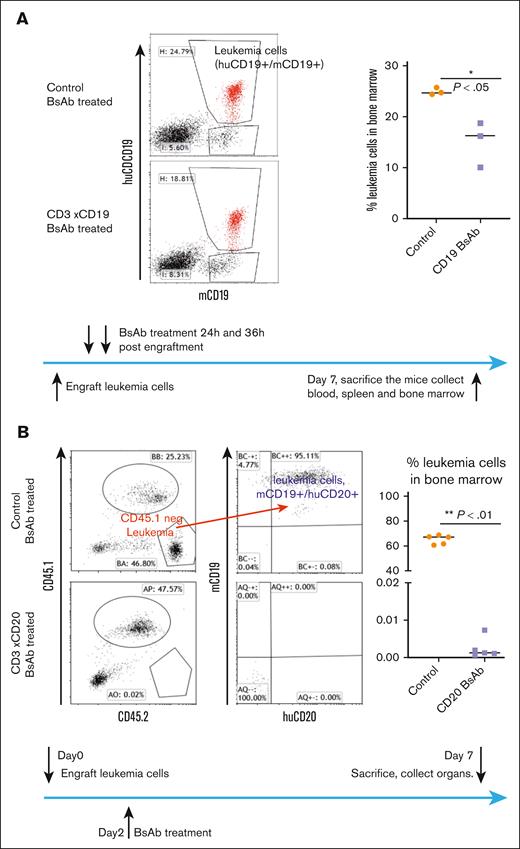
Next, we evaluated T-BsAb treatment in the E2A leukemia-engrafted huCD3-Tg mouse model. First, we engrafted huCD3-Tg mice with E2A leukemia cells expressing human target antigens (huCD19, huCD20, and huCD123). Engrafted mice were treated with specific T-BsAbs or irrelevant control T-BsAbs. On day 7, we euthanized the mice and evaluated the tumor burden. Initially, we tested blinatumomab (a CD3xCD19 BsAb) in huCD19+ leukemia-engrafted mice. We found that although the blinatumomab treatment group could not completely eradicate the leukemia cells, they did show a significant reduction in bone marrow tumor burden compared with that in the control group (Figure 3A). Blinatumomab has a short half-life of only ∼2 hours in mice in vivo.18 Because continuous infusion (as in human patients) is not feasible, 2 doses of IV injection only maintained therapeutic levels of T-BsAbs for several hours. Thus, its somewhat limited therapeutic efficacy is likely because of inferior pharmacokinetics.
T-BsAb treatment demonstrated therapeutic efficacy against leukemia cells expressing the corresponding human antigens in huCD3-Tg mice. (A) huCD3-Tg mice were engrafted with huCD19+ E2A-PBX1 cells. On day 2, mice were injected twice with blinatumomab. Leukemia cell burden in the bone marrow were evaluated on day 7. Leukemia cells were identified as cells double positive for both CD19 and huCD19. Left: representative FACS plot. Right: scatter plot showing individual data points and the median. (B) huCD3/20 dual transgenic mice (bred to express CD45.1) were engrafted with huCD20 expressing E2A-PBX1 cells. Two days later, mice were injected with CD20 X CD3 T-BsAb and were then euthanized at day 7. Leukemia cell burden in the bone marrow was evaluated. Leukemia cells were identified based on negative staining for congenic marker CD45.1 and for being double positive for both CD19 and huCD20. Left: representative FACS plot. Right: scatter plot showing individual data points and the median.
T-BsAb treatment demonstrated therapeutic efficacy against leukemia cells expressing the corresponding human antigens in huCD3-Tg mice. (A) huCD3-Tg mice were engrafted with huCD19+ E2A-PBX1 cells. On day 2, mice were injected twice with blinatumomab. Leukemia cell burden in the bone marrow were evaluated on day 7. Leukemia cells were identified as cells double positive for both CD19 and huCD19. Left: representative FACS plot. Right: scatter plot showing individual data points and the median. (B) huCD3/20 dual transgenic mice (bred to express CD45.1) were engrafted with huCD20 expressing E2A-PBX1 cells. Two days later, mice were injected with CD20 X CD3 T-BsAb and were then euthanized at day 7. Leukemia cell burden in the bone marrow was evaluated. Leukemia cells were identified based on negative staining for congenic marker CD45.1 and for being double positive for both CD19 and huCD20. Left: representative FACS plot. Right: scatter plot showing individual data points and the median.
In collaboration with Xencor Inc, we repeated the experiments outlined in Figure 3B and supplemental Figure 2 using their half-life–extended BsAbs (∼1 week in murine models19,20), Xmab 13676 (targeting huCD20), and Xmab 14045 (targeting huCD123). E2A leukemia cells expressing human CD20 or CD123 were used to engraft recipient mice. To faithfully model the clinical context of B-cell malignancies, in which antigens like CD20 are present on both malignant and normal B-cell lineages, we tested the CD20 BsAb using huCD3/20 dual transgenic mice (Figure 3B). These mice demonstrated appropriate B-cell lineage–specific expression of huCD20,12 making them an ideal model for studying CD20-targeted therapies. Our results demonstrate that both CD20 and CD123 BsAbs efficiently eradicated engrafted leukemia cells bearing their respective target antigens, as shown in Figures 3B and supplemental Figure 2.
Human antigen expression in leukemia cells triggers immune response, leading to reduced levels and rejection over time
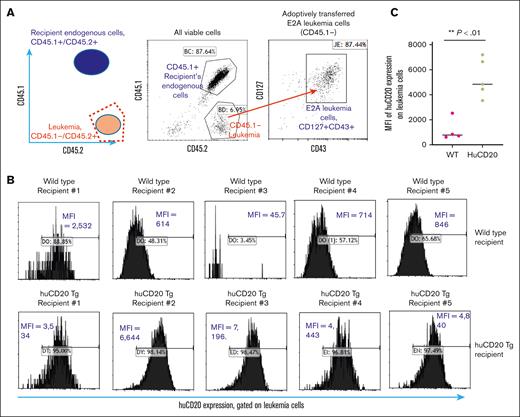
While working with this model, we observed that the ectopic expression of human CD20 by E2A leukemia cells diminished over time after engraftment in huCD3e-Tg mice (Figure 4B, upper panel). This phenomenon was observed for E2A-PBX1 leukemia cells expressing other human antigens such as CD123 (supplemental Figure 3). The reduction in human antigen level became apparent after 1 week after engraftment, which is in line with the induction of an adaptive immune response. This result suggests an immune response against the specific human antigen, which will be perceived as foreign in immune-competent mice. To further characterize the reduced human antigen expression, we monitored the change in huCD20 expression level in E2A-huCD20 leukemia cells after engraftment in huCD20-Tg recipients vs in wild-type recipients. The huCD20-Tg mice, obtained from Mark Shlomchik, express human CD20 on B-lineage cells.12 We hypothesized that huCD20-Tg mice would recognize human CD20 as self and not develop an immune response against it. The huCD20-Tg mice were crossed to CD45.1 congenic mice, allowing for easy discrimination of the engrafted leukemia cells from recipient endogenous cells (Figure 4A). We observed that 8 days after engraftment, the huCD20 expression level was significantly reduced in E2A leukemia cells after being transferred into wild-type recipients but not after being transferred into huCD20-Tg recipients (Figure 4B-C). As shown in supplemental Figure 4, we also observed that the leukemia engraftment level (as measured by the percentage of CD45.2/45.2 homozygous, CD127+/CD43+ leukemia cells) tended to be lower in wild-type recipients (average 3.1% circulating leukemia cells) compared with huCD20-Tg recipients (average 8.6% circulating leukemia cells). The expression levels of other surface markers, such as CD127 and CD43, by engrafted E2A leukemia cells were not found to be different between huCD20-Tg recipients and wild-type recipients (supplemental Figure 5). This result further suggests that the immune reaction is specific for the ectopically expressed human cell surface antigen CD20.
After engraftment into wild-type B6 recipient mice, the expression of huCD20 by leukemia cells was diminished over time, whereas engraftment into huCD20 transgenic recipient mice did not affect the leukemia engraftment nor expression of huCD20. (A) Schema of how to distinguish engrafted E2A-PBX1 leukemia cells (CD45.2 homozygous) from recipient endogenous cells (CD45.1/CD45.2 heterozygous) using congenic markers (B) Human CD20-expressing E2A-PBX1 leukemia cells were engrafted into wild-type and huCD20 transgenic recipient mice. After 8 days, peripheral blood samples were collected, and the expression of huCD20 was measured in leukemia cells using flow cytometry. We observed a reduction in huCD20 expression on leukemia cells in the wild-type recipients (upper panel), whereas no change was seen in the huCD20 transgenic mice (lower panel). (C) The statistical analysis of panel B showed that the downregulation of huCD20 expression level is statistically significant in wild-type mice compared with that in huCD20-Tg mice.
After engraftment into wild-type B6 recipient mice, the expression of huCD20 by leukemia cells was diminished over time, whereas engraftment into huCD20 transgenic recipient mice did not affect the leukemia engraftment nor expression of huCD20. (A) Schema of how to distinguish engrafted E2A-PBX1 leukemia cells (CD45.2 homozygous) from recipient endogenous cells (CD45.1/CD45.2 heterozygous) using congenic markers (B) Human CD20-expressing E2A-PBX1 leukemia cells were engrafted into wild-type and huCD20 transgenic recipient mice. After 8 days, peripheral blood samples were collected, and the expression of huCD20 was measured in leukemia cells using flow cytometry. We observed a reduction in huCD20 expression on leukemia cells in the wild-type recipients (upper panel), whereas no change was seen in the huCD20 transgenic mice (lower panel). (C) The statistical analysis of panel B showed that the downregulation of huCD20 expression level is statistically significant in wild-type mice compared with that in huCD20-Tg mice.
T-BsAbs causes a temporary sequestration of lymphocytes from circulation whereas activation of T cells takes place in secondary lymphoid organs
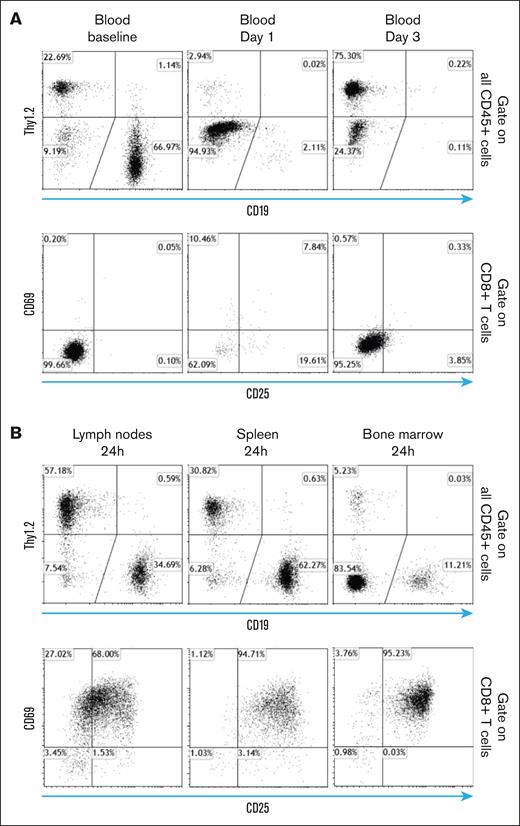
An interesting observation made during clinical trials with T-BsAbs targeting B-cell malignancies was the rapid disappearance of both T and B cells from the blood within hours. T cells reappeared in circulation in 2 to 3 days, and their numbers returned to baseline in ∼1 week.21 This finding has been interpreted as a result of the redistribution of T cells during activation, but no experimental evidence had been provided to support this claim. This important phenomenon was recapitulated in our CD3 and target antigen double-humanized model. As shown in Figure 5A (middle upper panel), T and B cells disappeared from the peripheral blood 24 hours after T-BsAb treatment. T cells, but not B cells reappeared in the blood by day 3 after BsAb treatment (Figure 5A, left panel). We also conducted similar experiments using both single transgenic and wild-type mice (data not shown). These mice did not exhibit T-cell sequestration from circulating blood. Such findings underscores that this T-cell sequestration phenomenon is specifically caused by the interaction between T cells and target cells induced by the BsAb.
Temporary sequestration of T cells in secondary lymphoid organs in which they undergo activation during the priming phase of T-BsAb treatment. (A) huCD3/20 double transgenic mice received IV injection of CD3 × CD20 T-BsAb. At the indicated time points after injection, blood samples were obtained. Percentage of B cells, T cells, and the expression of T-cell activation markers were analyzed by flow cytometry. Upper panel: gate on all CD45+ cells. Lower panel: gate on CD8+ T cells. (B) Mice and treatment were the same as described for panel A. Mice were euthanized 24 hours after treatment, and the lymph nodes, spleen, and bone marrow were removed. Percentage of B cells, T cells, and the expression of T-cell activation markers were analyzed by flow cytometry. Upper panel: gate on all CD45+ cells. Lower panel: gate on CD8+ T cells.
Temporary sequestration of T cells in secondary lymphoid organs in which they undergo activation during the priming phase of T-BsAb treatment. (A) huCD3/20 double transgenic mice received IV injection of CD3 × CD20 T-BsAb. At the indicated time points after injection, blood samples were obtained. Percentage of B cells, T cells, and the expression of T-cell activation markers were analyzed by flow cytometry. Upper panel: gate on all CD45+ cells. Lower panel: gate on CD8+ T cells. (B) Mice and treatment were the same as described for panel A. Mice were euthanized 24 hours after treatment, and the lymph nodes, spleen, and bone marrow were removed. Percentage of B cells, T cells, and the expression of T-cell activation markers were analyzed by flow cytometry. Upper panel: gate on all CD45+ cells. Lower panel: gate on CD8+ T cells.
We then studied the percentage and activation status of T cells in secondary lymphoid organs during the period when T cells disappeared from the circulating blood, ∼24 hours after T-BsAb treatment. As shown in Figure 5B (upper panel), the percentage of T and B cells in these organs were consistent with the expected values from normal wild-type mice, and the majority of these T cells expressed activation markers such as CD69/CD25 (Figure 5B, lower panel). These findings provided convincing experimental evidence that, after B-cell–targeting BsAb treatment, the temporary disappearance of T cells from the peripheral blood is indeed because of their redistribution to secondary lymphoid organs, in which they experience near-uniform activation. By the time these newly activated T cells reentered blood circulation ∼3 days afterward, their expression of acute activation markers such as CD69 and CD25 were already diminished. In contrast, B cells, which were targeted and eliminated by T cells redirected by CD20 BsAb, did not return to the blood circulation. This phenomenon likely explains why clinical correlative studies, primarily relying on blood samples, tend to markedly underestimate the true extent of T-cell activation.
The CD3/Target antigen double-humanized model allows for development of high tumor burden, for which T-BsAb treatment induces a CRS mirroring observations in human patients
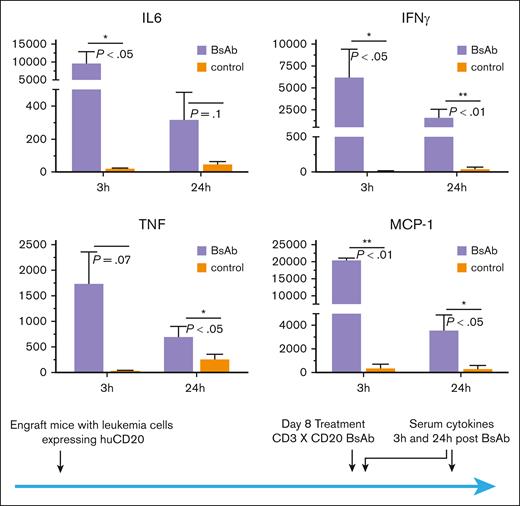
As mentioned earlier, humanization of the target antigen avoids T-BsAb–independent rejection of engrafted tumor cells, thus enabling the development of a high tumor burden that recapitulates human relapsed/refractory leukemia. Therefore, we engrafted CD3/CD20 double-humanized mice with human CD20-expressing E2A leukemia cells and allowed 8 days for the leukemia to grow into a high tumor burden before treating it with CD20 BsAbs. Evaluation of serum cytokines from these mice revealed significantly elevated levels of IL-6, MCP-1, IFN-γ, and tumor necrosis factor α (Figure 6), 3 hours after BsAb treatment. At 24 hours after treatment, serum cytokine levels decreased but remained elevated above baseline in mice with a high tumor burden. The patterns of the cytokine response in these mice recapitulated those seen in human patients treated with T-BsAbs such as blinatumomab, and the levels are similar to those observed in patients who suffered CRS.7,21,22
Elevated levels of inflammatory cytokines were detected after T-BsAb treatment in huCD3/CD20 double transgenic mice with a high tumor burden. Human CD3/CD20 double transgenic mice were engrafted with huCD20-expressing E2A-PBX1 leukemia cells. T-BsAbs (CD3 × CD20) were administered 8 days after leukemia engraftment to allow for growth into a high tumor burden. Serum was obtained at 3 hours and 24 hours after T-BsAb treatment, and cytokine levels were analyzed by the BD Cytometric Bead Array assay, using the Mouse Inflammation Kit.
Elevated levels of inflammatory cytokines were detected after T-BsAb treatment in huCD3/CD20 double transgenic mice with a high tumor burden. Human CD3/CD20 double transgenic mice were engrafted with huCD20-expressing E2A-PBX1 leukemia cells. T-BsAbs (CD3 × CD20) were administered 8 days after leukemia engraftment to allow for growth into a high tumor burden. Serum was obtained at 3 hours and 24 hours after T-BsAb treatment, and cytokine levels were analyzed by the BD Cytometric Bead Array assay, using the Mouse Inflammation Kit.
Discussion
T-BsAbs have emerged as a promising tool in cancer immunotherapy, stepping into the limelight and gaining attention as the next big thing after CAR T cells.23 T-BsAbs activate T cells with unique features, which, in turn, are associated with key clinical issues related to T-BsAb therapy. For example, patients with a high tumor burden, and thus elevated antigen levels, may experience rapid, high-affinity activation of numerous T cells, likely contributing to the development of CRS.
The primary objectives in this field are to enhance clinical efficacy and reduce toxicities, which necessitate a deeper understanding of the T-BsAb–induced immune response in vivo. A reliable animal model that accurately reflects the main characteristics of such immune response is crucial for acquiring further mechanistic insights and evaluating new treatment strategies. To this end, we have developed a humanized CD3 murine model expressing human CD3 on endogenous T cells and human target antigens (CD20 or CD19) on syngeneic tumor cells as well as endogenous B-lineage cells. This immunocompetent model provides a fully matched, native microenvironment and secondary lymphoid systems for T-cell interaction. By cohumanizing target antigens expressed by endogenous cell subsets, we can avoid BsAb-independent rejection of target antigen–expressing cells and facilitate the development of disseminated leukemia that mimics disease patterns and target antigen distributions observed in human acute leukemias. Moreover, our model reproduces the dynamic changes in the immune system resulting from T-BsAb treatment, including the development of CRS with serum cytokine levels and patterns closely resembling those observed in human patients with refractory/relapsed ALL.
Several groups have used humanized CD3 murine models to examine the effectiveness of T-BsAbs. However, a significant limitation of these studies is the lack of a suitable tumor model that can replicate disseminated leukemias, which are the most common clinical indication for T-BsAb treatment. Some research used localized tumors induced by subcutaneous injection,24-26 whereas other studies did not use a tumor model and instead had target antigens transgenically expressed by normal cell populations (eg, huCD20 expressed by B cells, or CLL-1 expressed by myeloid cells).27,28 As a result, the tumor/antigen burden in such models is relatively small and/or confined, whereas the majority of human leukemia/lymphoma cases present with high tumor burdens and widespread/systemic involvement. Treating such diseases with B-cell–targeting T-BsAbs leads to immediate, nearly uniform activation of almost all T cells, which is vital in the pathophysiology of CRS and contributes to treatment failure.29-31 In addition, the pattern of antigen distribution (ie, localized vs systemic) also affects the quality of the T-cell response. Systemic exposure to antigens tends to cause immune suppression, and antigen presentation by circulating leukemia cells and/or nonactivated B cells often induces T-cell tolerance.32-34 Therefore, the translational value and clinical relevance of data derived from models with localized, low tumor burden might be limited for leukemia/lymphoma treatment. Nonetheless, syngeneic models with disseminated high tumor/target antigen burden have not been previously reported before.
In this study, we observed a rapid immune response against humanized target antigens (eg, huCD20) expressed on the surface of syngeneic leukemia cells, which led to diminished expression and impaired engraftment of the leukemia cells, preventing the development of a high tumor burden (Figure 4; supplemental Figures 3 and 4). It is well established that ectopic expression of foreign antigens, such as fluorescent proteins, can trigger immune responses against them.35-37 Despite this, fluorescent proteins and luciferase have been extensively used to label syngeneic leukemia/lymphoma cells, successfully engrafting immune-competent recipient mice to establish systemic disease.38-40 However, the immune response against huCD20 observed in this study significantly compromised leukemia engraftment. We believe that surface-expressed foreign antigens tend to be more immunogenic than intracellular antigens, particularly when expressed by circulating blood cells. It has been reported that cell surface antigens, such as the human nerve growth factor receptor, are more immunogenic and lead to an accelerated and more comprehensive rejection of T cells expressing human nerve growth factor receptor in comparison to those expressing green fluorescent protein.37 Our findings align with these observations, emphasizing the importance of avoiding non-BsAb–mediated immune rejection of leukemia cells provoked by surface-expressed foreign antigens. Consequently, we modified our model system to include humanized versions of both CD3 and the target antigen (CD20). We demonstrated that cohumanizing the recipient mice with the target antigen (CD20) effectively prevented the development of anti-huCD20 immunity. This approach is essential for optimal leukemia cell engraftment and for achieving a high tumor/antigen burden in similar syngeneic mouse models.
An intriguing clinical observation with blinatumomab therapy is the temporary disappearance of T cells from circulation. This has been previously attributed to the redistribution of T cells into secondary lymphoid organs, as T cells typically reappear in the blood within several days.21 This phenomenon has been observed in classical antigen-driven immune responses;41,42 however, an overall decrease in blood T-cell numbers is not observed because only a small fraction of antigen-specific T cells is involved. In the case of T-BsAb therapy, all CD3-expressing T cells can be activated, regardless of antigen specificity, resulting in a much more extensive sequestration scale.
Many critical events, such as immune synapse formation, activation marker upregulation, and acquisition of effector functions occur within secondary lymphoid organs during this sequestration period. Consequently, key phenotypic and functional features of newly activated T cells cannot be observed by solely examining circulating T cells in blood samples. For instance, the extent of BsAb–induced T-cell activation is likely greatly underestimated in blinatumomab clinical trials. The percentage of CD69+ CD8 T cells in the peripheral blood was found to increase to an average of only 49% after blinatumomab treatment from a baseline of 20%.21 Our study provided evidence that B-cell targeting T-BsAb treatment induces extensive T-cell activation with nearly uniform upregulation of CD69. However, this occurs when T cells are sequestered in secondary lymphoid organs and would be overlooked if only peripheral blood samples were studied. Furthermore, the blockade of checkpoint molecules such as cytotoxic T lymphocyte–associated protein 4 and programmed cell death protein 1 has been studied in clinical trials to enhance blinatumomab's efficacy and has shown great promise, with preliminary data boasting a doubling of complete remission rates from ∼40% to 80% in patients with ALL.43,44 However, significant upregulation of checkpoint molecules on T cells has never been reported in clinical correlative studies of blinatumomab nor other T-BsAbs. This is again likely because of the redistribution of exhausted T cells to secondary lymphoid organs and tumor sites, as seen in the case of upregulated programmed cell death protein 1 expression in tumor-infiltrating but not circulating T cells in patients with solid tumors.45 Thus, our findings emphasize the importance of mechanistic studies using animal models, which can provide easy access to organ samples, and are invaluable for this type of research.
Acknowledgments
The huCD20 transgenic mice were kindly provided by Mark Shlomchik at the University of Pittsburg. The E2A-PBX1 leukemia cell line were provided by Terry J. Fry at the University of Colorado. The CD3xCD20 and CD3xCD123 BsAbs were provided by Xencor Inc.
This work was supported by the American Cancer Society through a mentored research scholar grant MRSG-19-033-01 (M.L.) and the American Society of Hematology scholar award (M.L.).
Authorship
Contribution: M.L., N.M., and J.B. conceived and designed the experiments; L.W., M.L., and V.L. performed experiments and prepared figures and the manuscript; and M.L., N.M., and J.B. analyzed the data and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Meixiao Long, The Ohio State University Comprehensive Cancer Center, 400 West 12 Ave, Columbus, OH 43220; email: Meixiao.long@osumc.edu.
References
Author notes
Data and renewable materials will be available upon request from the corresponding author, Meixiao Long (meixiao.long@osumc.edu).
The full-text version of this article contains a data supplement.